Abstract
Background:
Vagus nerve stimulation (VNS) paired with forelimb motor training enhances reorganization of movement representations in the motor cortex. Previous studies have shown an inverted-U relationship between VNS intensity and plasticity in other brain areas, such that moderate intensity VNS yields greater cortical plasticity than low or high intensity VNS. However, the relationship between VNS intensity and plasticity in the motor cortex is unknown.
Objective:
In this study we sought to test the hypothesis that VNS intensity exhibits an inverted-U relationship with the degree of motor cortex plasticity in rats.
Methods:
Rats were taught to perform a lever pressing task emphasizing use of the proximal forelimb musculature. Once proficient, rats underwent five additional days of behavioral training in which low intensity VNS (0.4 mA), moderate intensity VNS (0.8 mA), high intensity VNS (1.6 mA), or sham stimulation was paired with forelimb movement. 24 hours after the completion of behavioral training, intracortical microstimulation (ICMS) was used to document movement representations in the motor cortex.
Results:
VNS delivered at 0.8 mA caused a significant increase in motor cortex proximal forelimb representation compared to training alone. VNS delivered at 0.4 mA and 1.6 mA failed to cause a significant expansion of proximal forelimb representation.
Conclusion:
Moderate intensity 0.8 mA VNS optimally enhances motor cortex plasticity while low intensity 0.4 mA and high intensity 1.6 mA VNS fail to enhance plasticity. Plasticity in the motor cortex exhibits an inverted-U function of VNS intensity similar to previous findings in auditory cortex.
Keywords: Vagus nerve stimulation, motor training, ICMS, plasticity, cortical reorganization, motor cortex
Introduction
Vagus nerve stimulation (VNS) has recently emerged as a method of enhancing rehabilitation for a wide range of neurological disorders affecting motor function including stroke, traumatic brain injury, and spinal cord injury[1–11]. Recovery is thought to be associated with plasticity in central networks after injury[12,13]. VNS is believed to promote recovery by inducing plasticity in networks activated during rehabilitation[12]. Thus, increasing the amount of VNS-mediated plasticity could lead to enhanced recovery.
VNS drives rapid engagement of the neuromodulators acetylcholine and norepinephrine, which act synergistically to strengthen synaptic connections in activated circuits[14–18]. Repeatedly pairing VNS with a sensory or motor event drives robust, targeted cortical plasticity[1,19–22]. For example, repeatedly pairing VNS with forelimb training increases forelimb representation in the motor cortex[22].
A number of stimulation parameters influence the magnitude of plasticity driven by VNS in the auditory cortex, but the effect of these parameters on motor cortex plasticity remains largely unexplored[23]. Increasing VNS intensity drives monotonic increases in neural activity in the locus coeruleus (LC), an area necessary for the effects of VNS on central nervous system[18,24–26]. Thus, VNS intensity and plasticity may be linearly related, where higher intensities of VNS yield greater plasticity. Alternatively, studies in auditory cortex and hippocampus reveal an inverted-U relationship between VNS intensity and plasticity, where low and high intensity VNS drive little to no plasticity, while moderate intensity VNS significantly enhances plasticity[23,27–29]. Here we sought to test the hypothesis that motor cortex plasticity in rats exhibits an inverted-U relationship to VNS intensity.
Methods
All experimental procedures, statistical comparisons, and exclusion criteria were preregistered before beginning data collection (https://osf.io/3bxgc/).
Subjects
Forty-six female Sprague-Dawley rats weighing approximately 250 grams were used in this experiment. All rats were housed in a reversed 12:12 hour light-dark cycle. Rats were food restricted on weekdays during behavioral shaping and training with ad libitum access to food on weekends. All rats were maintained at or above 85% body weight. All handling, housing, stimulation, and surgical procedures were approved by The University of Texas at Dallas Institutional Animal Care and Use Committee.
Behavioral Training
Rats were trained to perform an automated lever pressing task[30]. The behavioral training apparatus consisted of an acrylic cage with a slot located at the front right for access to a lever positioned outside the cage (Fig. 1A). The slot was situated so that rats were only able to use their right forelimb to reach for the lever. A potentiometer affixed to the lever recorded the angle of the lever relative to the horizontal. The lever had a range of motion of 13°, and any lever depression exceeding 9.5° was considered a press. A spring provided approximately 28 grams of resistance to bring the lever back to a horizontal position after it had been depressed. An audio cue signaled successful presses. All rats were trained to depress the lever twice in rapid succession. If the second press occurred within 500 ms of the first, the trial was recorded as a success and a food pellet was delivered (45 mg dustless precision pellet, BioServ, Frenchtown, NJ) (Fig. 1B). If the lever was not depressed a second time or the second press occurred longer than 500 ms after the first press, the trial was recorded as a failure and no food pellet was delivered.
Figure 1. Lever Pressing Task and Experimental design.
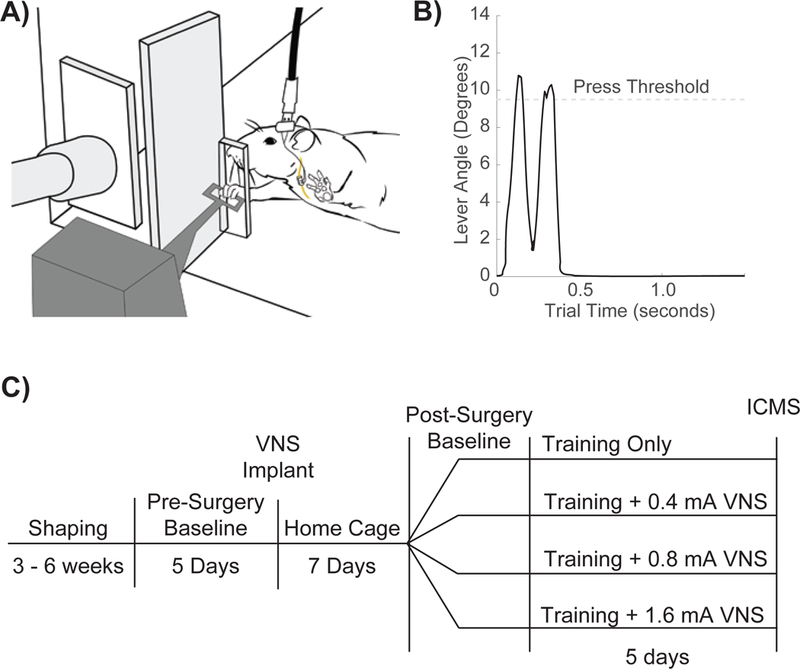
(A) Illustration of rat performing the lever pressing task. The stimulating cable plugged into the headmounted -connector, the subcutaneous stimulation leads and nerve cuff, and the vagus nerve are shown. (B) Representative trial depicting a double press. (C) Timeline of experimental design.
Behavioral shaping occurred in stages. Early in shaping, the lever extended into the behavioral cage and a single press was required for a food pellet to be dispensed. The lever was moved back gradually until the tip of the lever was positioned 2.5 cm away from the cage. Finally, a second press within 500 ms of the first press was required for a food pellet to be dispensed. Rats performed the task for two 30 minute periods five days a week, with each 30- minute session being separated by at least 2 hours. Rats received a supplemental 10 grams of food pellets if they did not receive at least 100 pellets in a day. Once proficient at the task, rats were implanted with VNS cuffs and recovered for 7 days before returning to behavioral testing.
Surgical Implantation
Rats were implanted with a stimulating cuff on the left cervical vagus nerve as described in previous studies[4–7]. Rats were anesthetized with ketamine hydrochloride (50 mg/kg, i.p.), xylazine (20 mg/kg, i.p.), and acepromazine (5 mg/kg, i.p.), and were placed in a stereotactic apparatus. An incision was made down the midline of the head to expose the skull. Bone screws were inserted into the skull at points surrounding the lamboid suture and over the cerebellum. A two-channel connector was mounted to the screws using acrylic.
An incision was made on the left side of the neck and the overlying musculature was blunt dissected to reveal the vagus nerve. The nerve was gently dissected away from the carotid artery. A cuff electrode was implanted surrounding the vagus nerve with 2 leads tunneled subcutaneously to connect with a 2-channel connector fixed with acrylic to the skull. Nerve activation was confirmed by observation of a ≥ 5% drop in blood oxygen saturation in response to a 10 s stimulation train of 30 Hz VNS consisting of 800 µ A, 100 µs biphasic pulses, as in previous studies. The head and neck incisions were then sutured. Rats received subcutaneous injections of 4 mL 50:50 0.9% saline 5% dextrose solution and sustained release Buprenorphine (0.3 mg/kg). A seven-day recovery period followed surgery and rats were given one Baytril tablet per day (2 mg/tablet, BioServ, Frenchtown, NJ).
Vagus Nerve Stimulation
After surgery rats were randomly sorted into four groups that received lever training where successful presses were paired with 0.4 mA VNS (n = 9), 0.8 mA VNS (n = 7), 1.6 mA VNS (n = 8), or sham stimulation (n = 9) (Fig. 1C). All rats, regardless of experimental group, were connected via headmount-connector to a stimulation cable. In the initial sessions after implantation rats were allowed to acclimate to being attached to stimulating cables until they performed at least 100 successful trials per day. Once acclimated, rats then underwent five days of training and received VNS according to their group. VNS consisted of a 500 ms train of 100 µs biphasic pulses at a frequency of 30 Hz with an amplitude of either 0.4 mA, 0.8 mA, 1.6 mA, or 0 mA, as appropriate for each group. During daily VNS sessions, a digital oscilloscope (PicoScope 2204A, PP906, Pico Technology) was used to monitor cuff impedance to ensure nerve cuff functionality.
Intracortical Microstimulation Mapping
Within 24 hours of their last behavioral session rats underwent intracortical microstimulation (ICMS) as previously described[22,31–34]. Rats were anesthetized with ketamine hydrochloride (70 mg/kg, i.p.) and xylazine (5 mg/k, i.p.). Toe-pinch response and whisking were used to determine when supplemental doses were needed in order to maintain a constant state of anesthesia for the procedure. To evaluate nerve cuff functionality, nerve activation was confirmed by observation of a ≥ 5% drop in blood oxygen saturation in response to a 10 s stimulation train of 30 Hz VNS consisting of 800 µA, 100 µ s biphasic pulses. Rats that failed to demonstrate stimulation-induced depression in oxygen saturation were excluded, as defined in the pre-registration.
Rats were placed in a stereotactic apparatus and a craniotomy and duratomy were performed to expose the left motor cortex (4 mm to −3 mm AP and 0.25 mm to 5 mm ML). A cisternal drain was created to minimize cortical swelling. A tungsten electrode with an impedance of approximately 0.7 MΩ (FHC, Tungsten Microelectrode - UEWMEGSEBN3M) was lowered into the brain to a depth of 1.8 mm. Stimulation sites were then chosen at random on a grid with sites set 500 µm apart from each other. Sites were at least 1 mm away from the previous site whenever possible. Stimulations consisted of a 40 ms pulse train of monophasic 200 µs cathodal pulses delivered at a frequency of 286 Hz. ICMS was conducted blinded with two experimenters, as previously described[22,35]. The first experimenter placed the electrode and recorded the data for each site. The second experimenter, blinded to group and electrode location, delivered stimulations and classified movements. Stimulation was increased from 20 µA until a movement was observed or until a maximum of 250 µA was reached. Movements were classified into the following categories: proximal forelimb, distal forelimb, head, and hindlimb.
Subject Exclusion
All subject exclusion criteria was preregistered before data collection began (https://osf.io/3bxgc/). 33 subjects were analyzed in the final results of the study out of a total of 46 subjects. Of the 13 subjects not used in final analysis, 2 subjects were excluded due to loss of function of nerve cuffs, 6 subjects were excluded due to head-connector failure during behavioral training, and 5 subjects were excluded due to death during or immediately after VNS implantation or before ICMS surgery.
Statistics
Statistical methods and comparisons were preregistered and defined a priori (https://osf.io/3bxgc/). The primary outcome of this study was area of motor cortex generating proximal forelimb movements. All other movement representations and behavioral performance data were analyzed as secondary outcome measures. A one-way ANOVA was used to compare experimental group representation sizes. Behavioral data was compared using one-way ANOVA. Bonferroni corrected unpaired two-tailed t-tests at an alpha of 0.0083 were used to identify any between group differences, as appropriate. All data are reported as mean ± SEM. “*” indicates Bonferroni-corrected significant differences in all figures.
Results
We sought to evaluate the effect of varying stimulation intensity on VNS-dependent plasticity in motor cortex. To do so, rats were shaped on an automated lever-pressing task emphasizing the proximal forelimb musculature. Once proficient, rats underwent five additional days of behavioral training in which low intensity VNS (0.4 mA), moderate intensity VNS (0.8 mA), high intensity VNS (1.6 mA), or sham stimulation was paired with forelimb movement (Fig. 1). Following completion of behavioral training, intracortical microstimulation (ICMS) was used to document movement representations in the motor cortex.
Moderate intensity 0.8 mA VNS enhances plasticity in motor cortex
Previous studies indicate that moderate intensity VNS paired with training drives substantial reorganization of cortical networks [23,27–29]. Group analysis of proximal forelimb representation area revealed a significant effect between groups (one-way ANOVA, F[3,29] = 8.03, p = 4.79 × 10−4). Moderate intensity 0.8 mA VNS paired with motor training significantly increased proximal forelimb representation compared to equivalent motor training without VNS (0.8 mA VNS: 2.86 ± 0.59 mm2; sham: 0.75 ± 0.27 mm2, Unpaired t-test, t(14) = −3.48, p = 3.69 × 10−3) (Fig. 2A, Fig. 3). These results are consistent with previous studies and demonstrate that pairing moderate intensity VNS with forelimb training enhances plasticity in motor cortex[22,35].
Figure 2. Moderate intensity VNS enhances plasticity in motor cortex.
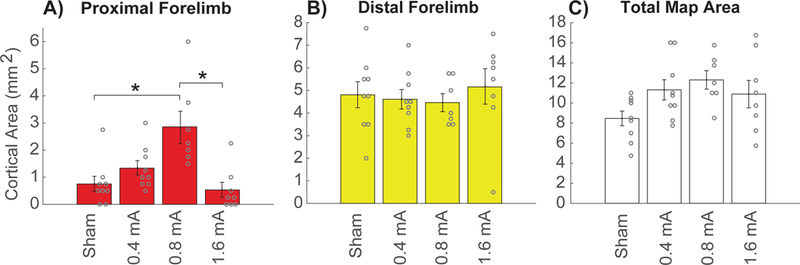
(A) Moderate intensity 0.8 mA VNS paired with forelimb motor training significantly increases the movement representation of the proximal forelimb in motor cortex compared to equivalent motor training without VNS (Sham). Low intensity 0.4 mA VNS and high intensity 1.6 mA VNS both fail to increase proximal forelimb representation compared to Sham. (B) No difference was observed in the area of distal forelimb representation across groups, indicating that VNS-dependent plasticity is specific to the trained movement. (C) No change in the total area of motor cortex was observed across groups. Circles depict individual subjects. Bars represent mean ± SEM. * denotes significant differences using Bonferroni-corrected p < 0.0083.
Figure 3. Average motor cortex movement representations.
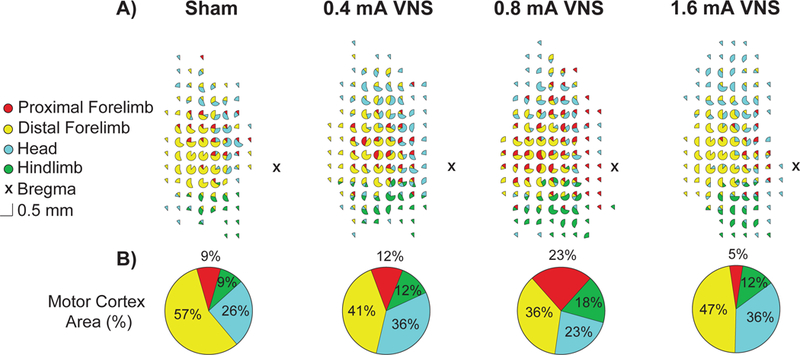
(A) Cumulative representations from all ICMS maps expressed as a percentage of representations observed at each electrode penetration for each group. (B) Average percentage of the total map devoted to each movement representation. Moderate intensity 0.8 mA VNS paired with forelimb training significantly increases the amount of motor cortex that represents the proximal forelimb compared equivalent training paired with high intensity 1.6 mA VNS.
Low intensity 0.4 mA VNS and high intensity 1.6 mA VNS fail to enhance plasticity in motor cortex
Next, we evaluated the effects of low intensity 0.4 mA VNS and high intensity 1.6 mA VNS paired with motor training. Low intensity VNS failed to increase proximal forelimb representation compared to equivalent motor training without VNS (0.4 mA VNS: 1.33 ± 0.26 mm2, sham: 0.75 ± 0.27 mm2, Unpaired t-test, t(16) = −1.54, p = 0.14) (Fig. 2A). Similarly, high intensity VNS also failed to increase proximal forelimb representation compared to equivalent motor training without VNS (1.6 mA VNS: 0.53 ± 0.27 mm2, sham: 0.75 ± 0.27 mm2, Unpaired t-test, t(15) = 0.56, p = 0.58). High intensity VNS also resulted in significantly less proximal forelimb representation than moderate intensity VNS (1.6 mA VNS: 0.53 ± 0.27 mm2, 0.8 mA VNS: 2.86 ± 0.59 mm2, Unpaired t-test, t(13) = 3.71, p = 2.60 × 10−3). Together these results suggest that VNS intensity has an inverted-U relationship with the magnitude of plasticity, consistent with previous studies in auditory cortex[23].
VNS-mediated plasticity is specific to the trained movement
Group analysis of other movement representations showed no significant differences between groups (one-way ANOVA, distal forelimb: F[3,29] = 0.28, p = 0.84, sham: 4.81 ± 0.56 mm2, 0.4 mA VNS: 4.61 ± 0.41 mm2, 0.8 mA VNS: 4.46 ± 0.38 mm2, 1.6 mA VNS: 5.16 ± 0.76 mm2; head: F[3,29] = 2.29, p = 0.10, sham: 2.17 ± 0.44 mm2, 0.4 mA VNS: 4.08 ± 0.66 mm2, 0.8 mA VNS: 2.82 ± 0.41 mm2, 1.6 mA VNS: 4.00 ± 0.74 mm2; hindlimb: F[3,29] = 2.14, p = 0.12, sham: 0.75 ± 0.20 mm2, 0.4 mA VNS: 1.36 ± 0.44 mm2, 0.8 mA VNS: 2.18 ± 0.54 mm2, 1.6 mA VNS: 1.34 ± 0.35 mm2) (Fig. 2B). Additionally, no differences in total motor cortex area were observed (one-way ANOVA, F[3,29] = 2.56, p = 0.07) (Fig. 2C). Group analysis of average response thresholds also showed no significant differences between groups (one-way ANOVA, F[3,29] = 2.05, p = 0.13). These results confirm that VNS-dependent enlargement of cortical movement representations is restricted to the paired movement and does not broadly influence cortical representations.
VNS does not alter behavioral performance
We tested whether differences in behavioral performance could account for observed VNS-dependent increase in proximal forelimb representation. Between-group analysis during behavioral training revealed no significant differences in total successful trials (one-way ANOVA, F[3,29] = 0.42, p = 0.74) total trials (one-way ANOVA, F[3,29] = 1.31, p = 0.29) success rate (one-way ANOVA, F[3,29] = 0.75, p = 0.53) nor inter-press-interval (one-way ANOVA, F[3,29] = 2.49, p = 0.08). Group analysis also revealed no differences in the number of stimulations received between groups during behavioral training (one-way ANOVA, F[3,29] = 0.99, p = 0.41) (Fig. 4A) or the average time between stimulations (one-way-ANOVA, F[3,29] = 1.19, p = 0.33) (Fig. 4B) which had a mean of 11.06 ± 1.13 seconds. These results suggest that expansion of map representations driven by moderate intensity VNS cannot be ascribed to changes in motivation, motor performance, or the amount of stimulations administered during training.
Figure 4. Amount of training or stimulation cannot explain moderate intensity VNS-dependent enhancement of plasticity.
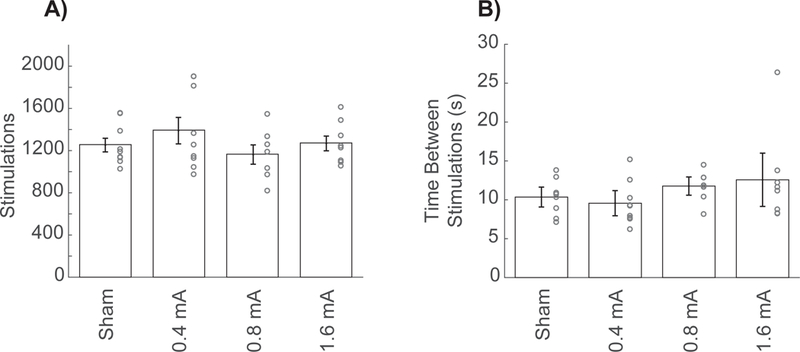
(A) No difference in the total number of stimulations received was observed across groups. (B) Additionally, no difference in the timing between stimulations was observed across groups. Together, these findings indicate that differences in the amount of stimulation or the timing between stimulations cannot account for the increased in proximal forelimb representation driven by moderate intensity 0.8 mA VNS. Circles depict individual subjects. Bars represent mean ± SEM.
Discussion
Repeatedly pairing VNS with motor or sensory training yields robust, specific cortical plasticity[1,19–22]. However, little is known about the stimulation parameters that most effectively drive VNS-dependent enhancement of plasticity in motor cortex. In this study, we report that the intensity of VNS paired with forelimb training exhibits an inverted-U relationship with plasticity, such that moderate intensity stimulation yields greater motor cortical reorganization than low or high intensity stimulation. VNS-dependent enhancement of plasticity is specific to the trained movement, with no changes observed in the representation of untrained movements. Differences in behavioral performance or number of stimulations administered cannot account for the observed enhancement of plasticity. These results provide evidence to guide the selection of parameters for VNS applications that aim to enhance motor cortical plasticity and promote recovery of motor function after neurological injury[1,2,4–11].
Consistent with findings reported here, previous studies of VNS document an inverted-U relationship between stimulation intensity and memory or plasticity in other brain regions, with moderate intensity VNS being most effective[23,27,36]. In the auditory cortex, low intensity 0.4 mA VNS was comparably effective to moderate intensity 0.8 mA VNS, while here we report that mA VNS fails to significantly enhance plasticity in motor cortex. It is possible that the difference in the effect of low intensity VNS arises from fundamental differences in auditory and motor cortices. Alternatively, this discrepancy could be explained by differences in interval between stimulations. In studies evaluating plasticity in auditory cortex, stimulations occur at a fixed interval of 30 seconds. In the present study, the interval between stimulations is variable and depends on the task performance, but averages approximately 11 seconds. The interval between stimulations is a major determinant of the degree of VNS-dependent plasticity, and reducing the amount of time between stimulations reduces VNS efficacy in auditory cortex[37]. Thus, the shorter interval in this study may account for the absence of plasticity using low intensity VNS and raises the possibility that lengthening the time between stimulations could restore VNS-dependent enhancement of motor cortex plasticity at low intensity stimulation. The results from the current study corroborate previous reports in which high intensity 1.6 mA VNS fails to enhance plasticity or memory[23,27–29].
While the vagus nerve mediates parasympathetic activity via descending projections, ascending projections are able to communicate information regarding arousing events from the periphery to the central nervous system. Ascending activation of the vagus nerve can occur in response to both beneficial stimuli, such as eating and digestion, or stimuli associated with a negative valence, such as stress, fear, and inflammation[38,39]. Vagal signals resulting from these events are able to enhance memory and promote learning, and several studies have demonstrated that the vagus nerve is required for peripherally-mediated enhancement of memory because the effect is blocked by vagal transection[29,40]. The locus coeruleus (LC), a major source of cortical norepinephrine, demonstrates rapid, phasic activation and releases norepinephrine in response to increasing intensities of VNS[14,18,25]. Similar to the effects of vagotomy on memory enhancement, lesions of the LC block central effects of VNS, suggesting norepinephrine plays a major role in VNS efficacy[26]. Thus, the vagus nerve is positioned to influence plasticity in response to a variety of peripheral stimuli and control of neuromodulatory networks by ascending vagal projections likely underlies VNS-dependent plasticity observed in response to electrical stimulation.
A variety of neuronal mechanisms could account for the inverted-U relationship observed between VNS intensity and enhancement of plasticity. VNS promotes plasticity by engaging multiple neuromodulatory networks during training[18,25]. Activation of these opposing neuromodulator actions or desensitization of receptors provide a mechanistic basis for the inverted-U. One potential explanation reflects the activation of opposing processes with different activation thresholds. In this case, VNS at moderate intensities sufficiently activates a low–threshold, pro-plasticity process and avoids activation of a high-threshold, anti-plasticity process, resulting in robust enhancement of plasticity (Fig. 5). Low stimulation intensities would fail to sufficiently activate the pro-plasticity process, precluding effective plasticity. At high stimulation intensities, activation of the anti-plasticity process would dominate and similarly prevent the enhancement of plasticity. Adrenergic receptors, which are activated by VNS-dependent norepinephrine release, express features that could underlie these opposing systems. Moderate intensities of VNS may result in sufficient norepinephrine release to engage higher-affinity α2-receptors and promote potentiation, whereas high intensity stimulation may increase norepinephrine levels further to activate lower-affinity β-receptors and oppose potentiation. Indeed, this concentration dependent control of the polarity of plasticity by adrenergic receptors has been described[41]. In addition to norepinephrine, several other neuromodulatory systems have been linked to the action of VNS in the central nervous system, including serotonin and acetylcholine [42–44]. Similar principles governing these neuromodulatory systems could also give rise to the inverted-U effect of VNS on plasticity.
Figure 5. Model of the Inverted-U relationship between VNS intensity and cortical plasticity.
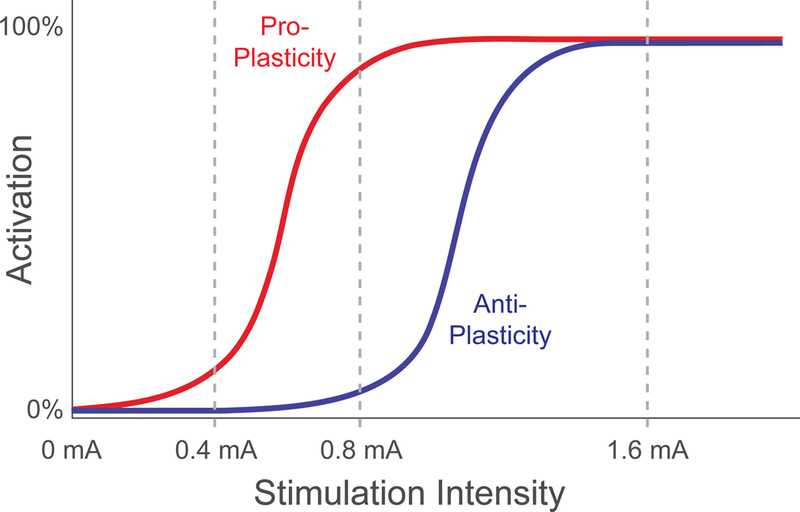
One potential explanation to account for the inverted-U relationship between VNS intensity and enhancement of plasticity relies on engagement of two opposing processes. At low stimulation intensities, VNS fails to drive sufficient activation of a low-threshold, pro-plasticity process (red) and thus fails to drive plasticity. At moderate stimulation intensities, VNS activates the low-threshold pro-plasticity process and avoids activation of a high-threshold, anti-plasticity process (blue), resulting in robust enhancement of plasticity. At high stimulation intensities, the anti-plasticity process dominates and prevents effective enhancement of plasticity.
Alternatively, a single desensitizing system could explain the inverted-U response. Moderate intensity stimulation would provide sufficient activation with minimal desensitization, while high levels of stimulation would result in overactivation and reduction of the response. G-protein coupled receptors, such as those which likely mediate the response to VNS-dependent engagement of the noradrenergic, cholinergic, and serotonergic systems, exhibit notable desensitization and may explain the inverted-U response to VNS[45]. Other effects of VNS on cortical neurons, including desynchronization and activation of hyperpolarizing currents, could potentially produce the network consequences observed in this study[44,46,47]. However, since cortical reorganization was assessed the day after the cessation of VNS, these neuronal effects would need to engender plasticity in order to mediate the lasting changes in movement representations. While all of these models can account for the inverted-U, they are not mutually exclusive and various other systems likely contribute. Future studies directed at manipulating the activation of neuromodulatory networks could provide insight into the neuronal mechanisms that underlie VNS-dependent enhancement of plasticity. Additionally, a clear understanding of activation of these neuromodulatory systems may lead to pharmacological manipulations to potentiate the effects of VNS.
At moderate intensity stimulation, the reorganization of cortical movement representations is likely produced by temporally-precise engagement of neuromodulatory activation by VNS. During performance of the behavioral task in this study, networks in motor cortex generate activity to produce movement of the proximal forelimb muscles. Delivery of VNS concurrent with movement-related neural activity provides precisely-timed neuromodulatory feedback, which likely facilitates canonical spike-timing-dependent plasticity mechanisms to enhance plasticity within the active motor networks[17,48,49]. The degree of activation of these neuromodulatory networks is contingent upon the intensity of VNS[18], and results from the present study support the notion that moderate intensity VNS produces favorable neuromodulatory activation to facilitate plasticity. This VNS-dependent enhancement of plasticity forms the basis for the use of VNS in treatment of movement disorders.
A number of studies in preclinical models and humans demonstrate that VNS paired with rehabilitative training supports recovery in a wide range of neurological disorders including stroke, traumatic brain injury, spinal cord injury[1–11]. Recovery after these injuries is thought to be dependent on plasticity in central networks after injury[12,13]. Indeed, VNS paired with rehabilitative training drives large-scale synaptic reorganization in motor control networks after stroke and spinal cord injury[10,12]. The VNS-dependent plasticity in corticospinal, corticorubral, and propriospinal networks likely underpins the improvements in recovery of function. Consequently, there is great interest in identifying paradigms that maximize plasticity and thereby yield greater recovery. The present study characterizes the effect of stimulation intensity across a range of parameters and establishes a framework for future studies to directly evaluate the effect of varying VNS intensity on plasticity and recovery after neurological injury. Ultimately, these findings may facilitate determination of optimal parameters for clinical application.
Supplementary Material
Research Highlights.
Recovery after neurological injury is thought to be dependent on plasticity
Moderate intensity VNS paired with motor training enhances motor cortex plasticity
Low and high intensity VNS paired with motor training fail to enhance plasticity
The intensity of stimulation is a critical factor in VNS-dependent plasticity
Optimizing stimulation paradigms may enhance VNS efficacy in clinical populations
Acknowledgements
We would like to thank Tanya Danaphongse and David Pruitt for surgical support, and Christine Mai Shedd, Aaron Kuo, Yun-Yee Tsang, Prathima Kandukuri, Ayushi Bisaria, Stephanie Abe, Alissar Zammam, Naser Asfoor, Eric Bellinghausen, Nicole Pillai, and Joanna John for behavioral training. We would also like to thank Sadmaan Sarker, Peyton Demetrovich, Joy Mong, and Helena Zhang for support in ICMS. Further thanks goes to Kimi Rahebi, Lena Lynn Sadler, and Matt Buell for vagus nerve cuff construction.
Sources of Funding
This work was supported by the National Institutes of Health R01NS085167 and R01NS094384 and by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Electrical Prescriptions (ElectRx) program under the auspices of Dr. Eric Van Gieson through the Space and Naval Warfare Systems Center, Pacific Cooperative Agreement No. N66001-15-2-4057 and the DARPA BTO Targeted Neuroplasticity Training (TNT) program under the auspices of Dr. Tristan McClure-Begley through the Space and Naval Warfare Systems Center, Pacific Grant/Contract No. N66001-17-2-4011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
MPK has a financial interesting in MicroTransponder, Inc., which is developing VNS for stroke and tinnitus. All other authors declare no conflicts of interest.
References
- [1].Hays SA, Rennaker RL, Kilgard MP. Targeting Plasticity with Vagus Nerve Stimulation to Treat Neurological Disease. Prog Brain Res 2013;207:275–99. doi: 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, et al. Vagus Nerve Stimulation Delivered with Motor Training Enhances Recovery of Function after Traumatic Brain Injury. J Neurotrauma 2016;33:871–9. doi: 10.1089/neu.2015.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kimberley TJ, Pierce D, Prudente CN, Francisco GE, Yozbatiran N, Smith P, et al. Vagus Nerve Stimulation Paired With Upper Limb Rehabilitation After Chronic Stroke. Stroke 2018;49:2789–92. doi: 10.1161/STROKEAHA.118.022279. [DOI] [PubMed] [Google Scholar]
- [4].Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis 2013;60:80–8. doi: 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- [5].Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, et al. Vagus Nerve Stimulation Delivered During Motor Rehabilitation Improves Recovery in a Rat Model of Stroke. Neurorehabil Neural Repair 2014;28:698–706. doi: 10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hays SA, Khodaparast N, Ruiz A, Sloan AM, Hulsey DR, Rennaker RL, et al. The timing and amount of vagus nerve stimulation during rehabilitative training affect poststroke recovery of forelimb strength. Neuroreport 2014;25:682–8. doi: 10.1097/WNR.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, et al. Vagus Nerve Stimulation During Rehabilitative Training Improves Functional Recovery After Intracerebral Hemorrhage. Stroke 2014;45:3097–100. doi: 10.1161/STROKEAHA.114.006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, et al. Vagus Nerve Stimulation During Rehabilitative Training Improves Forelimb Recovery After Chronic Ischemic Stroke in Rats. Neurorehabil Neural Repair 2016;30:676–84. doi: 10.1177/1545968315616494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hays SA, Ruiz A, Bethea T, Khodaparast N, Carmel JB, Rennaker RL, et al. Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol Aging 2016;43:111–8. doi: 10.1016/j.neurobiolaging.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL, et al. Vagus Nerve Stimulation Enhances Stable Plasticity and Generalization of Stroke Recovery. Stroke 2018;49:710–7. doi: 10.1161/STROKEAHA.117.019202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, et al. Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Ischemic Stroke. Stroke 2016;47:143–50. doi: 10.1161/STROKEAHA.115.010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ganzer PD, Darrow MJ, Meyers EC, Solorzano BR, Ruiz AD, Robertson NM, et al. Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. Elife 2018;7:1–19. doi: 10.7554/eLife.32058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pruitt DT, Danaphongse TT, Schmid AN, Morrison RA, Kilgard MP, Rennaker RL, et al. Traumatic Brain Injury Occludes Training-Dependent Cortical Reorganization in the Contralesional Hemisphere. J Neurotrauma 2017;34:2495–503. doi: 10.1089/neu.2016.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res 2006;1119:124–32. doi: 10.1016/j.brainres.2006.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dorr AE. Effect of Vagus Nerve Stimulation on Serotonergic and Noradrenergic Transmission. J Pharmacol Exp Ther 2006;318:890–8. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- [16].Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- [17].Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, et al. Neuromodulators Control the Polarity of Spike-Timing-Dependent Synaptic Plasticity. Neuron 2007;55:919–29. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 2017;289:21–30. doi: 10.1016/j.expneurol.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011;470:101–4. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shetake JA, Engineer ND, Vrana WA, Wolf JT, Kilgard MP. Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Exp Neurol 2012;233:342–9. doi: 10.1016/j.expneurol.2011.10.026. [DOI] [PubMed] [Google Scholar]
- [21].Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP. Pairing Speech Sounds With Vagus Nerve Stimulation Drives Stimulus-specific Cortical Plasticity. Brain Stimul 2015;8:637–44. doi: 10.1016/j.brs.2015.01.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, et al. Repeatedly Pairing Vagus Nerve Stimulation with a Movement Reorganizes Primary Motor Cortex. Cereb Cortex 2012;22:2365–74. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- [23].Borland MS, Vrana WA, Moreno NA, Fogarty EA, Buell EP, Sharma P, et al. Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity. Brain Stimul 2016;9:117–23. doi: 10.1016/j.brs.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Castoro MA, Yoo PB, Hincapie JG, Hamann JJ, Ruble SB, Wolf PD, et al. Excitation properties of the right cervical vagus nerve in adult dogs. Exp Neurol 2011;227:62–8. doi: 10.1016/j.expneurol.2010.09.011. [DOI] [PubMed] [Google Scholar]
- [25].Hulsey DR. Neuromodulatory pathways required for targeted plasticity therapy The University of Texas at Dallas, 2018. [Google Scholar]
- [26].Krahl SE, Clark KB, Smith DC, Browning RA. Locus Coeruleus Lesions Suppress the Seizure-Attenuating Effects of Vagus Nerve Stimulation. Epilepsia 1998;39:709–14. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- [27].Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training Unilateral Vagal Stimulation Enhances Retention Performance in the Rat. Neurobiol Learn Mem 1995;63:213–6. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- [28].Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Posttraining Electrical Stimulation of Vagal Afferents with Concomitant Vagal Efferent Inactivation Enhances Memory Storage Processes in the Rat. Neurobiol Learn Mem 1998;70:364–73. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- [29].Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 1999;2:94–8. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- [30].Hays SA, Khodaparast N, Sloan AM, Fayyaz T, Hulsey DR, Ruiz AD, et al. The bradykinesia assessment task: An automated method to measure forelimb speed in rodents. J Neurosci Methods 2013;214:52–61. doi: 10.1016/j.jneumeth.2012.12.022. [DOI] [PubMed] [Google Scholar]
- [31].Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, et al. Functional Organization of Adult Motor Cortex Is Dependent upon Continued Protein Synthesis. Neuron 2003;40:167–76. doi: 10.1016/S0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- [32].Neafsey EJ, Sievert C. A second forelimb motor area exists in rat frontal cortex. Brain Res 1982;232:151–6. doi: 10.1016/0006-8993(82)90617-5. [DOI] [PubMed] [Google Scholar]
- [33].Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, et al. The organization of the rat motor cortex: A microstimulation mapping study. Brain Res Rev 1986;11:77–96. doi: 10.1016/0165-0173(86)90011-1. [DOI] [PubMed] [Google Scholar]
- [34].Pruitt DT, Schmid AN, Danaphongse TT, Flanagan KE, Morrison RA, Kilgard MP, et al. Forelimb training drives transient map reorganization in ipsilateral motor cortex. Behav Brain Res 2016;313:10–6. doi: 10.1016/j.bbr.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, et al. Reorganization of Motor Cortex by Vagus Nerve Stimulation Requires Cholinergic Innervation. Brain Stimul 2016;9:174–81. doi: 10.1016/j.brs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zuo Y, Smith DC, Jensen RA. Vagus nerve stimulation potentiates hippocampal LTP in freely-moving rats. Physiol Behav 2007;90:583–9. doi: 10.1016/j.physbeh.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Borland MS, Engineer CT, Vrana WA, Moreno NA, Engineer ND, Vanneste S, et al. The Interval Between VNS-Tone Pairings Determines the Extent of Cortical Map Plasticity. Neuroscience 2018;369:76–86. doi: 10.1016/j.neuroscience.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schwartz MW. Central Nervous System Regulation of Food Intake. Obesity 2006;14:1S– 8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- [39].Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- [40].Williams CL, Jensen RA. Effects of vagotomy on leu-enkephalin-induced changes in memory storage processes. Physiol Behav 1993;54:659–63. doi: 10.1016/0031-9384(93)90073-O. [DOI] [PubMed] [Google Scholar]
- [41].Salgado H, Köhr G, Treviño M. Noradrenergic ‘Tone’ Determines Dichotomous Control of Cortical Spike-Timing-Dependent Plasticity. Sci Rep 2012;2:417. doi: 10.1038/srep00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Manta S, El Mansari M, Debonnel G, Blier P. Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int J Neuropsychopharmacol 2013;16:459–70. doi: 10.1017/S1461145712000387. [DOI] [PubMed] [Google Scholar]
- [43].Manta S, Dong J, Debonnel G, Blier P. Optimization of vagus nerve stimulation parameters using the firing activity of serotonin neurons in the rat dorsal raphe. Eur Neuropsychopharmacol 2009;19:250–5. doi: 10.1016/j.euroneuro.2008.12.001. [DOI] [PubMed] [Google Scholar]
- [44].Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience 2011;189:207–14. doi: 10.1016/j.neuroscience.2011.05.024. [DOI] [PubMed] [Google Scholar]
- [45].Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 2004;27:107–44. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- [46].Chase MH, Nakamura Y, Clemente CD, Sterman MB. Afferent vagal stimulation: Neurographic correlates of induced eeg synchronization and desynchronization. Brain Res 1967;5:236–49. doi: 10.1016/0006-8993(67)90089-3. [DOI] [PubMed] [Google Scholar]
- [47].Zagon A, Kemeny AA. Slow Hyperpolarization in Cortical Neurons: A Possible Mechanism Behind Vagus Nerve Simulation Therapy for Refractory Epilepsy? Epilepsia 2000;41:1382–9. doi: 10.1111/j.1528-1157.2000.tb00113.x. [DOI] [PubMed] [Google Scholar]
- [48].Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends Neurosci 2012;35:715–22. doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].He K, Huertas M, Hong SZ, Tie X, Hell JW, Shouval H, et al. Distinct Eligibility Traces for LTP and LTD in Cortical Synapses. Neuron 2015;88:528–38. doi: 10.1016/j.neuron.2015.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


