Abstract
Transcranial static magnetic field stimulation (tSMS) is a novel non-invasive brain stimulation technique that has been shown to locally increase alpha power in the parietal and occipital cortex. We investigated if tSMS locally increased alpha power in the left or right prefrontal cortex, as the balance of left/right prefrontal alpha power (frontal alpha asymmetry) has been linked to emotional processing and mood disorders. Therefore, altering frontal alpha asymmetry with tSMS may serve as a novel treatment to psychiatric diseases. We performed a crossover, double-blind, sham-controlled pilot study to assess the effects of prefrontal tSMS on neural oscillations. Twenty-four right-handed healthy participants were recruited and received left dorsolateral prefrontal cortex (DLPFC) tSMS, right DLPFC tSMS, and sham tSMS in a randomized order. Electroencephalography data were collected before (2 minutes eyes-closed, 2 minutes eyes-open), during (10 minutes eyes-open), and after (2 minutes eyes-open) stimulation. In contrast to our hypothesis, neither left nor right tSMS locally increased frontal alpha power. However, alpha power increased in occipital cortex during left DLPFC tSMS. Right DLPFC tSMS increased post-stimulation fronto-parietal theta power, indicating possible relevance to memory and cognition. Left and right DLPFC tSMS increased post-stimulation left hemisphere beta power, indicating possible changes to motor behavior. Left DLPFC tSMS also increased post-stimulation right frontal beta power, demonstrating complex network effects that may be relevant to aggressive behavior. We concluded that DLPFC tSMS modulated the network oscillations in regions distant from the location of stimulation and that tSMS has region specific effects on neural oscillations.
Keywords: tSMS, Static magnetic field, Neodymium magnet, Electroencephalography, Alpha oscillations, Alpha asymmetry, Theta Oscillations, Beta Oscillations, Beta Asymmetry
Graphical abstract
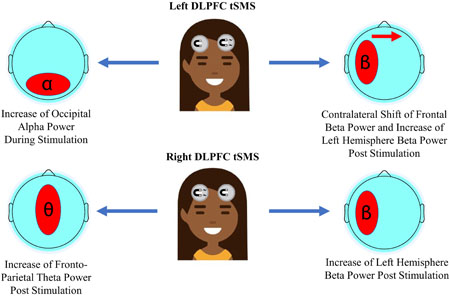
We applied transcranial static magnetic field stimulation to the dorsolateral prefrontal cortex and measured changes to neural oscillations. Contrary to prior findings of studies in the visual cortex, alpha power was unchanged at the site of stimulation, but we observed other alterations in the alpha, beta, and theta band. Our results indicate that the effect of transcranial static magnetic field stimulation on neural oscillations may be specific to the region stimulated.
Introduction
Alpha oscillations are prominent brain rhythms implicated in a variety of cognitive processes (Klimesch, 2012). In the context of psychiatric illnesses, frontal alpha oscillations, which have been linked to creative ideation and emotional processing (Harmon-Jones & Gable, 2017; Caroline Lustenberger, Boyle, Foulser, Mellin, & Fröhlich, 2015), are of particular interest. Specifically, the balance of alpha activity of the left and right frontal cortex, termed alpha asymmetry, predicts susceptibility to and may be a marker of depression and other mental disorders (Jesulola, Sharpley, Bitsika, Agnew, & Wilson, 2015). Approach and withdrawal behavior are believed to be lateralized to the left and right frontal cortex respectively (Harmon-Jones & Gable, 2017). Under the assumption that alpha oscillations represent hypoactivity of underlying cortex, higher left-than-right alpha power is thought to reflect a bias towards withdrawal related behaviors and emotions, whereas higher right-than-left alpha power reflect a bias towards approach related behaviors and emotions. Several studies have identified the dorsolateral prefrontal cortex (DLPFC) as a source of alpha oscillations contributing to frontal alpha asymmetry and associated emotional processing (Koslov, Mendes, Pajtas, & Pizzagalli, 2011; Pizzagalli, Sherwood, Henriques, & Davidson, 2005). Therefore, it is of interest to discover methods of altering frontal alpha oscillations and shift the balance of activity between the left and right frontal cortex. Transcranial static magnetic field stimulation (tSMS) is a novel non-invasive brain stimulation, which was reported to increase local alpha power when applied to parietal and occipital cortex (Carrasco-López et al., 2017; Gonzalez-Rosa et al., 2015). Yet, it remained unknown if tSMS modulates frontal alpha oscillations. To address this gap, we studied if alpha activity in the left or right frontal cortex can be altered with tSMS.
tSMS involves applying a moderate-strength static magnetic field (~100–200 mT) to the scalp. Magnetic fields of this strength have been shown to reduce neuronal firing rate both in vitro and in vivo, indicating that tSMS decreases neural excitability (Aguila, Cudeiro, & Rivadulla, 2014; Azanza & Del Moral, 1995). In humans, the effects of tSMS were demonstrated first by decreasing the amplitude of motor-evoked potentials (MEP) elicited by transcranial magnetic stimulation (TMS) pulses applied to the motor cortex (Oliviero et al., 2011). Two recent studies have demonstrated that tSMS increases alpha power at the site of the magnet when applied to the scalp above occipital and parietal cortex (Carrasco-López et al., 2017; Gonzalez-Rosa et al., 2015). TMS experiments have indicated an inverse relationship between occipital alpha and excitability of occipital cortex (Sauseng, Klimesch, Gerloff, & Hummel, 2009). Therefore, the increase of occipital alpha by tSMS was interpreted as further evidence of an inhibitory effect of tSMS on underlying cortex (Gonzalez-Rosa et al., 2015). Thus far it has not been demonstrated whether tSMS similarly increases frontal alpha power when applied to the frontal cortex. Given that frontal alpha oscillations have been localized to the DLPFC and alpha activity is assumed to be a signature of hypoactivity, one would predict that inhibiting the DLPFC with tSMS would lead to an increase in the amplitude of frontal alpha oscillations (Harmon-Jones & Gable, 2017; Koslov et al., 2011; Pizzagalli et al., 2005).
We performed a crossover, double blind, and sham-controlled pilot study of tSMS to modulate frontal alpha oscillations in healthy participants. All participants received, left, right, and sham tSMS to the DLPFC in a repeated-measures design. To our knowledge, this is the first investigation of DLPFC tSMS on neural oscillations, and the first combination of tSMS with high-density electroencephalography (EEG). Contrary to our hypothesis, tSMS did not locally increase frontal alpha power. Instead, left DLPFC tSMS increased occipital alpha oscillations during stimulation, both left and right DLPFC tSMS increased left hemisphere beta power post-stimulation, left DLPFC tSMS increased right frontal beta power post-stimulation, and right DLPFC tSMS increased theta oscillations along a fronto-parietal network post-stimulation. Our findings indicate a location-specific effect of tSMS on neural oscillations and warrant further investigation of DLPFC tSMS.
Materials and Methods
Participants
This study was performed at the University of North Carolina at Chapel Hill (UNC-CH) (ClinicalTrials.gov, NCT03244501) and was approved by the Biomedical Institutional Review Board of UNC-CH. All participants were recruited from the UNC-CH community and signed written consent prior to participation. Participants were 24 healthy, right-handed individuals (13 males and 11 females) aged 18–70 years old (26.54±12.28). Exclusion criteria were being under 18 years of age, a history of neurological or psychiatric illness, family history of psychopathology, brain implants/devices, implanted metal or pacemakers, history of brain surgery or traumatic brain injury, pregnancy, and use of hormonal birth control. This study was registered on ClinicalTrials.gov as NCT03244501.
Transcranial Static Magnetic Field Stimulation
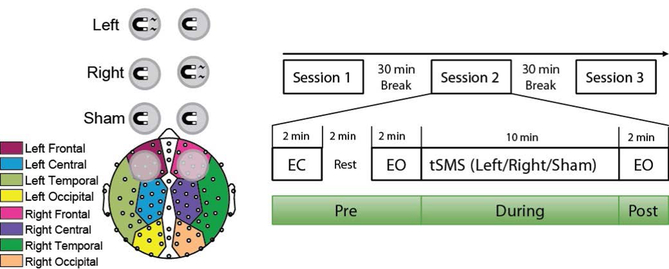
The magnet used for tSMS was a cylindrical nickel plated (Ni-Cu-Ni) NdFeB magnet 2.5 inches (6.35 cm) in diameter, 1 inch (2.54 cm) thick, and a mass of 603.4 g (model DY8X0-N52; K&J Magnetics, Inc., Pipersville, PA). The magnet is magnetized axially with a surface field strength of 0.46 Tesla on the cylindrical axis and has a pull force of 376.4 lbs. Modelling of tSMS magnetic fields found a magnet-to-cortex distance of ~1.2 cm when placed directly on the scalp above DLPFC (Tharayil, Goetz, Bernabei, & Peterchev, 2017). The EEG net used adds about another centimeter between the magnet and scalp, leading to a distance of ~2.2 cm from the cortex and a magnetic field strength of ~193 mT at the cortex, with a magnetic field gradient of ~9.5 mT/mm as calculated from the K&J Magnetic Calculator (https://www.kjmagnetics.com/fieldcalculator.asp) and confirmed with a DC Gaussmeter (model GM1-ST; AlphaLab Inc., Salt Lake City, UT). This magnet is of comparable size and magnetic strength to those used in prior tSMS studies (Carrasco-López et al., 2017; Gonzalez-Rosa et al., 2015). The south side of the magnet was always placed facing the scalp, though prior research indicated that tSMS-dependent effects on cortical excitability are polarity independent (Oliviero et al., 2011). Therefore, our choice of facing the south side towards the scalp was arbitrary. A custom-made nonferrous brass cylinder of the same size and mass as the real magnet was used for sham stimulation. A thin white cloth was used to hide color differences from the experimenter and participant to maintain integrity of the double-blind. Left DLPFC stimulation consisted of an active magnet placed over the F3 channel and a sham magnet placed over the F4 channel, right DLPFC stimulation consisted of an active magnet placed over F4 channel and a sham magnet placed over F3 channel, and sham stimulation consisted of two sham magnets placed over F3 and F4 channels based on the 10–20 coordinate system. All magnets were held in place on the scalp using custom-made nonmetal stands.
Study Procedure
We used a crossover, double-blind, and sham-controlled design. Participants attended one experimental session of three stimulation blocks during which they received left-DLPFC, right-DLPFC, or sham tSMS. The three blocks were separated by 30 minutes breaks to avoid outlasting effects of the stimulation from prior blocks since previous studies had shown that there were no outlasting effects of tSMS after 10 minutes (Carrasco-López et al., 2017; Gonzalez-Rosa et al., 2015; Oliviero et al., 2011). EEG data were recorded using a 128-channel Hydrocel Geodesic Sensor Net and Netamps 410 amplifier (EGI Inc., Eugene, OR) at a sampling rate of 1kHz. Electrode Cz and one electrode between Cz and Pz were used as the reference and as the ground, respectively. All instructions and experimental tasks were implemented in Presentation (Neurobehavioral Systems, Inc., Berkeley, CA).
At the beginning of each stimulation block, resting-state EEG data were recorded with two minutes eyes-closed, followed by two minutes rest, and finally two minutes eyes-open (Pre-Stim). Participants were instructed by a computer voice to fixate on a crosshair and open or close their eyes while staying relaxed. An experimenter then set up the magnets on the participant’s scalp according to randomized codes. Participants were asked to fixate on a crosshair during 10 minutes of stimulation while EEG data were recorded (During-Stim). The experimenter then removed the magnets, and two minutes eyes-open EEG data was recorded (Post-Stim). Participants read a book provided to them (The Great Gatsby, F. Scott Fitzgerald) during the 30 minutes break before the subsequent stimulation blocks.
There were six possible stimulations orders (left-right-sham, left-sham-right, right-left-sham, right-sham-left, sham-left-right, and sham-right-left) that were randomized and balanced across the 24 participants. After the final stimulation block, the participants and experimenter reported what order of stimulations they believed the participant received. The participants and experimenter responses did not significantly differ from chance (participants: 12.5% of correct guesses, Pearson χ2 test, p=0.557; experimenter: 12.5% of correct guesses, Pearson χ2 test, p=0.557). Participants also did not identify the sham stimulation more often than chance, indicating they could not distinguish between sham and active stimulation (37.5% of correct guesses, Pearson χ2 test,p=0.665).
Behavioral Assessments
Prior to their study visit, participants completed an online questionnaire of relevant behavioral measures. Behavioral inhibition/approach measures (BIS/BAS) and the Buss Perry Aggression Questionnaire were included as they have both been found to correlate with frontal alpha asymmetry (Buss & Perry, 1992; Carver, 1994; Harmon-Jones & Allen, 1997, 1998). The State Trait Anxiety Inventory (STAI, Trait Version) and Social Interaction Anxiety Scale (SIAS) were also included for exploratory analyses (Spielberger, 1983). The State Version of the STAI was completed by participants at the beginning of their study visit before the first stimulation session.
EEG and Statistical Analyses
EEG and statistical analyses were performed blind to the stimulation condition of participants. All data analyses were performed by EEGLAB (Delorme & Makeig, 2004) and custom-made scripts in MATLAB. First, data were down-sampled to 250 Hz with anti-aliasing filtering and band-pass filtered from 1–50 Hz. Second, the data were preprocessed by an artifact subspace reconstruction algorithm (Mullen et al., 2013). Third, bad channels discovered in the prior step were removed and interpolated followed by re-referencing to the common average. Lastly, infomax independent component analysis (ICA) was performed to remove artefacts caused by eye-blinks, eye movements, muscle activity, heartbeats, and channel noise. All ICA components were visually inspected and components were selected manually for rejection. These initial pre-processing steps were carried out for the entire EEG dataset.
EEG data were epoched into 2-second windows. Epochs were visually inspected in the temporal domain and bad epochs containing abnormal spikes or high-frequency noise were removed. Power spectral density (PSD) was computed by Welch’s method with a 2-second window and a 12.5% overlap resulting in a PSD with 0.5 Hz resolution. Individual alpha frequency (IAF), defined as the frequency of peak power in the occipital alpha band (8–12) Hz from 2 minutes eyes-closed EEG data, was determined for each participant during each of the three stimulation periods. Peak alpha frequencies did not differ between frontal and occipital channels (Supplementary Figure 1, paired samples t-test t46=1.220 p=0.229). The alpha power was obtained by averaging the PSD around the IAF (IAF 2 Hz ≤IAF ≤IAF + 2 Hz). Alpha power during and post-stimulation was normalized to the baseline eyes-open period by the formulas
Thirty-eight electrodes outside of the scalp and eight electrodes on the midline were excluded. Midline electrodes were excluded because we were primarily concerned with lateral effects of stimulation. The remaining 82 electrodes were divided into eight regions: left/right frontal, central, temporal, and occipital to investigate potential lateral effects of stimulation (Figure 1). Alpha power of these regions was calculated by averaging the alpha power of each electrode within that region as calculated above.
Figure 1.
Placement of magnets for DLPFC static magnetic field stimulation (left) and diagram of study session (right). Magnet with bolt represents active magnet, whereas magnet without bolt represents the non-ferrous sham magnet. Eighty-two EEG electrodes were divided into eight regions: left/right frontal, temporal, central, and occipital. All participants received left DLPFC, right DLPFC, and sham DLPFC stimulation with order randomized across participants (EO = Eyes Open, EC = Eyes Closed).
An ANOVA was performed for alpha power with within-subjects factor “stimulation” (left, right, sham), and within-subjects factor “region” (left/right frontal, central, temporal, occipital). Follow-up ANOVAs and paired samples t-tests with Bonferroni correction were used for multiple comparison by multiplying all p-values by the number of t-tests performed following a given ANOVA. Violations to sphericity were tested with Mauchly’s test and corrected with Huynh-Feldt correction if necessary. Additionally, similar ANOVAs were performed for the log-transformed baseline EO data, to verify the assumption that there were no baseline differences between stimulation conditions.
If group differences were discovered, a more conservative approach was used to isolate significant clusters of electrodes using a bootstrap permutation method (C. Lustenberger, M. Murbach, et al., 2015; C. Lustenberger, Wehrle, Tushaus, Achermann, & Huber, 2015; Maric et al., 2017; Nichols & Holmes, 2002; Plante et al., 2016). Paired t-tests are performed between groups for each electrode, and 5,000 permutations of participants are performed to create a distribution of t-values for each electrode. If the original t-value was at the 97.5th percentile on either tail, the electrode was considered significant. The largest cluster of significant electrodes for each permutation was also calculated. If the original analysis revealed a cluster larger than the 95th percentile of this cluster distribution, it was considered a significant cluster.
To elucidate the spatial topography of tSMS effects, a Laplacian filter was applied to the pre-processed EEG data using the spherical spline method implemented in the Current Source Density (CSD) MATLAB toolbox (Perrin, Pernier, Bertrand, & Echallier, 1989). Laplacian filtered EEG data were statistically analyzed as described above.
The alpha power at F3 and F4 were averaged across the three stimulation sessions for the eyes-open and eyes-closed pre-stim condition. Frontal alpha asymmetry (FAA) was calculated by the following formula
FAA was calculated for IAF defined alpha (IAF ± 2 Hz) and fixed frequency alpha (8–12 Hz) to produce four measures of FAA (FAAIAF,EO, FAAIAF,EC, FAA8–12,EO, FAA8–12,EC). Pearson’s correlation coefficient was calculated between each FAA measure and all behavioral tests.
Results
EEG Analysis
In alignment with our assumption about the lack of carry-over effects between stimulation conditions, there were no baseline differences between stimulation conditions for all frequency bands and brain regions (all p’s>0.14). Therefore, any changes to oscillation power across stimulation conditions reflects the effect of stimulation rather than differences in baseline power.
We first calculated the change in the alpha power during each stimulation relative to the eyes-open baseline period (IAF±2Hz, log normalized). There was no main effect of stimulation, but an interaction between stimulation and region (F14,322=1.732, p=0.048, corrected p=0.108, Table 1). This interaction was not present post-stimulation (p=0.583). To further elucidate this interaction, region was split into two factors: region (Frontal, Central, Temporal, Occipital), and hemisphere (left, right) as depicted in Figure 1. Contrary to our hypothesis, hemisphere was not part of the interaction (p=0.200) indicating the effect of tSMS was not specific to the hemisphere targeted.
Table 1.
Summary of ANOVA results for surface EEG data. Region(8) refers to a single factor with levels left/right frontal, central, temporal, and occipital. If significant effects were discovered, this was split into two factors, Region(4: frontal, central, temporal, occipital) and Hemisphere. Frequency bands outside of the alpha band were examined as part of an exploratory analysis and only trend level and significant findings are shown. Blank cells indicate analyses not performed due to lack of significance or trend in prior analyses. Significant p-values are bolded in black, while trends are bolded in red.
| Response | Factor(s) | During Stimulation | Post Stimulation |
|---|---|---|---|
| IAF±2Hz | Stimulation | F 2, 46=1.389, p=0.260, corrected p=0.260 | F 2, 46=0.202, p=0.818 |
| Stimulation * Region (8) | F 14, 322=1.732, p=0.048, corrected p 0.108 | F 14, 322=0.878, p=0.583, corrected p=0.528 | |
| Stimulation * Region (4) * Hemisphere | F 6, 138=1.450, p=0.200 | -- | |
| Stimulation * Region (4) | F 6, 138=2.127, p=0.054, corrected p=0.096 | -- | |
| IAF±2Hz, Left Stimulation | Region (4) | F 3,69=5.055, p=0.003, corrected p=0.006 | -- |
| IAF±2Hz, Right Stimulation | F 3, 69=0.188, p=0.904 corrected p=0.845 | -- | |
| IAF±2Hz, Sham Stimulation | F 3,69=0.237, p=0.870 corrected p=0.766 | -- | |
| Alpha (8–12 Hz) | Stimulation * Region (8) | F 14,322=1.425, p=0.139, corrected p=0.204 | F 14,322=0.806, p=0.663, corrected p=0.594 |
| Theta (4–8 Hz) | Stimulation | F 2, 46=1.857, p=0.168 | F 2, 46=3.073, p=0.056 |
| Beta (12–30 Hz) | Stimulation * Hemisphere | F 2, 46=1.608, p=0.211 | F 2, 46=4.815, p=0.013 |
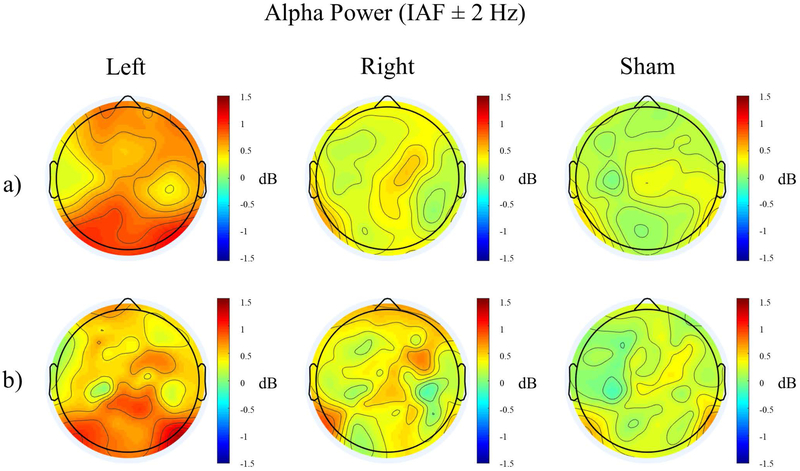
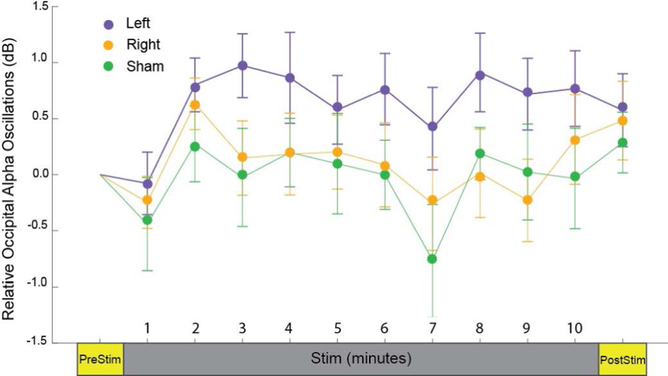
One-way ANOVAs were performed for each of the four regions with within-subjects factor “stimulation”, with no significant differences between groups for any region (all p’s>0.12). Therefore, one-way ANOVAs for each stimulation group with within-subjects factor “region” were performed, revealing significant differences in alpha power in different regions only in the left stimulation condition (Left: F3,69=5.055, p=0.003, corrected p=0.006). T-tests revealed significantly higher occipital alpha power than all other brain regions for the left stimulation condition (Figure 2a, Supplementary Figure 2; Occipital - Frontal: t47=2.965, p=0.028; Occipital - Central: t47=3.627, p=0.004; Occipital - Temporal: t47=3.492, p=0.006). To better localize these changes in alpha power, we performed Laplacian filtering (Figure 2b). There was no stimulation * region interaction (p=0.094, Table 2). To observe the time-course of the changes in alpha oscillation power, the period during stimulation was divided into ten one-minute segments and average occipital alpha power (IAF±2 Hz, determined from scalp topography in Figure 2a) was calculated for each stimulation condition (Figure 3). Error bars signify the standard error of the mean. By the first minute of stimulation, occipital alpha power was higher in the left stimulation condition than right or sham and remained higher for the duration of the stimulation. This effect disappeared post-stimulation, with little difference in occipital alpha power between groups. The large error bars explain the lack of significant differences between groups.
Figure 2.
Topographical distribution of relative alpha power (IAF ± 2 Hz) during stimulation for the three stimulation conditions without (a) and with (b) Laplacian filtering.
Table 2.
Summary of ANOVA results for Laplacian EEG data. Significant p-values are bolded in black, while trends are bolded in red.
| Response | Factor(s) | During Stimulation | Post Stimulation |
|---|---|---|---|
| IAF±2Hz | Stimulation | F 2, 46=1.248, p=0.297 | F 2, 46=0.223, p=0.800 |
| Stimulation * Region (8) | F 14, 322=1.544, p=0.094, corrected p=0.178 | F 14, 322=0.394 p=0.976, corrected p=0.921 | |
| Theta (4–8 Hz) | Stimulation | F 2, 46=1.467, p=0.241 | F 2, 46=2.576, p=0.087 |
| Beta (12–30 Hz) | Stimulation * Hemisphere | F 2, 46=0.847, p=0.435 | F 2, 46=2.972, p=0.061 |
Figure 3.
Time-course of relative occipital alpha power (IAF ± 2 Hz) during three stimulation conditions. Error bars denote standard error of the mean.
We note as that contrary to IAF analysis, there was no stimulation * region interaction for the fixed frequency alpha band (8–12 Hz) (p=0.139, Table 1). This is likely to reflect the increase of variability when using fixed frequency bands as opposed to IAF defined alpha.
As previous studies have noted changes in peak alpha frequency, a one-way ANOVA for peak alpha frequency with within-subjects factor “Stimulation” was performed. There were no differences across the three stimulation conditions (F2,46=0.865, p=0.428).
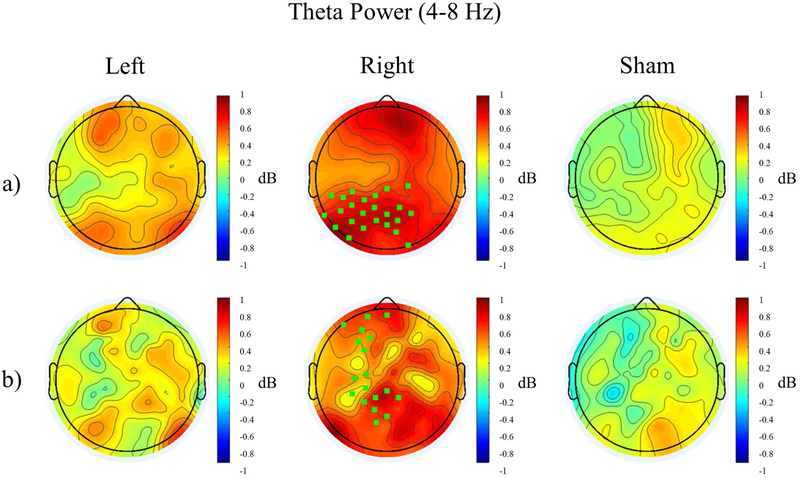
In further exploratory analysis, we investigated the effects on the following additional frequency bands: delta (1–4 Hz), theta (4–8 Hz), beta (12–30 Hz), and gamma (30–50 Hz). Separate ANOVAs were performed for the change in oscillation power during stimulation relative to eyes-open baseline and the change in power after stimulation relative to eyes-open baseline. Hemisphere was included as a factor only if there was a region * stimulation interaction with eight regions. We here report only trend-level and significant findings from these analyses (change in theta power and beta power post-stimulation, Table 1). In the post-stimulation condition, there was a trend-level main effect of stimulation in the theta band (F2,46=3.073, p=0.056). Post-hoc t-tests between the stimulation conditions revealed significant or trend-level differences between all groups, with theta power being greatest in the right stimulation condition, followed by the left stimulation condition, and lowest in the sham stimulation condition (Figure 4a, Supplementary Figure 3, Left - Sham: t191=2.326, p=0.063; Right - Sham: t191=5.657, p<0.001; Left - Right: t191=−4.062, p<0.001). Using a bootstrap permutation analysis, we identified a cluster of left-parietal electrodes that had higher theta power after right stimulation than sham stimulation (green square electrodes, middle topography in Figure 4a). Similarly, Laplacian filters showed a trend-level main effect of stimulation (F2,46=2.576, p=0.087). Follow-up paired t-tests confirmed higher global theta power for the right stimulation condition compared to left and sham stimulation (Right - Sham: t191=5.093, p<0.001; Right - Left: t191=−4.037, p<0.001). Furthermore, bootstrap analysis revealed that theta power was increased along left frontal, central, and parietal electrodes for the right stimulation condition compared to sham (green square electrodes, middle topography in Figure 4b).
Figure 4.
Topographical distribution of Relative Theta Power (4–8 Hz) post stimulation for the three stimulation conditions without (a) and with (b) Laplacian filtering. Electrodes significantly different from sham are highlighted in green.
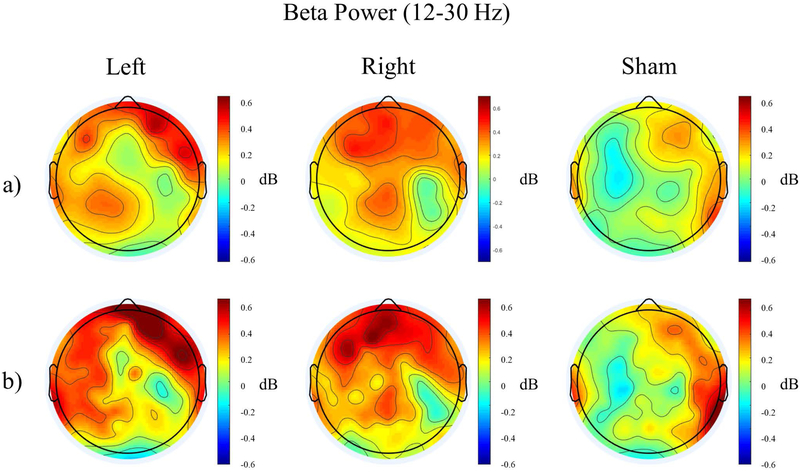
There was a stimulation * hemisphere interaction post-stimulation in the beta band (F2,46=4.815, p=0.013). Paired t-tests between all stimulation-hemisphere pairs revealed that left-hemisphere beta power was higher post left stimulation than post sham stimulation (t95=3.145, p=0.033). Similarly, left-hemisphere beta power was higher post right stimulation than post sham stimulation (t95=3.189, p=0.029). In the post-sham condition, beta power in the left hemisphere were lower than in the right hemisphere (t95=−4.551, p<0.001, Figure 5a, Supplementary Figure 4). Bootstrap permutation analysis did not find any clusters of electrodes differing between groups, indicating that changes in beta power were not well-localized. Laplacian filtering improved localization of this beta power increase, and revealed a similar trend of a stimulation * hemisphere interaction (F2,46=2.972, p=0.061). Follow-up t-tests revealed trend-level findings, with left-hemisphere beta power higher post- left and right stimulation than post sham stimulation (Left vs Sham: t95=2.935, p=0.063 ; Right vs. Sham: t95=2.707, p=0.121 ) and beta power lower in the left hemisphere than in the right hemisphere post-sham stimulation (t95=−3.559, p=0.009). Again, bootstrap permutation analysis did not reveal any significant clusters of electrodes (Figure 5b).
Figure 5.
Topographical distribution of Relative Beta Power (12–30 Hz) post stimulation for the three stimulation conditions without (a) and with (b) Laplacian filtering.
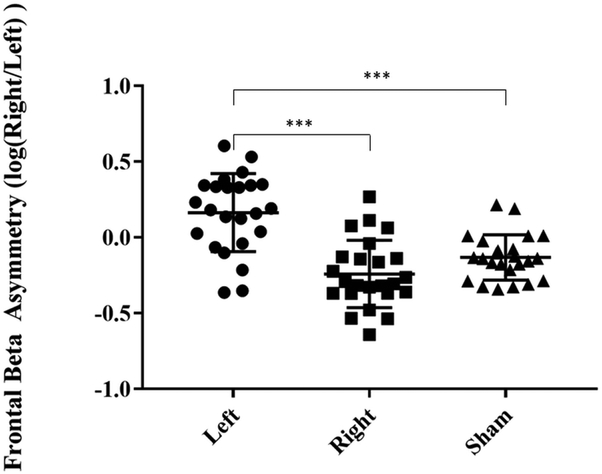
When calculating frontal beta asymmetry akin to frontal alpha asymmetry, an ANOVA revealed significant differences between groups (F2,46=26.46, p<0.001). Follow up paired t-tests showed that post left stimulation frontal beta asymmetry was higher (higher right beta) than both post right stimulation and post sham stimulation (Left - Sham: t46=5.835, p<0.001; Left – Right: t46=4.860, p<0.001) and post right stimulation frontal beta asymmetry was lower (higher left beta) than post sham stimulation (t46=−2.007, p=0.154, Figure 6) but failed to reach statistical significance.
Figure 6.
Frontal beta asymmetry post-stimulation with Laplacian filtering in the three stimulation conditions. Frontal beta asymmetry is calculated by taking the logarithmic ratio of beta power of the right and left frontal cortex, thus a higher value indicates higher right frontal beta oscillations. Error bars indicate standard deviation (*** p<0.001).
Behavioral Analysis
A summary of behavioral data collected is tabulated in Table 3. The four frontal alpha asymmetries (FAAIAF,EO, FAAIAF,EC, FAA8–12,EO, FAA8–12,EC) were correlated to the STAI (state and trait), SIAS, Buss Perry Aggression Questionnaire, and BISBAS using Pearson’s R. Even without correcting for multiple comparisons, there were no significant correlations between any of the FAA measures and the behavioral measures (all p>0.16, r2<0.09).
Table 3.
Summary of collected behavioral measures. Ranges for each measure are shown in parentheses.
| Scale | Subscale | Mean | Standard Deviation |
|---|---|---|---|
| State Trait Anxiety Inventory (STAI) | State (20–80) | 29.38 | 7.41 |
| Trait (20–80) | 37.83 | 8.65 | |
| Social Interaction Anxiety Scale (SIAS, 0–80) | 23.00 | 13.19 | |
| Behavioral Approach System (BAS) | Drive (4–16) | 10.71 | 2.24 |
| Fun Seeking (4–16) | 11.75 | 2.20 | |
| Reward Responsiveness (5–20) | 17.08 | 2.04 | |
| Total (13–52) | 39.54 | 5.09 | |
| Behavioral Inhibition System (BIS, 7–28) | 19.38 | 3.73 | |
| Buss Perry Aggression | Physical Aggression (9–45) | 17.21 | 6.12 |
| Verbal Aggression (5–25) | 14.58 | 4.51 | |
| Anger (7–35) | 13.71 | 4.76 | |
| Hostility (8–40) | 18.63 | 6.35 | |
| Total (29–145) | 64.13 | 17.08 |
Discussion
General Findings
In this study, we sought to alter the balance of frontal alpha oscillations through exposure to static magnetic fields. In contrast to our hypothesis, neither left nor right DLPFC tSMS increased alpha power at the site of the magnet, and left DLPFC tSMS actually increased occipital alpha power. This effect was specific to during stimulation, similar to effects observed for occipital tSMS (Gonzalez-Rosa et al., 2015).
Furthermore, exploratory analysis revealed changes in the theta and beta bands post-stimulation. Beta power in the left hemisphere increased after left and right DLPFC tSMS relative to sham, but no significant electrode clusters were discovered using a bootstrap permutation approach. The cluster approach looks for clusters of electrodes that are individually significant. Given the small size and diffuse nature of the beta increase, this effect was likely not significant when measured for independent electrodes and thus no significant clusters were discovered. Laplacian filtering confirmed an increase of left hemisphere beta power for left and right DLPFC stimulation, and also suggested that left DLPFC stimulation increased right frontal beta power, evidenced by significant changes to frontal beta asymmetry. The discrepancy between scalp-level and Laplacian-filtered EEG data may be due to the low spatial resolution of scalp EEG, and the Laplacian filter may have better localized the source of beta increase.
Global theta power increased after right and left DLPFC tSMS relative to sham, with a greater increase in the right DLPFC tSMS condition. A cluster of left-frontal electrodes were found to differ between right and sham stimulation, indicating the most consistent changes in theta power in this region. Laplacian filtering produced similar results, but revealed an increase in theta power along frontal, central, and parietal electrodes post right DLPFC stimulation.
This highlights that tSMS may induce offline effects on neural oscillations even when there are no observable online changes. Inconsistency between online and offline effects have been reported for other non-invasive brain stimulation techniques, indicating the need to further study the relationship between online and offline effects of tSMS (Veniero, Vossen, Gross, & Thut, 2015).
Limitations
As any scientific study, the work presented here has several limitations. Most importantly, this was a pilot study and thus not designed to establish statistical significance for small effect sizes. Sphericity was violated in several cases, with Huynh-Feldt corrected p-values being non-significant. Sphericity testing is not mentioned in prior tSMS findings of parietal alpha power increase, and thus future tSMS studies should ensure this step to avoid Type-1 errors (Carrasco-Lopez et al., 2017). Furthermore, we used a resting-state task as in prior tSMS studies, however the open-ended nature of resting-state tasks do not control for cognitive processes and resulted in drowsiness in some of our participants. Using a better-defined task to control for cognitive processes may reduce variability and increase the detectability of differences between stimulation conditions. Towards this end, tSMS has been applied to the motor cortex while participants performed an acoustic oddball task (Kufner, Bruckner, & Kammer, 2017a). No differences were found in post-stimulation MEP in contrast to prior studies that stimulated participants in the resting-state, revealing potential negation of tSMS effects by cognitive tasks (Foffani & Dileone, 2017; Kufner, Bruckner, & Kammer, 2017b). Further studies should include multiple cognitive tasks to investigate this effect and find a task which decreases variability without negating the effects of stimulation.
Our study did not find any association between frontal alpha asymmetry and behavioral measures, in contrast to prior findings of an association between FAA and BISBAS and Buss Perry Aggression (Harmon-Jones & Allen, 1997, 1998). However, these two findings were seen in an all-female and children-only samples respectively, as opposed to our gender and age-diverse sample. Given the possible importance of gender, age, and other factors in the association between frontal alpha asymmetry and depression, our study was not optimized to investigate the link between frontal alpha asymmetry and behavioral measures (Jesulola et al., 2015; van der Vinne, Vollebregt, van Putten, & Arns, 2017).
The differences in results observed between our study and previous studies could be potentially explained by the difference in magnetic field at the cortical surface. Our use of the Geodesic Sensor Nets caused the magnet to be an additional centimeter from the scalp. The magnet-to-cortex distance for occipital tSMS is ~1.6 cm (Tharayil et al., 2017). Assuming prior occipital tSMS research had negligible distance between the magnet and the scalp, this means our setup had ~5 mm greater cortex-to-scalp distance than prior research. Increased scalp-to-cortex distance not only decreases the strength of the magnetic field at the cortex, but also reduces focality, and thus should be carefully considered in future tSMS studies (Tharayil et al., 2017). Furthermore, prior studies assumed a magnet-to-cortex distance of 2–3 cm at the occipital cortex, meaning the actual magnetic field strength at the brain may have been higher than the presumed 120–200 mT (Carrasco-López et al., 2017; Gonzalez-Rosa et al., 2015). Given that magnetic field permeation in tissue is negligibly different from in air, magnetic strength at the cortex may be easily calculated using structured MR scans of participants and distance of the magnet from the scalp. The dose-response curve for tSMS is currently unclear, highlighting the need for careful consideration of magnetic field strength and use of modelling to inform future studies.
Despite the uncertainties regarding dosage, we find this an unlikely explanation for why DLPFC tSMS did not locally increase alpha power given the lack of any trend towards such an effect. This indicates possible issues with two assumptions maintained in our design. The first assumption was that static magnetic fields would inhibit underlying neurons when applied to the frontal cortex. The inhibitory effects of tSMS have been observed when applied to motor and visual cortex (Aguila et al., 2014; Oliviero et al., 2011), thus it is unknown whether this applies generally to all cortical regions in vivo. Techniques such as fMRI and TMS-EEG may be used in the future to assess whether DLPFC tSMS similarly inhibits neural tissue under the magnet.
Furthermore, the alpha asymmetry theory rests on the assumption that alpha oscillations measured through frontal EEG electrodes indicate hypoactivity of underlying neural tissue (Cook, O’Hara, Uijtdehaage, Mandelkern, & Leuchter, 1998; Harmon-Jones & Gable, 2017). Due to the issue of volume conduction with EEG, alpha oscillations measured at frontal electrodes do not necessarily reflect alpha activity generated in the frontal cortex. It is unknown whether the alpha oscillation occurs ubiquitously in the brain, and frontal alpha activity has been found to be considerably lower than posterior alpha using magnetoencephalography (MEG), which does not suffer from volume conduction as much as EEG does (Huang et al., 2014). Further studies should utilize techniques such as MEG and EEG with participant MRI/fMRI to improve source localization of frontal alpha asymmetry. Furthermore, the inverse association between alpha oscillations and cortical excitability is founded on TMS research in the visual and motor cortex (Romei et al., 2008; Sauseng et al., 2009). It remains uncertain how frontal alpha activity relates to cortical excitability, therefore techniques such as TMS-EEG should be used to elucidate this relationship. Unilateral hand contraction has been shown to alter frontal alpha asymmetry and approach behavior (Harmon-Jones, 2006), indicating the mu rhythm may be a possible source of 8–12 Hz activity measured by frontal electrodes. Indeed, the precentral gyrus has been indicated as a source of scalp-level alpha asymmetry (Smith, Cavanagh, & Allen, 2018).
Future Work and Implications
An increase of occipital alpha during left DLPFC tSMS may reflect a relationship between activity of the left DLFPC and occipital alpha oscillations. Previous studies have observed negative correlations between frontal activity and occipital alpha, particularly PET-EEG studies that observed a negative correlation between regional cerebral blood flow (rCBF) of the left dorsomedial prefrontal cortex and occipital alpha rhythm (Goldman, Stern, Engel, Cohen, & Cohen, 2002; Sadato et al., 1998). Nonetheless, in our study we targeted the DLPFC, and it is yet unclear how tSMS would impact other measures of brain activity such as the blood-oxygen level dependent response or rCBF. Future work may elaborate the effect of tSMS on these other measures of brain activity. Occipital alpha oscillations play a central role in visual attention, and the impact of left DLPFC tSMS on this process should be assessed in future studies (Klimesch, 2012). As all of our participants were right-handed, an increase of left-hemisphere beta power may indicate changes to motor activity of the dominant hand (Khanna & Carmena, 2017). Concurrent frontal tSMS with electromyographic recordings of the hand and other physiological measurements could test this hypothesis and search for a mechanism relating DLPFC activity to hand movement. Beta frequencies are believed to indicate cortical inhibition mediated by GABAergic neurotransmission, and thus it is seemingly contradictory that frontal tSMS would increase an inhibitory biomarker on the contralateral side (Hall, Barnes, Furlong, Seri, & Hillebrand, 2010; Muthukumaraswamy et al., 2013; van Lier, Drinkenburg, van Eeten, & Coenen, 2004). This may reflect post-stimulation homeostasis, connections between left and right DLPFC, or other network interactions induced by tSMS. Frontal beta asymmetry has been related to trait aggression, with higher right frontal beta being associated with higher aggression, suggesting that frontal tSMS may be potentially able to modulate aggressive behaviors (Hofman & Schutter, 2012). Further studies will be necessary to clarify the mechanism of beta power increase and possible influences to behavior.
Theta oscillations in the frontal and parietal cortex have been implicated in higher-order cognitive processes including memory, attention, and executive functioning, hinting at the exciting possibility that right DLPFC tSMS may modulate cognitive processes (Kawasaki, Kitajo, & Yamaguchi, 2014; Rajan et al., 2018; Scholz, Schneider, & Rose, 2017). A simultaneous increase of theta oscillations in the frontal and parietal cortex may indicate activation of a fronto-parietal network which have been particularly implicated in attentional processes and general intelligence (Jung & Haier, 2007; Scolari, Seidl-Rathkopf, & Kastner, 2015). Further research will be required to confirm whether right DLPFC tSMS facilitates or disrupts natural activity of this network and can indicate whether it may have any therapeutic potential.
Conclusions
Despite consistent evidence of decreased cortical excitability from tSMS in several cortical areas (Aguila et al., 2014; Azanza & Del Moral, 1995; Carrasco-López et al., 2017; Gonzalez-Rosa et al., 2015; Oliviero et al., 2011), DLPFC tSMS failed to locally increase alpha oscillations. Nonetheless, left DLPFC tSMS increased occipital alpha oscillations during stimulation, both left and right DLPFC tSMS increased left hemisphere beta power post-stimulation, left DLPFC tSMS increased right frontal beta power post-stimulation, and right DLPFC tSMS increased theta oscillations along a fronto-parietal network post-stimulation. These findings suggest that tSMS interacts with cortical oscillations in more complex ways than we had initially hypothesized, and may have implications for modulation of aggressive behavior and cognition.
Supplementary Material
Acknowledgements
The authors thank Morgan Alexander, Juliann Mellin, Jhana Parikh, and Julianna Prim for their assistance in data collection. We also thank Ehsan Negahbani for assistance with double-blind study design.
Funding
This study was funded by the department of Psychiatry at UNC Chapel Hill and supported in part by the National Institute of Mental Health of the National Institutes of Health under Award Numbers R01MH101547 and R01MH111889.
Abbreviations:
- DLPFC
, dorsolateral prefrontal cortex
- EEG
electroencephalography
- MEG
magnetoencephalography
- tSMS
transcranial static magnetic field stimulation
Footnotes
Conflict of Interest: FF is the lead inventor of IP filed on the topics of brain stimulation by UNC. The clinical studies performed in the Frohlich Lab have originally received a designation as conflict of interest with administrative considerations that was subsequently removed. FF is the founder, CSO and majority owner of Pulvinar Neuro LLC. The study presented here is unrelated to Pulvinar Neuro. AS, SeA, and SmA have no financial conflicts.
Data Sharing
Upon acceptance of the manuscript, the raw data is available by request to the corresponding author.
References
- Aguila J, Cudeiro J, & Rivadulla C (2014) Effects of Static Magnetic Fields on the Visual Cortex: reversible Visual Deficits and Reduction of Neuronal Activity. Cerebral Cortex, 26, 628–638. [DOI] [PubMed] [Google Scholar]
- Azanza MJ, & Del Moral A (1995) Neuron firing frequency dependence on the static magnetic field intensity. Journal of Magnetism and Magnetic Materials, 140, 144–144. [Google Scholar]
- Buss AH, & Perry M (1992) The aggression questionnaire. J Pers Soc Psychol, 63, 452–459. [DOI] [PubMed] [Google Scholar]
- Carrasco-Lopez C, Soto-Leon V, Cespedes V, Profice P, Strange BA, Foffani G, & Oliviero A (2017) Static Magnetic Field Stimulation over Parietal Cortex Enhances Somatosensory Detection in Humans. J Neurosci, 37, 3840–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994) Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319–333. [Google Scholar]
- Cook IA, O’Hara R, Uijtdehaage SH, Mandelkern M, & Leuchter AF (1998) Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr Clin Neurophysiol, 107, 408–414. [DOI] [PubMed] [Google Scholar]
- Foffani G, & Dileone M (2017) No modulatory effects by tSMS when delivered during a cognitive task. Brain Stimulation, 10, 867–867. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Cohen MS, & Cohen MS (2002) Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport, 13, 2487–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rosa JJ, Soto-Leon V, Real P, Carrasco-Lopez C, Foffani G, Strange BA, & Oliviero A (2015) Static Magnetic Field Stimulation over the Visual Cortex Increases Alpha Oscillations and Slows Visual Search in Humans. J Neurosci, 35, 9182–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SD, Barnes GR, Furlong PL, Seri S, & Hillebrand A (2010) Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco-magnetoencephalography. Hum Brain Mapp, 31, 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E (2006) Unilateral right-hand contractions cause contralateral alpha power suppression and approach motivational affective experience. Psychophysiology, 43, 598–603. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, & Allen JJ (1997) Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. J Abnorm Psychol, 106, 159–163. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, & Allen JJ (1998) Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. J Pers Soc Psychol, 74, 1310–1316. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, & Gable PA (2017) On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: An updated review of the evidence. Psychophysiology, 55, 1–23. [DOI] [PubMed] [Google Scholar]
- Hofman D, & Schutter DJ (2012) Asymmetrical frontal resting-state beta oscillations predict trait aggressive tendencies and behavioral inhibition. Soc Cogn Affect Neurosci, 7, 850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MX, Huang CW, Robb A, Angeles A, Nichols SL, Baker DG, Song T, Harrington DL, Theilmann RJ, Srinivasan R, Heister D, Diwakar M, Canive JM, Edgar JC, Chen YH, Ji Z, Shen M, El-Gabalawy F, Levy M,McLay R, Webb-Murphy J, Liu TT, Drake A, Lee RR (2014) MEG source imaging method using fast L1 minimum-norm and its applications to signals with brain noise and human resting-state source amplitude images. Neuroimage, 84, 585–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesulola E, Sharpley CF, Bitsika V, Agnew LL, & Wilson P (2015) Frontal alpha asymmetry as a pathway to behavioural withdrawal in depression: Research findings and issues. Behavioural Brain Research, 292, 56–67. [DOI] [PubMed] [Google Scholar]
- Jung RE, & Haier RJ (2007) The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci, 30, 135–187. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Kitajo K, & Yamaguchi Y (2014) Fronto-parietal and fronto-temporal theta phase synchronization for visual and auditory-verbal working memory. Front Psychol, 5, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna P, Carmena JM (2017) Beta band oscillations in motor cortex reflect neural population signals that delay movement onset. eLife, 6, e24573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W (2012) Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci, 16, 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslov K, Mendes WB, Pajtas PE, & Pizzagalli DA (2011) Asymmetry in Resting Intracortical Activity as a Buffer to Social Threat. Psychological Science, 22, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufner M, Bruckner S, & Kammer T (2017a) No modulatory effects by transcranial static magnetic field stimulation of human motor and somatosensory cortex. Brain Stimulation, 10, 703–710. [DOI] [PubMed] [Google Scholar]
- Kufner M, Bruckner S, & Kammer T (2017b) Reply to the letter to the editor on “No modulatory effects by tSMS when delivered during a cognitive task”. Brain Stimulation, 10, 868–869. [DOI] [PubMed] [Google Scholar]
- Lustenberger C, Boyle MR, Foulser AA, Mellin JM, & Fröhlich F (2015) Functional role of frontal alpha oscillations in creativity. Cortex, 67, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustenberger C, Murbach M, Tushaus L, Wehrle F, Kuster N, Achermann P, & Huber R (2015) Inter-individual and intra-individual variation of the effects of pulsed RF EMF exposure on the human sleep EEG. Bioelectromagnetics, 36, 169–177. [DOI] [PubMed] [Google Scholar]
- Lustenberger C, Wehrle F, Tushaus L, Achermann P, & Huber R (2015) The Multidimensional Aspects of Sleep Spindles and Their Relationship to Word-Pair Memory Consolidation. Sleep, 38, 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric A, Lustenberger C, Werth E, Baumann CR, Poryazova R, & Huber R (2017) Intraindividual Increase of Homeostatic Sleep Pressure Across Acute and Chronic Sleep Loss: A High-Density EEG Study. Sleep, 40, zsx122. [DOI] [PubMed] [Google Scholar]
- Mullen T, Kothe C, Chi YM, Ojeda A, Kerth T, Makeig S, Cauwenberghs G, Jung T-P (2013) Real-time modeling and 3D visualization of source dynamics and connectivity using wearable EEG 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC): IEEE, Osaka, Japan, 2184–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Myers JF, Wilson SJ, Nutt DJ, Lingford-Hughes A, Singh KD, & Hamandi K (2013) The effects of elevated endogenous GABA levels on movement-related network oscillations. Neuroimage, 66, 36–41. [DOI] [PubMed] [Google Scholar]
- Nichols TE, & Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp, 15, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero A, Mordillo-Mateos L, Arias P, Panyavin I, Foffani G, & Aguilar J (2011) Transcranial static magnetic field stimulation of the human motor cortex. The Journal of Physiology, 589, 4949–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, & Echallier JF (1989) Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol, 72, 184–187. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, & Davidson RJ (2005) Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychol Sci, 16, 805–813. [DOI] [PubMed] [Google Scholar]
- Plante DT, Goldstein MR, Cook JD, Smith R, Riedner BA, Rumble ME, Jelenchick L, Roth A, Tononi G, Benca RM, Peterson MJ (2016) Effects of partial sleep deprivation on slow waves during non-rapid eye movement sleep: A high density EEG investigation. Clin Neurophysiol, 127, 1436–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Siegel SN, Liu Y, Bengson J, Mangun GR, & Ding M (2018) Theta Oscillations Index Frontal Decision-Making and Mediate Reciprocal Frontal-Parietal Interactions in Willed Attention. Cereb Cortex, bhy149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, & Thut G (2008) Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex, 18, 2010–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Nakamura S, Oohashi T, Nishina E, Fuwamoto Y, Waki A, & Yonekura Y (1998) Neural networks for generation and suppression of alpha rhythm: a PET study. Neuroreport, 9, 893–897. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gerloff C, & Hummel FC (2009) Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia, 47, 284–288. [DOI] [PubMed] [Google Scholar]
- Scholz S, Schneider SL, & Rose M (2017) Differential effects of ongoing EEG beta and theta power on memory formation. PLoS One, 12, e0171913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolari M, Seidl-Rathkopf KN, & Kastner S (2015) Functions of the human frontoparietal attention network: Evidence from neuroimaging. Curr Opin Behav Sci, 1, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Cavanagh JF, Allen JJ (2017) Intracranial source activity (eLORETA) related to scalp‐level asymmetry scores and depression status. Psychophysiology, 55, e13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Tharayil JJ, Goetz SM, Bernabei JM, & Peterchev AV (2017) Field Distribution of Transcranial Static Magnetic Stimulation in Realistic Human Head Model. Neuromodulation: Technology at the Neural Interface, 21, 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vinne N, Vollebregt MA, van Putten M, & Arns M (2017) Frontal alpha asymmetry as a diagnostic marker in depression: Fact or fiction? A meta-analysis. Neuroimage Clin, 16, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lier H, Drinkenburg WH, van Eeten YJ, & Coenen AM (2004) Effects of diazepam and zolpidem on EEG beta frequencies are behavior-specific in rats. Neuropharmacology, 47, 163–174. [DOI] [PubMed] [Google Scholar]
- Veniero D, Vossen A, Gross J, & Thut G (2015) Lasting EEG/MEG Aftereffects of Rhythmic Transcranial Brain Stimulation: Level of Control Over Oscillatory Network Activity. Front Cell Neurosci, 9, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.