Abstract
Extensive research conducted in the last three decades has identified the roles for the main bioactive sphingolipids, namely ceramide, sphingosine, and sphingosine 1-phosphate (S1P) as key regulators of cellular homeostasis, growth and death. One of the major groups of enzymes in the ceramide pathway, ceramidases, convert ceramide into sphingosine and fatty acids, with sphingosine being further metabolized to S1P. Thus, these enzymes play important roles in the network controlling the functions associated with these bioactive sphingolipids.
Among the family of ceramidases, neutral ceramidase (nCDase), which is named according to its optimal pH for catalytic activity, has received increased attention in the last decade. The goal of this review is to provide a brief background on bioactive sphingolipids and the ceramidases. We then describe more recent advances on nCDase, specifically the resolution of its crystal structure and understanding its roles in cell biology and physiology.
Keywords: Sphingolipids, Akt, Sphingosine, cell proliferation, cancer, structure, catalysis
1-. Introduction
Most lipids, predominantly found in biologic membranes, are comprised of four general types of lipids: glycerophospholipids, in which the hydrophobic regions are composed of two fatty acids joined to glycerol; sphingolipids, in which a single fatty acid is joined to a fatty amine, usually sphingosine; sterols, compounds characterized by a rigid system of four fused hydrocarbon rings; and archaebacterial tetraether lipids, in which two very long alkyl chains are ether-linked to glycerol at both ends (Lehninger et al., 2008).
The biological functions of lipids are extremely diverse and are dictated by their specific chemistry. Some lipids such as the triacylglycerols serve as the principal storage forms of energy while others, like phospholipids and sterols, act as major structural components of biological membranes. Additional lipids play crucial roles in diverse functions such as enzyme cofactors, electron carriers, pigments, and emulsifying agents in the digestive tract. On the other hand, other lipids, usually present in relatively small quantities, function as signaling and regulatory molecules within and between cells, and these are dubbed as bioactive.
Sphingolipids are composed of one molecule of the long-chain amino alcohol sphingosine (also called 4-sphingenine) or one of its derivatives or analogs (collectively called sphingoid bases), one molecule of a long-chain fatty acid, and a polar head group. When a fatty acid is attached in amide linkage on the C-2, the resulting compound is called a ceramide. Ceramide then serves as the structural precursor of all sphingolipids, which are derived by modifications of the 1-OH group of ceramide (Hannun and Obeid, 2018). When the head group is joined by a glycosidic linkage, the compounds formed are either glucosylceramide (cerebroside), galactosylceramide, lactosylceramide, or more complex glycosphingolipids and gangliosides. When the head group is instead joined by a phosphodiester, the compound becomes a sphingomyelin, or less commonly a ceramide phosphoethanolamine.
Ceramide is the central hub in the synthesis of sphingolipids (Hannun and Obeid, 2011). Ceramide can be generated via its structural components in a multi-step pathway called the de novo pathway. The first step of this synthesis occurs by the condensation of serine and palmitate, catalyzed by the serine-palmitoyl transferase. Ceramide can also be generated by degradation of sphingomyelin (or other complex glycosphingolipids) as well as by the recycling of sphingosine generated in the endolysosomal system from the breakdown of complex sphingolipids. Additionally, ceramide can be formed by the reverse action of ceramidases. Finally, ceramides can also be taken up from exogenous sources (Hannun and Obeid, 2008; Kitatani et al., 2008)
The breakdown of complex sphingolipids involves sphingomyelinases and various glycosidases that trim the glycosphingolipids. These enzymes ultimately generate ceramide which can be further broken down by the action of ceramidases. Thus, ceramidases are critical for completing the catabolism of sphingolipids. They are also functionally very significant as they regulate the interconversion of key bioactive sphingolipids: ceramide, sphingosine, and sphingosine 1-phosphate (S1P). These lipids serve as key regulators of cellular homeostasis and responses. A recent review by Wang et al in 2018 summarizes the emerging role of ceramide and S1P as cell signaling hubs in the pathophysiology of several neurodegenerative diseases (Wang and Bieberich, 2018). In this work the authors showed that modulation of sphingolipid metabolism alters many cell biological processes such as ER stress, autophagy, protein and lipid transport, exosome secretion and neurotoxic protein spreading, neuroinflammation, and mitochondrial dysfunction.
More specifically, sphingolipids and sphingolipid metabolism have been involved in cancer, especially breast cancer showing a mechanism that can operate in breast cancer cells and fibroblast involves of S1P and its receptor, S1P2 (Pyne et al., 2018).
Thus far, five ceramidases, encoded by five different genes, have been identified in humans: acid ceramidase (AC), neutral ceramidase (nCDase), and alkaline ceramidases 1 to 3 (ACER1, ACER2 and ACER3). This classification refers to the optimal pH for catalytic activity of each of these enzymes (Canals and Hannun, 2013; Coant et al., 2017). AC, which is localized to the lysosomal compartment, has been associated with Farber’s disease when it is deficient congenitally. It is also involved in the regulation of cell viability and the response to stress agents, especially chemotherapeutics (Tan et al., 2017). Neutral ceramidase, which is primarily localized to the plasma membrane (but also Golgi and mitochondria) and primarily expressed in the small intestine and colon, is involved in digestion, and has been implicated in colon carcinogenesis (Garcia-Barros et al., 2016). ACERs 1–3 belong to a closlery related family, first identified in yeast (Mao et al., 2000a; Mao et al., 2000b). ACER1, which can be found in the endoplasmic reticulum and is highly expressed in the skin, plays an important role in keratinocyte differentiation (Sun et al., 2008). ACER2, localized to the Golgi complex and highly expressed in the placenta, is involved in programed cell death in response to DNA damage (Uchida et al., 2010). ACER3, also localized to the endoplasmic reticulum and the Golgi complex, is ubiquitously expressed, and is involved in motor coordination-associated Purkinje cell degeneration (Wang et al., 2015). These have been more extensively described in Coant et al (Coant et al., 2017).
This review is conceived as an update from Coant et al, 2017 (Coant et al., 2017) with a major focus on the biochemistry and structure as well as functions of nCDase.
2-. Basics of nCDase
Discovery and structure.
The full name of neutral ceramidase (nCDase) is N-acylsphingosine amidohydrolase 2 with an Enzyme Commission of 3.5.1.23. It was cloned and purified by El-Bawab between 1999 and 2000 (El Bawab et al., 1999; El Bawab et al., 2000). This led to the realization that nCDase is a member of an extended family of enzymes across species, including some bacteria (Kita et al., 2000). The initial cloning report identified nCDase as a protein of 763 amino acids. Interestingly nCDase was later cloned by another group as a longer protein of 782 amino acids (Zhu et al., 2014) called ASAH2B. The authors suggested that this protein was coded by the same gene but with an alternative transcription start site.
The enzyme is highly expressed in the kidneys, liver, small intestines and colon with lower expression in the brain, lung, and heart. Developing and investigating the nCDase deficient mice, Kono et al found that the mice are viable with no obvious deficiency under normal breeding conditions. However, deeper investigation revealed some key roles for nCDase.
The first role was identified by measuring stool content and composition in nCDase deficient mice. Kono et al (Kono et al., 2006) showed that nCDase deficient mice were not able to degrade dietary sphingolipids and concluded that nCDase regulates the levels of bioactive sphingolipid metabolites in the intestinal tract.
Early studies on nCDase suggested a role of nCDase in chemotherapy. Wu et al. found that gemcitabine treated cells have an increase of the levels of specific ceramides, and this was attributed to a reduction of nCDase expression. The increased ceramide was also implicated in suppression of cell growth (Wu et al., 2009).
Several other studies also helped elucidate the biological functions of nCDase. Among the major discoveries about nCDase, was the finding that nitric oxide decreases nCDase expression in mesangial cells (Franzen et al., 2002). In another study, it was found that UV-B irradiation decreased nCDase activity in keratinocytes, and ceramidase inhibition or siRNA-mediated suppression sensitized keratinocytes to low-dose-UVB-induced apoptosis (Uchida et al., 2010). Other studies showed that ATRA down-regulated nCDase expression at the message level, resulting in less protein and activity in SH-SY5Y (Tanaka et al., 2012) and that nCDase downregulation induces a decrease of cell growth and neuronal differentiation.
Studies from Snider et al also suggested that nCDase may protect against inflammation using a dextran sodium sulfate (DSS) mouse model. In that study, nCDase deficient mice treated with DSS showed a paradoxical elevation of sphingosine and an increase of S1P. Later, in 2012, Tanaka et al. found a role for nCDase in neuronal differentiation by inducing ceramide accumulation (Tanaka et al., 2012). Then, in 2014, Novgorodov et al. investigated the role of nCDase in traumatic brain injury. They showed an activation of neutral ceramidase after traumatic brain injury that correlated with an increase in sphingomyelin species and sphingosine The knock-out mice in this study was partly protected from brain injury.
Further studies on nCDase deficient mice showed also that those mice are protected against colon cancer induced by azoxymethane (AOM). A study from Garcia-Barros et al showed that nCDase inhibition induces a delay in tumor growth and increased ceramide in a xenograft model (Garcia-Barros et al., 2016).
Very recently, a nCDase was identified by Shi et al. in the planthopper (Nilaparvata lugens). This enzyme uses C12-ceramide as preferred substrate and displays optimal activity at pH6. The knock down of this enzyme enhanced survival of the female planthopper under high (32C) or low (22C) temperature (Shi et al., 2018). It is particularly exciting to see that distant homologues from nCDase are found in taxon all over evolution reinforcing a crucial role for ceramide. This is also illustrated by a new study from Zhong et al founding nCDase in Amorphophallus muelleri (Zhong et al., 2018).
3-. Updates on Biochemistry and structure
We (Airola et al., 2015) have recently been able to purify and crystalize human neutral ceramidase. This work allowed us to propose a structure and a possible mechanism for the catabolism of ceramide by this enzyme. We first purified an active version of neural ceramidase, in an insect cell model, that consisted of the extracellular region of human nCDase (residues 99–780) and lacked the short intracellular region of nCDase (residues 1–12), the transmembrane domain (residues 12–34), and the flexible O-glycosylated mucin box (residues 34–99). The activity was measured using the NBD ceramide activity assay displaying a Km and Kcat in the range of those previously described for the enzyme purified from rat brain (El Bawab et al., 1999).
The diffraction pattern from the crystals allowed the proposal of a structure of the protein at a 2.6Å resolution. The overall structure showed a catalytic domain (residues 99–626), a short linker (627–641), and an immunoglobulin (IG)-like domain (642–780). In comparison with the distantly homologous bacterial CDase (bCDase), in the human nCDase, a 30-residue subdomain insert replaces a 6-residue span of bCDase. This insertion seems to stabilize two alpha helixes of the active site. This subdomain conformation is aided by two internal disulfide bridges, formed by four cysteines that are conserved in eukaryotes.
Importantly, the structure revealed the active site of human neutral ceramidase as a narrow, 20 Å deep, hydrophobic pocket with a Zn 2+ ion at the base. Hydrophobic residues line the pocket from outside to inside with one side of the active site cavity formed by the η2-α8 helices, which have a divergent amino-acid sequence in comparison to bCDase. In this structure, a cluster of polar residues lies at the base of the pocket that coordinates Zn2+ and interacts with a bound phosphate. Zn-dependent amidases have been described as having three sidechains that coordinate to the proximal side of Zn2+ and have in addition a Glu, Asp, or His residues, with the remaining two Zn-coordination sites occupied by water molecules (Hernick and Fierke, 2005). Interestingly, for nCDase, an alternate model was proposed that shows four protein sidechains coordinating the Zn2+ with a phosphate occupying the fifth Zn-coordination site. Specifically, His194, His303, and Glu540 occupy the expected, proximal Zn-coordinating positions, while Tyr579 coordinates to the opposite, distal face of the Zn2+ below phosphate.
In the solved structure, a phosphate is present in the active site of the enzyme, in tetrahedral conformation; thus, mimicking the transition state of ceramide during its hydrolysis. Therefore, we were able to predict the interaction of the enzyme with ceramide in the active site. Moreover, His196, Arg257, Tyr579, and Tyr591 were identified as playing critical roles in catalysis and stabilizing the transition state of ceramide hydrolysis. The proposed model of ceramide hydrolysis by nCDase in membranes is presented figure 1.
Figure 1:
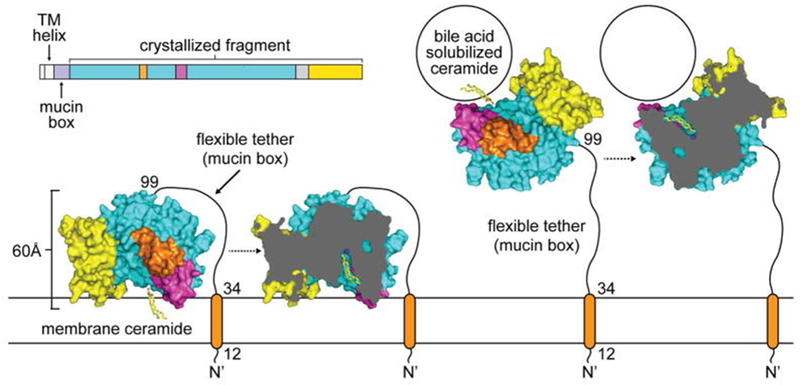
Model of ceramide hydrolysis by nCDase in membranes and bile-acid micelles as proposed by Airola et al. Schematic of ceramide hydrolysis by the membrane-tethered human nCDase involves extracting ceramide from membranes (left) or bile-acid micelles (right) into the deep hydrophobic pocket. The flexible tether would allow human nCDase to hydrolyze ceramide in these two different physiological forms. This figure is reproduced from Airola et al., 2015 with permission.
In a chemical biology approach, Bhabak in 2013, developed new ceramidase assay using FRET (fluorescence resonance energy transfer) technology. In an elegant manner, they used the transfer of fluorescence from NBD (on the sphingosine backbone) to Nile Red (on the fatty acid chain of ceramide). They were able with this probe to follow the activity of neutral ceramidase (Bhabak et al., 2013).
4-. Roles in signaling and Cancer
Cell biology.
Sundaram et al studied the ffects of loss of nCDase on necroptosis induced by nutrient-deprivation. In this study, they treated wild type (WT) and nCDase−/− Mouse embryonic fibroblast (MEFs) with 2-Deoxyglucose (2DG) and antimycin A (AA) 2DG/AA, and cell death was measured by LDH release from the cells. Their results showed that nCDase −/− MEFs have a reduced percentage of LDH release and therefore concluded that that nCDase−/− MEFs were protected from the 2DG/AA model of necroptosis. Investigating further, they found that MEFs from nCDase deficient mice present an increase of autophagic flux and more specifically mitophagy when subjected to the 2DG/AA model of necroptosis. They showed as well that inhibition of autophagy reversed this phenotype. This allowed them to suggest that inhibition of nCDase may enhance cell survival by increasing the clearance of damaged mitochondria via mitophagy.
In a recent study, our group showed that inhibition of nCDase in colorectal cancer (CRC) cells induces a decrease of phosphorylation of GSK3β, which activates the kinase. In turn, activated GSK3ß phosphorylated ß-catenin, resulting in a significant decrease in its levels (Coant et al., 2018). In additional studies, it was found that inhibition of nCDase resulted in dephosphorylation and inactivation of Akt, which was responsible for the loss of phosphorylation of GSK3ß and the loss of ß-catenin. In order to dis sect the signaling pathway involved, we over expressed a constitutively active AKT (AKT-DD, phospho mimic) and showed that this mutant was able to overcome the growth suppressive effects of nCDase inhibition in CRC cells. Thus, we concluded and that maintenance of basal activity of AKT requires the presence and activity of nCDase. A model of intra cellular signaling is presented in figure 2
Figure 2:
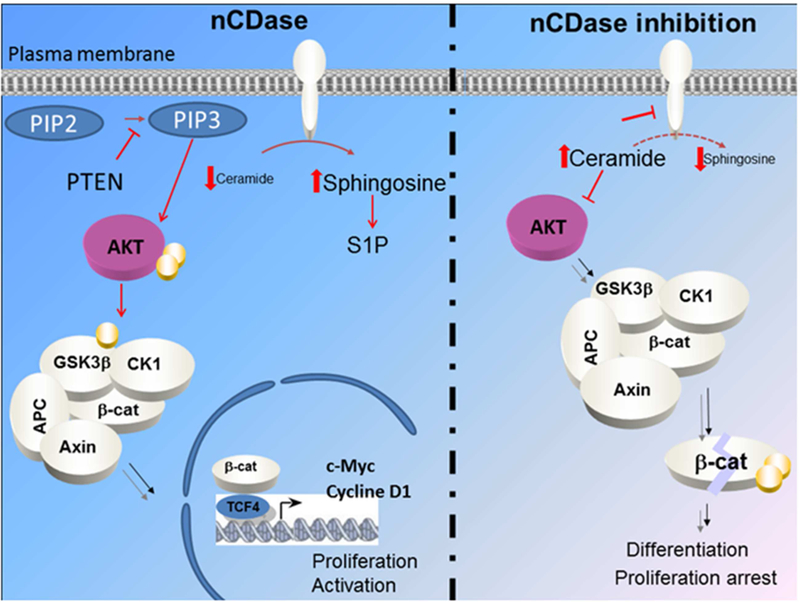
Proposed model of regulation of AKT dependent CRC cell growth by nCDase as described by Coant et al. This figure is reproduced from Coant et al., 2015 with permission.
Interestingly, as previously reported, nCDase inhibition induces a growth delay of xenograft tumors from CRC cell lines (Garcia-Barros et al., 2016). In the study from Coant et al, we showed that xenograft tumors from constitutively active AKT cells became resistant to nCDase inhibition. Together, those results suggest that endogenous ceramide may serve to attenuate activation of AKT and that nCDase has a key function in controlling that function of ceramide.
The subcellular localization of nCDase has been investigated in multiple studies, with demonstration of its existence at the plasma membrane but also in the mitochondria as well as in endosomes (El Bawab et al., 2000 ; Hwang et al., 2005,#40; Kono et al., 2006; Lundgren et al., 2001; Olsson et al., 2004). In a very recent study on subcellular localization and functions of nCDase in CRC cells from our group (Sakamoto et al., 2018), nCDase was found to be located in both the plasma membrane and the Golgi apparatus using a tagged overexpressing construct, see figure 3. Functionally, cells overexpressing nCDase were protected from cell death induced by the short chain C6-ceramide. They were also protected from Golgi fragmentation induced by C6-ceramide. In order to pursue the functions of nCDase in CRC and the compartmentalized pathways of sphingolipid metabolism, we expressed a Golgi-targeted bacterial SMase (bSMase) and bacterial ceramidase (bCDase) and were able to modulate Golgi fragmentation and cell survival, suggesting a role of nCDase in the Golgi.
Figure 3:
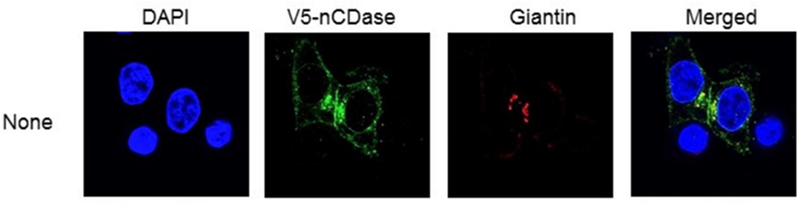
Localization of nCDase in HCT116 cells as described by Sakamoto et al. showing localization of nCDase in the Golgi apparatus. This figure is reproduced from Sakamoto et al 2018 with permission.
Additional studies investigated the role of nCDase in palmitate-induced β cell death. Previous studies showed that ceramide or ceramide analogues impaired insulin production in pancreatic β-cells (Veret et al., 2014). Pharmacological approaches aimed at inhibiting ceramide synthesis implicated de novo synthesis of ceramide in inhibition of glucose-stimulated insulin gene expression by palmitate (Kelpe et al., 2003) (Veret et al., 2014). Zhu et al showed that treatment of INS-1 cells with a mix of TNF-α, IL-1β and IFN-γ induced the production of exosomes expressing nCDase. The author isolated those exosomes and showed that they could protect INS-1 cells or rat primary Langerhans islets against apoptosis induced by high dose cytokines. In order to investigate the cellular mechanism, they treated the cells with an S1P or S1PR2 blocking antibody and were able to block the anti-apoptotic effect of nCDase-containing exosome (Zhu et al., 2014). Therefore, they concluded that nCDase protects from apoptosis of β pancreatic cells induced by high dose cytokine.
In another study, the same group investigated the effect of palmitate treatment on insulin response in H4IIEC3 hepatocytes. Treating cells with palmitate induced an attenuation of the insulin response on phosphorylation of IRS2, AKT, PI3k and GSK3β as well as glucose analogue intake. They showed that the nCDase-containing exosomes were able to block these effects of palmitate. The authors hypothesized that this effect of nCDase-containing exosomes was mediated by a decrease of ceramide mediated by the action of nCDase (Zhu et al., 2016). Likewise, the same group showed that, in INS-1 cells, nCDase-containing exosomes blocked apoptosis induced by palmitate. They also hypothesized that this effect was mediated by a decrease of ceramide or an increase of sphingosine or S1P (Tang et al., 2017). Conversely, in the latest publication from this group, they showed that palmitate treatment induced a decrease of nCDase activity as well as expression at the message and protein. This was accompanied by a decrease in cell viability. Interestingly, overexpressing nCDase prevented the decrease in cell viability induced by palmitate and a nCDase siRNA (Luo et al., 2017)
5-. Concluding remarks.
New studies have significantly advanced our knowledge on the structure and function of nCDase. Resolving the crystal structure of the enzyme opens the door for a better understanding of its mechanism of action as well as a way to generate better inhibitors. From a cell biology point of view, interesting work on nCDase and its localization in the Golgi apparatus will allow future discovery and better understanding of new roles in regulating various biological processes such as cell proliferation, differentiation, apoptosis, and autophagy. From a physiological point of view, work on nCDase’s role in diabetes and metabolic disease opens a new field of potential research.
Acknowledgements.
The authors wish to thank Ayanna Lewis, MD for careful read of the manuscript. This work was supported in part by NIH grant CA172517.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We declare no conflict of interest.
References
- Airola MV, Allen WJ, Pulkoski-Gross MJ, Obeid LM, Rizzo RC, Hannun YA, 2015. Structural Basis for Ceramide Recognition and Hydrolysis by Human Neutral Ceramidase. Structure 23(8), 1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhabak KP, Hauser A, Redmer S, Banhart S, Heuer D, Arenz C, 2013. Development of a novel FRET probe for the real-time determination of ceramidase activity. Chembiochem : a European journal of chemical biology 14(9), 1049–1052. [DOI] [PubMed] [Google Scholar]
- Canals D, Hannun YA, 2013. Novel chemotherapeutic drugs in sphingolipid cancer research. Handbook of experimental pharmacology(215), 211–238. [DOI] [PMC free article] [PubMed]
- Coant N, Garcia-Barros M, Zhang Q, Obeid LM, Hannun YA, 2018. AKT as a key target for growth promoting functions of neutral ceramidase in colon cancer cells. Oncogene 37(28), 3852–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coant N, Sakamoto W, Mao C, Hannun YA, 2017. Ceramidases, roles in sphingolipid metabolism and in health and disease. Advances in biological regulation 63, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bawab S, Bielawska A, Hannun YA, 1999. Purification and characterization of a membrane-bound nonlysosomal ceramidase from rat brain. J Biol Chem 274(39), 27948–27955. [DOI] [PubMed] [Google Scholar]
- El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA, 2000. Molecular cloning and characterization of a human mitochondrial ceramidase. J Biol Chem 275(28), 21508–21513. [DOI] [PubMed] [Google Scholar]
- Franzen R, Fabbro D, Aschrafi A, Pfeilschifter J, Huwiler A, 2002. Nitric oxide induces degradation of the neutral ceramidase in rat renal mesangial cells and is counterregulated by protein kinase C. J.Biol.Chem 277(48), 46184–46190. [DOI] [PubMed] [Google Scholar]
- Garcia-Barros M, Coant N, Kawamori T, Wada M, Snider AJ, Truman JP, Wu BX, Furuya H, Clarke CJ, Bialkowska AB, Ghaleb A, Yang VW, Obeid LM, Hannun YA, 2016. Role of neutral ceramidase in colon cancer. FASEB journal : official publication of the Federation of American Societies for Experimental Biology [DOI] [PMC free article] [PubMed]
- Hannun YA, Obeid LM, 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature reviews. Molecular cell biology 9(2), 139–150. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM, 2011. Many ceramides. J Biol Chem 286(32), 27855–27862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM, 2018. Sphingolipids and their metabolism in physiology and disease. Nature reviews. Molecular cell biology 19(3), 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernick M, Fierke CA, 2005. Zinc hydrolases: the mechanisms of zinc-dependent deacetylases. Archives of biochemistry and biophysics 433(1), 71–84. [DOI] [PubMed] [Google Scholar]
- Hwang YH, Tani M, Nakagawa T, Okino N, Ito M, 2005. Subcellular localization of human neutral ceramidase expressed in HEK293 cells. Biochemical and biophysical research communications 331(1), 37–42. [DOI] [PubMed] [Google Scholar]
- Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V, 2003. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem 278(32), 30015–30021. [DOI] [PubMed] [Google Scholar]
- Kita K, Okino N, Ito M, 2000. Reverse hydrolysis reaction of a recombinant alkaline ceramidase of Pseudomonas aeruginosa. Biochim Biophys Acta 1485(2–3), 111–120. [DOI] [PubMed] [Google Scholar]
- Kitatani K, Idkowiak-Baldys J, Hannun YA, 2008. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cellular signalling 20(6), 1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M, Dreier JL, Ellis JM, Allende ML, Kalkofen DN, Sanders KM, Bielawski J, Bielawska A, Hannun YA, Proia RL, 2006. Neutral ceramidase encoded by the Asah2 gene is essential for the intestinal degradation of sphingolipids. J Biol Chem 281(11), 7324–7331. [DOI] [PubMed] [Google Scholar]
- Lehninger A, Nelson D, Cox M, 2008. Lehninger Principles of Biochemistry
- Freeman WH. Lundgren P, Nilsson A, Duan RD, 2001. Distribution and properties of neutral ceramidase activity in rat intestinal tract. Digestive diseases and sciences 46(4), 765–772. [DOI] [PubMed] [Google Scholar]
- Luo F, Feng Y, Ma H, Liu C, Chen G, Wei X, Mao X, Li X, Xu Y, Tang S, Wen H, Jin J, Zhu Q, 2017. Neutral ceramidase activity inhibition is involved in palmitate-induced apoptosis in INS-1 cells. Endocrine journal 64(8), 767–776. [DOI] [PubMed] [Google Scholar]
- Mao C, Xu R, Bielawska A, Obeid LM, 2000a. Cloning of an alkaline ceramidase from Saccharomyces cerevisiae. An enzyme with reverse (CoA-independent) ceramide synthase activity. J Biol Chem 275(10), 6876–6884. [DOI] [PubMed] [Google Scholar]
- Mao C, Xu R, Bielawska A, Szulc ZM, Obeid LM, 2000b. Cloning and characterization of a Saccharomyces cerevisiae alkaline ceramidase with specificity for dihydroceramide. J Biol Chem 275(40), 31369–31378. [DOI] [PubMed] [Google Scholar]
- Olsson M, Duan RD, Ohlsson L, Nilsson A, 2004. Rat intestinal ceramidase: purification, properties, and physiological relevance. American journal of physiology. Gastrointestinal and liver physiology 287(4), G929–937. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, El Buri A, Adams DR, Pyne S, 2018. Sphingosine 1-phosphate and cancer. Advances in biological regulation 68, 97–106. [DOI] [PubMed] [Google Scholar]
- Sakamoto W, Coant N, Canals D, Obeid LM, Hannun YA, 2018. Functions of neutral ceramidase in the Golgi apparatus. Journal of lipid research [DOI] [PMC free article] [PubMed]
- Shi XX, Huang YJ, Begum MA, Zhu MF, Li FQ, Zhang MJ, Zhou WW, Mao C, Zhu ZR, 2018. A neutral ceramidase, NlnCDase, is involved in the stress responses of brown planthopper, Nilaparvata lugens (Stal). Scientific reports 8(1), 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Xu R, Hu W, Jin J, Crellin HA, Bielawski J, Szulc ZM, Thiers BH, Obeid LM, Mao C, 2008. Upregulation of the human alkaline ceramidase 1 and acid ceramidase mediates calcium-induced differentiation of epidermal keratinocytes. J Invest Dermatol 128(2), 389–397. [DOI] [PubMed] [Google Scholar]
- Tan SF, Pearson JM, Feith DJ, Loughran TP Jr., 2017. The emergence of acid ceramidase as a therapeutic target for acute myeloid leukemia. Expert opinion on therapeutic targets 21(6), 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Tamiya-Koizumi K, Hagiwara K, Ito H, Takagi A, Kojima T, Suzuki M, Iwaki S, Fujii S, Nakamura M, Banno Y, Kannagi R, Tsurumi T, Kyogashima M, Murate T, 2012. Role of down-regulated neutral ceramidase during all-trans retinoic acid-induced neuronal differentiation in SH-SY5Y neuroblastoma cells. Journal of biochemistry 151(6), 611–620. [DOI] [PubMed] [Google Scholar]
- Tang S, Luo F, Feng YM, Wei X, Miao H, Lu YB, Tang Y, Ding DF, Jin JF, Zhu Q, 2017. Neutral Ceramidase Secreted Via Exosome Protects Against Palmitate-Induced Apoptosis in INS-1 Cells. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association 125(2), 130–135. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Houben E, Park K, Douangpanya S, Lee YM, Wu BX, Hannun YA, Radin NS, Elias PM, Holleran WM, 2010. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J Invest Dermatol 130(10), 2472–2480. [DOI] [PubMed] [Google Scholar]
- Veret J, Bellini L, Giussani P, Ng C, Magnan C, Le Stunff H, 2014. Roles of Sphingolipid Metabolism in Pancreatic beta Cell Dysfunction Induced by Lipotoxicity. Journal of clinical medicine 3(2), 646–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Bieberich E, 2018. Sphingolipids in neurodegeneration (with focus on ceramide and S1P). Advances in biological regulation [DOI] [PMC free article] [PubMed]
- Wang K, Xu R, Schrandt J, Shah P, Gong YZ, Preston C, Wang L, Yi JK, Lin CL, Sun W, Spyropoulos DD, Rhee S, Li M, Zhou J, Ge S, Zhang G, Snider AJ, Hannun YA, Obeid LM, Mao C, 2015. Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain. PLoS genetics 11(10), e1005591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BX, Zeidan YH, Hannun YA, 2009. Downregulation of neutral ceramidase by gemcitabine: Implications for cell cycle regulation. Biochim Biophys Acta 1791(8), 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Liu E, Yang C, Diao Y, Harijati N, Liu J, Hu Z, Jin S, 2018. Gene cloning of a neutral ceramidase from the sphingolipid metabolic pathway based on transcriptome analysis of Amorphophallus muelleri. PloS one 13(3), e0194863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Kang J, Miao H, Feng Y, Xiao L, Hu Z, Liao DF, Huang Y, Jin J, He S, 2014. Low-dose cytokine-induced neutral ceramidase secretion from INS-1 cells via exosomes and its anti-apoptotic effect. The FEBS journal 281(12), 2861–2870. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Zhu R, Jin J, 2016. Neutral ceramidase-enriched exosomes prevent palmitic acid-induced insulin resistance in H4IIEC3 hepatocytes. FEBS open bio 6(11), 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coant N, Hannun YA, Neutral ceramidase: Advances in mechanisms, cell regulation, and roles in cancer, Advances in Biological Regulation, doi: 10.1016/j.jbior.2018.10.005 [DOI] [PMC free article] [PubMed]


