Abstract
Cerebral and cardiac dysfunction cause morbidity and mortality in post-cardiac arrest syndrome (PCAS) patients. Predicting clinical outcome is necessary to provide the optimal level of life support for these patients. In this pilot study, we examined whether plasma ATP and adenylate levels have value in predicting clinical outcome in PCAS patients. In total, 15 patients who experienced cardiac arrest outside the hospital setting and who could be reanimated were enrolled in this study. Healthy volunteers (n=8) served as controls. Of the 15 PCAS patients, 8 died within 4 days after resuscitation. Of the 7 survivors, 2 lapsed into vegetative states, 1 survived with moderate disabilities, and 4 showed good recoveries. Arterial blood samples were drawn immediately after successful resuscitation and return of spontaneous circulation (ROSC). The concentrations of ATP and other adenylates in plasma were assessed with high performance liquid chromatography. PCAS patients had significantly higher ATP levels than healthy controls. Plasma ATP levels correlated with lactate levels, Acute Physiology and Chronic Health Evaluation (APACHE) II scores, and the time it took to regain spontaneous circulation (time-to-ROSC). Plasma adenylate levels in patients who died after resuscitation were significantly higher than in survivors. Based on our results and receiver operating characteristic curve analysis, we conclude that plasma adenylate levels may help predict outcome in PCAS patients.
Keywords: ATP, lactate, AMP, ADP, adenosine, out-of-hospital cardiac arrest
INTRODUCTION
About 100,000 patients each year experience out-of-hospital cardiac arrest in Japan alone (1, 2). A meta-analysis has shown a 1-month survival of only 4.7% in these patients (3). Patients who respond to reanimation with a return of spontaneous circulation (ROSC) after cardiac arrest often suffer cerebral and cardiac dysfunction due to prolonged whole-body ischemia (4). This phenomenon is referred to as post-cardiac arrest syndrome (PCAS) and involves anoxic brain injury, post-cardiac arrest myocardial dysfunction, and systemic ischemia/reperfusion (IR) injury. Post-resuscitation abnormalities in PCAS patients resemble the immune and coagulation disorders observed in sepsis patients (5, 6). The inflammatory response caused by wide-spread cell death and tissue injury shares similarities with the inflammatory response to microbial infections (7, 8).
Despite the advances in post-resuscitative care, many patients with PCAS experience poor neurological outcome. In the meta-analysis mentioned above, satisfactory outcome was obtained in little more than 2% of all patients who suffered cardiac arrest outside of the hospital setting (3). Predicting outcome is important to decide whether basic or advanced life support is needed to increase chances of survival and full recovery of PCAS patients (9, 10). Therefore, it is necessary to develop robust and timely prognostic tools that can accurately predict clinical outcome and inform treatment strategies that best benefit each individual PCAS patient. However, this task is difficult because routine interventions such as sedation and target temperature management (TTM) of patients, that are necessary at the onset of clinical care, interfere with neurological assessment strategies (11). With this in mind, the American Heart Association recently recommended the use of multiple factors to predict outcome of patients with cardiac arrest and to wait with final prognostication for at least 72 hours after completion of TTM (11).
Several studies with the goal of identifying parameters that help predict outcome in PCAS patients concluded that an eye witness of the cardiopulmonary arrest and the time to the return of spontaneous circulation (time-to-ROSC), ventricular fibrillation as first recorded rhythm, and cardiac causes have predictive values (12–14). In addition, many biomarkers have been examined as possible aids in the prognostication of PCAS patients, including neuron-specific enolase and S-100B. However, additional predictive markers are required as none of the individual biomarkers that are available to date allow accurate prediction of outcome with sufficient confidence (12).
Hypoxia and cell damage cause the release of ATP into the extracellular space, where ATP and its breakdown products contribute to inflammatory processes and immune cell activation (7, 8). ATP release and the activation of ATP receptors on the cell surfaces of neutrophils, T cells, and other immune cells play essential roles in the upregulation of cellular immune responses (15). Thus, ATP that is released into the circulation of PCAS patients may promote inflammation and aggravate organ damage by potentiating the inflammatory response to injury. Here we show that cardiopulmonary arrest results in the release of ATP into the circulation of PCAS patients and that plasma adenylate levels in PCAS patients correspond with clinical outcome.
METHODS
Study design
This is a prospective observational study of post-cardiac arrest patients admitted to the Emergency Department of Urayasu Hospital following an out-of-hospital cardiac arrest event with subsequent ROSC. The study was approved by the institutional review board of Juntendo University, Urayasu Hospital, Chiba, Japan (IRB approval # 23-19) and conducted in compliance with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all participants or a legal representative.
Study population
Inclusion criteria for the study were: age ≥ 18 years, out-of-hospital cardiac arrest with subsequent return of spontaneous circulation, comatose at the time of enrollment (Glasgow Coma Scale < 8). Exclusion criteria were pregnancy, traumatic cardiac arrest, stroke, terminal illnesses such as malignancies or liver cirrhosis, and the inability to obtain informed consent at the time of resuscitation.
Data collection
Demographic and other patient-related data that were collected included age, gender, past medical history, and the nature of the events that led to cardiac arrest. Specifically, information on whether the cardiac arrest was witnessed by a bystander, when the event occurred, whether bystanders provided CPR, and the initial cardiac rhythm. Vital signs and laboratory data including arterial blood gases, lactate levels, and biomarkers of liver and muscle damage were acquired as soon as possible after ROSC was established. The Acute Physiology and Chronic Health Evaluation (APACHE) II score was assessed when patients were admitted to the intensive care unit (16). Outcome measures were in-hospital mortality and neurologic function at the time of hospital discharge. Neurologic function was scored using Glasgow–Pittsburgh cerebral performance categories (CPC) as previously defined (17). The following CPC scores were used:
CPC1 - good performance
CPC2 - moderate disability
CPC3 - severe disability
CPC4 - vegetative state
CPC5 - brain death or death
PCAS patient management
Comprehensive management was performed according to the post cardiac arrest treatment algorithm proposed by the American Heart Association (4), ensuring adequate oxygenation and ventilation, support of circulation, timely initiation of TTM, and coronary angiography (CAG) as needed. The TTM protocol included cooling of core temperature to 34°C for 24 h using an Arctic Sun® 5000 temperature management system (IMI, Saitama, Japan) under general anesthesia with midazolam, fentanyl, and vecuronium bromide followed by a 48-h rewarming phase. Patients who did not regain circulatory stability with adequate fluid therapy and high-dose inotropic drug therapy (continuous infusion of epinephrine >0.5 mg/h) were excluded from TTM. Percutaneous cardiopulmonary support was applied for intractable ventricular fibrillation (VF) with defibrillation and medication. The intra-aortic balloon pump was used for acute coronary syndrome with cardiogenic shock.
High performance liquid chromatography (HPLC)
Arterial blood samples were drawn with a heparinized syringe using arterial lines within 15 min after ROSC. Concentrations of adenine compounds (ATP, ADP, AMP, and adenosine) in plasma samples were assessed by high-performance liquid chromatography (HPLC) as previously described (18). All blood sample preparation procedures were carried out at 0°C to prevent the hydrolytic breakdown of adenine compounds by ectonucleotidases and related enzymes that are ubiquitously present in human blood. After a two-step centrifugation method to separate blood cells and platelets from plasma, plasma samples (200 μl) were stabilized by precipitating plasma proteins with 8 M perchloric acid (10 μl; Sigma-Aldrich, St. Louis, MO). The resulting samples were neutralized, lipids were removed, and adenylates were converted to etheno-derivatives as previously described (18). Samples were analyzed using a Waters HPLC system (Waters, Milford, MA) and a Waters 474 fluorescence detector.
ELISA assay
A human Th1/Th2 10-plex assay kit from Meso Scale Diagnostics (Rockville, MD) was used to assess plasma cytokine levels.
Statistical analysis
Data are expressed as means ± SEM unless otherwise indicated. Differences between two groups were tested for statistical significance using unpaired two-tailed Student’s t tests for normally distributed data and the Mann-Whitney test for not normally distributed data. Multiple group comparisons were done with one-way ANOVA followed by post-hoc Tukey’s test when data were normally distributed and Kruskal-Wallis analysis and post-hoc Dunn’s test when data were not normally distributed. Associations between two parameters were analyzed with the Pearson’s correlation test. The receiver operating characteristic (ROC) curve analysis was used to evaluate the prognostic value (defined by the area under the curve, AUC) of parameters to predict survival or death. AUCs were compared with the DeLong test. Statistical analyses were performed with SigmaPlot (Systat Software Inc., San Jose, CA). Differences were considered statistically significant at p<0.05.
RESULTS
Patient demographics, treatment, and clinical outcome
About 300 patients with out-of-hospital cardiac arrest (OHCA) are admitted annually to the emergency department at Juntendo University, Urayasu Hospital in Chiba, Japan. In the study period, 60 of these patients were successfully resuscitated to regain spontaneous circulation. These patients were admitted to the intensive care unit (ICU) and informed consent to participate in this study was sought. Due to the acute nature of this study, informed consent could be secured from 15 patients only. These patients included 13 male and 2 female patients with a combined average age of 69±4.5 years. In 9 cases, cardiac arrest was of cardiogenic origin, which included 4 cases of ST elevation myocardial infarction (STEMI). Most of the remaining patients who were admitted with non-cardiac reasons for cardiopulmonary arrest had central airway obstructions (Table 1). Of the 15 patients who were enrolled, 10 received TTM and 5 patients were maintained at normothermia (36–37°C). The overall in-hospital mortality was 53%. The 8 patients who died were assigned a cerebral performance category (CPC) score of 5. Of the 7 surviving patients, 4 had good neurological outcome (CPC score: 1), 1 patient had moderate disabilities (CPC score: 2), and 2 patients lapsed into a vegetative state (CPC score: 4).
Table 1.
Demographics, treatments, and clinical course of PCAS patients
| Age | Sex | Eye witness | Initial cardiac rhythm | Bystander CPR | Time-to-ROSC (min) | Cause of cardiac arrest | Therapeutic hypothermia | CAG/PCI | CPC score | Time of death |
|---|---|---|---|---|---|---|---|---|---|---|
| 33 | M | Yes | Asystole | Yes | 60 | Cardiogenic (STEMI) | Yes | Yes/Yes | 1 | - |
| 65 | M | Yes | VF | Yes | 10 | Cardiogenic (STEMI) | Yes | Yes/Yes | 1 | - |
| 61 | M | Yes | VF | Yes | 5 | Cardiogenic (STEMI) | Yes | Yes/Yes | 1 | - |
| 95 | M | Yes | VF | Yes | 17 | Asphyxia | Yes | No | 1 | - |
| 49 | M | Yes | PEA | No | 34 | Cardiogenic | Yes | Yes/No | 2 | - |
| 71 | M | Yes | VF | Yes | 43 | Cardiogenic (STEMI) | Yes | Yes/Yes | 4 | - |
| 91 | F | No | VF | No | 35 | Cardiogenic | No | No | 4 | - |
| 60 | M | No | PEA/VF | No | 75 | Cardiogenic | Yes | No | 5 | 33 h |
| 71 | M | No | Asys/Refractory VF | No | 68 | Asphyxia (CO poisoning) | Yes | No | 5 | day 3 |
| 53 | M | Yes | VF | No | 60 | Cardiogenic (Arrhythmia) | Yes | Yes/No | 5 | day 3 |
| 93 | M | Yes | Yes | 51 | Asphyxia | No | No | 5 | 13 h | |
| 72 | M | No | PEA | Yes | 50 | Asphyxia | No | No | 5 | 8 h |
| 76 | F | No | PEA | No | 65 | Asphyxia | No | No | 5 | day 1 |
| 84 | M | No | No | 86 | Unknown | No | No | 5 | 3 h | |
| 63 | M | No | Asys/PEA | No | 74 | Cardiogenic | Yes | No | 5 | 4 h |
Asys, asystole/pulseless electrical activity; CAG, coronary angiography; CPC, cerebral performance categories; CPR, cardiopulmonary resuscitation; PCAS, post-cardiac arrest syndrome; PCI, percutaneous coronary intervention; PEA, pulseless electrical activity; ROSC, return of spontaneous circulation; STEMI, ST-elevation myocardial infarction; VF, ventricular fibrillation
Plasma ATP levels in PCAS patients are significantly higher than in healthy controls
Blood samples from all PCAS patients were obtained within 15 min of ROSC and immediately processed for HPLC analysis. Blood samples from healthy volunteers were collected and processed in the same fashion and served as controls. In the sampling method we employed, we avoided the use of commonly used additives such as dipyridamole and erythro-6-amino-9-(2-hydroxy-3-nonyl)- purine hydrochloride (EHNA) in order to minimize distortions of actual plasma adenylate levels. We found that this method is suitable for monitoring plasma ATP levels in critical care patients over a time course of several days (Supplemental Figure, Supplemental Digital Content 1). PCAS patients had significantly higher ATP levels than healthy controls (Fig. 1A). The wide distribution of ATP levels in the PCAS patient cohort suggested that differences in the degree of ischemia and reperfusion injury, tissue damage, or inflammation could be associated with the amount of ATP released into the circulation of these patients following cardiac arrest. However, while elevated plasma levels of ALT, AST, LDH, and CK indicated liver and skeletal muscle damage in most patients, these markers of tissue damage did not correlate with circulating ATP levels (Table 2).
Fig. 1. PCAS patients who survive have lower plasma levels of ATP, ADP, and AMP than patients who die after resuscitation.
(A) Plasma concentrations of ATP in PCAS patients (n=15) were assessed by HPLC immediately after ROSC was regained. These levels were compared with plasma ATP concentrations in healthy controls (HC, n=8). Data are means ± SEM; ***p<0.001 (Mann-Whitney test). (B) Plasma concentrations of ATP, ADP, AMP, and adenosine (ADO) in PCAS patients were assessed with HPLC and grouped in non-survivors (n=8) and survivors (n=7) and compared to healthy controls (n=8). Data are shown as mean±SEM. Statistical comparisons were done with one-way ANOVA and post-hoc Tukey’s test or Kruskal-Wallis and post-hoc Dunn’s test when data were normally or not normally distributed, respectively; *p<0.05. (C) Plasma adenylate levels of two patients of each group were measured within 15 min of resuscitation and after 24 h.
Table 2.
Biomarkers of tissue damage, arterial lactate, time-to-ROSC, and APACHE II score of PCAS patients
| ALT (U/ml) | AST (U/ml) | LDH (U/ml) | CK (U/ml) | Lactate (mg/dl) | Time-to-ROSC (min) | APACHE II score | |
|---|---|---|---|---|---|---|---|
| Normal ranges | 8–42 | 13–33 | 119–229 | 60–287 | 3.2–11.3 | n. a. | 0–6 |
| All PCAS | 112.7±47.5 | 151.9±61.8 | 469.3±82.8 | 266.5±71.6 | 93±11 | 49±6 | 30±2 |
| Non-survivors | 55.1±20.7 | 83.1±37.2 | 405.9±102.5 | 119.5±42 | 117±15 | 66±4 | 36±2 |
| Survivors | 178.4±100.8 | 230.6±129.2 | 541.7±145.1 | 434.6±133.1 | 66±12* | 29±7* | 25±2* |
| CPC>3 | 63.7±17.8 | 98.7±31.2 | 405.5±81.4 | 181.4±74.1 | 104±14 | 61±5 | 34±2 |
| CPC<3 | 210.6±142.7 | 258.4±185.3 | 596.8±202.9 | 436.8±148.5 | 71±16 | 25±10# | 24±2# |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; CK, creatine kinase; APACE II, Acute Physiology and Chronic Health Evaluation II score; CPC, cerebral performance categories; PCAS, post-cardiac arrest syndrome; ROSC, return of spontaneous circulation; n. a., not applicable. Data represent mean ± SEM. Unpaired two-tailed t test and Mann-Whitney test were used for comparisons of normally and not normally distributed data, respectively.
p<0.05 survivors vs. non-survivors,
p<0.05 good vs. poor outcome.
Plasma adenylate levels in non-survivors are significantly higher than in survivors
Because ATP is unstable, we used HPLC to assess the circulating levels of ATP along with its breakdown products ADP, AMP, and adenosine in plasma samples. We found that the PCAS patients who died in the ICU had significantly higher levels of ATP and AMP in their circulation when compared to patients who survived (Fig. 1B, Table 2). These findings suggest that systemic ATP release is associated with mortality after the resuscitation of cardiac arrest patients. This notion is supported by follow-up measurements of plasma ATP concentrations in two patients of each group 24 h after resuscitation. The patients who survived showed a more substantial drop in combined plasma levels of ATP, ADP, and AMP when compared the non-survivors (Fig. 1C). Future studies with more patients will be needed to confirm these trends. Plasma levels of ATP, ADP, and AMP of healthy controls were significantly lower when compared to PCAS patients. Interestingly, adenosine levels in healthy controls were significantly higher than in PCAS patients (Fig. 1B). These findings suggest that most of the ATP that is released into the circulation of PCAS patients is converted to ADP and AMP, while a comparatively larger percentage of the extracellular ATP in the plasma of healthy subjects is converted to adenosine, an ATP derivative with anti-inflammatory properties (15).
Plasma lactate levels in PCAS patients correlate with plasma ATP levels
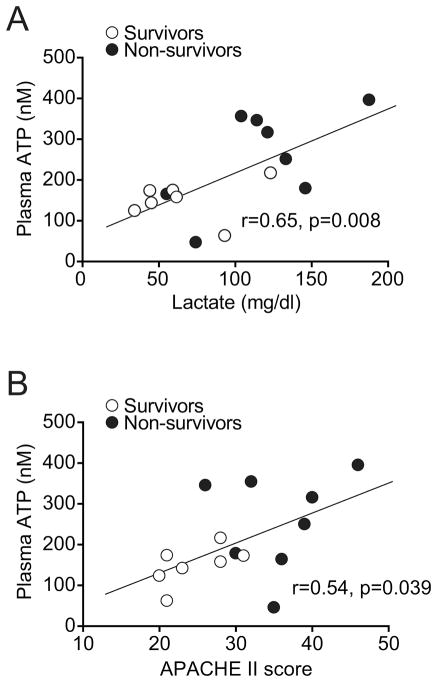
ATP and its breakdown products in the plasma of PCAS patients may be the result of ATP release from damaged cells and inflamed tissues. Lactate is a widely used marker of impaired metabolism and tissue perfusion that is associated with microcirculatory and mitochondrial dysfunctions (19). Similar to the plasma ATP levels in PCAS patients, plasma lactate levels in non-survivors were significantly higher than in survivors (Table 2). Interestingly, the lactate and ATP levels in the plasma of PCAS patients showed a significant correlation that supports the notion that ATP release is associated with impaired metabolism and/or ischemia and reperfusion injury following cardiopulmonary arrest (Fig. 2A).
Fig. 2. Plasma ATP correlates with lactate levels and APACHE II scores.
(A) Plasma lactate levels were assessed in blood samples obtained immediately after return of spontaneous circulation. (B) APACHE II scores were assessed after ROSC and on admission of the patients to the intensive care unit. Both parameters were correlated with the corresponding plasma ATP concentrations. Correlation plots and the linear regression line are shown (n=15; white symbols: survivors; black symbols: non-survivor; r: Pearson’s correlation coefficient).
Plasma ATP levels correlate with APACHE II scores
Like plasma ATP and lactate levels, APACHE II scores were also significantly higher in PCAS patients who died in the ICU when compared to patients who survived (Table 2). The APACHE II scores of survivors and non-survivors showed a significant correlation with the corresponding plasma ATP levels of PCAS patients (Fig. 2B). APACHE II scores also correlated with the sum of the concentration of ATP, ADP, and AMP in the plasma of these patients (data not shown). Due to the small sample size, it was not feasible to perform correlation analyses within patient subgroups.
Plasma adenylate and lactate levels correlate with time-to-ROSC
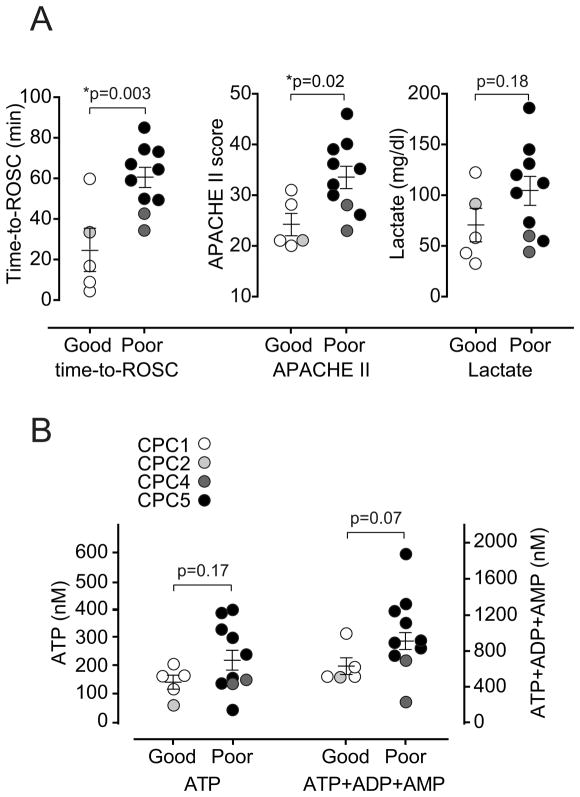
The average time required to achieve the return of spontaneous circulation (time-to-ROSC) in all cardiopulmonary arrest patients who were enrolled was ~50 min (Table 2). Successful reanimation of those PCAS patients who survived required significantly less time compared to the time-to-ROSC values of non-survivors (29 min vs. 66 min). Moreover, we found that patients with good neurological outcome (CPC<3) required less than half the time to regain spontaneous circulation when compared to patients with poor outcome (CPC>3; 25 min vs. 61 min). Interestingly, the time-to-ROSC values of all PCAS patients enrolled correlated with the sum of the concentrations of ATP, ADP, and AMP in the plasma of these patients and with their plasma lactate levels (Fig. 3, A and B). These correlations suggest that lactate and ATP levels in the circulation of PCAS patients are associated with the duration of ischemia and the metabolic stress caused by hypoxia and reperfusion following cardiopulmonary arrest.
Fig. 3. Plasma adenylate and lactate levels correspond with the duration of post cardiac arrest ischemia.
(A) The sum of ATP, ADP, and AMP plasma levels were correlated with the time that elapsed before the spontaneous circulation could be reestablished (time-to-ROSC). (B) Plasma lactate levels were correlated with corresponding time-to-ROSC values for each PCAS patient. Correlation plots and the linear regression line are shown (n=15; r: Pearson’s correlation coefficient).
Predicting mortality and morbidity in PCAS patients
The differences in plasma ATP and lactate levels, time-to-ROSC, and APACHE II scores between survivors and non-survivors suggest that these parameters may have predictive value for the assessment of PCAS patients. In order to test whether any of these parameters is suitable to predict neurological outcome, we separated all PCAS patients into two groups: patients with poor outcome (CPC>3) and patients with good outcome (CPC<3). These two groups differed significantly in their average time-to-ROSC values and APACHE II scores but not in their plasma lactate or ATP levels (Fig. 4). There was a trend towards lower combined levels of plasma ATP, ADP, and AMP in PCAS patients with good versus poor outcome. However, these differences did not reach statistical significance (Fig. 4B).
Fig. 4. Plasma adenylate levels as potential markers of outcome in PCAS patients.
PCAS patients were separated in two groups with good (CPC<3) and poor (CPC>3) neurological outcome and time-to-ROSC, plasma lactate levels, and APACHE II scores (panel A) or plasma ATP levels or the sum of plasma levels of ATP, ADP, and AMP (panel B) were compared. Each point shown represents an individual PCAS patient (open circles: CPC category 1; light gray circles: CPC category 2; dark gray circles: CPC category 4; black circles: CPC category 5). None of the patients fell in CPC category 3. Statistical analyses were done using unpaired two-tailed t test or the Mann-Whitney test for normally or not normally distributed data, respectively.
Adenylates, lactate, and cytokines as possible predictors of survival
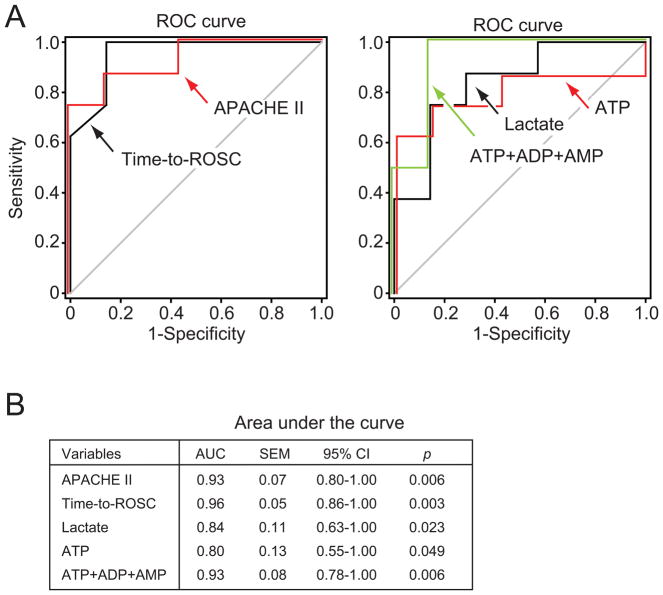
Based on our findings, time-to-ROSC and APACHE II scores are possible predictors of outcome. In agreement with this concept, we found that ROC curve analysis of both time-to-ROSC and APACHE II scores had a very high accuracy in predicting survival (AUC 0.96 and 0.93, respectively; Fig. 5). However, these parameters are not always available before treatment decisions must be implemented. ROC analysis revealed that plasma ATP values, the combined concentrations of ATP, ADP, and AMP in plasma, and plasma lactate levels were also good predictors of outcome in PCAS patients (Fig. 5). There were no significant differences between the AUC values of any of these plasma parameters and time-to-ROSC and APACHE II scores tested. However, since the small sample size did not allow multiple predictor analyses, future studies with larger patient cohort sizes will be necessary to confirm the predictive value and individual contributions of each of these parameters. We also examined cytokine levels in plasma samples of PCAS patients and found evidence that IFN-γ levels were significantly higher in non-survivors than in survivors (Table 3). Taken together, these findings suggest that analysis of plasma levels of ATP, its breakdown products, lactate, and cytokines could be used in combination to predict clinical outcome in PCAS patients.
Fig. 5. Plasma adenylate levels as predictors of survival in PCAS patients.
ROC curves for APACHE II and time-to-ROSC (left) were assessed as predictors of survival using Receiver Operating Characteristic (ROC) curves and compared to plasma ATP, lactate, and the sum of plasma ATP, ADP, and AMP levels (right). (B) Area under the curve (AUC) characteristics are shown. CI, confidence interval.
Table 3.
Th1/Th2 cytokine levels in plasma of PCAS patients (plasma concentrations in pg/ml)
| IFN-γ | IL-10 | IL-12p70 | IL-13 | IL-1β | IL-2 | IL-4 | IL-5 | IL-8 | TNF-α | |
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy controls | 0.1±0.0 | 0.5±0.2 | 5.1±4.7 | 3.0±1.4 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.2±0.1 | 1.8±1.1 | 0.6±0.1 |
| All PCAS | 82±26 | 261±57 | 122±23 | 148±18 | 61±15 | 91±26 | 80±11 | 114±15 | 253±39 | 242±42 |
| Non-survivors | 126±34 | 272±48 | 147±24 | 163±11 | 84±18 | 91±39 | 92±7 | 125±17 | 254±28 | 234±11 |
| Survivors | 20±19* | 247±130 | 87±43 | 126±40 | 29±21 | 91±37 | 64±25 | 99±28 | 251±93 | 253±107 |
| CPC>3 | 98±32 | 320±64 | 146±23 | 165±10 | 77±17 | 101±33 | 81±10 | 123±13 | 283±42 | 276±48 |
| CPC<3 | 33±31 | 87±46# | 48±46 | 94±61 | 13±13 | 64±37 | 78±41 | 86±49 | 163±85 | 141±68 |
CPC, cerebral performance categories; PCAS, post-cardiac arrest syndrome; Data: mean ± SEM;
p<0.05 survivors vs. non-survivors,
p<0.05 good vs. bad outcome.
Statistics were done using unpaired two-tailed t test or Mann-Whitney test if data were normally or not normally distributed, respectively.
DISCUSSION
We believe that this is the first study to show that ATP levels are elevated in the plasma of PCAS patients and that the concentrations of ATP and its breakdown products in the circulation of these patients correspond with the duration of ischemia and the clinical outcome following cardiac arrest. Regardless of the etiology of cardiac arrest in our study group, plasma adenylate levels of non-survivors were significantly higher than those of survivors. Like others before, we found that elevated lactate levels were associated with increased mortality in PCAS patients (19, 20). Ischemia as the consequence of reduced blood flow following cardiac arrest and the inflammatory response following ischemia and reperfusion are likely causes of the initial increase in plasma lactate levels (21). In our study, we found that plasma ATP levels of PCAS patients correlate with their plasma lactate levels and APACHE II scores (Fig. 2). It should be noted, however, that due to the small sample size that was available in this pilot study, we were not able to perform correlation analyses within patient subgroups. This should be done in future, larger scale studies to corroborate our current observations.
Anoxic/ischemic neurological cell damage remains the leading cause of death in PCAS patients. Accurate assessment of neurological damage and new prognostic methods are critical for the identification of patients who require special interventions in order to improve their recovery (22, 23). Based on our study, we propose that circulating plasma adenylate levels are useful parameters to help predict outcome in PCAS patients. This notion is supported by ROC curve analysis that suggests that these plasma adenylate levels in combination with lactate could be used to improve prediction of the clinical outcome in PCAS patients (Fig. 5). Further studies will be needed, however, to confirm our observations and to test the predictive power of plasma adenylates in larger patient cohort studies.
PCAS involves pathological changes similar to those seen in sepsis (6). Plasma cytokine levels were reported to be increased particularly in those PCAS patients who do not survive (5, 24). In our study, we found that IFN-γ plasma levels in non-survivors were higher than in survivors (Table 3). Taken together with the results shown above, these findings support the notion that ATP release promotes inflammation, which contributes to mortality in PCAS patients. Damage associated pattern molecules including ATP are known to promote inflammation and the activation of monocytes, neutrophils, and macrophages (25, 26). Activation of these phagocytes causes host tissue damage and multiple organ failure that contribute to morbidity and mortality following ischemia and reperfusion injury (25, 26). We have previously shown that extracellular ATP and autocrine feedback through ATP receptors are pivotal processes in the activation of neutrophils and other immune cells (27–30). Homeostasis of immune cell functions requires proper mitochondrial function, which could be impaired by hypoxia in PCAS patients (31, 32).
Our current findings that plasma ATP levels in non-survivors are significantly higher than in healthy controls and in PCAS patients who survive cardiac arrest support the notion that extracellular ATP promotes phagocyte activation, which is associated with IR-induced inflammation and host tissue damage. Thus, plasma ATP levels may allow the assessment of tissue damage and clinical outcome more reliably than standard markers of tissue damage, such as liver and muscle enzymes. This could be done with HPLC analysis as described in our study. However, this method is technically challenging and not feasible as a routine clinical laboratory test. However, novel methods such as those based on luminescent reagents or fluorescent probes previously described could be adopted for clinical use (33). In addition to its utility as a marker of cell damage and inflammation, ATP in the plasma of cardiopulmonary arrest patients might represent a therapeutic target for the treatment of PCAS patients. Removal of ATP or its conversion to anti-inflammatory adenosine using apyrase has been shown to attenuate tissue damage in animal models of ischemia-reperfusion injury (34, 35). Thus, it is possible that apyrase or recombinant ectonucleotidases to scavenge plasma ATP could help reduce inflammation and organ damage and thereby improve clinical outcome in PCAS patients. In summary, we conclude that plasma adenylates are potential predictors of outcome and possible therapeutic targets to reduce inflammation in PCAS patients. In order to test these concepts, additional preclinical trials and clinical studies with larger cohort sizes will be required.
Supplementary Material
Figure that demonstrates the feasibility of longitudinal monitoring of ATP plasma levels in critical care patients.
Acknowledgments
Source of Funding
This study was supported by the Japan Society for the Promotion of Science (23792086, 25462830; to Y.S.) and in part by grants from the National Institutes of Health (R01GM051477, R01GM060475, R01GM116162, R01AI080582, R01AI072287, and T32GM103702; to W.G.J.)
Abbreviations
- ADO
adenosine
- ADP
adenosine diphosphate
- AMP
adenosine monophosphate
- APACE II
Acute Physiology and Chronic Health Evaluation II score
- ATP
adenosine triphosphate
- CAG
coronary angiography
- CPA
cardio-pulmonary arrest
- CPC
cerebral performance categories
- HPLC
high performance liquid chromatography
- ICU
intensive care unit
- IR
ischemia/reperfusion
- OHCA
out-of-hospital cardiac arrest
- PCAS
post-cardiac arrest syndrome
- ROC curve
receiver operating characteristic curve
- ROSC
return of spontaneous circulation
- STEMI
ST-elevation myocardial infarction
- TTM
target temperature management
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Kitamura T, Iwami T, Kawamura T, Nagao K, Tanaka H, Berg RA, Hiraide A. Implementation Working Group for All-Japan Utstein Registry of the Fire and Disaster Management Agency: Time-dependent effectiveness of chest compression-only and conventional cardiopulmonary resuscitation for out-of-hospital cardiac arrest of cardiac origin. Resuscitation. 2011;82:3–9. doi: 10.1016/j.resuscitation.2010.09.468. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa M, Hori S, Adachi T, Miyazaki K, Inoue S, Suzuki M, Mori H, Nakazawa H, Aikawa N, Ogawa S. Adenosine triphosphate-sensitive potassium channels prevent extension of myocardial ischemia to subepicardium during hemorrhagic shock. Shock. 2008;30:178–183. doi: 10.1097/shk.0b013e318160d990. [DOI] [PubMed] [Google Scholar]
- 3.Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA. 2012;307:1161–1168. doi: 10.1001/jama.2012.294. [DOI] [PubMed] [Google Scholar]
- 4.Stub D, Bernard S, Duffy SJ, Kaye DM. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation. 2011;123:1428–1435. doi: 10.1161/CIRCULATIONAHA.110.988725. [DOI] [PubMed] [Google Scholar]
- 5.Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10:208–212. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- 6.Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 7.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 9.Dragancea I, Rundgren M, Englund E, Friberg H, Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation. 2013;84:337–342. doi: 10.1016/j.resuscitation.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Kurz MC, Schmicker RH, Leroux B, Nichol G, Aufderheide TP, Cheskes S, Grunau B, Jasti J, Kudenchuk P, Vilke GM, et al. Advanced vs. basic life support in the treatment of out-of-hospital cardiopulmonary arrest in the resuscitation outcomes consortium. Resuscitation. 2018 doi: 10.1016/j.resuscitation.2018.04.031. pii:S0300-9572(18)30192-8 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Taccone F, Cronberg T, Friberg H, Greer D, Horn J, Oddo M, Scolletta S, Vincent JL. How to assess prognosis after cardiac arrest and therapeutic hypothermia. Crit Care. 2014;18:202. doi: 10.1186/cc13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM, et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S465–482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corrada E, Mennuni MG, Grieco N, Sesana G, Beretta G, Presbitero P. Neurological recovery after out-of-hospital cardiac arrest: hospital admission predictors and one-year survival in an urban cardiac network experience. Minerva Cardioangiol. 2013;61:451–460. [PubMed] [Google Scholar]
- 14.Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 15.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 17.Cummins RO, Chamberlain DA, Abramson NS, Allen M, Baskett PJ, Becker L, Bossaert L, Delooz HH, Dick WF, Eisenberg MS, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation. 1991;84:960–975. doi: 10.1161/01.cir.84.2.960. [DOI] [PubMed] [Google Scholar]
- 18.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller BM, Dellinger RP. Lactate as a hemodynamic marker in the critically ill. Curr Opin Crit Care. 2012;18:267–272. doi: 10.1097/MCC.0b013e3283532b8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kliegel A, Losert H, Sterz F, Holzer M, Zeiner A, Havel C, Laggner AN. Serial lactate determinations for prediction of outcome after cardiac arrest. Medicine (Baltimore) 2004;83:274–279. doi: 10.1097/01.md.0000141098.46118.4c. [DOI] [PubMed] [Google Scholar]
- 21.Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88:1127–1140. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mongardon N, Dumas F, Ricome S, Grimaldi D, Hissem T, Pene F, Cariou A. Postcardiac arrest syndrome: from immediate resuscitation to long-term outcome. Ann Intensive Care. 2011;1:45. doi: 10.1186/2110-5820-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grunau B, Reynolds J, Scheuermeyer F, Stenstom R, Stub D, Pennington S, Cheskes S, Ramanathan K, Christenson J. Relationship between Time-to-ROSC and survival in out-of-hospital cardiac arrest ECPR candidates: When is the best time to consider transport to hospital? Prehosp Emerg Care. 2016;20:615–622. doi: 10.3109/10903127.2016.1149652. [DOI] [PubMed] [Google Scholar]
- 24.Braunstein M, Williamson M, Kusmenkov T, Landes J, Biberthaler P, Kanz KG, Böcker W, Bogner V. Significant cytokine mRNA expression changes immediately after initiation of cardiopulmonary resuscitation. Mediators Inflamm. 2017;2017:8473171. doi: 10.1155/2017/8473171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schofield ZV, Woodruff TM, Halai R, Wu MC, Cooper MA. Neutrophils-a key component of ischemia-reperfusion injury. Shock. 2013;40:463–470. doi: 10.1097/SHK.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 27.Sumi Y, Woehrle T, Chen Y, Bao Y, Li X, Yao Y, Inoue Y, Tanaka H, Junger WG. Plasma ATP is required for neutrophil activation in a mouse sepsis model. Shock. 2014;42:142–147. doi: 10.1097/SHK.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 29.Manohar M, Hirsh MI, Chen Y, Woehrle T, Karande AA, Junger WG. ATP release and autocrine signaling through P2X4 receptors regulate gammadelta T cell activation. J Leukoc Biol. 2012;92:787–794. doi: 10.1189/jlb.0312121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao Y, Ledderose C, Graf AF, Brix B, Birsak T, Lee A, Zhang J, Junger WG. mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J Cell Biol. 2015;210:1153–1164. doi: 10.1083/jcb.201503066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledderose C, Bao Y, Ledderose S, Woehrle T, Heinisch M, Yip L, Zhang J, Robson SC, Shapiro NI, Junger WG. Mitochondrial dysfunction, depleted purinergic signaling, and defective T cell vigilance and immune defense. J Infect Dis. 2016;213:456–464. doi: 10.1093/infdis/jiv373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ledderose C, Bao Y, Zhang J, Junger WG. Novel method for real-time monitoring of ATP release reveals multiple phases of autocrine purinergic signalling during immune cell activation. Acta Physiol (Oxf) 2015;213:334–345. doi: 10.1111/apha.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts V, Lu B, Rajakumar S, Cowan PJ, Dwyer KM. The CD39-adenosinergic axis in the pathogenesis of renal ischemia-reperfusion injury. Purinergic Signal. 2013;9:135–143. doi: 10.1007/s11302-012-9342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahim M, Wang X, Puyo CA, Montecalvo A, Huang HJ, Hachem RR, Andreetti C, Menna C, Chen R, Krupnick AS, et al. Human recombinant apyrase therapy protects against canine pulmonary ischemia-reperfusion injury. J Heart Lung Transplant. 2015;34:247–253. doi: 10.1016/j.healun.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure that demonstrates the feasibility of longitudinal monitoring of ATP plasma levels in critical care patients.