Abstract
Many preclinical studies examined cue-induced relapse to heroin and cocaine seeking in animal models, but most of these studies examined only one drug at a time. In human addicts, however, polydrug use of cocaine and heroin is common. We used a polydrug self-administration relapse model in rats to determine similarities and differences in brain areas activated during cue-induced reinstatement of heroin and cocaine seeking. We trained rats to lever press for cocaine (1.0 mg/kg/infusion, 3-h/d, 18 d) or heroin (0.03 mg/kg/infusion) on alternating days (9 d for each drug); drug infusions were paired with either intermittent or continuous light cue. Next, the rats underwent extinction training followed by tests for cue-induced reinstatement where they were exposed to either heroin- or cocaine-associated cues. We observed cue-selective reinstatement of drug seeking: the heroin cue selectively reinstated heroin seeking and the cocaine cue selectively reinstated cocaine seeking. We used Fos immunohistochemistry to assess cue-induced neuronal activation in different subregions of the medial prefrontal cortex (mPFC), dorsal striatum (DS), nucleus accumbens (NAc), and amygdala. Fos expression results indicated that only the prelimbic cortex (PL) was activated by both heroin and cocaine cues; in contrast, no significant cue-induced neuronal activation was observed in other brain areas. RNA in situ hybridization indicated that the proportion of glutamatergic and GABAergic markers in PL Fos-expressing cells were similar for the heroin and cocaine cue-activated neurons. Overall the results indicate that PL may be a common brain area involved in both heroin and cocaine seeking during polydrug use.
Keywords: Prefrontal cortex, Fos, drug self-administration, extinction, reinstatement, neuronal ensembles, polydrug, opioids, psychostimulants
Graphical Abstract
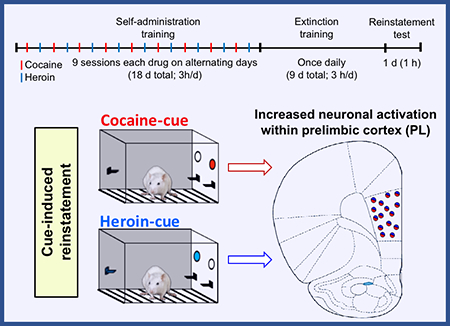
We used a polydrug self-administration model to study heroin and cocaine cue-induced reinstatement and observed drug cue-selective responding. Re-exposure to the cocaine-cue or heroin-cue induced Fos expression in a mixed population of glutamatergic and GABAergic neurons specifically in the PL area, with no differences in other brain areas investigated. Activation of PL may be a common substrate for cocaine and heroin cue-induced relapse in polydrug users.
Introduction
Many human addicts use both heroin and cocaine and are thus called polydrug users (Leri et al., 2003; John et al., 2018). Between 30 and 80% of heroin addicts, including those treated with methadone, also use cocaine (Kosten et al., 1986; Levin et al., 1996; Leri et al., 2003; Dobler-Mikola et al., 2005), and up to half of intravenous cocaine addicts also use heroin (Lauzon et al., 1994). Learned associations play an important role in addiction to both heroin and cocaine. With repeated experiences, addicts learn to associate drug taking with a variety of drug-associated stimuli, and over time these stimuli (cues) can induce craving and contribute to drug relapse (O’Brien et al., 1992). Drug relapse can be triggered by discrete cues (tools used to take drugs) and contextual cues (people, places and settings during drug taking) that were associated with drug taking (O’Brien et al., 1992; Yu et al., 2007; Crombag et al., 2008; De Pirro et al., 2018). Discrete cues directly related to drug taking (needles, powder) can promote both heroin and cocaine relapse in polydrug abusers (Childress et al., 1988; O’Brien et al., 1990; Carter & Tiffany, 1999; McHugh et al., 2014), but it is unknown whether similar or different neural substrates control cue-induced heroin versus cocaine seeking in polydrug abusers.
The behavioral and neurobiological mechanisms underlying cue- and context-induced relapse of heroin and cocaine seeking have been examined in both primate and rodent models using the extinction-reinstatement model (Shaham et al., 2003; Feltenstein & See, 2008; Venniro et al., 2016). In this model, animals are first trained to perform a response (usually lever pressing) to obtain drug, and then the drug is removed to extinguish the drug-reinforced responding. On test day, reinstatement of lever pressing is assessed in response to drug-associated contextual (environment) or discrete cues (lights or tone cues). In rats, pharmacological inactivation experiments and studies using the neural activity marker Fos indicate that medial prefrontal cortex (mPFC), dorsal and ventral striatum, as well as amygdala and ventral subiculum regions are involved in cue-induced reinstatement of heroin and cocaine seeking (See, 2005; Bossert et al., 2013). Some of these brain areas are common for both cue-induced heroin and cocaine seeking, including dorsal mPFC, nucleus accumbens (NAc) core, central and basolateral amygdala nuclei (CeA and BLA, respectively), and dorsal striatum (DS) (Kruzich & See, 2001; Fuchs & See, 2002; McLaughlin & See, 2003; Bossert et al., 2007; Di Ciano et al., 2008; Rogers et al., 2008). However, in each of these studies, the rats were trained to self-administer either heroin or cocaine separately, but not with both drugs.
Here, we used a polydrug (heroin/cocaine) self-administration procedure based on previous studies where rats were trained to self-administer heroin and cocaine in different sessions using different levers and different drug-associated cues (Leri & Stewart, 2001; Leri et al., 2004; Caprioli et al., 2008; Caprioli et al., 2009; Lenoir et al., 2012). Leri and Stewart demonstrated that after extinction of heroin and cocaine seeking, cocaine and heroin priming injections selectively reinstate responding on the lever associated with each drug (Leri & Stewart, 2001; Leri et al., 2004). Badiani and colleagues used their polydrug self-administration procedure to demonstrate that the drug environment plays a critical role in modulating: (1) the reinforcing effects of both drugs, (2) the choice between heroin and cocaine, and (3) cocaine and heroin priming-induced reinstatement of drug seeking (Caprioli et al., 2008; Caprioli et al., 2009; Badiani et al., 2011; Badiani, 2013; Montanari et al., 2015). We first determined whether cues paired selectively with either cocaine or heroin in rats trained to self-administer both drugs will reinstate drug-selective responses. Second, since the brain sites involved in reinstatement of polydrug use were not investigated in the above studies, we used the neural activity marker Fos (Morgan & Curran, 1991; Cruz et al., 2013; Cruz et al., 2015) to assess whether exposure to heroin- or cocaine-paired cues during the reinstatement test activate similar or different brain regions. Third, we assessed the cellular phenotypes of activated neurons in prelimbic (PL) and infralimbic (IL) cortex by co-labeling Fos mRNA with glutamate and GABA cell-type mRNAs.
Materials and methods
Many features of our methods, including apparatus, surgery, behavioral training, and cell labeling techniques are similar to those described in our previous studies (Bossert et al., 2011; Cruz et al., 2014; Caprioli et al., 2017).
Subjects
We used 27 male Long-Evans rats from Charles River Laboratories that weighed 300–350 g at the time of surgery. Rats were housed individually before and after surgery under a reverse 12 h light/dark cycle (lights off at 8:00 A.M.). Throughout the experiment, water was freely available in their home cages, but food was restricted to 20 g of Purina rat chow after each daily operant session. All procedures followed the guidelines outlined in the Guide for the care and use of laboratory animals (Ed 8; http://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf). We used 8 rats for RNAscope in situ hybridization and 19 rats for Fos immunohistochemistry.
Intravenous surgeries
We anesthetized the rats with isoflurane gas (5% for induction; 2–3% for maintenance). We attached silastic catheters to a modified 22-gauge cannula cemented to a polypropylene mesh (Small Parts), then inserted the catheter into the jugular vein and fixed the mesh to the mid-scapular region as described previously (Bossert et al., 2016; Adhikary et al., 2017; Caprioli et al., 2017; Venniro et al., 2017b). After surgery, we injected the rats with 2.5 mg/kg of ketoprofen (Butler Schein) to relieve pain and decrease inflammation. We allowed the rats 10–11 days to recover before cocaine and heroin self-administration training. During the recovery and training, we flushed the catheters every day with 4.25 mg/ml of gentamicin (APP Pharmaceuticals) dissolved in sterile saline.
Apparatus
We trained and tested the rats in standard Med Associates self-administration chambers located inside sound-attenuating cabinets. Each chamber had a red house-light and three levers: two retractable active levers on the right side and a nonretractable inactive lever on the left side located 9 cm above the grid floor. Lever presses on the active retractable levers activated the infusion pump and a white cue light above the lever, whereas lever presses on the inactive nonretractable lever had no programmed consequences.
Drugs
Stock solutions of cocaine-hydrochloride and heroin-hydrochloride in sterile saline were obtained from NIDA Pharmacy. (-)-Cocaine-HCl was diluted in sterile saline for a final dose of 1.0 mg/kg/infusion. Diamorphine (heroin)-HCl was diluted in sterile saline for a final dose of 0.03mg/kg/infusion.
Behavioral procedures
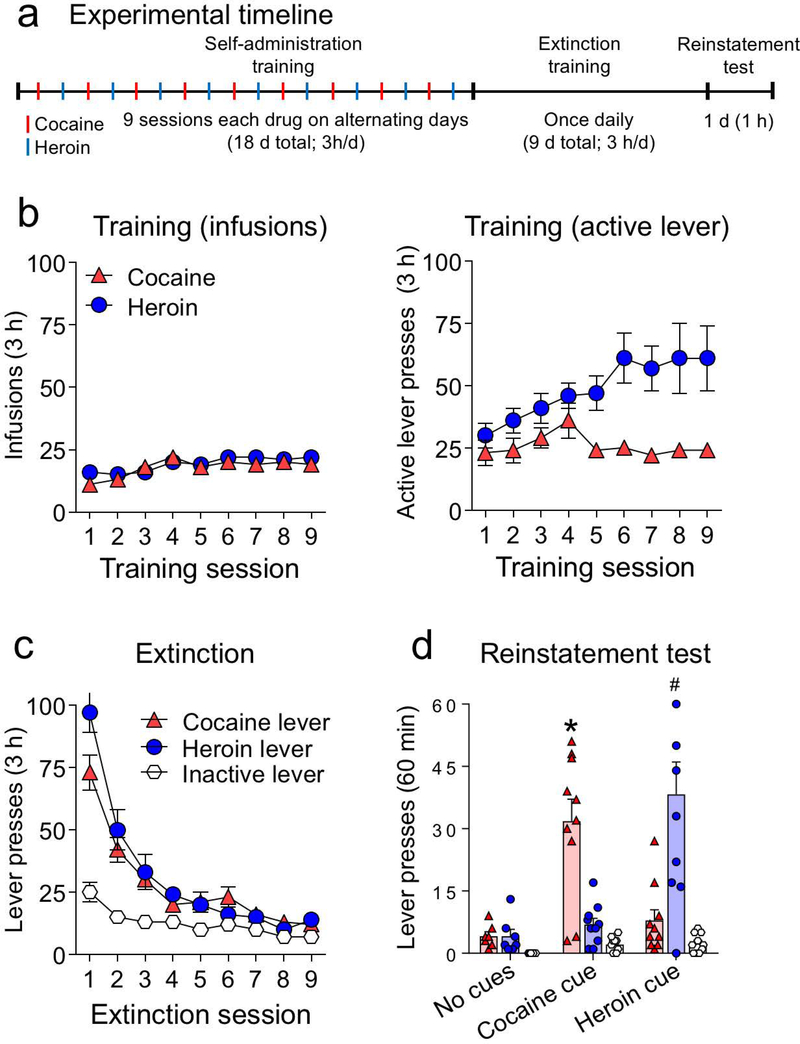
The experiment consisted of three phases: (1) self-administration training (3-h/day for 18 d; 9 d for each drug), (2) extinction training (3-h/day for 9 d), and (3) test for cue-induced reinstatement of cocaine- or heroin-seeking (60 min). The experimental timeline is shown in Fig. 1a.
Figure 1.
Cue-induced reinstatement of cocaine and heroin seeking (n = 27). (a) Experimental timeline for all rats. (b) Cocaine and heroin self-administration data during training. Left: number of drug infusions; right: number of active lever presses (mean ± SEM) for each drug over 9 alternating sessions. (c) Extinction data: number of nonreinforced active and inactive lever presses (mean ± SEM) over 9 daily sessions. (d) Reinstatement test: total number of active lever presses (mean ± SEM) for cocaine and heroin over the 60 min test (*p< 0.05 relative to the cocaine lever in the other groups, #p< 0.05 relative to the heroin lever in the other groups, n = 7–10 per group).
Phase 1: Cocaine and heroin self-administration training
We trained the 27 rats to self-administer cocaine or heroin on alternating days for a total of 9 days per drug. The sessions began with the extension of only one of the active levers and the illumination of the red house light, which remained on for the duration of the 3-h session. Active lever presses resulted in a 0.1 ml infusion of either cocaine (1.0 mg/kg/infusion) or heroin (0.03 mg/kg/infusion) and 3.5 s of either a continuous or intermittent white cue light located above the active lever (the doses were chosen based on preliminary experiments shown in Supplemental Fig. 1). The maximum number of infusions was limited to 60 per session. For cocaine, only two rats reached maximum infusions: on days 1 and 4 after 71 min and 171 min, respectively. For heroin, only one rat reached maximum infusions: on day 6 after 178 min. On these sessions, the rats remained in the chamber for full 180 min but the levers were retracted after the last 60th infusion. We trained the rats using a fixed-ratio-1 (FR-1) with a 20-s timeout reinforcement schedule in which rats did not receive a drug infusion after the active lever press. Inactive lever presses had no programmed consequences. The total number of infusions, cue presentations, active and inactive lever presses for all 18 self-administration sessions are summarized in Table S1 for each experimental group (No cues, Cocaine cue, and Heroin cue). The drugs, drug-paired active levers and cue lights were counterbalanced across groups. No rats were excluded from the experiment.
Phase 2: Extinction phase
All rats underwent 9 d of operant extinction training in the absence of the drugs and their drug-paired cues. During this phase, the nonretractable inactive lever and the two retractable active levers (previously paired with heroin and cocaine) were present, but active lever pressing led to no drug delivery nor cue light presentation. The house-light remained on for the duration of the 3-h sessions.
Phase 3: Cue-induced reinstatement test
Twenty-four hours after the final extinction session, we separated the rats into two groups to test for cue-induced reinstatement of either cocaine (n = 10) or heroin seeking (n = 10) under extinction conditions for 60 min. Test sessions for the heroin and cocaine groups started with 3.5 s of either heroin- or cocaine-cue presentation followed by extension of the two drug-associated active levers. Rats could press either heroin or cocaine levers in the same session, operationally defined as active lever presses under extinction conditions, but only one cue light (heroin- or cocaine-paired cue) was presented immediately after each lever press. The control no-test group (n = 7) underwent an extinction session as described for the extinction phase, but for only 60 min. Rats in the control and test groups were matched for their cocaine and heroin intake and number of active lever presses during the last 3 d of training and extinction phases, respectively. The inactive lever was always present during the test.
Fos immunohistochemistry assay
For Fos immunohistochemistry, 19 rats were perfused 90 min after the start of the testing session (Cruz et al., 2014). Rats were deeply anesthetized with isoflurane and perfused with 100 ml 0.1 M phosphate buffered saline (PBS) followed by 400 ml of 4% paraformaldehyde in PBS. The brains were post-fixed in paraformaldehyde for 90 min and transferred to 30% sucrose in PBS solution at 4 ºC for 2–3 d until they sank. Brains were frozen in powdered dry ice and kept at -80 ºC. Forty micron-thick coronal sections were cut in a cryostat between bregma levels +4.20 mm and +2.76 mm for cingulate 1 cortex (Cg1) and PL regions, +3.72 mm and +3.00 mm for IL region, +2.28 mm and +0.36 mm for dorsal striatum (DS) and NAc regions, and −1.92 and −2.76 for CeA and BLA (Paxinos & Watson, 2005). Free-floating sections were washed three times in PBS, blocked with 3% normal goat serum (NGS) in PBS containing 0.25% Triton X-100 (PBS-Tx) for 1 h, and incubated overnight at 4 ºC with anti-Fos antibody (1:8000 dilution; catalog #5348, Cell Signaling) in the same blocking solution. Sections were washed again with PBS and incubated for 2 h in biotinylated goat anti-rabbit secondary antibody (1:600 dilution; Vector Laboratories) in PBS-Tx and 1% NGS. After washing in PBS, sections were incubated for 1 h in avidin-biotin-peroxidase complex (ABC Elite kit; catalog #PK-6100, Vector Laboratories) in PBS containing 0.5% Triton X-100. Finally, sections were washed in PBS and developed in 3,3’-diaminobenzidine in the presence of hydrogen peroxide for approximately 2–3 min. Sections were mounted onto chromalum/gelatin-coated slides and dehydrated through a series of alcohol (30%, 60%, 90%, 95%, 100%, 100% ethanol) and cleared with Citrisolv (Fisher Scientific) before coverslipping with Permount (Fisher Scientific).
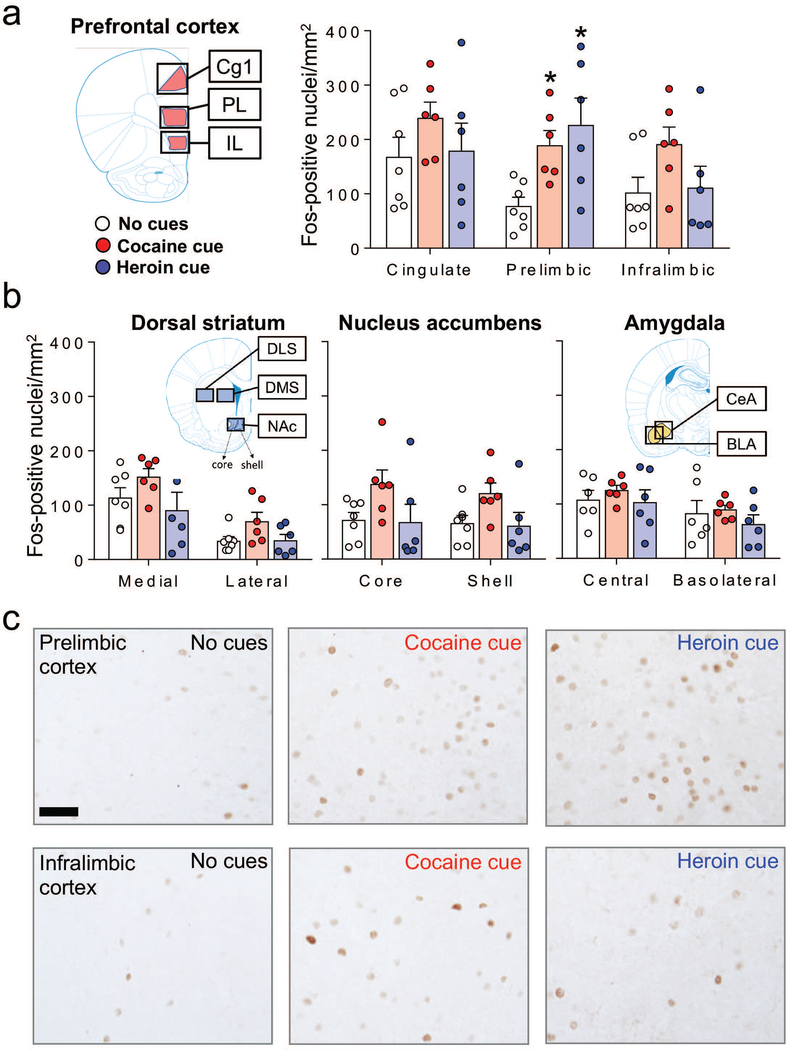
All images were digitally captured at 100X magnification using a digital camera attached to a Zeiss Axioskop 2 microscope (Carl Zeiss Microscopy). Bright-field images of Fos-immunopositive nuclei in the PL, IL, DS (medial and lateral), NAc (shell and core) were digitally captured using an Exi Aqua camera (QImaging) and iVision software for Macintosh, version 4.0.15 (Biovision). The microscope camera was updated after the analysis of the brain areas above. Therefore, images from Cg1 and amygdala regions (CeA and BLA) were digitally captured using a Dhyana 400DC camera and Mosaic software for Windows, version 1.4 (Tucsen). All sections for a given brain area were captured with the same equipment and software. The areas of the captured images for PL, IL, and each of the DS and NAc subregions were 1.060 mm2 (1.191 mm × 0.890 mm) using the Exi Aqua camera (QImaging) and 1.698 mm2 (1.304 mm × 1.302 mm) using the Dhyana 400DC camera for Cg1, CeA and BLA subregions. The location of the quantified areas within these images were selected based on the rat brain atlas (Paxinos & Watson, 2005) and indicated by overlays on brain coronal sections schematics in Figure 2a and 2b. The quantified areas were: dorsomedial striatum (DMS) and dorsolateral striatum (DLS), 1.06 mm2; PL, 0.796–1.06 mm2; IL, 0.647–1.06 mm2; NAc core, 0.480–0.654 mm2; NAc shell, 0.303–1.06 mm2; Cg1, 1.226–1.570 mm2; CeA, 0.174–1.127 mm2; BLA, 0.082–1.096 mm2. Two observers blind to the test conditions used automated counting software (Fiji) to count Fos-positive nuclei. A nuclei was considered Fos-positive when its intensity value was at least 122 on a 0–255 grayscale. As in our previous studies, we chose this threshold to count the most strongly labeled nuclei. We calculated Fos-positive nuclei per mm2 for each brain area by dividing the number of Fos-positive nuclei within the chosen area by the area in mm2, which accounts for different sizes of the quantified areas in different sections. The values of Fos-positive nuclei per mm2 were obtained from 2–3 sections (4–6 hemisphere images) per rat and averaged for a single n value per brain area for each rat.
Figure 2.
Cue-induced reinstatement of cocaine or heroin seeking is associated with Fos induction in the PL, but not in the other prefrontal cortical areas (Cg1 and IL), and not in the striatum or amygdala. (a) Number of Fos-positive nuclei/mm2 (mean ± SEM) in mPFC (Cg1, PL and IL subregions), (b) dorsal striatum (medial and lateral subregions), nucleus accumbens (core and shell subregions), and amygdala (CeA and BLA subregions) for the no cues (n = 6–7), cocaine cue (n = 6), and heroin cue (n = 6) groups. *p< 0.05 relative to the no cues group. Images for each brain region were captured from the areas indicated by the outer black boxes on the coronal section schematics. The specific sampling areas used for quantifying Fos-positive nuclei are indicated by the colored overlays. (c) Representative images of Fos-positive nuclei in PL and IL cortex. Scale bar is 50 μm.
RNAscope in situ hybridization (ISH) assay
For RNAscope ISH, 8 of the rats (that were trained and extinguished, above) were decapitated 60 min after cue-induced reinstatement with the heroin cue (n=4) or the cocaine cue (n=4). We used procedures similar to those previously described for RNAscope ISH (Wang et al., 2012; Rubio et al., 2015). The brains were snap frozen for 20 s in -50 ºC isopentane within 2–3 min of decapitation, wrapped in labeled alumina foil and stored at -80 ºC. Brains were equilibrated in a Cryostat (CM 3050S) to -20 ºC for 2 h, and 16 μm coronal sections were cut and thaw-mounted directly onto Super Frost Plus slides (Fisher). These slides were left at -20 ºC for 10 min and transferred to -80 ºC until ISH processing.
RNA ISH for Fos, Vglut1 (vesicular glutamate transporter 1), and Vgat (vesicular GABA transporter) mRNAs was performed manually according to the User Manual for Fresh Frozen Tissue using RNAscope Multiplex Fluorescence Reagent Kit (Advanced Cell Diagnostics). Briefly, the -80 ºC brain slides were transferred to slide racks and fixed by immersion in 10% neutral buffered formalin (Fisher Scientific) for 20 min at 4 ºC. Slides were rinsed two times in PBS and dehydrated two times each in 50% and 70% ethanol and twice in 100% ethanol. Slides were transferred to a new 100% ethanol container and kept at -20 ºC overnight. The slides were dried out at room temperature (22 ºC) for 10 min and a hydrophobic pen (ImmEdger Hydrophobic Barrier Pen, Vector Laboratories) was used to make a physical barrier surrounding the brain sections to incubate with the different RNAscope probes and reagents. We used the HybEZ Hybridization System from Advanced Cell Diagnostics. The protease solution (Pretreatment 4 solution) was incubated with sections at room temperature for 20 min. After washing off the protease solution, 1X target probes for specific RNAs (Fos, Vglut1, and/or Vgat) were applied to the brain sections and incubated at 40 ºC for 2 h in the HybEZ oven. Each RNAscope target probe contained a mixture of 20 ZZ oligonucleotide probes that bound to the target mRNA. These probes were as follows: Fos-C2 probe (Cat. Number 403591-C2); Vglut1-C1 probe (Slc17a7 gene, a marker of pyramidal glutamatergic projection neurons, Cat. Number 317001); and Vgat-C3 (Slc32a1 gene, a marker of GABAergic interneurons, Cat. Number 424541-C3).
Sections were then incubated with preamplifier and amplifier reagents by applying AMP1 (40 ºC for 30 min), AMP2 (40 ºC for 15 min), and AMP3 (40 ºC for 30 min). Sections were then incubated with the fluorescently labeled probes by selecting a specific combination of colors associated with each channel; green (Alexa 488 nm), orange (Atto 550 nm), and far-red (Atto 647 nm). We used AMP4 AltC to detect triplex Fos, Vglut1, and Vgat mRNAs in far-red, orange, and green, respectively. Finally, we incubated the sections for 20 s with DAPI to stain nuclei (blue). Between each step, we washed two times with 1X wash buffer supplied with the kit.
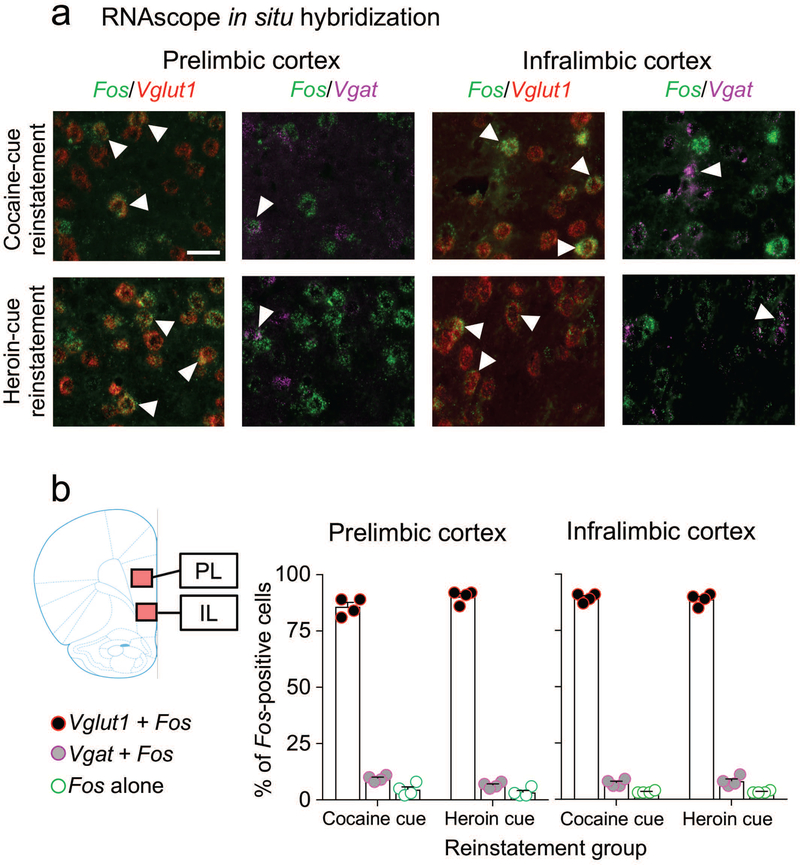
All images were digitally captured at 200X magnification using Rolera EM-C2 (QImaging) camera attached to a Nikon Eclipse E800 microscope and saved as a TIFF file for further quantification using ImageJ software. Sampled regions were chosen between bregma level +4.20 mm and +2.76 mm for PL and between +3.72 mm and +3.00 mm for IL (Paxinos & Watson, 2005). The areas of the captured images for PL and IL were 0.265 mm2 (0.595 mm × 0.445 mm) and indicated by overlays on brain coronal section schematics (Paxinos & Watson, 2005) in Figure 3a. We quantified labeling within the entire imaged areas indicated. We manually selected the Fos-positive cells based on a minimum number of positive pixels, as described previously (Rubio et al., 2015). We calculated the percentage of Fos-positive nuclei that were also co-labeled with Vglut1 or Vgat, or neither of them. Two to three sections (4–6 hemisphere images) per rat were counted by two observers blind to the test conditions and averaged for a single n value per brain area for each rat.
Figure 3.
Cellular phenotypes of Fos-positive neurons in mPFC after cue-induced reinstatement of cocaine or heroin seeking. (a) Triplex detection of Fos (green), Vglut1 (red), and Vgat (purple) mRNAs in PL and IL cortex after cue-induced reinstatement of cocaine or heroin seeking. Representative images of selected cells (Fos-positive, white arrows). Images on the left (Vglut1 + Fos) for each subregion show merged channels for Fos (green) and Vglut1 (red) signals. Images on the right (Vgat + Fos) for each subregion show the merged channels for Fos (green) and Vgat (purple) from the same brain sections and fields than the left side. (b) Graphs indicating the percentage of Fos-positive neurons (n = 8 rats, 4 per cue condition) that coexpressed Vglut1 (Vglut1 + Fos) or Vgat (Vgat + Fos) mRNA in the PL or IL subregions of the mPFC. A small number of Fos-positive neurons did not coexpress either Vglut1 or Vgat mRNA (Fos alone). Images of PL and IL were captured from the areas indicated in the coronal section schematic drawing. Fos-positive nuclei were quantified from the entire captured image indicated by the colored overlays. Scale bar is 30 μm.
Statistical analyses
The behavioral data were analyzed using multivariate ANOVAs (see Results for the description of the between- and within-subjects factors). We analyzed the data separately for the different phases (training, extinction, and reinstatement test) using SPSS v23 (GLM procedure) or GraphPad Prism v7 software. For Fos immunohistochemical data, we used a univariate ANOVA for each brain region (dorsolateral (DLS) and dorsomedial (DMS) striatum, NAc core and shell, Cg1, PL, IL, CeA, and BLA) with the between-subjects factor of Cue (no cues, cocaine cue, and heroin cue). We followed up on significant main effects and interaction effects (P < 0.05) using Fisher’s PLSD post-hoc test.
Results
Self-administration training and extinction phases
During the training phase, the rats demonstrated reliable cocaine and heroin self-administration, as indicated by the similar amount of infusions for the drugs (F1,26 = 0.736, p = 0.399), resulting in similar number of contingent drug cue-light presentations (Fig. 1b, left). A differential increase in active lever presses for heroin but not cocaine was seen after the fourth session (Fig. 1b, right). [Note: The results from preliminary experiments that tested different heroin and cocaine doses on numbers of infusions and cue-light presentations are shown in Supplemental Fig. 1.]
During the extinction phase, non-reinforced responding on the previously active cocaine- and heroin-paired levers was similar across sessions (Fig. 1c and Table 1). Repeated-measures ANOVA, which included the within-subjects factors of Levers (cocaine lever, heroin lever, and inactive lever) and Session, showed a significant interaction between Lever and Session (F16,416 = 15.99, p < 0.001). All rats reached the criterion for extinction of less than 15 lever presses per day on either the cocaine or heroin lever during the last 3 extinction sessions.
Table 1.
Statistical analysis for behavioral data (SPSS GLM). Partial Eta2 = proportion of explained variance.
| Figure/Phase | Factor name | F-value | p-value | Partial Eta2 |
|---|---|---|---|---|
| Figure 1b/Self-administration (SA) Training: Infusions | 1. Session within-subjects 2. Cue/drug within-subjects 3. Session × Cue/drug |
F8,208=8.99 F1,26=0.736 F8,208=1.62 |
p<0.001* p=0.399 p=0.122 |
0.257 0.028 0.059 |
| Figure 1b/SA Training: Active levers & inactive lever | 4. Session within-subjects 5. Lever within-subjects 6. Session × Lever |
F8,208=1.22 F3,78=16.44 F24,624=1.51 |
p=0.288 p<0.001* p=0.058 |
0.045 0.387 0.055 |
| Figure 1c/Extinction: Active levers & inactive lever | 7. Session within-subjects 8. Lever within-subjects 9. Session × Lever |
F8,208=71.12 F2,52=18.67 F16,416=15.99 |
p<0.001* p<0.001* p<0.001* |
0.732 0.418 0.381 |
| Figure 1d/Test | 10. Cue between subjects 11. Lever within-subjects 12. Cue × Lever |
F2,24=7.37 F2,48=16.26 F4,48=19.72 |
p=0.003* p<0.001* p<0.001* |
0.380 0.404 0.622 |
Cue-induced reinstatement
Exposure to either cocaine- or heroin-paired cues induced reinstatement on the cocaine- or heroin-associated lever. This selective cue-induced reinstatement was seen in the cocaine cue and heroin cue groups, but not in the no cues (extinction) group (Fig. 1d). Statistical analysis indicated a main effect of the between-subjects factor of Cue (no cues, cocaine cue, or heroin cue; F2,24 = 7.37, p = 0.003), a main effect of Lever (Actives, Inactive; F2,48 = 16.26, p< 0.001), and a significant interaction between the two factors (F4,48 = 19.72, p< 0.001). See also Table 1.
Fos expression during cue-induced reinstatement
We used Fos-immunohistochemistry to assess the number of activated neurons in different subregions within the mPFC (Cg1, PL and IL cortex) (Fig. 2a), DS (DMS, DLS), and NAc (core, shell), and Amygdala (CeA and BLA) (Fig. 2b). We found that exposure to the heroin or cocaine cues caused a significant increase in Fos expression in the PL but not the other brain regions (Fig. 2). The one-way ANOVA of Fos-IR neurons per mm2 showed a significant effect of Cue condition (heroin cue, cocaine cue, no cue) for the PL (F2,16=6.03, p=0.011) but not for the other brain regions (p values >0.05, see Table 2). Post-hoc Fisher PLSD showed that the number of Fos-IR neurons was higher in the heroin and cocaine cue groups than in the no-cue group (p<0.05).
Table 2.
Statistical analysis for Fos quantification (SPSS GLM univariate module). Partial Eta2 = proportion of explained variance
| Figure 2 | Factor name | F-value | p-value | Partial Eta2 | No cues vs. Cocaine cue | No cues vs. Heroin cue | Cocaine cue vs. Heroin cue |
|---|---|---|---|---|---|---|---|
| Figure 2b. Left & Center Fos quantification (one-way ANOVA of Group: No cues, Cocaine cue, Heroin cue) | DLS DMS NAc core NAc shell |
F2,16=2.94 F2,16=1.76 F2,16=2.51 F2,16=2.98 |
p=0.082 p=0.204 p=0.113 p=0.080 |
0.268 0.180 0.239 0.271 |
0.045 0.247 0.072 0.054 |
0.957 0.480 0.905 0.863 |
0.058 0.082 0.066 0.045* |
| Figure 2a. Fos quantification | PL IL Cg1 |
F2,16=6.03 F2,16=2.18 F2,16=0.94 |
p=0.011* p=0.145 p=0.410 |
0.430 0.214 0.106 |
0.025* 0.071 0.212 |
0.005* 0.849 0.842 |
0.437 0.113 0.306 |
| Figure 2b, right. Fos quantification | CeA BLA |
F2,15=0.43 F2,15=0.66 |
p=0.660 p=0.529 |
0.054 0.081 |
0.646 0.777 |
0.656 0.424 |
0.370 0.284 |
Phenotyping of Fos-expressing neurons in the PL and IL cortex after Heroin or Cocaine cue-induced reinstatement
We used RNAscope ISH for triple-labeling of Fos mRNA with glutamatergic and GABAergic cell type markers Vglut1 and Vgat mRNAs in the PL and IL in rats that reinstated lever pressing after cocaine (n = 4) or heroin (n = 4) cue presentation. Representative images for both subregions are shown in Fig. 3a. In the PL, most of the Fos-expressing neurons in the cocaine-cue group (86±2%) and heroin-cue group (90±1%) co-expressed the vesicular glutamate transporter 1 (Vglut1), while 9±1% in the cocaine cue group and 7±1% in the heroin cue group co-expressed the vesicular gamma-aminobutyric acid transporter (Vgat) (Fig. 3b, left). Only a minority of the Fos-positive cells in the cocaine cue (5±1%) and heroin cue (3±1%) groups did not co-express either Vglut1 or Vgat mRNAs (Fig. 3b, left). Similar results were seen in the IL cortex, where most of the Fos-expressing neurons in the cocaine cue (90±1%) and heroin cue (89±1%) groups co-expressed Vglut1, while 7±1% in the cocaine cue and 8±1% in the heroin cue groups co-expressed the GABAergic marker Vgat (Fig. 3b, right). The other Fos-positive cells in the cocaine cue (3%) and heroin cue (3%) groups did not co-express Vglut1 or Vgat mRNAs (Fig. 3b, right).
Overall, we found that cue-induced reinstatement of cocaine or heroin seeking activated PL neurons that are either glutamatergic or GABAergic. The distribution of phenotypes remained similar for the Fos-expressing neurons in the IL cortex, although the total number of Fos-expressing neurons were lower than in the PL (Fig. 2a).
Discussion
We used a polydrug self-administration model to study cue-induced reinstatement of heroin and cocaine seeking in rats that self-administered both drugs on alternate days. The discrete cue paired with heroin reinstated lever pressing on the heroin lever, but not on the cocaine lever. The discrete cue paired with cocaine reinstated lever pressing on the cocaine lever, but not in the heroin lever. Cue-induced reinstatement of heroin or cocaine seeking was associated with selective activation of the PL area of the mPFC, but not Cg1 or IL region, and none of the other areas under study (NAc core and shell, DLS, DMS, CeA and BLA). While exposure to the cocaine cue was associated with overall higher levels of Fos expression in most of the other brain areas, these differences were not statistically significant. Furthermore, cocaine- and heroin-paired cues induced Fos expression in similar proportions of glutamatergic and GABAergic neurons in PL. Thus, neuronal activity in the PL could be a common neural substrate mediating cue-induced relapse to heroin and cocaine seeking in polydrug users.
Relapse to cocaine and heroin seeking after polydrug self-administration
Previous studies using a polydrug self-administration model focused on drug priming-induced reinstatement in rats with a history of polydrug cocaine and heroin self-administration (Leri & Stewart, 2001; Leri et al., 2004; Montanari et al., 2015). Leri and Stewart (2001) reported that cocaine priming-induced reinstatement increased pressing on the cocaine-associated lever, but not the heroin-associated lever; in contrast, heroin priming-induced reinstatement increased pressing on the heroin-associated lever, but not on the cocaine lever. In a subsequent study, Leri et al. (2004) reported that chronic delivery (via Alzet minipumps) of the preferential mu opioid receptor agonist methadone during the extinction and reinstatement phases decreased both heroin priming-induced reinstatement of heroin seeking and cocaine priming induced-reinstatement of cocaine seeking. In contrast, chronic methadone exposure had no effect on stress-induced reinstatement, a manipulation that induced reinstatement on both the heroin- and cocaine-paired levers. More recently, Montanari et al. (2015) reported that cocaine priming reinstated cocaine seeking only when cocaine was previously self-administered outside the rat homecage environment but not in the homecage environment, while an opposite environmental context-modulated effect was observed for heroin priming. Based on these studies, these authors suggest that drug priming-induced reinstatement is mediated by learned associations between drug-induced interoceptive cues and drug-specific levers and contexts, and that they are likely mediated by neural substrates that are at least partly distinct for each drug (Badiani et al., 2011). Previous studies have also shown reliable context- and cue-induced reinstatement in rats trained to self-administer a mixture of heroin and cocaine (speedball) (Highfield et al., 2001; Crombag & Shaham, 2002). However, cue-induced reinstatement drug seeking was not assessed for cues associated separately with cocaine versus heroin, as in the present study.
Cue-induced reinstatement of heroin and cocaine seeking: similarities and differences in neural substrates
We found that discrete cues associated with each drug also become associated with drug-specific lever responding. The specificity of these cue-specific learned associations suggest that different neural substrates could be involved for each drug. However, our study and the previous literature indicate that there are both similar and different substrates for cue-induced cocaine and heroin seeking. To simplify the discussion of previous studies that used different terminologies for subregions of the PFC, we equate ventral mPFC with IL cortex, dorsal mPFC with PL cortex, while the cingulate cortex (Cg1) is considered a separate subregion from the dorsal mPFC. Here, we examined Fos expression induced only by cue-induced reinstatement; we did not compare our results with studies that examined Fos expression induced by other pharmacological (Zavala et al., 2007) or behavioral (Ciccocioppo et al., 2001) processes.
In the mPFC of rats trained to self-administer only cocaine, cue-induced reinstatement of cocaine seeking increased the number of Fos expressing neurons in PL (Kufahl et al., 2009; McGlinchey et al., 2016; James et al., 2018), IL (Kufahl et al., 2009), orbitofrontal and anterior cingulate cortices (Kufahl et al., 2009) and whole mPFC (Leong et al., 2017). These data are supported by inactivation experiments that demonstrate causal roles for PL (McLaughlin & See, 2003; McGlinchey et al., 2016; James et al., 2018), IL (Pockros et al., 2011) but see (McLaughlin & See, 2003), orbitofrontal cortex (Fuchs et al., 2004b), and anterior cingulate cortex (McLaughlin & See, 2003) in cue-induced reinstatement of cocaine seeking. In the mPFC of rats trained to self-administer only heroin, cue-induced heroin seeking increased the number of Fos-expressing neurons in whole mPFC (Koya et al., 2006) and orbitofrontal cortex (Koya et al., 2006; Fanous et al., 2012; Fanous et al., 2013). These data are supported by inactivation experiments that demonstrate causal roles for PL (Rogers et al., 2008), IL (Rogers et al., 2008), and orbitofrontal cortex (Fanous et al., 2012) in cue-induced reinstatement of heroin seeking. Together, these data indicate that similar mPFC neural substrates mediate cue-induced reinstatement of heroin and cocaine seeking when the drugs are assessed separately.
In mPFC of rats trained to self-administer both drugs in our study, cue-induced reinstatement was associated with increased numbers of Fos-expressing neurons only in the PL, but not Cg1 or IL. These data agree with that for cue-induced reinstatement of cocaine seeking (Kufahl et al., 2009; McGlinchey et al., 2016; James et al., 2018) and possibly for cue-induced reinstatement of heroin seeking within the mPFC (Koya et al., 2006). As mentioned above, inactivation experiments demonstrate a role for PL in cue-induced reinstatement of cocaine seeking (Kufahl et al., 2009; McGlinchey et al., 2016; James et al., 2018) as well as cue-induced reinstatement of heroin seeking (Rogers et al., 2008). However, for Fos expression in the IL and Cg1 cortices, cue-induced reinstatement of cocaine or heroin seeking did not increase Fos expression when assessed in rats trained with both drugs in our study.
Taken together, PL (but not IL or Cg1) is similarly activated during cue-induced reinstatement of drug seeking in rats trained with both heroin and cocaine, which is different from results that assessed in rats trained only with cocaine (Kufahl et al., 2009). The experimental conditions in the Kufahl study (2009) were similar to our study, with the exception that they increased the response requirements from an FR1 to FR11 reinforcement schedule during the latter part of their training phase. The intermittent reinforcement shcedule (FR11) likely increased persistence of responding under extinction conditions (Feldon & Gray, 1981) on test day compared to the continous reinforecemnt schedule in our study, potentially resulting in different anatomcial patterns of cortical activation.
In our study, we did not observe increased Fos expression in the DS or NAc subregions after cue-induced reinstatement of heroin or cocaine seeking. This is different from previous studies using rats trained to self-administer only one of these drugs. Cue-induced reinstatement of cocaine seeking increased the number of Fos-expressing neurons in DS (Kufahl et al., 2009), NAc core (Mahler & Aston-Jones, 2012; Leong et al., 2017) and shell (Mahler & Aston-Jones, 2012), and whole NAc (Kufahl et al., 2009). These data are supported by inactivation experiments that demonstrate causal roles for DLS (Fuchs et al., 2006) and accumbens core (Fuchs et al., 2004a; McGlinchey et al., 2016) but not for accumbens shell (Fuchs et al., 2004a) in cue-induced reinstatement of cocaine seeking. Disconnection experiments also indicate a role for PL-to-accumbens core projection, but not PL-to-shell projection, in cue-induced reinstatement of cocaine seeking (McGlinchey et al., 2016). Cue-induced reinstatement of heroin seeking did not increase Fos mRNA in the DS or NAc shell, but increased another activity-dependent gene ania-3 in the NAc core (Koya et al., 2006). Inactivation experiments support a causal role for DLS (Rogers et al., 2008) and accumbens core, but not shell, subregion in cue-induced reinstatement of heroin seeking (Bossert et al., 2006; Bossert et al., 2007; Rogers et al., 2008).
Taken together, when rats are trained separately, the drug cues may activate and utilize the DLS and NAc core, but our Fos expression data suggest this may be different when rats are trained with both drugs. The different DS Fos expression results between our study and those of Kufahl (2009) for cocaine may again be due to different experimental conditions as described above for mPFC. The different NAc Fos expression results between our study and those of Mahler et al. (2012) could be due to measuring Fos expression in different sets of NAc neurons. While we counted all Fos-expressing neurons in core and shell, they counted Fos expression in only those neurons that projected to the ventral tegmental area (VTA), which are primarily in dopamine receptor type-1 (D1-type) medium spiny neurons (MSN), and not in dopamine receptor type-2 (D2-type) neurons (Gerfen & Surmeier, 2011). Thus some of the differences between our results and those of Mahler et al. may be due to Fos expression in D2-type MSN in our study that mask increased Fos expression in D1-type neurons.
In our study, cue-induced reinstatement of heroin or cocaine seeking did not increase Fos expression in either the BLA or CeA. This finding is different from previous studies using rats trained to self-administer only one of these drugs. Cue-induced reinstatement of cocaine seeking increased the number of Fos-expressing neurons in the BLA (Kufahl et al., 2009; McGlinchey et al., 2016) and lateral amygdala, but see (Leong et al., 2017). The data for BLA are supported by inactivation experiments that demonstrate a role for this brain region in cue-induced cocaine seeking (McLaughlin & See, 2003). Fos expression has not previously been assessed in amygdala following cue-induced reinstatement of heroin seeking; however, inactivation experiments indicate a causal role for both the BLA and CeA in cue-induced reinstatement of heroin seeking (Fuchs & See, 2002; Rogers et al., 2008). Taken together, when rats are trained separately, the cocaine cues may activate and utilize the BLA and CeA, but our Fos expression data suggest this may be different when rats are trained with both drugs. The differences between Fos expression results in our study and those of Kufahl (2009) for cocaine may again be due to different experimental conditions as described above for mPFC. For the BLA, while Mahler et al. (2012) used very similar experimental conditions with cocaine to ours for the test and control groups, they assessed Fos expression in only those neurons that projected from BLA to the NAc, and therefore assessed Fos expression in only a subset of BLA neurons. Thus, results of Mahler et al. cannot be directly compared to our study.
Overall in our polydrug model, cue-induced reinstatement induces similar levels of Fos expression in the PL for heroin and cocaine cues. The Fos expression and inactivation studies using rats trained with one drug further support the PL as a common substrate for both heroin and cocaine cue-induced reinstatement. Thus, the PL may be a common substrate for heroin and cocaine cue-induced reinstatement in a polydrug model. Some of our cocaine cue-induced Fos expression results from rats trained with both drugs are different from studies using only cocaine-trained rats. In contrast, none of our heroin cue-induced Fos expression results disagree with previous studies that used only heroin-trained rats. The reasons for the differences in cocaine-trained rats could be due to different experimental conditions, as described above. Additionally, some of the negative results in our study, particularly for the cocaine condition, may be due to a low sample size in our study (n=6–7) that may not have been sufficient to detect significant group differences (e.g., IL, core, shell) for low to medium effect sizes.
When we looked further at cellular phenotypes of activated Fos mRNA-expressing cells in the PL (and IL) cortex using in situ hybridization, we found similar proportions of cells expressing the glutamatergic marker Vglut1 (86–90%) or the GABAergic marker Vgat (7–9%) after cue-induced reinstatement with the cocaine- or heroin-associated cues, similar to previous studies of cue- and context-specific reinstatement of food and drug seeking (Bossert et al., 2013; Fanous et al., 2013; Cruz et al., 2014; Warren et al., 2016). These data suggest that the same sets of neurons are activated by heroin and cocaine cues during the cue-induced reinstatement test. Alternatively, different sets of neurons may be activated by each cue during reinstatement testing. Single-unit recordings in self-administration models indicate that different drugs and natural rewards can activate different neurons within the same cortical areas (Guillem & Ahmed, 2018; Guillem et al., 2018), including the mPFC during exposure to cocaine and heroin exposure (Chang et al., 1998). More recently, Fos has been identified as a marker of neuronal ensembles that mediate specific learned associations in cue-induced heroin (Fanous et al., 2012), methamphetamine (Li et al., 2015; Caprioli et al., 2017; Venniro et al., 2017a) and nicotine (Funk et al., 2016) seeking and context-induced reinstatement of food and drug seeking (Bossert et al., 2011; Cruz et al., 2014; Rubio et al., 2015; Marchant et al., 2016). Different Fos-expressing neuronal ensembles within the same IL cortex have been shown to mediate distinct learned associations that mediate opposite effects on behavior using operant models similar to that in the current study (Suto et al., 2016; Warren et al., 2016). However, a recent study using an in situ hybridization strategy suggested that alcohol and saccharin cue-induced reinstatement neuronal ensembles are largely similar (Pfarr et al., 2018). Future studies employing ensemble-specific technologies (Cruz et al., 2013; Cruz et al., 2015) are required to distinguish whether the same or different Fos-expressing ensembles in PL cortex mediate cocaine versus heroin cue-induced reinstatement in our polydrug model.
Conclusions
We have used a polydrug self-administration procedure to demonstrate that cue-induced reinstatement of cocaine or heroin seeking is selective to the lever previously associated with the drug-specific cue and is associated with increased activity in the PL part of the mPFC but not in other mPFC areas, ventral or dorsal striatal areas, or the amygdala. We propose that the cue-selective response is likely mediated by activation of different neuronal ensembles within the PL.
Supplementary Material
Supplementary Figure 2 legend. Inactive lever presses during Cocaine and Heroin self-administration (n = 27). Number of inactive lever presses (mean ± SEM) for each drug over 9 alternating sessions during training.
Supplementary Figure 3 legend. Individual correlation between number of Fos-expressing neurons and lever pressings for Cocaine and Heroin lever. (a) No significant correlations were observed between Fos counts and lever presses for the Cocaine lever (R square = 0.043; P value = 0.693) or Fos counts and lever presses for the Heroin lever (R square = 0.056; P value = 0.651) in the Cocaine-cue reinstated rats. (b) No significant correlations were observed between Fos counts and lever presses for the Heroin lever (R square = 0.002; P value = 0.936) or Fos counts and lever presses for the Cocaine lever (R square = 0.004; P value = 0.904) in the Heroin-cue reinstated rats.
Supplementary Figure 1 legend. Experiments to determine the appropriate drug doses to produce similar numbers of infusions/cue light presentations for both cocaine and heroin. (a-c) Cocaine and heroin self-administration data during training (n = 22–24, n = 32–34, and n = 26–27, respectively). Left: number of infusions; right: number of active lever presses (mean ± SEM) for each drug over 9 alternating sessions.
Acknowledgements
This research was supported by the Intramural Research Program of the NIDA, NIH. We thank JK Hoots for assistance with IV catheter surgeries.
Funding and Disclosure
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the text of the paper. The research was supported by the NIDA Intramural Research Program (BH and YS).
Abbreviations:
- mPFC
medial prefrontal cortex
- Cg1
cingulate cortex
- PL
prelimbic cortex
- IL
infralimbic cortex
- NAc
nucleus accumbens
- DS
dorsal striatum
- DLS
dorsolateral striatum
- DMS
dorsomedial striatum
- CeA
central amygdala
- BLA
basolateral amygdala
- FR-1
fixed ratio-1
- PBS
phosphate-buffered saline
- NGS
normal goat serum
- Vglut1
vesicular glutamate transporter 1
- Vgat
vesicular GABA transporter
- AMP
fluorescent multiplex reagent reagent
- DAPI
4′,6-diamidino-2-phenylindole
- VTA
ventral tegmental area
- D1-type
dopamine receptor type 1
- D2-type
dopamine receptor type 2
- SEM
standard error of the mean
- Partial Eta2
proportion of explained variance
References
- Adhikary S, Caprioli D, Venniro M, Kallenberger P, Shaham Y & Bossert JM (2017) Incubation of extinction responding and cue-induced reinstatement, but not context- or drug priming-induced reinstatement, after withdrawal from methamphetamine. Addict Biol, 22, 977–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A (2013) Substance-specific environmental influences on drug use and drug preference in animals and humans. Curr Opin Neurobiol, 23, 588–596. [DOI] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D & Shaham Y (2011) Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci, 12, 685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Adhikary S, St Laurent R, Marchant NJ, Wang HL, Morales M & Shaham Y (2016) Role of projections from ventral subiculum to nucleus accumbens shell in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl), 233, 1991–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L & Shaham Y (2006) Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology, 31, 2197–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ & Shaham Y (2013) The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl), 229, 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E & Shaham Y (2007) Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci, 27, 12655–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT & Shaham Y (2011) Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci, 14, 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Dubla A, Lucantonio F, Nencini P & Badiani A (2009) Ambience and drug choice: cocaine- and heroin-taking as a function of environmental context in humans and rats. Biol. Psychiatry, 65, 893–899. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Paolone G, Lucantonio F, Bari A, Nencini P & Badiani A (2008) Opposite environmental regulation of heroin and amphetamine self-administration in the rat. Psychopharmacology, 198, 395–404. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT & Shaham Y (2017) Role of Dorsomedial Striatum Neuronal Ensembles in Incubation of Methamphetamine Craving after Voluntary Abstinence. J Neurosci, 37, 1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL & Tiffany ST (1999) Meta-analysis of cue-reactivity in addiction research. Addiction, 94, 327–340. [PubMed] [Google Scholar]
- Chang JY, Janak PH & Woodward DJ (1998) Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J Neurosci, 18, 3098–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R & O’Brien CP (1988) Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr, 84, 25–43. [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP & Weiss F (2001) Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A, 98, 1976–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E & Shaham Y (2008) Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci, 363, 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS & Shaham Y (2002) Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci, 116, 169–173. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Babin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y & Hope BT (2014) Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci, 34, 7437–7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Javier Rubio F & Hope BT (2015) Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction. Brain Res, 1628, 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y & Hope BT (2013) New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci, 14, 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pirro S, Galati G, Pizzamiglio L & Badiani A (2018) The Affective and Neural Correlates of Heroin versus Cocaine Use in Addiction Are Influenced by Environmental Setting But in Opposite Directions. J Neurosci, 38, 5182–5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW & Everitt BJ (2008) Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology, 33, 1413–1425. [DOI] [PubMed] [Google Scholar]
- Dobler-Mikola A, Hattenschwiler J, Meili D, Beck T, Boni E & Modestin J (2005) Patterns of heroin, cocaine, and alcohol abuse during long-term methadone maintenance treatment. J Subst Abuse Treat, 29, 259–265. [DOI] [PubMed] [Google Scholar]
- Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y & Hope BT (2012) Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci, 32, 11600–11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous S, Guez-Barber DH, Goldart EM, Schrama R, Theberge FR, Shaham Y & Hope BT (2013) Unique gene alterations are induced in FACS-purified Fos-positive neurons activated during cue-induced relapse to heroin seeking. J Neurochem, 124, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldon J & Gray JA (1981) The partial reinforcement extinction effect: influence of chlordiazepoxide in septal lesioned rats. Psychopharmacology (Berl), 74, 280–289. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW & See RE (2008) The neurocircuitry of addiction: an overview. Br. J. Pharmacol, 154, 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK & See RE (2006) Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci, 26, 3584–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC & See RE (2004a) Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl), 176, 459–465. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP & See RE (2004b) Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci, 24, 6600–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA & See RE (2002) Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology, 160, 425–433. [DOI] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Hope BT, Shaham Y & Le AD (2016) Role of Central Amygdala Neuronal Ensembles in Incubation of Nicotine Craving. J Neurosci, 36, 8612–8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR & Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci, 34, 441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K & Ahmed SH (2018) Preference for Cocaine is Represented in the Orbitofrontal Cortex by an Increased Proportion of Cocaine Use-Coding Neurons. Cereb Cortex, 28, 819–832. [DOI] [PubMed] [Google Scholar]
- Guillem K, Brenot V, Durand A & Ahmed SH (2018) Neuronal representation of individual heroin choices in the orbitofrontal cortex. Addict Biol, 23, 880–888. [DOI] [PubMed] [Google Scholar]
- Highfield D, Yap J, Grimm JW, Shalev U & Shaham Y (2001) Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology, 25, 320–331. [DOI] [PubMed] [Google Scholar]
- James MH, McGlinchey EM, Vattikonda A, Mahler SV & Aston-Jones G (2018) Cued Reinstatement of Cocaine but Not Sucrose Seeking Is Dependent on Dopamine Signaling in Prelimbic Cortex and Is Associated with Recruitment of Prelimbic Neurons That Project to Contralateral Nucleus Accumbens Core. Int J Neuropsychopharmacol, 21, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Zhu H, Mannelli P, Schwartz RP, Subramaniam GA & Wu LT (2018) Prevalence, patterns, and correlates of multiple substance use disorders among adult primary care patients. Drug Alcohol Depend, 187, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Gawin FH, Rounsaville BJ & Kleber HD (1986) Cocaine abuse among opioid addicts: demographic and diagnostic factors in treatment. Am J Drug Alcohol Abuse, 12, 1–16. [DOI] [PubMed] [Google Scholar]
- Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer AN, De Vries TJ & Smit AB (2006) Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J Neurochem, 98, 905–915. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ & See RE (2001) Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J. Neurosci, 21, RC155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN & Neisewander JL (2009) c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse, 63, 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon P, Vincelette J, Bruneau J, Lamothe F, Lachance N, Brabant M & Soto J (1994) Illicit use of methadone among i.v. drug users in Montreal. J Subst Abuse Treat, 11, 457–461. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Guillem K, Koob GF & Ahmed SH (2012) Drug specificity in extended access cocaine and heroin self-administration. Addict Biol, 17, 964–976. [DOI] [PubMed] [Google Scholar]
- Leong KC, Freeman LR, Berini CR, Ghee SM, See RE & Reichel CM (2017) Oxytocin Reduces Cocaine Cued Fos Activation in a Regionally Specific Manner. Int J Neuropsychopharmacol, 20, 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Bruneau J & Stewart J (2003) Understanding polydrug use: review of heroin and cocaine co-use. Addiction, 98, 7–22. [DOI] [PubMed] [Google Scholar]
- Leri F & Stewart J (2001) Drug-induced reinstatement to heroin and cocaine seeking: a rodent model of relapse in polydrug use. Exp Clin Psychopharmacol, 9, 297–306. [DOI] [PubMed] [Google Scholar]
- Leri F, Tremblay A, Sorge RE & Stewart J (2004) Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacology, 29, 1312–1320. [DOI] [PubMed] [Google Scholar]
- Levin FR, Foltin RW & Fischman MW (1996) Pattern of cocaine use in methadone-maintained individuals applying for research studies. J Addict Dis, 15, 97–106. [DOI] [PubMed] [Google Scholar]
- Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, Kennedy PJ, Liu QR, Cimbro R, Hope BT, Nestler EJ & Shaham Y (2015) Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci, 35, 8232–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV & Aston-Jones GS (2012) Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci, 32, 13309–13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Whitaker LR, Harvey BK, Kaganovsky K, Adhikary S, Hope BT, Heins RC, Prisinzano TE, Vardy E, Bonci A, Bossert JM & Shaham Y (2016) Role of Ventral Subiculum in Context-Induced Relapse to Alcohol Seeking after Punishment-Imposed Abstinence. J Neurosci, 36, 3281–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EM, James MH, Mahler SV, Pantazis C & Aston-Jones G (2016) Prelimbic to Accumbens Core Pathway Is Recruited in a Dopamine-Dependent Manner to Drive Cued Reinstatement of Cocaine Seeking. J Neurosci, 36, 8700–8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Park S & Weiss RD (2014) Cue-induced craving in dependence upon prescription opioids and heroin. Am J Addict, 23, 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J & See RE (2003) Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl), 168, 57–65. [DOI] [PubMed] [Google Scholar]
- Montanari C, Stendardo E, De Luca MT, Meringolo M, Contu L & Badiani A (2015) Differential vulnerability to relapse into heroin versus cocaine-seeking as a function of setting. Psychopharmacology (Berl), 232, 2415–2424. [DOI] [PubMed] [Google Scholar]
- Morgan JI & Curran T (1991) Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci, 14, 421–451. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT & Ehrman R (1992) Classical conditioning in drug-dependent humans. Ann N Y Acad Sci, 654, 400–415. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T & Ehrman R (1990) Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav, 15, 355–365. [DOI] [PubMed] [Google Scholar]
- Paxinos G & Watson C (2005) The rat brain in stereotaxic coordinates, Ed. 5 Academic Press, San Diego. [Google Scholar]
- Pfarr S, Schaaf L, Reinert JK, Paul E, Herrmannsdorfer F, Rossmanith M, Kuner T, Hansson AC, Spanagel R, Korber C & Sommer WH (2018) Choice for Drug or Natural Reward Engages Largely Overlapping Neuronal Ensembles in the Infralimbic Prefrontal Cortex. J Neurosci, 38, 3507–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros LA, Pentkowski NS, Swinford SE & Neisewander JL (2011) Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology (Berl), 213, 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Ghee S & See RE (2008) The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience, 151, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio FJ, Liu QR, Li X, Cruz FC, Leao RM, Warren BL, Kambhampati S, Babin KR, McPherson KB, Cimbro R, Bossert JM, Shaham Y & Hope BT (2015) Context-induced reinstatement of methamphetamine seeking is associated with unique molecular alterations in Fos-expressing dorsolateral striatum neurons. J Neurosci, 35, 5625–5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE (2005) Neural substrates of cocaine-cue associations that trigger relapse. Eur. J. Pharmacol, 526, 140–146. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H & Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology, 168, 3–20. [DOI] [PubMed] [Google Scholar]
- Suto N, Laque A, De Ness GL, Wagner GE, Watry D, Kerr T, Koya E, Mayford MR, Hope BT & Weiss F (2016) Distinct memory engrams in the infralimbic cortex of rats control opposing environmental actions on a learned behavior. Elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D & Shaham Y (2016) Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res, 224, 25–52. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, Cifani C, Marchant NJ, Yizhar O, Bossert JM, Chiamulera C, Morales M & Shaham Y (2017a) The Anterior Insular Cortex-->Central Amygdala Glutamatergic Pathway Is Critical to Relapse after Contingency Management. Neuron, 96, 414–427 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Shaham Y & Caprioli D (2017b) Incubation of Methamphetamine but not Heroin Craving After Voluntary Abstinence in Male and Female Rats. Neuropsychopharmacology, 42, 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ & Luo Y (2012) RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn, 14, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Mendoza MP, Cruz FC, Leao RM, Caprioli D, Rubio FJ, Whitaker LR, McPherson KB, Bossert JM, Shaham Y & Hope BT (2016) Distinct Fos-Expressing Neuronal Ensembles in the Ventromedial Prefrontal Cortex Mediate Food Reward and Extinction Memories. J Neurosci, 36, 6691–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang S, Epstein DH, Fang Y, Shi J, Qin H, Yao S, Le Foll B & Lu L (2007) Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacol Biochem Behav, 86, 485–492. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE & Neisewander JL (2007) Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience, 145, 438–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 2 legend. Inactive lever presses during Cocaine and Heroin self-administration (n = 27). Number of inactive lever presses (mean ± SEM) for each drug over 9 alternating sessions during training.
Supplementary Figure 3 legend. Individual correlation between number of Fos-expressing neurons and lever pressings for Cocaine and Heroin lever. (a) No significant correlations were observed between Fos counts and lever presses for the Cocaine lever (R square = 0.043; P value = 0.693) or Fos counts and lever presses for the Heroin lever (R square = 0.056; P value = 0.651) in the Cocaine-cue reinstated rats. (b) No significant correlations were observed between Fos counts and lever presses for the Heroin lever (R square = 0.002; P value = 0.936) or Fos counts and lever presses for the Cocaine lever (R square = 0.004; P value = 0.904) in the Heroin-cue reinstated rats.
Supplementary Figure 1 legend. Experiments to determine the appropriate drug doses to produce similar numbers of infusions/cue light presentations for both cocaine and heroin. (a-c) Cocaine and heroin self-administration data during training (n = 22–24, n = 32–34, and n = 26–27, respectively). Left: number of infusions; right: number of active lever presses (mean ± SEM) for each drug over 9 alternating sessions.