Abstract
Background:
Phthalates are known endocrine disruptors and peroxisome proliferator-activated receptor (PPAR) activators, potentially capable of promoting an obesogenic effect. Pregnant women are especially vulnerable to phthalate exposure due to physiological and metabolic changes during pregnancy, including those related to the metabolism of xenobiotics. Phthalate exposure during pregnancy has been associated with early gestational weight gain, however, its effect on long-term weight gain remains unclear. The aim of the present study was to evaluate the association between phthalate exposure during pregnancy and long-term changes in weight among women.
Methods:
Urinary phthalate concentrations, socioeconomic, anthropometry and information on diet and socioeconomic status were collected during pregnancy from 178 women from the Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT) birth cohort. Maternal body weight and diet information was also collected up to 5 times in the first year postpartum and twice during follow-up visits 5.2–10.7 years later. A path analysis was performed to assess associations between urinary phthalate metabolite levels during pregnancy and change in weight (kg) per year after delivery, including age, education, living with/without partner, parity, daily energy intake and breastfeeding duration.
Results:
The mean age at pregnancy was 27.3±5.9 years and mean body mass index during the first postpartum year was 27.07±4.22 kg/m2. On average, women gained 3.48 kg (0.52±0.84 kg/year). A unit increase in log-transformed mono-3-carboxypropyl phthalate (MCPP) was associated with 0.33 kg (95% CI: 0.09, 0.56) higher weight gain per year, and mono-benzyl phthalate (MBzP) with 0.21 kg (95% CI: −0.38, −0.03) lower weight gain per year.
Conclusion:
Exposure to certain phthalates during pregnancy may be associated with long-term weight change in women. More studies on the effects of phthalate exposure during pregnancy on women’s long-term health are required.
Keywords: phthalates, MCPP, MBZP, pregnancy, weight
1. Introduction
Pregnancy is a critical period for the development of obesity and overweight status among women due to dramatic metabolic changes that occur during this time. Long-term health effects related to high gestational weight gain (GWG) and postpartum weight retention include diabetes, cardiovascular disease, and other chronic illnesses (1, 2). Among some of the factors involved in postpartum weight retention are pre-gestational overweight or obesity status and excessive GWG, while longer breastfeeding duration and moderate exercise have been associated with decreased weight retention (3). However, the effects of prenatal exposure to phthalates and other potential obesogens on long-term maternal weight status have not been explored. In addition, pregnancy may be a period of increased susceptibility to the effects of phthalate exposure, as animal studies suggest that pregnancy-related changes in metabolism may result in longer biological residence times for some phthalates, potentially increasing the effect of a given exposure (4). In accordance with this, phthalates exposure during pregnancy has been associated with early GWG, however, the mechanism behind the effect of phthalates on weight gain is unclear (5).
Phthalates are a group of chemicals widely used in the manufacture of industrial and consumer products, such as PVC plastics and personal care products (6). Due to their non-covalent bonds to these products, they are easily released into the environment during their manufacture, use, and disposal (7). Phthalates are considered endocrine and metabolic disruptors with obesogenic functions (8, 9), as they are weakly estrogenic (10), anti-androgenic, and act as thyroid-axis antagonists, and PPAR activators (8). PPARs form heterodimers with the retinoid X receptor (RXR) (11) which play a role in glucose and triglyceride homeostasis, preadipocyte differentiation, and the expression of diverse adipogenic genes (9).
Adult women have higher concentrations of phthalate metabolites in their urine than men (12), possibly due to their greater use of personal care products (13). In pregnant women the use of personal care products has been reported as an important source of phthalate exposure (14). However, other sources of phthalate exposure among pregnant women have been found, including bottled water, food contact materials, hair dye or permanents, and the consumption of certain food items and medications (15–17).
In women, exposure to phthalates has been positively associated with body mass index (BMI) and waist circumference (WC) (18, 19) in cross-sectional studies, and long-term weight gain in a longitudinal analysis of US women in the Nurses’ Health Study (20). Other related health outcomes associated with phthalate exposure in humans include metabolic syndrome (21) and diabetes (22). Specifically in Mexican women, a case-control study showed that women with diabetes presented higher levels of mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP); mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), and lower levels of MBzP when compared to non-diabetic women (22).
There is a scarcity of studies regarding phthalate exposure during pregnancy and long-term health in women. To our knowledge, the only previous study evaluating phthalate exposure during pregnancy on maternal weight gain had a short follow-up period of 7 weeks (5). As a result, the current study aimed to evaluate the association between the phthalate exposure during pregnancy, a vulnerable life stage, and long term weight change over 5.2–10.7 years in women from the ELEMENT cohort study.
2. Methods
2.1. Study Population
The present study comprises information from a subsample of 250 women belonging to two of three sequentially-enrolled birth cohorts from the ELEMENT project that recruited women during the first trimester of pregnancy between 1997 and 2004. The ELEMENT project has been described in detail elsewhere (23, 24). The research protocol was approved by the Ethics and Research Committees of the National Institute of Public Health in Mexico and the University of Michigan School of Public Health.
Women in the current analysis were selected for a follow-up study of their children in 2010 based on the availability of maternal urine samples collected during pregnancy. Sociodemographic information was collected upon study enrollment, while biological samples, anthropometric, and dietary data were collected three times during pregnancy (1st, 2nd and 3rd trimester). Anthropometric measures and dietary data were collected again at up to five study visits during the first 12 months postpartum (1998–2005) and at two follow-up visits 5.2–10.7 years after the first postpartum year (2008–2011). Information about breastfeeding duration was collected during the child’s infancy visits (birth to 36 months) and parity information was collected 13.42±1.65 years after the first month postpartum (Supplementary Table 1).
2.2. Change in Weight and BMI
Weight was measured up to five times during the first year postpartum (1st, 3rd, 4th, 7th and 12th month, depending of the specific cohort design) and twice during follow-up visits (7.1±1.13 and 9.6±1.50 years after the last visit during the first year postpartum) using a hospital scale (accurate to 0.1 kg). Change in weight (kg) and BMI (kg/m2) per year were calculated for each woman with the following formula: where WBy = mean change in weight or BMI per year, WBf= mean weight or BMI in the follow-up visits (2008–2011), WBp= mean weight or BMI in the first year postpartum (1997–2005), Yf = earliest follow-up visit date, and Yp = last postpartum visit. WBf and WBp were calculated as arithmetic means using all available measurements for each woman and Yf −Yp was expressed in years.
2.3. Urinary Phthalate Measurement
Nine phthalate metabolites were measured in maternal urine samples collected during each trimester of pregnancy: monoethyl phthalate (MEP), MnBP, mono-isobutyl phthalate (MiBP), MBzP, MCPP, mono-2-ethylhexyl phthalate [MEHP], MEHHP, MEOHP and MECPP. All assays were performed at NSF International (Ann Arbor, MI) using high performance liquid chromatography and tandem mass-spectrometry as previously described (25, 26). The sum of di-2-ethylhexyl phthalate metabolites (∑DEHP) was calculated by adding the molar fractions of MEHP, MEHHP, MEOHP and MECPP; and the sum of dibutyl phthalate (∑DBP) was calculated by adding the molar fractions of MiBP and MnBP. This grouping was corroborated by a principal component analysis and factor analysis. To achieve unit comparability, ∑DEHP (nmol/ml) was multiplied by the molecular weight (MW) of MEHP (278.348 g/mol) and ∑DBP (nmol/ml) by the MW of MnBP (222.24 g/mol). The resulting units were ng/ml.
Phthalates measurements below the limit of detection (LOD) were substituted with LOD/√2 (25, 27). A correction for urinary specific gravity was also made (28, 29): where Pc = corrected phthalate concentration, P = measured phthalate concentration, SG = specific gravity of the sample, and 1.015 = median specific gravity of all samples collected.
2.4. Covariate Information
Dietary information was collected using a validated semi-quantitative questionnaire (30) administered during pregnancy at the 1st, 2nd and 3rd trimester visit; up to five times during the first year postpartum and at two follow-up visits 5.2–10.7 years after the first postpartum year. With the use of food composition tables and food frequency and quantity information collected via food frequency questionnaires (FFQ), daily energy intake (EI) (30) was calculated for pregnancy, postpartum, and follow-up periods for each woman. Breastfeeding duration was categorized as 1) never to <1 month, 2) 1 to <6 months, 3) 6 to <12 months and 4) 12 months or more. Education information was collected through questionnaires administered during pregnancy as the total number of years the participant attended school, and was categorized in four corresponding education level categories: 1) elementary school (1–6 years), 2) middle school (7–9 years), 3) high school (10–12 years) and 4) undergraduate/graduate school (>12 years). The parity rate variable was created by dividing the number of children the woman had after the study child (range=0 to 4), by the amount of time between the study birth and the date when parity information was collected (10.7 to 17. 6 years after the first month postpartum, mean 13.42±1.65). Calculating parity rate allowed us to account for the time between last weight measurements and parity report (4.95±0.92 years).
Marital status was classified in two categories: 1) living with a partner (married or in free-union); and 2) not living with a partner (single, separated, or divorced). Socioeconomic status (SES) during pregnancy was estimated using a validated scale consisting of thirteen questions on housing quality, services, material goods and head of household education (Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública, AMAI version 13×6). This scale classifies households into six SES categories (A/B, C+, C, D+, D, E; with A/B being the highest category) using hierarchical trees (31, 32). Although information about postpartum physical activity was collected for some ELEMENT participants, this variable was not included in the present analysis due to the small number of participants with this data (n=5) in the final sample.
2.5. Statistical analysis
Preliminary analysis of the data included frequency measures of categorical variables such as education level, SES, breastfeeding duration, and marital status. For continuous variables, the presence of outliers was evaluated and distributional statistics such as standard deviation and variance, skewness and kurtosis, and additionally for phthalates, geometric means were calculated. Logarithmic transformations, zero-skewness Box–Cox and zero-skewness log-transformations were evaluated for phthalate concentrations to achieve normality. Due to the similarity of the distributions achieved, logarithmic transformations were performed in order to achieve better interpretability.
Mixed-effects models were used to assess changes in log-transformed urinary phthalate concentrations across pregnancy. Path analysis with maximum likelihood estimation was used to analyze association patterns between phthalate exposure (∑DEHP, ∑DPB, MBzP, MCPP and MEP) and weight change per year. A path analysis is a type of structural equation model used to evaluate associations. This model is specified by the simultaneous inclusion of multiple observed independent and dependent variables, allowing the estimation of total, direct, and indirect effects through the use of several multiple linear regressions (33). In this model, a direct effect is the unmediated influence of a variable on other variable, an indirect effect is the effect that is mediated by other variables, and the total effect is the sum of both direct and indirect effects (33). This approach allowed us to include log-transformed geometric mean and specific gravity corrected phthalate metabolite concentrations for each individual across pregnancy, while also accounting for correlations among phthalate groupings and metabolites.
The choice of covariates and paths in the conceptual model was based on previous literature regarding the predictors of weight change in women [breastfeeding, education and SES (3)] and phthalate exposure [dietary sources (15), education (16), age, marital and socioeconomic status (34)]. Age, education level, breastfeeding duration, SES, marital status, parity rate and energy intake were included as part of the association pattern to evaluate the direct and indirect effects between phthalate exposures and weight change per year. Age, education level and energy intake were considered confounding variables. Variables and paths were included a priori based on the conceptual model. Paths not statistically significant to the model were eliminated. All analyses were performed using STATA SE version 14.
3. RESULTS
Among the initial subset of 250 ELEMENT women, 229 had phthalate measurements from at least one trimester of pregnancy. Of these 229, 205 had complete information on postpartum weight and weight at follow-up, of which 178 had complete information on age, breastfeeding duration, education level, marital status, energy intake and parity rate. This final analytic sample was not statistically different from the 229 women with phthalate measurements, or from the original cohorts, in terms of baseline sociodemographic characteristics, daily energy intake, breastfeeding duration, and body weight in the first year postpartum (data not shown).
Baseline sociodemographic characteristics are summarized in Table 1. The mean age at pregnancy was 27.3±5.9 years. As of 13.42±1.65 years after the index birth, 56.2% had not had additional children, 32.0% had one additional child, and 11.8% had between 2 and 4 more children. The mean education was 11.0±2.9 years, and 84% of participants were low to middle SES. Mean BMI was 27.1 kg/m2 during the first year postpartum and 28.6 kg/m2 at the follow-up visits, and mothers gained an average of 0.52 kg, or a 0.22 unit increase in BMI, per year of follow-up (Table 2).
Table 1.
Baseline sociodemographic characteristics
| n | % | Mean | SD | Range | |
|---|---|---|---|---|---|
| 178 | 27.32 | 5.88 | 14–44 | ||
| Parity (number of children)a | 178 | 0.58 | 0.76 | 0–4 | |
| Parity rate (number of children/year)a | 178 | 0.04 | 0.06 | 0–0.37 | |
| Energy intake in pregnancy (kcal) | 178 | 1912.80 | 536.01 | 859.96–3792.98 | |
| Energy intake postpartum (kcal) | 178 | 1797.96 | 484.67 | 789.08–3342.58 | |
| Education | 178 | 11.03 | 2.86 | 2–20 | |
| Elementary school | 11 | 6.18 | |||
| Middle school | 56 | 31.46 | |||
| High school | 83 | 46.63 | |||
| Undergraduate and graduate school | 28 | 15.73 | |||
| SESb | 169 | ||||
| A/B | 0 | 0.00 | |||
| C+ | 5 | 2.96 | |||
| C | 22 | 13.02 | |||
| D+ | 52 | 30.77 | |||
| D | 60 | 35.50 | |||
| E | 30 | 17.75 | |||
| Marital status | 178 | ||||
| Living with a partner | 159 | 89.33 | |||
| Living with no partner | 19 | 10.67 | |||
| Breastfeeding duration | 178 | ||||
| 0 months | 11 | 6.18 | |||
| 1–6 months | 67 | 37.64 | |||
| 7–12 months | 48 | 26.97 | |||
| > 12 months | 52 | 29.21 |
SD: standard deviation, SES: socioeconomic status.
Note:
The variable parity and parity rate only includes the number of children the woman had 13.42±1.65 years after the index child.
A/B is the highest SES category.
Table 2.
Anthropometric measurements by follow-up period (n=178)
| Mean | SD | Range | |
|---|---|---|---|
| Height (m) | 1.54 | 0.06 | 1.37–1.72 |
| Mean weight during first year postpartum (kg) | 63.79 | 10.55 | 43.05–96.13 |
| Mean BMI during first year postpartum (kg/m2) | 27.07 | 4.22 | 17.69–41.61 |
| Mean weight during follow-up visits (kg) | 67.27 | 11.44 | 44.6–108.5 |
| Mean BMI during follow-up visits (kg/m2) | 28.56 | 4.63 | 18.34–43.06 |
| Weight change (kg)a | 3.48 | 5.70 | −17.08–26.43 |
| Weight change (kg) per year | 0.52 | 0.84 | −2.82–3.57 |
| BMI change (kg/m2)a | 1.49 | 2.40 | −6.78–9.95 |
| BMI change (kg/m2) per year | 0.22 | 0.35 | −1.13–1.43 |
SD: standard deviation, BMI: body mass index.
Note:
The period considered for the weight and BMI change was 7.1±1.2 years (5.2–10.7 years).
Geometric mean concentrations of phthalate metabolites corrected for specific gravity are presented in Supplementary Table 2. Comparing phthalate concentrations across pregnancy we found that MEOHP concentrations in the second and third trimester, and MEHP, MECPP, MEHHP, MEOHP MiBP, MBzP, and ∑DEHP concentrations in the third trimester were significantly higher in comparison to the first trimester. There were no statistically significant differences across pregnancy for the rest of the phthalates evaluated (Supplementary Table 3).
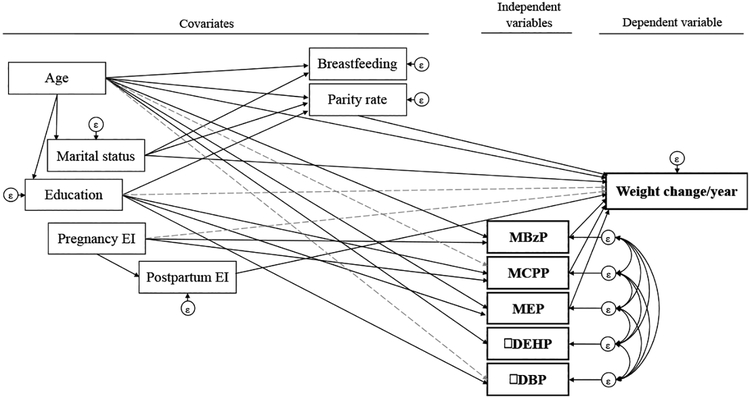
The resulting path model for evaluating associations among geometric means of phthalate metabolites across pregnancy, weight change per year, and covariates is illustrated in Figure 1. The main direct and indirect effect coefficients for weight change per year from the path model are presented in Table 3. Overall, the path analysis model explained 61.96% of the total variance of the weight change per year. Based on total effects, a one unit increase in log-transformed MCPP concentration during pregnancy was significantly associated with a 0.33 kg increase in weight gain per year (95% CI:0.09,0.56) during an average period of 7.1±1.2 years (5.2–10.7 years). In contrast, a one unit increase in log-transformed of MBzP was associated with a 0.21 kg decrease in weight gain per year (95% CI: −0.38, −0.03) during the same time period. Similarly, a one unit increase in log-transformed of MEP was associated with 0.07 kg (95% CI: −0.20, 0.05) lower weight gain per year, although this difference did not reach statistical significance. The total effects did not deviate from the direct effects (Table 3).
Figure 1. Final path model of the relation between phthalate exposures and weight change per year.
EI: energy intake. Notes: continuous lines indicate direct effects, while the dotted gray lines represent indirect effects. Age, marital status and education information were collected during pregnancy. The breastfeeding lasted up to 36 months after delivery; weight change was calculated for the period between the first year postpartum and the follow-up visits (5 to 10 years, mean 7.1±1.2) and parity rate was measured 4.95±0.92 years after the last weight measurements.
Table 3.
Main direct and indirect effects for the Path model (n=178)
| Direct effects | Indirect effects | |||||
|---|---|---|---|---|---|---|
| Paths | β | 95% CI | p value | β | 95% CI | p value |
| Effects on weight change/yeara | ||||||
| MBzP | −0.21 | −0.38,−0.03 | 0.02* | |||
| MCPP | 0.33 | 0.09,0.56 | 0.007* | |||
| MEP | −0.07 | −0.20,0.05 | 0.24 | |||
| Age | −0.02 | −0.05,0.0001 | 0.05 | −0.002 | −0.01,0.01 | 0.78 |
| Educationb | ||||||
| Middle school | 0.15 | 0.002, 0.30 | 0.047* | |||
| High school | 0.19 | 0.02,0.36 | 0.03* | |||
| Undergraduate/graduate school | 0.22 | 0.03,0.41 | 0.03* | |||
| Parity ratec | 1.01 | −1.34,3.37 | 0.40 | |||
| Pregnancy EId | 0.01 | −0.003,0.03 | 0.11 | |||
| Postpartum EId | 0.02 | −0.0003,0.05 | 0.05 | |||
| Marital statuse | −0.22 | −0.62,0.18 | 0.27 | 0.03 | −0.04,0.10 | 0.43 |
p<0.05. CI: confidence interval, EI: energy intake.
Notes:
The period considered for the weight change was 7.1±1.2 years (5.2–10.7 years).
Elementary school is the reference group in education.
The variable parity rate only includes the number of children the woman had 13.42±1.65 years after the index child.
The energy intake unit is 100 kcal.
Living with a partner is the reference group for marital status.
Based on the total effects, higher education was positively associated with annual weight change, with increases of 0.15, 0.19, and 0.22 kg/year for middle school, high school, and college, respectively, compared to the elementary school category (p<0.05). Similarly, each increase of 100 kcal in daily energy intake during the postpartum period was marginally associated with a 0.02 kg increase in weight per year (p=0.053). Also, a year increase in age at pregnancy was associated with a decrease of 0.02 kg per year in weight during follow-up. Parity rate, marital status and daily energy intake during pregnancy were not significantly associated with weight change rate (p>0.05) (Table 3). Although SES and EI during the follow-up were initially considered as part of the path model, they were not included in the final model due minor contributions when level of education, and pregnancy and postpartum EI were included, respectively. Path coefficients for remaining endogenous variables are presented in Supplementary Table 4.
Finally, in a complementary analysis including the nine individual phthalates, we observed that the lack of statistical significance of the association between ∑DEHP, ∑DBP and weight change/year could be explained by the contradicting and non-statistically significant effects of the metabolites that compose these groups, with the exception of MiBP (Supplementary Table 5). The goodness of fit of both path analyses was evaluated.
4. Discussion
In the present study, we aimed to explore the association of phthalate exposure during pregnancy with long-term weight gain among Mexican women from the ELEMENT birth cohort. We observed that urinary MCPP concentrations during pregnancy were associated with greater weight gain, while MBzP was associated with lower weight gain, over an average of 7 years after the first year postpartum.
To our knowledge, one previous study evaluated phthalate exposure and long-term weight gain in adult women (20). Contrary to our results, in a sample of U.S. women 32–79 years old from the Nurses’ Health Study (NHS) and NHS II, higher urinary concentrations of MBzP, as well as phthalic acid, bisphenol A and ∑DBP metabolites, were significantly associated with increased annual weight gain during a ten year period. A mean weight gain of 2.09 kg over this period was reported, which is lower than the mean weight gain of 3.5 kg over a smaller period of time (7.1 years) found in the present study (20). One potential explanation for the discrepancies between this study and our findings is the timing of exposure measurement, as our study measured phthalate exposure during pregnancy whereas the NHS study included non-pregnant women. In addition, women participating in NSH and NSH II were older than women in our study.
A previous study in the LIFECODES pregnancy cohort evaluated associations between urinary phthalate metabolites in the first trimester and early GWG in the first to second trimester (5). It was found that the highest two quartiles of MBzP and MEP concentrations were associated with lower GWG, compared to the lowest two MBzP and MEP quartiles. In contrast, MCPP concentrations were non-linearly associated with higher GWG, but this association was not statistically significant (5). Although this study only described associations of phthalates with early GWG, they observed similar relationships of MBzP, MCPP and MEP with maternal weight gain to those observed in the present study (5). Additionally, GWG has been associated with long-term weight gain. For example, a birth cohort study of English women found that women who had GWG above the recommendation had 3 times the risk of high central adiposity and being overweight 16 years after delivery (35).
Cross-sectional associations between phthalate exposure and adiposity have been evaluated in various populations with inconsistent results. In contrast to our findings, in the NHANES 1999–2002, MEP quartiles were positively associated with BMI and WC in adolescent girls (p<0.05) and in 20–59 year old women (p=0.1) (18). Similar to the NHANES study, a cross-sectional study of Chinese adults reported that MBzP concentrations were associated with increased odds of obesity in women aged over 45 years; however, MBzP was also associated with decreased odds of central obesity measured by WC (36). Moreover, in a cross-sectional study of adults aged over 20 years in the NHANES 1999–2006, MBzP was inversely associated with lean mass (37), a contributor to the overall weight. Similarly, inverse associations between MBzP and weight gain in infants and school-aged children have been previously described (38, 39). However, as these findings have been in children and non-pregnant adults and only consider cross-sectional exposure, the generalizability to exposure during pregnancy and long-term weight in women is unclear.
Pregnancy may be a life stage during which women are particularly vulnerable to the effects of phthalate exposure. First, pregnancy is a dynamic process related to several physiological changes that affect the pharmacodynamics of xenobiotics, such as phthalates, in the maternal body. In a physiologically-based pharmacokinetic (PBPK) model, pregnant rats presented a reduced capacity for glucuronide conjugation due to a decrease in glucuronyltransferase compared to male rats. This reduction may subsequently cause an increase in free mono-butyl phthalate (MnBP) residence time, in both maternal and fetal plasma, suggesting longer exposure (4). Second, dramatic changes in metabolism occur during pregnancy, including increases in maternal fat stores and changes in insulin sensitivity that prepare the body for the increased energy demands of gestation and lactation (2). During the postpartum period, metabolism, hormone concentrations, and body weight typically return to pre-pregnancy levels, but approximately 20 percent of women retain excessive weight from pregnancy to one year post-delivery (40, 41). Factors such as breastfeeding duration, diet, social support, sleep deprivation, and depression are thought to play a role in postpartum weight retention, but the effects of environmental exposures have not been explored.
Phthalate exposure during pregnancy could potentially have an impact on long-term maternal weight through endocrine disruption or interference with PPAR activation. Evidence of relationships between phthalate exposures and alterations in hormone levels during pregnancy has been previously described. For example, a birth cohort study in Puerto Rico reported an inverse association between MCPP and free triiodothyronine (T3), and a positive association between MBzP and thyroid-stimulating hormone (TSH) during pregnancy (42). Although the potential impact of subclinical alterations during pregnancy remains understudied, it is known that thyroid hormones are involved in the regulation of basal metabolism and thermogenesis, lipid and glucose metabolism, and food intake (43). TSH, T3 and free T3 concentrations have been positively associated with weight, body fat, BMI, WC and waist/hip ratio in both euthyroid men and non-pregnant women (44).
Phthalates are also known activators of PPARα, PPARβ, and PPARγ, which are involved in glucose and fatty acid homeostasis (45). PPARs form heterodimers with RXR (11), the signaling of which affects glucose and triglyceride disposition, promotes preadipocyte differentiation, and promotes the expression of diverse adipogenic genes (9). During pregnancy, PPARs also play a role in regulating inflammation, angiogenesis, and oxidative stress (46). In vitro studies suggest that the molecular weight of phthalate metabolites may play a role in PPAR activation, as large chain monoesters induced a greater adipogenic effect, an indicator of PPARγ activation, in 3T3-L1 fibroblasts (45). This might explain the lack of statistical significance in the association of MEP with weight change in the current study, as MBzP and MCPP are high molecular weight metabolites and MEP is a low molecular weight metabolite.
The current study has a number of limitations. Phthalate metabolites were measured three times during pregnancy and summarized as a proxy of overall exposure in this period. Due to the short biological half-lives of phthalates and temporal variability of urinary phthalate levels during pregnancy (15, 47, 48), this measure may not fully characterize exposure. Furthermore, the lack of information on diet quality is a limitation, as phthalates have been associated with intake of spices, meat, and organic and dairy products in previous studies of pregnant women (15, 49). Likewise, in a study of NHANES 2003–2010 participants, energy and fat consumption from fast food were associated with higher exposures to diisononyl phthalate (DiNP), a parent compound of MCPP, in comparison with non-consumers (50). In the present study, we addressed this issue by adjusting for mean energy intake during pregnancy and the first postpartum year, which has been proposed as a valid method to control confounding in epidemiological studies due to the association of energy intake with disease risk, physical activity, body weight, and metabolic efficiency (51).
Furthermore, information regarding parity after the index ELEMENT child was collected an average of 4.95±0.92 (range: 3 to 7) years after the last maternal weight measurement. However, information on births specifically during the study period was unavailable, and it is likely that the majority of births occurred within the first few years post ELEMENT recruitment as these were prime child-bearing years. In addition, we expressed parity as a rate to adjust for varying follow-up times. In addition, our analysis met the linearity and normality assumptions of structural equation models (52), so our results should not be affected by these limitations. Specifically, the normality of the response variable and the linearity of associations between the response variable and the continious independent variables were verified using statistical tests and graphical methods. Furthermore, interactions (derived from the conceptual model) between some of the covariates were evaluated, but were not statistically significant.
A major strength of the present study is that it is based on mothers from a long-standing birth cohort study, which permits the evaluation of long-term maternal health. To our knowledge, this is the first study to evaluate the association between phthalate exposure during pregnancy and subsequent long-term changes in weight in adult women. Additionally, we used path analysis, which has greater statistical power than traditional regression models due to the consideration of relationships between covariates (52), including correlations between multiple phthalate metabolite levels during pregnancy.
5. Conclusion
We observed significant relationships between select urinary phthalate metabolite concentrations in pregnancy and long-term changes in maternal weight. Exposure to MCPP during pregnancy was associated with higher than average weight gain, while MBzP exposure was associated with lower than average weight gain over several years of follow-up. Further studies are needed to confirm these findings, and to explore the long-term effects of prenatal phthalate exposure on maternal health.
Supplementary Material
Highlights.
Metabolism and lifestyle changes promote postpartum weight retention in women.
Prenatal MCPP exposure is associated with higher long-term weight gain in women.
Prenatal MBzP exposure is associated with lower long-term weight gain in women.
Acknowledgements
We thank the study team at the American British Cowdray Medical Center and at the Instituto Nacional de Perinatología for allow us use their research facilities.
Funding
This study was supported by the National Institute of Environmental Health Sciences/National Institutes of Health (NIEHS/NIH) [grants R01ES007821, P01 ES022844]; U.S. Environmental Protection Agency (EPA) [grant RD 83543601]; and Consejo Nacional de Ciencia y Tecnología [grant 37210-M, 41912-M, 29192-M]. This study was also supported and partially funded by the National Institute of Public Health/Ministry of Health of Mexico.
Abbreviations
- ELEMENT
Early Life Exposure in Mexico to Environmental Toxicants
- MEHP
mono-2-ethylhexyl phthalate
- MECPP
mono(2-ethyl-5-carboxypentyl) phthalate
- MEHHP
mono(2-ethyl-5-hydroxyhexyl) phthalate
- MEOHP
mono(2-ethyl-5-oxohexyl) phthalate
- MnBP
mono-butyl phthalate
- MiBP
mono-isobutyl phthalate
- MBzP
mono-benzyl phthalate
- MCPP
mono-3-carboxypropyl phthalate
- MEP
monoethyl phthalate
- ∑DBP
sum of di-n-butyl phthalate metabolites
- ∑DEHP
sum of di(2-ethylhexyl) phthalate metabolites
- DEP
diethyl phthalate
- DiNP
di-isononyl phthalate
- DiBP
diisobutyl-phthalate
- DOP
di-n-octyl phthalate
- SG
specific gravity
- MW
molecular weight
- LOD
limit of detection
- PPAR
peroxisome proliferator-activated receptor
- RXR
retinoid X receptor
- PBPK
physiologically-based pharmacokinetic
- TSH
thyroid-stimulating hormone
- T3
free triiodothyronine
- BMI
body mass index
- GWG
gestational weight gain
- WC
waist circumference
- FFQ
food frequency questionnaires
- SES
socioeconomic status
- AMAI
Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública
- NHS
Nurses’ Health Study
- NHANES
National Health and Nutrition Examination Survey
- NIEHS/NIH
National Institute of Environmental Health Sciences/National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 2000;22(2):261–74. [DOI] [PubMed] [Google Scholar]
- 2.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clinical obstetrics and gynecology. 2007;50(4):938–48. [DOI] [PubMed] [Google Scholar]
- 3.Endres LK, Straub H, McKinney C, Plunkett B, Minkovitz CS, Schetter CD, et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol. 2015;125(1):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clewell RA, Kremer JJ, Williams CC, Campbell JL Jr., Andersen ME, Borghoff SJ Tissue exposures to free and glucuronidated monobutylyphthalate in the pregnant and fetal rat following exposure to di-n-butylphthalate: evaluation with a PBPK model. Toxicol Sci. 2008;103(2):241–59. [DOI] [PubMed] [Google Scholar]
- 5.Bellavia A, Hauser R, Seely EW, Meeker JD, Ferguson KK, McElrath TF, et al. Urinary phthalate metabolite concentrations and maternal weight during early pregnancy. Int J Hyg Environ Health. 2017;220(8):1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62(11):806–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wittassek M, Koch HM, Angerer J, Bruning T. Assessing exposure to phthalates - the human biomonitoring approach. Mol Nutr Food Res. 2011;55(1):7–31. [DOI] [PubMed] [Google Scholar]
- 8.De Coster S, van Larebeke N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health. 2012;2012:713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grun F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord. 2007;8(2):161–71. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Xu S, Tan T, Lee ST, Cheng SH, Lee FW, et al. Toxicity and estrogenic endocrine disrupting activity of phthalates and their mixtures. Int J Environ Res Public Health. 2014;11(3):3156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, et al. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008;456(7220):350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2017. In: Services USDoHah, editor. 2017. [Google Scholar]
- 13.CDC. Phthalates Atlanta, GA, USA: Centers for Disease Control and Prevention; 2016. [December 23, 2016:[ [Google Scholar]
- 14.Buckley JP, Palmieri RT, Matuszewski JM, Herring AH, Baird DD, Hartmann KE, et al. Consumer product exposures associated with urinary phthalate levels in pregnant women. J Expo Sci Environ Epidemiol. 2012;22(5):468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, et al. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ Int. 2014;62:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Shi M, Chen B, Lin J, Yang S, Zhu B, et al. [Levels of phthalate internal exposure levels in pregnant women and influencing factors]. Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49(11):998–1004. [PubMed] [Google Scholar]
- 17.Hernandez-Diaz S, Su YC, Mitchell AA, Kelley KE, Calafat AM, Hauser R. Medications as a potential source of exposure to phthalates among women of childbearing age. Reprod Toxicol. 2013;37:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaghjyan L, Sites S, Ruan Y, Chang SH. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National Health and Nutrition Examination Survey 1999–2004. Int J Obes (Lond). 2015;39(6):994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes (Lond). 2014;38(12):1532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James-Todd TM, Huang T, Seely EW, Saxena AR. The association between phthalates and metabolic syndrome: the National Health and Nutrition Examination Survey 2001–2010. Environ Health. 2016;15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svensson K, Hernandez-Ramirez RU, Burguete-Garcia A, Cebrian ME, Calafat AM, Needham LL, et al. Phthalate exposure associated with self-reported diabetes among Mexican women. Environ Res. 2011;111(6):792–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ettinger AS, Lamadrid-Figueroa H, Tellez-Rojo MM, Mercado-Garcia A, Peterson KE, Schwartz J, et al. Effect of calcium supplementation on blood lead levels in pregnancy: a randomized placebo-controlled trial. Environ Health Perspect. 2009;117(1):26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu H, Tellez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, et al. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect. 2006;114(11):1730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva MJ, Samandar E, Preau JL Jr., Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860(1):106–12. [DOI] [PubMed] [Google Scholar]
- 26.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 1990;5(1). [Google Scholar]
- 28.Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect. 2008;116(2):173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahar MS, Soliman AS, Colacino JA, Calafat AM, Battige K, Hablas A, et al. Urinary bisphenol A concentrations in girls from rural and urban Egypt: a pilot study. Environ Health. 2012;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez-Avila M, Romieu I, Parra S, Hernandez-Avila J, Madrigal H, Willett W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998;40(2):133–40. [DOI] [PubMed] [Google Scholar]
- 31.AMAI. Avances del Comité de Niveles Socioeconómicos. Comité de Niveles Socioeconómicos. Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública, A.C; 2000.
- 32.López H Nivel Socieconómico AMAI. In: AMAI, editor.: INEGI; 2008. [Google Scholar]
- 33.Schumacker RE, Lomax RG. A Beginner’s Guide to Structural Equation. Third ed: Routledge: Taylor & Francis Group; 2010. [Google Scholar]
- 34.Wenzel AG, Brock JW, Cruze L, Newman RB, Unal ER, Wolf BJ, et al. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere. 2018;193:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser A, Tilling K, Macdonald-Wallis C, Hughes R, Sattar N, Nelson SM, et al. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC). Am J Clin Nutr. 2011;93(6):1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong R, Zhou T, Chen J, Zhang M, Zhang H, Wu M, et al. Gender- and Age-Specific Relationships Between Phthalate Exposures and Obesity in Shanghai Adults. Arch Environ Contam Toxicol. 2017;73(3):431–41. [DOI] [PubMed] [Google Scholar]
- 37.Corbasson I, Hankinson SE, Stanek EJ 3rd, Reeves KW. Urinary bisphenol-A, phthalate metabolites and body composition in US adults, NHANES 1999–2006. Int J Environ Health Res. 2016;26(5–6):606–17. [DOI] [PubMed] [Google Scholar]
- 38.Kasper-Sonnenberg M, Koch HM, Wittsiepe J, Wilhelm M. Levels of phthalate metabolites in urine among mother-child-pairs - results from the Duisburg birth cohort study, Germany. Int J Hyg Environ Health. 2012;215(3):373–82. [DOI] [PubMed] [Google Scholar]
- 39.Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, et al. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect. 2015;123(10):1022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collings R, Hill B, Skouteris H. The influence of psychological factors on postpartum weight retention 12 months post-birth. Journal of reproductive and infant psychology. 2018;36(2):177–91. [DOI] [PubMed] [Google Scholar]
- 41.Gunderson EP, Rifas-Shiman SL, Oken E, Rich-Edwards JW, Kleinman KP, Taveras EM, et al. Association of fewer hours of sleep at 6 months postpartum with substantial weight retention at 1 year postpartum. Am J Epidemiol. 2008;167(2):178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-Gonzalez LO, Del Toro LV, et al. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol. 2015;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Longhi S, Radetti G. Thyroid function and obesity. J Clin Res Pediatr Endocrinol. 2013;5 Suppl 1:40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontenelle LC, Feitosa MM, Severo JS, Freitas TE, Morais JB, Torres-Leal FL, et al. Thyroid Function in Human Obesity: Underlying Mechanisms. Horm Metab Res. 2016;48(12):787–94. [DOI] [PubMed] [Google Scholar]
- 45.Bility MT, Thompson JT, McKee RH, David RM, Butala JH, Vanden Heuvel JP, et al. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci. 2004;82(1):170–82. [DOI] [PubMed] [Google Scholar]
- 46.Ganss R Maternal Metabolism and Vascular Adaptation in Pregnancy: The PPAR Link. Trends in endocrinology and metabolism: TEM. 2017;28(1):73–84. [DOI] [PubMed] [Google Scholar]
- 47.Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of Urinary Phthalate Metabolite and Bisphenol A Concentrations before and during Pregnancy. Environmental Health Perspectives. 2012;120(5):739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watkins DJ, Sanchez BN, Tellez-Rojo MM, Lee JM, Mercado-Garcia A, Blank-Goldenberg C, et al. Impact of phthalate and BPA exposure during in utero windows of susceptibility on reproductive hormones and sexual maturation in peripubertal males. Environmental health : a global access science source. 2017;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serrano SE, Karr CJ, Seixas NS, Nguyen RH, Barrett ES, Janssen S, et al. Dietary phthalate exposure in pregnant women and the impact of consumer practices. Int J Environ Res Public Health. 2014;11(6):6193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zota AR, Phillips CA, Mitro SD. Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003–2010. Environ Health Perspect. 2016;124(10):1521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–8S; discussion 9S-31S. [DOI] [PubMed] [Google Scholar]
- 52.VanderWeele TJ. Invited commentary: structural equation models and epidemiologic analysis. Am J Epidemiol. 2012;176(7):608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.