Abstract
Background
Phthalates are ubiquitous endocrine disrupting chemicals present in a wide variety of consumer products. However, the personal characteristics associated with phthalate exposure are unclear.
Objectives
We sought to describe personal, behavioral, and reproductive characteristics associated with phthalate metabolite concentrations in an ongoing study nested within the Women’s Health Initiative (WHI).
Materials and Methods
We measured thirteen phthalate metabolites in two or three archived urine samples collected in 1993–2001 from each of 1,257 WHI participants (2,991 observations). We fit multivariable generalized estimating equation models to predict urinary biomarker concentrations from personal, behavioral, and reproductive characteristics.
Results
Older age was predictive of lower concentrations of monobenzyl phthalate (MBzP), monocarboxyoctyl phthalate (MCOP), mono-3-carboxypropyl phthalate (MCPP), and the sum of di-n-butyl phthalate metabolites (ΣDBP). Phthalate metabolite concentrations varied by race/region, with generally higher concentrations observed among non-Whites and women from the West region. Higher neighborhood socioeconomic status predicted lower MBzP concentrations, and higher education predicted lower monoethyl phthalate (MEP) and higher concentrations of the sum of metabolites of di-isobutyl phthalate (ΣDiBP). Overweight/obesity predicted higher MBzP, MCOP, monocarboxynonyl phthalate (MCNP), MCPP, and the sum of metabolites of di(2-ethylhexyl) phthalate (ΣDEHP) and lower MEP concentrations. Alcohol consumption predicted higher concentrations of MEP and ΣDBP, while current smokers had higher ΣDBP concentrations. Better diet quality as assessed by Healthy Eating Index 2005 scores predicted lower concentrations of MBzP, ΣDiBP, and ΣDEHP.
Conclusion
Factors predictive of lower biomarker concentrations included increased age and healthy behaviors (e.g. lower alcohol intake, lower body mass index, not smoking, higher quality diet, and moderate physical activity). Racial group (generally higher among non-Whites) and geographic regions (generally higher in Northeast and West compared to South regions) also were predictive of phthalate biomarker concentrations.
Introduction
Increasing scientific evidence supports the endocrine-disrupting and potentially carcinogenic effects of some phthalates, chemicals used in plastics and in a wide variety of consumer products either to increase the flexibility of the plastic or as solvents (e.g. vinyl flooring, cosmetics, medical supplies, medications) (Koch and Calafat 2009). Although nearly all U.S. residents have detectable concentrations of phthalate metabolites in their urine, the concentrations vary widely (Silva et al. 2004).
Phthalate exposure can occur through inhalation, ingestion, or transdermal absorption because phthalates are not chemically bound to plastics and thus can be easily released into the environment (Centers for Disease Control and Prevention 2014). Phthalates are rapidly metabolized in the body into their monoesters, a step that can increase their bioactivity (Frederiksen et al. 2007). High molecular weight phthalates undergo further oxidation; phthalate metabolites may also be glucuronidated (Frederiksen et al. 2007; Koch and Calafat 2009; Silva et al. 2004). Because metabolites are eliminated nearly completely in urine within 24–28 hours after exposure (Frederiksen et al. 2007), measurement of urinary phthalate metabolites gives an accurate assessment of recent exposure to phthalates; such measurements would reflect both ongoing, and potentially low-level exposures, as well as changing exposures over time. Indeed, prior work has established the moderate within-person variability of urinary phthalate biomarker concentrations (Adibi et al. 2008; Braun et al. 2012; Cantonwine et al. 2014; Peck et al. 2010; Townsend et al. 2013; Watkins et al. 2014).
Extensive research has documented the endocrine-disrupting effects of phthalates. Phthalates may have important, adverse effects on female reproductive function, although data are not entirely consistent (Kay et al. 2013). Previous studies have related urinary concentrations of various phthalate biomarkers to adverse human health effects in women including endometriosis (Buck Louis et al. 2013; Upson et al. 2013; Weuve et al. 2010), elevated body mass index (BMI) (Hatch et al. 2008; Trasande et al. 2012) and weight gain (Song et al. 2014), diabetes (James-Todd et al. 2012) and insulin resistance (Trasande et al. 2013), and breast cancer (Holmes et al. 2014;)Lopez-Carrillo et al. 2010), although a recent study reported no association between urinary phthalate biomarkers and breast cancer risk (Parada et al. 2018). Exposure to phthalates may be related to increased risk of a wide range of chronic health outcomes.
Predictors of phthalate exposure are not well understood, though phthalate metabolite concentrations have been associated with diet (Cantonwine et al. 2014; Colacino et al. 2010), personal care product use (Cantonwine et al. 2014; Duty et al. 2005; Parlett et al. 2013), medications (Hauser et al. 2004; Hernandez-Diaz et al. 2009), and elevated BMI and body fat (Hatch et al. 2008; Lind et al. 2012). We sought to describe personal, behavioral, and reproductive characteristics associated with phthalate metabolite concentrations in an ongoing study nested within the Women’s Health Initiative (WHI).
Materials and Methods
Study Population
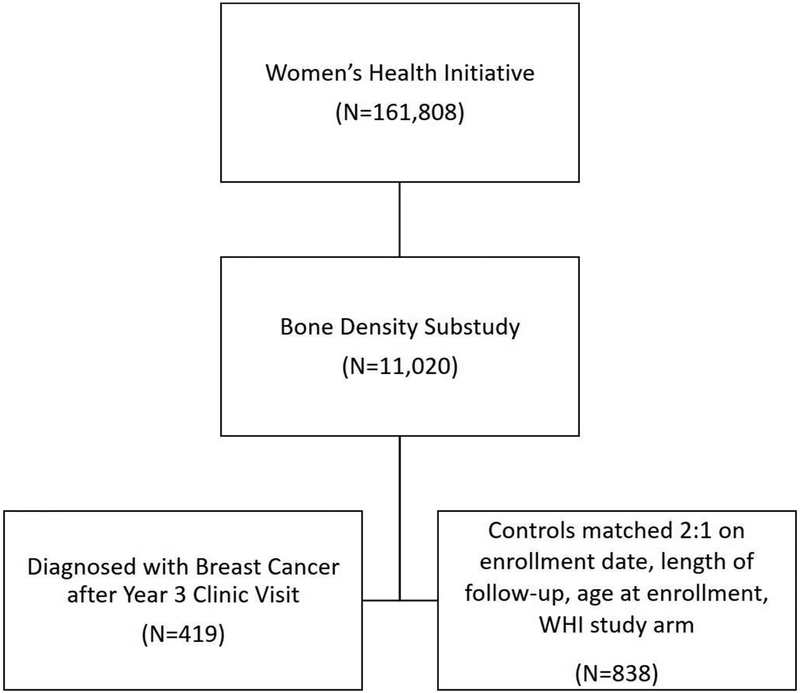
Participants in the WHI were recruited from 40 clinical centers nationwide in the United States between October 1, 1993 and December 21, 1998, and details relevant to participant recruitment have been reported previously (Women’s Health Initiative 1998). Briefly, the WHI consisted of three clinical trials (CT): postmenopausal hormone therapy (HT; N=27,347), dietary modification (DM; N=48,835), and calcium/vitamin D supplementation (CaD; N=36,282); participants were able to take part in more than one trial. Women who were either not interested or not eligible for the CT were enrolled in an observational study (OS; N=93,676). A total of 161,808 women participated in the WHI. All participants were between the ages of 50 and 79 at the time of enrollment. Women at three WHI clinical sites (N=11,020) also participated in a bone mineral density substudy, and these were the only sites at which urine samples were collected.
Participants in this analysis were those selected as either cases (N=419) or controls (N=838) for a nested case-control study of phthalate exposure and breast cancer risk within the WHI (Figure 1). Eligible women for the parent study were those without a self-reported history of cancer, except for non-melanoma skin cancer, and who had sufficient urine available from baseline and the year 3 clinic visit. Cases were all adjudicated cases of invasive breast cancer occurring among women at the three bone density substudy sites after AV3 and during their WHI follow-up through 2013. Cases diagnosed prior to AV3 were excluded. The final (AV3) urine sample was provided an average of 6.2 years (SD 4.3) prior to breast cancer diagnosis among cases. Controls were selected using incidence density matching from among eligible participants who were not diagnosed with breast cancer or any other cancer, except for nonmelanoma skin cancer, during WHI follow-up and had urine sample available. Those eligible were individually matched on enrollment date, length of follow-up, age at enrollment, and WHI study arm in a 2:1 ratio to cases. When more than two eligible controls existed as a potential match for a case, two controls were randomly selected from the pool of eligible controls.
Figure 1.
Illustration of selection of study population included in present analysis.
First morning void urine samples were collected at home and processed within 30 minutes after participants arrived at each clinic visit. The samples were frozen and stored until they were thawed and processed for this analysis. WHI recommended, but did not require, the use of polypropylene urine collection containers, which are phthalate-free. Two clinical centers did not use the specific containers recommended, and the composition of the containers that were used is unknown. However, all sites used polypropylene centrifuge tubes and cryovials. Concerns over contamination are minimal, however, because we measured metabolites (which reflect endogenous exposure and are unlikely to originate from external contamination) rather than the parent phthalate (which would be the contaminant from the collection vial). The present analysis included urine samples collected at baseline (N=1,257) and at the year 3 (AV3) clinic visit (N=1,191); additionally, we included urine samples collected from the year 1 (AV1) clinic visit for women enrolled in the WHI CT (N=570).
All participants provided written informed consent upon enrollment into the WHI. The WHI was approved by institutional review boards (IRB) at each clinical center. Additionally IRB approval for the present study was obtained from the University of Massachusetts Amherst. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory in the analysis of samples did not constitute engagement in human subjects research.
Data Ascertainment
Participants provided data at an initial enrollment clinic visit and at the year 3 clinic visit; additionally, WHI CT participants provided data at the year 1 clinic visit. Pertinent to this analysis, data were collected via self-reported questionnaire on age, race, educational attainment, marital status, occupation, alcohol use, smoking duration and amount, total physical activity, hormone therapy (HT) use, pregnancy history, breastfeeding history, infertility history, hysterectomy, and other medical history. Because race/ethnicity was strongly related to U.S. census region due to the nature of the WHI study design for the bone density centers, we created a joint race/region variable for use in this analysis. We included conditions that have been related to phthalate exposure in prior studies (thyroid disease, based on reported associations between phthalate exposure and thyroid hormone levels (Boas et al. 2012; Meeker and Ferguson 2011), and diabetes (Sun et al. 2014)) or that are treated with medications known to contain high concentrations of phthalates (Hauser et al. 2004; Hernandez-Diaz et al. 2009) (stomach ulcer, diverticulitis, and ulcerative colitis). Height and weight were measured by trained study personnel and used to calculate BMI. Medication use and dietary intake are complex exposures that have been previously associated with phthalate exposure and will be addressed in separate analyses; however, we did explore menopausal hormone therapy use and a measure of diet quality as predictors of phthalate metabolites in the current analysis. The Healthy Eating Index 2005 (HEI-2005) score was calculated as a measure of diet quality based on the U.S. Department of Agriculture 2005 Dietary Guidelines for Americans from self-reported food frequency questionnaire data (George et al. 2014; Guenther et al. 2008); higher scores on the HEI-2005 reflect a better quality diet as defined by the USDA 2005 Dietary Guidelines (e.g. consuming the recommended amounts of fruits versus not consuming any fruit). We utilized a previously calculated assessment of neighborhood socioeconomic status (Griffin et al. 2013). Updated data on BMI, alcohol use, smoking status, physical activity, hysterectomy, and hormone therapy use were available at years 1 and 3 and were incorporated in the analysis.
Quantification of Urinary Phthalate Metabolites and Creatinine
WHI followed a standard urine collection, processing, and storage protocol at the three clinical centers that collected urine samples. Urine samples were collected at home and processed within 30 minutes after participants arrived at their clinic visit. Urine samples were centrifuged for 5 minutes at 1330 x g and 1.8mL aliquots were frozen and then shipped to McKesson Bioservices packed in dry ice via overnight FedEx where they were stored at −70 °C.
A panel of thirteen phthalate metabolites was measured in baseline, year 1, and year 3 urine samples at the CDC: mono-ethyl phthalate (MEP), mono-n-butyl phthalate (MBP), monohydroxybutyl phthalate (MHBP), mono-isobutyl phthalate (MiBP), mono-hydroxyisobutyl phthalate (MHiBP), monobenzyl phthalate (MBzP), mono(3-carboxypropyl) phthalate (MCPP), four DEHP metabolites (mono(2-ethylhexyl) phthalate [MEHP], mono(2-ethyl-5-hydroxyhexyl) phthalate [MEHHP], mono(2-ethyl-5-oxohexyl) phthalate [MEOHP], mono(2-ethyl-5-carboxypentyl) phthalate [MECPP]), mono-carboxyoctyl phthalate (MCOP), and monocarboxynonyl phthalate (MCNP). Phthalate biomarkers were measured after enzymatic hydrolysis of the conjugated metabolites followed by on-line solid phase extraction coupled to high performance liquid chromatography-electrospray ionization-isotope dilution tandem mass spectrometry. Complete details of the assay are published online at https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/PHTHTE_H_MET_Phthalates.pdf. The limits of detection (LODs) were in the low ng/mL range (0.2 ng/mL – 0.6 ng/mL). We imputed phthalate metabolite concentrations below the limit of detection (LOD) to a value equal to the LOD divided by the square root of two, following the protocol used for NHANES (Hornung and Reed 1990): MBP (n=2, 0.07%), MEHP (n=19, 0.63%), MHBP (n=13, 0.43%), MHiBP (n=47, 1.56%), MiBP (n=14, 0.46%); all concentrations of MBzP, MCNP, MCOP, MCPP, MECPP, MEHHP, MEOHP, MEP, and creatinine were >LOD. Study samples were randomly distributed through the analytical batches, with cases and matched controls analyzed together. A blinded 10% quality control sample was included, and estimated CVs were as follows: MBP 5.4%, MBzP 6.1%, MCNP 4.7%, MCOP 6.3%, MCPP 5.8%, MECPP 4.3%, MEHHP 5.4%, MEHP 19.5%, MEOHP 6.0%, MEP 3.1%, MHBP 9.0%, MHiBP 21.9%, MiBP 10.3%. All laboratory staff were masked to the identity, disease status, and demographic and risk factor characteristics of the samples. Creatinine was also measured by using an enzymatic assay at CDC on a Roche Modular P Chemistry Analyzer (Indianapolis, IN). The LOD for creatinine was 1 mg/dL and the CV was 2.5%.
Statistical Analysis
Phthalate metabolite concentrations were natural log transformed to improve normality. Phthalate metabolites were analyzed individually and using summary scores. For metabolites of the same parent compound, we created a summary biomarker by dividing each metabolite of a single parent by its molecular weight and then summing across metabolites (Hauser et al. 2016; Watkins et al. 2014). Sum of di-n-butyl phthalate (ΣDBP) was calculated as the molar sum of MBP and MHBP, sum of di-isobutyl phthalate (ΣDiBP) was calculated as the molar sum of MiBP and MHiBP, and sum of di(2-ethylhexyl) phthalate (ΣDEHP) was calculated as the molar sum of MEHP, MEHHP, MEOHP, and MECPP. Pearson correlation coefficients were calculated to evaluate correlations among the phthalate biomarker concentrations, using the individual replicate measures.
We fit generalized estimating equation (GEE) models, using an identity link and exchangeable correlation, to explore the characteristics that predict phthalate exposures. . This modeling approach allowed for each observation to contribute to the analysis as many replicate measurements as available and included updated covariate information for each timepoint (e.g. age, BMI) as appropriate. We initially fit single predictor models for each phthalate biomarker concentrations including creatinine and a single covariate. We considered the following possible predictors: age (continuous, years), race and clinical site (white/Northeast, non-white/Northeast, white/South, non-white/South, white/West, non-white/West), education (<high school, high school/some college, college degree and higher), marital status (single/divorced/widowed, married/marriage-like relationship), occupation (managerial/professional, technical/sales/administrative, service/labor, homemaker only), BMI (underweight/normal [<25 kg/m2], overweight [25-<30 kg/m2], obese [≥30 kg/m2]), current alcohol intake (non-drinker, <1 drink per week, 1–6 drinks per week, ≥7 drinks per week), smoking status (never smoked, past smoker, current smoker), total physical activity (continuous, MET-h/wk) unopposed estrogen use (never, past, current), estrogen + progestin use (never, past, current), any hormone therapy use (never, past, current), parity (nulliparous, 1–3 children, ≥4 children), breastfeeding duration (never breastfed, 1–6 months, 7–12 months, 13–23 months, ≥24 months), infertility (no, yes), hysterectomy (no, yes), ever had thyroid disease (no, yes), ever had diabetes (no, yes), ever had stomach ulcer (no, yes), ever had diverticulitis (no, yes), ever had ulcerative colitis (no, yes), healthy eating index score (continuous), and neighborhood SES index (continuous).
We then fit multivariable regression models considering first all predictors with a p value less than 0.25 from the bivariate GEE models. We used a backward selection approach to select a final, parsimonious model for each phthalate biomarker where all variables significant at the p≤0.05 level were retained; age was included in all models regardless of statistical significance. We calculated predicted means and 95% confidence intervals (CI) for each variable based on the final parsimonious models, with all covariates held at their means. For categorical variables, we calculated the mean for each level of the variable; for continuous variables, we calculated means at the midpoint value of each quartile. As a sensitivity analysis, we repeated our analyses among the subsample of participants selected as controls. All analyses were performed using Stata version 15.0 (Stata Corp, College Station, TX).
Results
Table 1 describes the personal, behavioral, and reproductive characteristics of the study group included in these analyses at WHI enrollment. Participants were, on average, 62.5 years old (SD 6.9), and the majority was of white race (83.2%). Participants were nearly evenly distributed between enrollment in the Clinical Trial (47.3%) and Observational Study (52.7%) and across BMI categories. Participants had a low level of current alcohol use (32.8% current non-drinkers) and smoking (6.2% current smokers), a high level of current HT use (38.6%), and a high neighborhood SES score (73.1, SD 8.7).
Table 1.
Descriptive characteristics of study group, N=1257
| Characteristic | Mean (SD) |
|---|---|
| Age, years | 62.5 (6.9) |
| Neighborhood SES Index | 73.1 (8.7) |
| Healthy eating index score | 66.8 (10.9) |
| Total physical activity, MET-h/week | 12.0 (14.4) |
| Characteristic | N (%) |
| Race/Region | |
| White/Northeast | 467 (37.2) |
| White/South | 215 (17.1) |
| White/West | 363 (28.9) |
| Non-white/Northeast | 28 (2.2) |
| Non-white/South | 104 (8.3) |
| Non-white/West | 80 (6.4) |
| WHI Study Participation | |
| Clinical trial(s) | 595 (47.3) |
| Observational study | 662 (52.7) |
| Education level | |
| <High school | 345 (27.4) |
| High school/Some college | 456 (36.3) |
| College degree and higher | 450 (35.8) |
| Marital status | |
| Single/divorced/widowed | 405 (32.2) |
| Married/married like relationship | 852 (67.8) |
| Occupation | |
| Managerial/Professional | 371 (29.5) |
| Technical/Sales/Admin | 300 (23.9) |
| Service/Labor | 182 (14.5) |
| Homemaker only | 121 (9.6) |
| Body mass index, kg/m2 | |
| Underweight/Normal, <25 kg/m2 | 419 (33.3) |
| Overweight, 25-<30 kg/m2 | 449 (35.7) |
| Obese, ≥30 kg/m2 | 381 (30.3) |
| Alcohol intake | |
| Non-drinker | 412 (32.8) |
| <1 drink/week | 431 (34.3) |
| 1–6 drinks/week | 288 (22.9) |
| ≥7 drinks/week | 117 (9.3) |
| Smoking status | |
| Never Smoked | 698 (55.5) |
| Past Smoker | 461 (36.7) |
| Current Smoker | 78 (6.2) |
| Unopposed estrogen use | |
| Never | 814 (64.8) |
| Past | 138 (11.0) |
| Current | 303 (24.1) |
| Estrogen + Progestin use | |
| Never | 983 (78.2) |
| Past | 92 (7.3) |
| Current | 182 (14.5) |
| Any hormone therapy use | |
| Never | 584 (46.5) |
| Past | 186 (14.8) |
| Current | 485 (38.6) |
| Parity | |
| Nulliparous | 128 (10.2) |
| 1–3 children | 718 (57.1) |
| ≥4 children | 405 (32.2) |
| Breastfeeding duration | |
| Never breastfed | 554 (44.1) |
| 1–6 months | 357 (28.4) |
| 7–12 months | 146 (11.6) |
| 13–23 months | 112 (8.9) |
| ≥24 months | 79 (6.3) |
| Ever thyroid disease | 286 (22.8) |
| Ever diabetes | 59 (4.7) |
| Ever stomach ulcer | 92 (7.3) |
| Ever diverticulitis | 80 (6.4) |
| Ever ulcerative colitis | 18 (1.4) |
Abbreviations used: MET, metabolic equivalent; SD, standard deviation; SES, socioeconomic status; WHI, Women’s Health Initiative
Descriptive statistics of phthalate biomarkers concentrations are presented in Table 2, calculated from all measurements of phthalate biomarker concentrations in samples from baseline, year 1, and year 3 using GEE models to account for repeated measurements within a participant. Exposure to phthalates was common but highly variable. For example, MEP was detected in 100% of samples with a broad range of concentrations (12.7 ng/mL, 5th percentile – 773.0 ng/mL, 95th percentile). Concentrations of phthalate metabolites from the same parent compound (e.g. DiBP) were highly correlated with one another (e.g. MHiBP and MiBP, r=0.95). MBzP was highly correlated with MHBP concentrations (r=0.74). MCNP was significantly correlated with MCOP (r=0.40) and MCPP (r=0.36), and MCOP and MCPP also were highly correlated (r=0.60). Concentrations of other phthalate biomarkers were not strongly correlated with one another.
Table 2.
Descriptive statistics of phthalate biomarker concentrationsa
| Phthalate Biomarker | Geometric Mean | 5th Percentile | 25th Percentile | 75th Percentile | 95th Percentile |
|---|---|---|---|---|---|
| MEP, ng/mL | 77.7 | 12.7 | 31.8 | 163.0 | 773.0 |
| MBzP, ng/mL | 11.2 | 1.8 | 5.9 | 22.0 | 58.5 |
| MCOP, ng/mL | 3.8 | 0.9 | 2.1 | 6.5 | 18.5 |
| MCNP, ng/mL | 2.9 | 0.8 | 1.6 | 4.7 | 14.0 |
| MCPP, ng/mL | 3.1 | 0.7 | 1.7 | 5.4 | 13.6 |
| ΣDBP, μmol/Lb | 0.114 | 0.018 | 0.059 | 0.23 | 0.657 |
| ΣDiBP, μmol/Lb | 0.013 | 0.002 | 0.006 | 0.025 | 0.076 |
| ΣDEHP, μmol/Lb | 0.185 | 0.037 | 0.101 | 0.331 | 0.925 |
Statistics were calculated on natural log-transformed values and were back-transformed to original scale for presentation; includes measurements from baseline, year 1, and year 3
ΣDBP was calculated as the molar sum of MBP and MHBP; ΣDiBP was calculated as the molar sum of MiBP and MHiBP; ΣDEHP was calculated as the molar sum of MEHP, MEHHP, MEOHP, and MECPP
Abbreviations used: MEP, mono-ethyl phthalate; MBzP, monobenzyl phthalate; MCOP, monocarboxyoctyl phthalate; MCNP, monocarboxynonyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; DBP, di-n-butyl phthalate; DiBP, di-isobutyl phthalate; DEHP, di(2-ethylhexyl) phthalate
Table 3 presents the results of our parsimonious, multivariable regression modeling of MEP, MBzP, MCOP, MCNP, and MCPP concentrations. Predictors of MEP concentrations were race/region (higher among non-whites), education level (lower among college educated women), BMI (lower among obese women), and current alcohol intake (higher with increased intake). MBzP concentrations were inversely related to age. Non-white/Northeast and non-white/South participants had lower MBzP compared to white/Northeast, while MBzP was highest among white/West. Increased BMI was positively associated with MBzP, while higher neighborhood SES and higher quality diet were associated with lower MBzP. Increased parity and history of hysterectomy were both associated with higher MBzP concentrations. MCOP concentrations were negatively associated with age and were highest among white/Northeast women and lowest among non-white/South women. Overweight and obese women also had higher MCOP concentrations compared to underweight/normal weight women. MCNP concentrations were lowest in non-white/South and white/South women compared to other race/region groups and were positively associated with BMI category. Physical activity was positively associated with MCNP concentrations, while history of hysterectomy was associated with lower MCNP concentrations. MCPP was inversely associated with age, higher among Whites compared to non-whites, positively associated with BMI category, and positively associated with physical activity.
Table 3.
Predictors of urinary concentrations of MEP, MBzP, MCOP, MCNP, and MCPP in generalized estimating equation modelsa
| MEP | MBzP | MCOP | MCNP | MCPP | |
|---|---|---|---|---|---|
| Predictors | Predicted Mean (95% CI) ng/mL | ||||
| Age, yearsb | |||||
| 54.0 | 77.4 (70.7 – 84.7) | 12.5* (11.7 – 13.4) | 4.1* (3.9 – 4.3) | 2.9 (2.7 – 3.1) | 3.3* (3.1 – 3.5) |
| 60.6 | 77.9 (73.6 – 82.5) | 11.6* (11.1 – 12.1) | 3.9* (3.8 – 4.1) | 2.9 (2.7 – 3.0) | 3.1* (3.0 – 3.2) |
| 66.1 | 78.4 (74.3 – 82.7) | 10.9* (10.5 – 11.3) | 3.8* (3.7 – 3.9) | 2.8 (2.7 – 2.9) | 2.9* (2.9 – 3.1) |
| 5.5 | 79.2 (71.6 – 87.5) | 9.8* (9.1 – 10.5) | 3.6* (3.3 – 3.8) | 2.7 (2.6 – 2.9) | 2.7* (2.5 – 2.9) |
| Race/Region; N (%) | |||||
| White/Northeast (referent) | 75.6 (69.4 – 82.3) | 10.8 (10.2 – 11.5) | 4.6 (4.4 – 4.8) | 3.3 (3.1 – 3.4) | 3.1 (3.0 – 3.3) |
| White/South | 81.6 (72.0 – 92.5) | 11.0 (10.1 – 12.1) | 3.2* (3.0 – 3.4) | 2.4* (2.2 – 2.7) | 2.7* (2.5 – 2.9) |
| White/West | 66.5* (60.3 – 73.4) | 13.2* (12.3 – 14.2) | 3.8* (3.6 – 4.0) | 2.8* (2.7 – 3.0) | 3.4* (3.2 – 3.7) |
| Non-white/Northeast | 105.7 (74.6 – 149.8) | 7.5* (5.8 – 9.7) | 3.4* (2.8 – 4.2) | 3.1 (2.5 – 3.9) | 2.1* (1.7 – 2.7) |
| Non-white/South | 113.2* (94.4 – 135.7) | 8.4* (7.3 – 9.6) | 2.7* (2.4 – 3.0) | 1.9* (1.7 – 2.1) | 2.0* (1.8 – 2.2) |
| Non-white/West | 100.2* (81.3 – 123.4) | 11.1 (9.5 – 12.9) | 4.0* (3.6 – 4.6) | 2.9 (2.5 – 3.3) | 3.1 (2.7 – 3.6) |
| Normalized SES Index scoreb | |||||
| 48.6 | 13.0* (11.5 – 14.8) | ||||
| 71.5 | 11.3* (10.9 – 11.7) | ||||
| 76.7 | 10.9* (10.5 – 11.4) | ||||
| 86.9 | 10.2* (9.5 – 11.1) | ||||
| Education level | |||||
| Less than high school (referent) | 89.4 (80.9 – 98.7) | ||||
| High school/Some college | 82.1 (75.4 – 89.5) | ||||
| College degree and higher | 67.2* (61.6 – 73.3) | ||||
| Body mass index | |||||
| Underweight/Normal, <25 kg/m2 (referent) | 81.5 (74.4 – 89.2) | 10.3 (9.7 – 11.0) | 3.4 (3.3 – 3.6) | 2.5 (2.4 – 2.7) | 2.8 (2.6 – 3.0) |
| Overweight, 25-<30 kg/m2 | 81.8 (75.4 – 88.8) | 11.6* (11.0 – 12.3) | 3.9* (3.7 – 4.1) | 2.9* (2.7 – 3.0) | 3.0* (2.8 – 3.2) |
| Obese, ≥30 kg/m2 | 71.6* (65.4 – 78.3) | 11.6* (10.8 – 12.3) | 4.2* (4.0 – 4.5) | 3.1* (2.9 – 3.3) | 3.2* (3.0 – 3.4) |
| Alcohol intake | |||||
| Non-drinker (referent) | 67.0 (61.6 – 72.8) | ||||
| <1 drink/week | 82.7* (75.9 – 90.0) | ||||
| 1–6 drinks/week | 86.7* (78.0 – 96.3) | ||||
| ≥7 drinks/week | 96.0* (82.3 – 112.0) | ||||
| Healthy Eating Index scoreb | |||||
| 45.6 | 12.4* (11.4 – 13.4) | ||||
| 63.7 | 11.3* (10.9 – 11.8) | ||||
| 71.7 | 10.9* (10.4 – 11.3) | ||||
| 82.6 | 10.3* (9.6 – 11.0) | ||||
| Total physical activity, MET- hr/wkb | |||||
| 0.9 | 2.7* (2.6 – 2.9) | 2.9* (2.8 – 3.0) | |||
| 4.7 | 2.8* (2.7 – 2.9) | 2.9* (2.8 – 3.0) | |||
| 12.4 | 2.8* (2.7 – 2.9) | 3.0* (2.9 – 3.1) | |||
| 67 | 3.3* (2.9 – 3.7) | 3.7* (3.2 – 4.1) | |||
| Number of children | |||||
| Nulliparous (referent) | 10.6 (9.4 – 11.9) | ||||
| 1–3 children | 11.0 (10.4 – 11.5) | ||||
| ≥4 children | 11.8* (11.0 – 12.6) | ||||
| History of hysterectomy | |||||
| No (referent) | 10.9 (10.4 – 11.5) | 3.0 (2.9 – 3.1) | |||
| Yes | 11.5* (10.9 – 12.1) | 2.6* (2.5 – 2.8) | |||
| Number of observations/Number of participants | 2426/1247 | 2907/1224 | 2991/1257 | 2349/1185 | 2351/1185 |
Phthalate metabolite levels were natural log transformed for analysis; predicted means have been back-transformed for presentation
Predicted means are calculated for the midpoints of each quartile for continuous variables
P value≤0.05 for comparison to referent group (categorical variables) or for one unit change (continuous variables)
Abbreviations used: MEP, mono-ethyl phthalate; MBzP, monobenzyl phthalate; MCOP, monocarboxyoctyl phthalate; MCNP, monocarboxynonyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MET, metabolic equivalent; SES, socioeconomic status
Predictors of the summary measures of exposure to DBP, DiBP, and DEHP in multivariable regression models are shown in Table 4. ΣDBP concentrations were inversely associated with age. Participants from the West region had higher ΣDBP concentrations, with non-white/West women having the highest concentrations. Women reporting current alcohol intake of 1–6 drinks per week had higher estimated ΣDBP concentration than non-drinkers. Likewise, current smokers had higher ΣDBP concentrations as compared to never smokers. ΣDBP concentration was higher among women with a hysterectomy and lower among those self-reporting diabetes. ΣDiBP concentration was lowest among white/South women and positively associated with education level. Overweight women had significantly higher ΣDiBP concentration as compared to the underweight/normal and obese women. Better diet quality was predictive of lower ΣDiBP concentrations. Women who used unopposed estrogen either in the past or currently had higher ΣDiBP concentration than never users. ΣDEHP concentrations were lower among women from the South region. BMI category was positively associated with ΣDEHP concentration, while better diet quality was negatively associated with ΣDEHP. ΣDEHP concentrations also were lower among women with a history of diverticulitis compared to those without this condition.
Table 4.
Predictors of exposure to DBP, DiBP, and DEHP estimated as molar sums of their urinary metabolites in generalized estimating equation modelsa
| ΣDBP | ΣDiBP | ΣDEHP | |
|---|---|---|---|
| Predictors | Predicted Mean (95% CI) μol/L | ||
| Age, yearsb | |||
| 54.0 | 0.127* (0.118 – 0.136) | 0.013 (0.013 – 0.014) | 0.189 (0.178 – 0.202) |
| 60.6 | 0.119* (0.113 – 0.124) | 0.013 (0.013 – 0.014) | 0.185 (0.178 – 0.193) |
| 66.1 | 0.112* (0.108 – 0.117) | 0.013 (0.012 – 0.013) | 0.182 (0.176 – 0.189) |
| 75.5 | 0.102* (0.094 – 0.111) | 0.012 (0.012 – 0.013) | 0.177 (0.165 – 0.189) |
| Race/Region; N (%) | |||
| White/Northeast (referent) | 0.110 (0.103 – 0.118) | 0.013 (0.012 – 0.014) | 0.192 (0.181 – 0.203) |
| White/South | 0.106 (0.096 – 0.116) | 0.011* (0.010 – 0.012) | 0.161* (0.147 – 0.176) |
| White/West | 0.128* (0.118 – 0.138) | 0.014 (0.013 – 0.015) | 0.202 (0.189 – 0.215) |
| Non-white/Northeast | 0.099 (0.075 – 0.132) | 0.012 (0.009 – 0.015) | 0.158 (0.126 – 0.200) |
| Non-white/South | 0.097 (0.084 – 0.112) | 0.014 (0.012 – 0.016) | 0.133* (0.118 – 0.149) |
| Non-white/West | 0.141* (0.119 – 0.167) | 0.014 (0.012 – 0.017) | 0.204 (0.177 – 0.235) |
| Education level | |||
| Less than high school (referent) | 0.012 (0.011 – 0.013) | ||
| High school/Some college | 0.013* (0.012 – 0.014) | ||
| College degree and higher | 0.014* (0.013 – 0.015) | ||
| Body mass index | |||
| Underweight/Normal, <25 kg/m2 (referent) | 0.012 (0.011 – 0.013) | 0.160 (0.151 – 0.171) | |
| Overweight, 25-<30 kg/m2 | 0.014* (0.013 – 0.015) | 0.192* (0.182 – 0.203) | |
| Obese, ≥30 kg/m2 | 0.013 (0.012 – 0.014) | 0.198* (0.186 – 0.210) | |
| Alcohol intake | |||
| Non-drinker (referent) | 0.107 (0.100 – 0.114) | ||
| <1 drink/week | 0.114 (0.107 – 0.122) | ||
| 1–6 drinks/week | 0.130* (0.120 – 0.142) | ||
| ≥7 drinks/week | 0.115 (0.102 −0.130) | ||
| Smoking status | |||
| Never smoked (referent) | 0.110 (0.104 – 0.116) | ||
| Past smoker | 0.119 (0.112 – 0.128) | ||
| Current smoker | 0.132* (0.112 – 0.156) | ||
| Healthy Eating Index scoreb | |||
| 45.6 | 0.014* (0.013 – 0.015) | 0.205* (0.190 – 0.222) | |
| 63.7 | 0.013* (0.013 – 0.014) | 0.186* (0.179 – 0.193) | |
| 71.7 | 0.013* (0.012 – 0.013) | 0.178* (0.172 – 0.185) | |
| 82.6 | 0.012* (0.011 – 0.013) | 0.168* (0.158 – 0.179) | |
| Unopposed estrogen use | |||
| Never (referent) | 0.012 (0.012 – 0.013) | ||
| Past | 0.014* (0.013 – 0.015) | ||
| Current | 0.014* (0.013 – 0.015) | ||
| History of hysterectomy | |||
| No (referent) | 0.110 (0.104 – 0.116) | ||
| Yes | 0.120* (0.113 – 0.127) | ||
| Diabetes | |||
| Never (referent) | 0.116 (0.111 – 0.121) | ||
| Ever | 0.095* (0.079 – 0.115) | ||
| Diverticulitis | |||
| Never (referent) | 0.185 (0.179 – 0.192) | ||
| Ever | 0.162* (0.142 – 0.184) | ||
| Number of observations/Number of participants | 2409/1233 | 2932/1244 | 2495/1077 |
Phthalate metabolite levels were natural log transformed for analysis; predicted means have been back-transformed for presentation
Predicted means are calculated for the midpoints of each quartile for continuous variables
P value≤0.05 for comparison to referent group (categorical variables) or for one unit change (continuous variables)
Abbreviations used: DBP, di-n-butyl phthalate; DiBP, di-isobutyl phthalate; DEHP, di(2-ethylhexyl) phthalate
Although all data and urine samples from breast cancer cases were collected prior to their diagnosis (average 6.2 years prior), we repeated our analysis in the subset of women selected as controls. When fitting the same statistical models developed among the full cohort, the magnitude and direction of associations were nearly identical among the subset of controls as in the full sample. We also repeated our model building process restricting to the subset of controls. In general, we found very similar results to those obtained with the full sample (Supplementary Tables 1 & 2). Some differences were noted, however. MEP concentrations were also predicted by smoking (positive) and higher diet quality score (negative), while BMI was not predictive of MEP concentration among controls. Hysterectomy was not predictive of MBzP concentrations among controls. MCOP was predicted by smoking status (positive). MCNP was not predicted by hysterectomy status, though a positive association with estrogen + progestin use and a negative association with stomach ulcer were observed among controls. MCPP concentrations in controls were not predicted by BMI category, though a negative association with SES was observed. The model for ΣDBP among controls did not include smoking status or diabetes. Finally, the model for ΣDEHP did not include diverticulitis, but a positive association with thyroid disease was observed. The models for ΣDiBP among controls and the full sample were identical.
Discussion
In a multi-ethnic population of postmenopausal women, we observed many common exposures that predicted urinary phthalate metabolite concentrations. It is noteworthy that predictors of some phthalate biomarkers (MCOP, MCNP, and MCPP) were generally similar, perhaps reflecting commonalities in sources of exposure to the parent compounds, which are commonly used in soft plastics (e.g. tubing, food packaging) (Committee on the Health Risks of Phthalates 2008). Notably, concentrations of MBzP, MCOP, MCPP, and ΣDBP were all inversely associated with age, though age was not significantly associated with other biomarker concentrations. The lower concentrations of some phthalate biomarkers observed in older age groups may result from differences in exposure patterns and/or slower metabolism of phthalates with older age, though additional work would be useful in confirming and understanding these associations. Race/region was a significant predictor of most phthalate biomarkers. Interestingly, concentrations of many biomarkers were highest among participants from the West, and lowest among women from the South; these findings suggest that patterns of exposure may differ across regions and/or the differing racial/ethnic backgrounds of participants from those regions. Consistent with prior reports (Centers for Disease Control and Prevention 2018), concentrations of MEP were highest among non-white women. Higher neighborhood SES was predictive of lower MBzP, while higher education was predictive of lower MEP and higher ΣDiBP; these demographic factors were not predictive of other phthalate metabolites in multivariable models. Although the exact mechanisms and/or sources responsible are unclear, our results suggest variation in phthalate exposure across many demographic factors.
BMI was positively associated with MBzP, MCOP, MCNP, MCPP, and ΣDEHP, though was inversely associated with MEP. These findings are in agreement with prior work (Buser et al. 2014; Hatch et al. 2008; Hatch et al. 2010; Yaghjyan et al. 2015). Phthalates may act as obesogens and contribute to the development of obesity; there is some evidence that some phthalates can trigger adipogenesis through activation of peroxisome proliferator-activated receptors (Feige et al. 2007; Hurst and Waxman 2003). However, phthalate biomarker concentrations in the obese may instead be elevated because of their obesity, either due to differences in metabolism of these compounds or from differential exposure patterns among the obese. Future prospective studies would be useful to establish whether elevated phthalate biomarker concentrations are a cause or an effect of obesity. MEP and ΣDBP were higher among women consuming alcohol versus non-drinkers. Current smoking predicted higher ΣDBP concentrations. A higher quality diet was predictive of lower MBzP, ΣDiBP, and ΣDEHP concentrations. Recent studies have reported presence of phthalates in food (Schecter et al. 2013; Serrano et al. 2014). Concentrations of phthalates in food groups vary across studies, but meats and dairy appear to be common sources of phthalates, including DEHP, DBP, butylbenzyl phthalate, and DiBP (Schecter et al. 2013; Serrano et al. 2014). Physical activity was positively associated with concentrations of MCNP and MCPP. A prior study in a sample of Australian men reported slightly higher total phthalate metabolite concentration associated with insufficient physical activity, although this association did not attain statistical significance (Bai et al. 2015). The mechanisms linking physical activity and phthalate exposure and/or metabolism are unclear and merit further study.
Unopposed estrogen use was positively associated with ΣDiBP concentrations. This finding was surprising given that HT formulations have not been reported to contain phthalates (Hauser et al. 2004; Hernandez-Diaz et al. 2009). Interestingly, women reporting a hysterectomy had higher concentrations of MBzP, MCNP, and ΣDBP. Few medical conditions were predictive of urinary phthalate biomarker concentrations; diabetes was inversely associated with ΣDBP and diverticulitis was inversely associated with ΣDEHP. Based on previous reports of phthalate exposure via medications and medical equipment (Gimeno et al. 2014; Green et al. 2005; Hauser et al. 2004; Hernandez-Diaz et al. 2009), we had anticipated that these chronic conditions would be associated with higher phthalate biomarker concentrations, contrary to what we observed; the observed associations may result from misclassification due to our self-reported data or from our definition of exposed as “ever” having these conditions as opposed to currently having them. Interestingly, reproductive characteristics (age at menarche, parity, breastfeeding duration, and infertility) were generally not predictive of phthalate biomarker concentrations, except for a positive association observed between parity and MBzP concentrations. The link between parity and MBzP is not clear, and will require confirmation in future studies. The overall lack of predictive value of the reproductive characteristics, which are factors determined many years prior to sample collection, supports the conclusion that measured phthalate metabolite concentrations are determined by very recent exposures (i.e. within the past day).
It is important to consider these results in the context of relevant limitations. First, our study group was comprised of postmenopausal women selected for a nested case-control study of breast cancer within a large, prospective cohort study. Thus, the population is highly selected and may not reflect the general population of U.S. women, although we did observe similar phthalate metabolite concentrations and patterns of association with age and race as reported by contemporary NHANES measurements. Additionally, we observed similar patterns of association when we restricted our analyses to women selected as controls, suggesting that undiagnosed disease and/or risk factor patterns associated with future disease did not appreciably influence our results. Third, phthalate metabolites exhibit considerable within-person variability (Townsend et al. 2013), which may result in exposure misclassification and bias our results towards the null. Thus, we may have failed to identify some weaker predictors of the urinary phthalate biomarkers examined, and the associations we observed may actually be stronger than we report. This may especially be true for lifetime exposures, given that measured phthalate metabolite concentrations reflect only very recent exposure which may not represent typical values in an individual. Additionally, some observed differences between groups were very small (e.g. ΣDiBP: 0.012 ng/mL underweight/normal, 0.013 ng/mL overweight, and 0.014 ng/mL obese) and may be statistically significant, yet not clinically or analytically meaningful. Also, we did not adjust for multiple comparisons; thus, some observed associations may reflect type I error. However, the general consistency of associations across phthalate biomarkers provides reassurance that the associations we observed were not due to chance alone. Finally, given the complexity of assessing medications and diet as exposures, we evaluated only postmenopausal HT use and a summary measure of diet quality in the present study. It is likely that additional features of diet and/or medication use are related to phthalate exposure, and these will be comprehensively evaluated in future work.
Our work is strengthened by the large sample size and repeated measurements (1,257 women and 2,991 observations in total) of a broad panel of phthalate biomarkers using first morning void urine samples, which are not often available within large epidemiologic cohorts. The laboratory that quantified the phthalate metabolites is highly experienced with such measurements in biological specimens, and the overall reliability of the assays was excellent. Our work was also enhanced by the wide range of data collected in the WHI, which allowed us to evaluate a broad panel of personal, behavioral, and reproductive characteristics for their association with urinary phthalate metabolite concentrations.
Although use of some phthalates has declined in recent years (Zota et al. 2014), phthalate exposure remains ubiquitous in the United States and globally. Understanding the factors that are predictive of phthalate exposure, as well as the consequences of such exposures, is critical for identifying potential high-risk groups with elevated exposure and for protecting public health. Our findings identified a number of factors associated with lower phthalate biomarker concentrations, including older age and engaging in “healthy behaviors” including lower alcohol intake, lower BMI, not smoking, and higher quality diet. We also observed substantial differences in phthalate biomarker concentrations across racial groups and geographic regions. Importantly, while such factors were predictive of urinary phthalate metabolite concentrations, the differences in predicted mean concentrations were often very small across groups defined by these variables. Thus, biological measurements of urinary phthalate metabolites would strengthen future epidemiologic studies.
Supplementary Material
Highlights.
Healthy behaviors (e.g. lower alcohol intake, lower body mass index, not smoking, higher quality diet, and moderate physical activity) were generally associated with lower phthalate biomarker concentrations.
Phthalate biomarker concentrations varied substantially across racial groups and geographic regions.
These finds may help to identify high-risk groups with elevated phthalate exposure.
Acknowledgments
Funding:
This work was supported by the National Institute of Environmental Health Sciences (R01ES024731). The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
The authors wish to acknowledge the contributions of the following institutions and individuals: WHI Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston- Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota, Minneapolis, MN) Karen L. Margolis CDC: Xiaoyun Ye, Manori Silva, Ella Samandar, Jim Preau, Tao Jia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Financial Interests
The authors declare they have no actual or potential competing financial interests.
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
References
- [Anonymous]. Centers for Disease Control and Prevention Fourth Report on Human Exposure to Environmental Chemicals, 2009. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; https://www.cdc.gov/exposurereport/. [Google Scholar]
- [Anonymous]. 1998. Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials 19(1): 61–109, [DOI] [PubMed] [Google Scholar]
- Bai PY, Wittert GA, Taylor AW, Martin SA, Milne RW, Shi Z. 2015. The association of socio-demographic status, lifestyle factors and dietary patterns with total urinary phthalates in Australian men. PLoS One 10(4): e0122140, 4398403: 4398403, 10.1371/journal.pone.0122140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, Main KM. 2012. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 355(2): 240–248, 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Peterson CM, Chen Z, Croughan M, Sundaram R, Stanford J, et al. 2013. Bisphenol A and phthalates and endometriosis: the Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertil Steril 100(1): 162–169 e161–162, 3700684: 3700684, 10.1016/j.fertnstert.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser MC, Murray HE, Scinicariello F. 2014. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007–2010. Int J Hyg Environ Health 217(6): 687–694, 10.1016/j.ijheh.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, et al. 2014. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ Int 62: 1–11, 3874859: 3874859, 10.1016/j.envint.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2014. National Biomonitoring Program, Biomonitoring Summaries-DiDP. Available: http://www.cdc.gov/biomonitoring/DiDP_BiomonitoringSummary.html. Accessed January 7, 2014.
- Colacino JA, Harris TR, Schecter A. 2010. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environmental health perspectives 118(7): 998–1003, 2920922: 2920922, 10.1289/ehp.0901712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on the Health Risks of Phthalates. 2008. Phthalates and Cumulative Risk Assessment: The Task Ahead. Washington D.C.: National Academies Press. [PubMed] [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, Hauser R. 2005. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environmental health perspectives 113(11): 1530–1535, 1310914: 1310914, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Rossi D, Zoete V, Metivier R, Tudor C, et al. 2007. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem 282(26): 19152–19166, 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Skakkebaek NE, Andersson AM. 2007. Metabolism of phthalates in humans. Mol Nutr Food Res 51(7): 899–911, 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- George SM, Ballard-Barbash R, Shikany JM, Caan BJ, Freudenheim JL, Kroenke CH, et al. 2014. Better postdiagnosis diet quality is associated with reduced risk of death among postmenopausal women with invasive breast cancer in the women’s health initiative. Cancer Epidemiol Biomarkers Prev 23(4): 575–583, 4091724: 4091724, 10.1158/1055-9965.EPI-13-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno P, Thomas S, Bousquet C, Maggio AF, Civade C, Brenier C, et al. 2014. Identification and quantification of 14 phthalates and 5 non-phthalate plasticizers in PVC medical devices by GC-MS. J Chromatogr B Analyt Technol Biomed Life Sci 949–950: 99–108, 10.1016/j.jchromb.2013.12.037. [DOI] [PubMed] [Google Scholar]
- Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, et al. 2005. Use of di(2-ethylhexyl) phthalate-containing medical products and urinary levels of mono(2-ethylhexyl) phthalate in neonatal intensive care unit infants. Environmental health perspectives 113(9): 1222–1225, 1280405: 1280405, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin BA, Eibner C, Bird CE, Jewell A, Margolis K, Shih R, et al. 2013. The relationship between urban sprawl and coronary heart disease in women. Health Place 20: 51–61, 3594054: 3594054, 10.1016/j.healthplace.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther PM, Reedy J, Krebs-Smith SM. 2008. Development of the Healthy Eating Index-2005. J Am Diet Assoc 108(11): 1896–1901, 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, et al. 2008. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health 7: 27, 2440739: 2440739, 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EE, Nelson JW, Stahlhut RW, Webster TF. 2010. Association of endocrine disruptors and obesity: perspectives from epidemiological studies. Int J Androl 33(2): 324–332, 3005328: 3005328, 10.1111/j.1365-2605.2009.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Duty S, Godfrey-Bailey L, Calafat AM. 2004. Medications as a source of human exposure to phthalates. Environmental health perspectives 112(6): 751–753, 1241971: 1241971, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, et al. 2016. Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Women Undergoing in Vitro Fertilization: Results from the EARTH Study. Environ Health Perspect 124(6): 831–839, 4892919: 4892919, 10.1289/ehp.1509760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Diaz S, Mitchell AA, Kelley KE, Calafat AM, Hauser R. 2009. Medications as a potential source of exposure to phthalates in the U.S. population. Environmental health perspectives 117(2): 185–189, 2649218: 2649218, 10.1289/ehp.11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. 2003. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci 74(2): 297–308, 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- James-Todd T, Stahlhut R, Meeker JD, Powell SG, Hauser R, Huang T, et al. 2012. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environmental health perspectives 120(9): 1307–1313, 3440117: 3440117, 10.1289/ehp.1104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay VR, Chambers C, Foster WG. 2013. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol 43(3): 200–219, 3604737: 3604737, 10.3109/10408444.2013.766149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Calafat AM. 2009. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci 364(1526): 2063–2078, 2873011: 2873011, 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PM, Roos V, Ronn M, Johansson L, Ahlstrom H, Kullberg J, et al. 2012. Serum concentrations of phthalate metabolites are related to abdominal fat distribution two years later in elderly women. Environ Health 11: 21, 3379932: 3379932, 10.1186/1476-069X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Carrillo L, Hernandez-Ramirez RU, Calafat AM, Torres-Sanchez L, Galvan-Portillo M, Needham LL, et al. 2010. Exposure to phthalates and breast cancer risk in northern Mexico. Environ Health Perspect 118(4): 539–544, 2854732: 2854732, 10.1289/ehp.0901091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. 2011. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environmental health perspectives 119(10): 1396–1402, 3230451: 3230451, 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlett LE, Calafat AM, Swan SH. 2013. Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol 23(2): 197–206, 10.1038/jes.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, et al. 2013. Phthalate concentrations and dietary exposure from food purchased in New York State. Environmental health perspectives 121(4): 473–494, 3620091: 3620091, 10.1289/ehp.1206367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 13(1): 43, 4050989: 4050989, 10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. 2004. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 112(3): 331–338, 1241863: 1241863, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q. 2014. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes (Lond), 10.1038/ijo.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, et al. 2014. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environmental health perspectives 122(6): 616–623, 4050512: 4050512, 10.1289/ehp.1307201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MK, Franke AA, Li X, Hu FB, Eliassen AH. 2013. Within-person reproducibility of urinary bisphenol A and phthalate metabolites over a 1 to 3 year period among women in the Nurses’ Health Studies: a prospective cohort study. Environ Health 12(1): 80, 10.1186/1476-069X-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Blustein J. 2012. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA 308(11): 1113–1121, 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- Trasande L, Spanier AJ, Sathyanarayana S, Attina TM, Blustein J. 2013. Urinary phthalates and increased insulin resistance in adolescents. Pediatrics 132(3): e646–655, 10.1542/peds.2012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upson K, Sathyanarayana S, De Roos AJ, Thompson ML, Scholes D, Dills R, et al. 2013. Phthalates and risk of endometriosis. Environ Res 126: 91–97, 10.1016/j.envres.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Eliot M, Sathyanarayana S, Calafat AM, Yolton K, Lanphear BP, et al. 2014. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol 48(15): 8881–8890, 4123928: 4123928, 10.1021/es501744v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Hauser R, Calafat AM, Missmer SA, Wise LA. 2010. Association of exposure to phthalates with endometriosis and uterine leiomyomata: findings from NHANES, 1999–2004. Environmental health perspectives 118(6): 825–832, 2898860: 2898860, 10.1289/ehp.0901543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghjyan L, Sites S, Ruan Y, Chang SH. 2015. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National Health and Nutrition Examination Survey 1999–2004. Int J Obes (Lond) 39(6): 994–1000, 4962699: 4962699, 10.1038/ijo.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. 2014. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environmental health perspectives 122(3): 235–241, 3948032: 3948032, 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.