Abstract
Among 1306 patients with primary myelofibrosis (PMF), we sought to identify risk factors that predicted leukemic transformation (LT) in the first 5 years of disease and also over the course of the disease. 149 (11%) LT were documented; patients who subsequently developed LT (n = 149), compared to those who remained in chronic phase disease (n = 1,157), were more likely to be males (p = 0.02) and display higher circulating blasts (p = 0.03), ASXL1 (p = 0.01), SRSF2 (p = 0.001) and IDH1 (p = 0.02) mutations. Logistic regression analysis identified IDH1, ASXL1 and SRSF2 mutations, very high-risk karyotype, age > 70 years, male sex, circulating blasts ≥ 3%, presence of moderate or severe anemia and constitutional symptoms, as predictors of LT in the first 5 years of diagnosis. Time-to-event Cox analysis confirmed LT prediction for IDH1 mutation (HR 4.3), circulating blasts ≥ 3% (HR 3.3), SRSF2 mutation (HR 3.0), age > 70 years (HR 2.1), ASXL1 mutation (HR 2.0) and presence of moderate or severe anemia (HR 1.9). HR-based risk point allocation resulted in a three-tiered LT risk model: high-risk (LT incidence 57%; HR 39.3, 95% CI 10.8–114), intermediate-risk (LT incidence 17%; HR 4.1, 95% CI 2.4–7.3) and low-risk (LT incidence 8%). The current study provides a highly discriminating LT predictive model for PMF.
Introduction
Primary myelofibrosis (PMF) is an aggressive myeloid malignancy currently listed under the World Health Organization (WHO) category of myeloproliferative neoplasms (MPN)1. PMF represents a stem cell-derived clonal expansion of myeloid cells that often harbor one of three driver mutations, including JAK2, CALR and MPL. PMF is morphologically characterized by abnormal megakaryocyte proliferation that is often accompanied by reticulin fibrosis. Patients with PMF typically display severe anemia, marked hepatosplenomegaly and profound constitutional symptoms. Other complications of the disease include cachexia, thrombosis, bleeding and leukemic transformation (LT). Overall survival (OS) in PMF is estimated at 6 years and can range between a few months to over 20 years, depending on the presence or absence of specific clinical and genetic risk factors2–4. Current treatment in PMF includes allogeneic stem cell transplant (allo-SCT), which is the only treatment modality with the potential to cure the disease or prolong survival5. Other treatment approaches in PMF are mostly palliative and include drug therapy (e.g. JAK2 inhibitors), splenectomy and involved field radiation therapy6.
Taking the above into consideration, the primary objective in developing a treatment strategy for the individual patient with PMF is to establish the timing of allo-SCT. The particular task is often accomplished by considering risk level, according to previously established risk models for OS. At present, these include the genetically-inspired prognostic scoring system (GIPSS)3 and the mutation- and karyotype-enhanced prognostic scoring system (MIPSS70 + version 2.0)4. GIPSS relies on genetic risk factors only, including karyotype, driver mutations and other mutations, including ASXL1, SRSF2 and U2AF1 Q157. MIPSS70 + version 2.0 utilizes the same genetic risk factors used in GIPSS but also incorporates three specific clinical risk factors, including constitutional symptoms, presence of severe/moderate anemia and ≥ 2% circulating blasts. The main objective for the current study was to develop a robust LT predictive model that complements GIPSS and MIPSS70 + version 2.0 and thus further facilitates treatment decision-making in PMF; in this regard, it is to be recalled that, in the context of GIPSS/MIPSS70 + , leukemia-free survival (LFS) was previously shown to be affected by karyotype, SRSF2 and ASXL1 mutations, platelet count < 100 × 109/l and circulating blasts ≥ 2%3,7.
Methods
The current study was approved by the institutional review board of the Mayo Clinic, Rochester, MN, USA. The study population consisted of consecutive patients with PMF seen at our institution between April 26, 1976 and November 21, 2017. Diagnoses of PMF and LT were confirmed by both clinical and bone marrow examinations, in line with the 2016 WHO criteria; specifically, LT required presence of ≥ 20% blasts in the peripheral blood (PB) or bone marrow (BM)1. Data was collected retrospectively corresponding to the time of first referral which in the majority of cases was at the time of or within the first year of diagnosis. All patients were followed until death or last follow-up as assessed by medical records or through direct contact with patients or their physicians. Data collection was updated as of April 2018. The determination of prognostically relevant mutations was made by next generation sequencing (NGS)-derived mutation information8,9. Cytogenetics data were analyzed using standard techniques and reported in conformity with the International System for Human Cytogenetic Nomenclature criteria10.
Variables evaluated included those that are currently listed in MIPSS707, MIPSS70 + version 2.04 and GIPSS3, as well as age ( ≤ 70 vs > 70 years) and sex. Constitutional symptoms were defined as:1 weight loss > 10% of baseline during the year before the diagnosis, or2 unexplained excessive sweats, or3 fever persisting for at least a month11. Karyotype was designated as favorable, unfavorable or very high-risk (VHR), according to the recently published revised three-tiered cytogenetics risk model;12 VHR karyotype was defined as chromosomal abnormalities with single/multiple abnormalities of −7, i(17q), inv(3)/3q21, 12p−/12p11.2, 11q−/11q23, or other autosomal trisomies not including + 8/ + 9 (e.g., + 21, + 19)12. Sex-adjusted values for hemoglobin were categorized as severe anemia, defined by hemoglobin levels of < 8 g/dl in women and < 9 g/dl in men, and moderate anemia, defined by hemoglobin levels of 8–9.9 g/dl in women and 9–10.9 g/dl in men13. High molecular risk (HMR) mutations included ASXL1, SRSF2, U2AF1 Q157, IDH1/2 and EZH214,15.
Statistical analyses considered clinical and laboratory data collected at the time of initial PMF diagnosis or Mayo Clinic referral point. Continuous variables are presented as median (range) and categorical variables as frequency (percentage). The differences in the distribution of continuous variables between categories were compared using the Mann–Whitney or Kruskal–Wallis test. Categorical variables were compared using the χ2 test. Logistic regression statistics was employed in order to identify predictors of LT at 5 years (i.e., early events) from initial diagnosis/referral; in the particular method, patients with documented LT within 5 years were “uncensored” while those followed up for at least 5 years, without developing LT, were “censored”; the analysis excluded patients without LT and not followed for at least 5 years. In addition, Cox regression analysis was performed to identify risk factors for overall leukemia-free survival (LFS). The Kaplan–Meier method was used to construct time-to-leukemia curves, which were compared by the log-rank test. P values of < 0.05 were considered significant. In order to develop LT predictive model, HR-based risk point allocation was employed and predictive accuracy was compared to those of GIPSS and MIPSS70 + version 2.0, using Akaike Information Criterion (AIC) and receiver operating characteristic (ROC) curve-derived area under the curve (AUC) estimates. The JMP® Pro 13.0.0 software from SAS Institute, Cary, NC, USA, was used for all calculations.
Results
The current study included 1306 consecutive patients with PMF (median age 65 years, range 19-92; 63% males) seen at the Mayo Clinic between April 26, 1976 and November 21, 2017. Details of presenting clinical and laboratory features are outlined in Table 1. Among evaluable patients, sex-adjusted moderate or severe anemia was present in 54% of the patients at time of PMF diagnosis, thrombocytopenia < 100 × 109/l in 23%, leukocytosis > 25 × 109/l in 15%, circulating blasts ≥ 3% in 17%, constitutional symptoms in 29%, thrombosis history in 16%, VHR karyotype in 6% and other unfavorable karyotype in 17%. Driver mutation distribution was 67% JAK2, 16% CALR type 1/like, 3% CALR type 2/like, 6% MPL and 8% triple-negative (Table 1). Also in evaluable patients, mutational frequencies were 41% for ASXL1, 14% SRSF2, 2% IDH1, 4% IDH2, 4% EZH2, 15% U2AF1 and 10% U2AF1 Q157. DIPSS risk distribution was evaluable in 1265 patients and included 9% high risk, 39% intermediate-2 risk, 37% intermediate-1 risk and 15% low risk (Table 1). GIPSS risk distribution was evaluable in 560 patients and showed 25% high risk, 30% intermediate-2 risk, 35% intermediate-1 risk and 9% low risk (Table 1). MIPSS70 + version 2.0 was evaluable in 513 patients and showed 20% very high risk, 41% high risk, 19% intermediate risk, 16% low risk and 4% very low risk.
Table 1.
Clinical and laboratory characteristics, at time of initial diagnosis of primary myelofibrosis, of 1306 patients, stratified by whether or not they developed leukemic transformation during their clinical course
| Variables | All patients (n = 1306) | Patients who transformed into acute myeloid leukemia during their clinical course (n = 149) | Patients who remained in chronic phase disease at last follow-up (n = 1157) | P value |
|---|---|---|---|---|
| Age in years; median (range) | 65 (19–92) | 64 (32–85) | 65 (19–92) | 0.2 |
| Age > 70 years; n (%) | 382 (29) | 35 (23) | 347 (30) | 0.1 |
| Males; n (%) | 820 (63) | 106 (71) | 714 (62) | 0.02 |
| Hemoglobin, g/dl; median (range) “N” evaluable = 1298 | 10.2 (3.8–17.5) | 10.2 (6.1–15.2) | 10.3 (3.8–17.5) | 0.7 |
| Hemoglobin < 10 g/dl; n (%) “N” evaluable = 1298 | 608 (47) | 69 (48) | 539 (47) | 0.8 |
| Sex and severity adjusted anemia categories | 0.6 | |||
| “N” evaluable = 1298 | ||||
| Mild/no anemia; n (%) | 591 (46) | 63 (44) | 528 (46) | |
| Moderate/severe anemia; n (%) | 707 (54) | 81 (56) | 626 (54) | |
| Transfusion dependent; n (%) “N” evaluable = 1299 | 417 (32) | 38 (26) | 379 (33) | 0.1 |
| Platelets, ×109/l; median (range) “N” evaluable = 1299 | 225 (6–2400) | 202 (10–2399) | 230 (6–2400) | 0.09 |
| Platelets < 100 × 109/l; n (%) “N” evaluable = 1299 | 294 (23) | 38 (26) | 256 (22) | 0.2 |
| Leukocytes, ×109/l; median (range) “N” evaluable = 1298 | 8.8 (0.8–249) | 10 (1.1–249) | 8.8 (0.8–236) | 0.5 |
| Leukocytes > 25 × 109/l; n (%) “N” evaluable = 1298 | 189 (15) | 23 (16) | 166 (14) | 0.6 |
| Circulating blasts %; median (range) “N” evaluable = 1283 | 0 (0–18) | 1 (0–18) | 0 (0–18) | 0.03 |
| Circulating blasts ≥ 3%; n (%) “N” evaluable = 1283 | 217 (17) | 34 (24) | 183 (16) | 0.02 |
| Palpable splenomegaly; n (%) “N” evaluable = 1260 | 902 (72) | 94 (70) | 808 (72) | 0.6 |
| Bone marrow fibrosis grade (2 or above); n (%) “N” evaluable = 793 | 646 (81) | 82 (79) | 564 (82) | 0.4 |
| Constitutional symptoms; n (%) “N” evaluable = 1302 | 375 (29) | 45 (31) | 330 (29) | 0.5 |
| History of any thrombosis at or prior to diagnosis; n (%) “N” evaluable = 1299 | 208 (16) | 15 (11) | 193 (17) | 0.05 |
| History of venous thrombosis at or prior to diagnosis; n (%) “N” evaluable = 1298 | 92 (7) | 5 (4) | 87 (8) | 0.08 |
| History of arterial thrombosis at or prior to diagnosis; n (%) “N” evaluable = 1299 | 136 (10) | 12 (8) | 124 (11) | 0.4 |
| Karyotype | 0.2 | |||
| “N” evaluable = 1218 | ||||
| Favorable; n (%) | 931 (76) | 91 (71) | 840 (77) | |
| Unfavorable; n (%) | 212 (17) | 26 (20) | 186 (17) | |
| VHR; n (%) | 75 (6) | 12 (9) | 63 (6) | |
| DIPSS risk stratification | 0.005 | |||
| “N” evaluable = 1265 | ||||
| High risk; n (%) | 111 (9) | 7 (6) | 104 (9) | |
| Intermediate risk-2; n (%) | 501 (39) | 50 (42) | 451 (39) | |
| Intermediate risk-1; n (%) | 466 (37) | 54 (46) | 412 (36) | |
| Low risk; n (%) | 187 (15) | 7 (6) | 180 (16) | |
| GIPSS risk stratification | 0.07 | |||
| “N” evaluable = 560 | ||||
| High risk; n (%) | 142 (25) | 21 (29) | 121 (25) | |
| Intermediate risk-2; n (%) | 169 (30) | 29 (40) | 140 (29) | |
| Intermediate risk-1; n (%) | 198 (35) | 18 (25) | 180 (37) | |
| Low risk; n (%) | 51 (9) | 4 (6) | 47 (10) | |
| MIPSS70 + version 2.0 risk stratification | 0.02 | |||
| “N” evaluable = 513 | ||||
| Very high risk; n (%) | 104 (20) | 15 (17) | 89 (21) | |
| High risk; n (%) | 209 (41) | 49 (56) | 160 (38) | |
| Intermediate risk; n (%) | 97 (19) | 9 (10) | 88 (21) | |
| Low risk; n (%) | 80 (16) | 13 (15) | 67 (16) | |
| Very low risk; n (%) | 23 (4) | 2 (2) | 21 (5) | |
| Driver mutational status | 0.06 | |||
| “N” evaluable = 897 | ||||
| JAK2; n (%) | 603 (67) | 48 (54) | 555 (69) | |
| CALR type 1/like; n (%) | 149 (16) | 18 (20) | 121 (15) | |
| CALR type 2/like; n (%) | 31 (3) | 4 (4) | 27 (3) | |
| MPL; n (%) | 54 (6) | 7 (8) | 47 (6) | |
| Triple-negative; n (%) | 70 (8) | 12 (13) | 58 (7) | |
| ASXL1 mutated; n (%) “N” evaluable = 596 | 246 (41) | 41 (55) | 205 (39) | 0.01 |
| SRSF2 mutated; n (%) “N” evaluable = 597 | 83 (14) | 21 (27) | 62 (12) | 0.001 |
| IDH1 mutated; n (%) “N” evaluable = 479 | 9 (2) | 4 (6) | 5 (1) | 0.02 |
| IDH2 mutated; n (%) “N” evaluable = 479 | 18 (4) | 5 (8) | 13 (3) | 0.07 |
| EZH2 mutated; n (%) “N” evaluable = 452 | 17 (4) | 2 (3) | 15 (4) | 0.9 |
| U2AF1 mutated; n (%) “N” evaluable = 579 | 88 (15) | 11 (15) | 77 (15) | 0.9 |
| U2AF1 Q157 mutated; n (%) “N” evaluable = 579 | 57 (10) | 6 (8) | 51 (10) | 0.8 |
| Allogeneic stem cell transplant; n (%) | 68 (6) | 4 (3) | 64 (6) | 0.2 |
| Follow-up in years; median (range) | 3.2 (0–31) | 3.1 (0.3–20.2) | 3.2 (0–31) | 0.9 |
| Deaths; n (%) | 922 (71) | 142 (95) | 780 (67) | <0.0001 |
DIPSS dynamic international prognostic scoring system, GIPSS genetically-inspired prognostic scoring system, MIPSS70 + Version 2.0 mutation-enhanced international prognostic scoring system, VHR very high-risk karyotype
Bold values indicates significance indicator
Median follow-up was 3.2 years (range 0-31); during this time, a total of 149 (11%) cases of LT were documented. Comparison of clinical and laboratory features, recorded at the time of initial PMF diagnosis, between the patients who subsequently developed LT (n = 149) and those who remained in chronic phase disease at last follow-up (n = 1,157) reveled the former to be more likely to be males (p = 0.02) and display higher incidence of excess circulating blasts (p = 0.03), ASXL1 (p = 0.01), SRSF2 (p = 0.001) and IDH1 (p = 0.02) mutations (Table 1).
We employed two separate statistical methods in order to assess the risk of developing LT (Table 2). The first method involved binary outcome analysis using logistic regression, in order to calculate the odds of developing LT in the first 5 years of disease (elaborated further in the Methods section). In univariate analysis, the logistic 5-year risk of LT was predicted by age > 70 years, male sex, moderate or severe anemia, thrombocytopenia < 100 × 109/l, leukocytosis of > 25 × 109/l, circulating blasts ≥ 3%, constitutional symptoms, VHR karyotype, absence of CALR type 1/like and mutations affecting ASXL1, SRSF2, IDH1 and IDH2 (odds ratio (OR) and 95% CI are provided in Table 2); multivariable logistic regression confirmed the independent prognostic contribution of IDH1 mutation (OR 78.4), VHR karyotype (OR 57.6), ASXL1 mutation (OR 15.1), age > 70 years (OR 13.3), SRSF2 mutation (OR 8.5), male sex (OR 6.9), circulating blasts ≥ 3% (OR 5.4), presence of sex-adjusted moderate or severe anemia (OR 3.6) and constitutional symptoms (OR 3.1). A parallel time-to-event Cox analysis confirmed inferior LFS in patients with IDH1 mutation (HR 4.3), SRSF2 mutation (HR 3.0), ASXL1 mutation (HR 2.0), circulating blasts ≥ 3% (HR 3.3), age > 70 years (HR 2.1), and presence of sex-adjusted moderate or severe anemia (HR 1.9).
Table 2.
Univariate and multivariable analysis of clinical and genetic predictors of leukemic transformation in 1306 patients with primary myelofibrosis
| Predictors of leukemic transformation in the first 5 years of diagnosis (Logistic regression analysis) | Risk factors for leukemia-free survival (Cox analysis) | |||
|---|---|---|---|---|
| Variables | Univariate analysis P value (OR, 95% CI) | Multivariable analysis P value (OR, 95% CI) | Univariate analysis P value (HR, 95% CI) | Multivariable analysis P value (HR, 95% CI) |
| Age in years | <0.001 | 0.01 (1.02, 1–1.03) | ||
| Age > 70 years | 0.003 (2.1, 1.3–3.3) | <0.001 (13.3, 3.5–51.2) | 0.4 (1.2, 0.8–1.7) | 0.03 (2.1, 1.1–3.8) |
| Gender (Male) | <0.001 (2.8, 1.7–4.6) | 0.01 (6.9, 1.6–30.2) | 0.002 (1.7, 1.2–2.5) | |
| Sex and severity adjusted anemia | ||||
| “N” evaluable = 1298 | ||||
| Moderate/Severe anemia | < 0.001 (3.1, 2.0–4.9) | 0.02 (3.6, 1.2–10.7) | < 0.001 (1.8, 1.3–2.6) | 0.02 (1.9, 1.1–3.3) |
| No/Mild anemia | Reference | Reference | ||
| Platelets, ×109/l “N” evaluable = 1299 | <0.001 | 0.05 (0.2, 0.04–1.03) | ||
| Platelets<100 × 109/l “N” evaluable = 1299 | <0.001 (3.6, 2.1–6.1) | 0.001 (1.9, 1.3–2.8) | ||
| Leukocytes, ×109/l “N” evaluable = 1298 | <0.001 | <0.001 (17, 3.9–51.4) | ||
| Leukocytes > 25 × 109/l “N” evaluable = 1298 | 0.002 (3.4, 1.8–6.3) | 0.01 (1.8, 1.1–2.8) | ||
| Circulating blasts % “N” evaluable = 1283 | <0.001 | <0.001 (18.5, 7.3–41.6) | ||
| Circulating blasts ≥ 3% “N” evaluable = 1283 | <0.001 (3.6, 2.2–6.1) | 0.009 (5.5, 1.5–19.6) | <0.001 (2.6, 1.7–3.7) | 0.001 (3.3, 1.6–6.2) |
| Palpable splenomegaly “N” evaluable = 1260 | 0.4 (0.8, 0.5–1.3) | 0.3 (0.8, 0.6–1.2) | ||
| Bone marrow fibrosis grade (2 or above) “N” evaluable = 793 | 0.5 (0.8, 0.4–1.5) | 0.6 (0.9, 0.6–1.5) | ||
| Constitutional symptoms “N” evaluable = 1302 | <0.001 (2,5, 1.5–3.9) | 0.04 (3.1, 1.0–9.2) | 0.009 (1.6, 1.1–2.3) | |
| Any thrombosis at or prior to diagnosis “N” evaluable = 1299 | 0.3 (0.7, 0.3–1.4) | 0.08 (0.6, 0.4–1.1) | ||
| Venous thrombosis at or prior to diagnosis “N” evaluable = 1298 | 0.2 (0.5, 0.1–1.4) | 0.04 (0.4, 0.2–1) | ||
| Arterial thrombosis at or prior to diagnosis “N” evaluable = 1299 | 0.8 (1.1, 0.5–2.2) | 0.7 (0.9, 0.5–1.6) | ||
| Presence of very high-risk (VHR) karyotype | <0.001 (10.9, 3.7–32.3) | 0.005 (57.6, 3.3–994) | <0.001 (3.6, 1.9–6.3) | |
| Karyotype | ||||
| “N” evaluable = 1218 | ||||
| VHR | <0.001 (12, 4–35.7) | <0.001 (3.9, 2–7) | ||
| Unfavorable | 0.08 (1.6, 0.9–2.9) | 0.05 (1.6, 1–2.9) | ||
| Favorable | Reference | Reference | ||
| Driver mutational status | ||||
| “N” evaluable = 897 | ||||
| JAK2 | 0.7 (0.9, 0.5–1.5) | 0.2 (0.7, 0.5–1.1) | ||
| CALR | 0.2 (0.6, 0.3–1.3) | 0.9 (0.9, 0.6–1.6) | ||
| MPL | 0.5 (1.3, 0.4–3.7) | 0.5 (1.3, 0.5–2.6) | ||
| Triple-negative | 0.05 (2.2, 0.9–4.7) | 0.06 (1.9, 0.9–3.3) | ||
| CALR type 1/like absent | 0.045 (2.2, 1.0–4.9) | 0.7 (1.1, 0.7–1.8) | ||
| Mutations | ||||
| ASXL1 mutated “N” evaluable = 596 | <0.001 (3.8, 2–7.2) | <0.001 (15.1, 4.0–56.7) | <0.001 (2.2, 1.4–3.5) | 0.01 (2.0, 1.2–3.3) |
| SRSF2 mutated “N” evaluable = 597 | <0.001 (8.04, 3.7–17.2) | 0.002 (8.5, 2.2–32.2) | <0.001 (3.5, 2.1–5.7) | 0.001 (3.0, 1.6–5.3) |
| IDH1 mutated “N” evaluable = 479 | 0.005 (23.5, 2.5–217) | 0.002 (78.4, 5.3–1153) | 0.006 (6.2, 1.9–15.4) | 0.03 (4.3, 1.2–11.7) |
| IDH2 mutated “N” evaluable = 479 | 0.002 (9.9, 2.3–43.7) | 0.01 (3.1, 1.1–7.1) | ||
| EZH2 mutated “N” evaluable = 452 | 0.4 (1.9, 0.4–9.7) | 0.7 (1.3, 0.2–4.2) | ||
| U2AF1 mutated “N” evaluable = 579 | 0.09 (2, 0.9–4.6) | 0.3 (1.4, 0.7–2.7) | ||
| U2AF1 Q157 mutated “N” evaluable = 579 | 0.3 (1.8, 0.6–5.9) | 0.5 (1.4, 0.5–3) | ||
Abbreviation: OR odds ratio, HR hazard ratio, CI confidence interval
Bold values indicates significance indicator
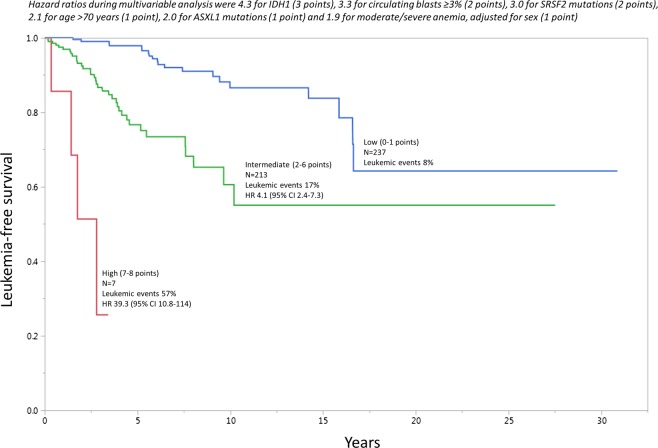
Using the results from the Cox time-to-event multivariable analysis, a predictive model for LT was devised, with point allocations commensurate with HR values; IDH1 (HR 4.3; 3 points), circulating blasts ≥ 3% (HR 3.3; 2 points), SRSF2 mutations (HR 3.0; 2 points), age > 70 years (HR 2.1; 1 point), ASXL1 mutations (HR 2.0; 1 point) and sex-adjusted moderate or severe anemia (HR 1.9; 1 point). A total of 456 patients were informative for all six independent predictors of LT; subsequently a three-tiered LT risk stratification was developed: high-risk (7–8 adverse points; LT incidence 57%; HR 39.3, 95% CI 10.8–114), intermediate-risk (2–6 adverse points; LT incidence 17%; HR 4.1, 95% CI 2.4–7.3) and low-risk (0–1 adverse points; LT incidence 8%) (Fig. 1). AIC and AUC analysis confirmed the superior performance of the new LT predictive model (AIC 598; AUC 0.83), compared to GIPSS (AIC 778; AUC 0.78) and MIPSS70 + version 2.0 (AIC 931; AUC 0.79) for predicting LFS (Fig. 2).
Fig. 1.
Leukemia-free survival among 456 patients with primary myelofibrosis who were informative for independent predictors of leukemic transformation (i.e., IDH1, SRSF2 and ASXL1 mutations; age > 70 years; circulating blasts ≥ 3%; moderate/severe anemia)
Fig. 2.
Predictive accuracy of the new risk model for leukemic transformation in primary myelofibrosis, compared to previously established overall survival risk models, including the genetically-inspired prognostic scoring system (GIPSS) and karyotype- and mutation-enhanced prognostic scoring system (MIPSS70 + version 2.0)
Discussion
Leukemic transformation (LT) is a dreaded complication of myeloproliferative neoplasms (MPN); reported 10-year estimates of LT incidence range from 0.7–3% for ET, 2.3–14.4% for PV and 10-20 % for PMF2,16–18. In a recent communication of 410 patients with post-MPN LT, recruited from the Mayo Clinic (n = 248) and multiple centers in Italy (n = 162), median survival was only 3.6 months and post-LT survival was independently affected by unfavorable karyotype, platelet count < 100 × 109/l, age > 65 years and transfusion need at time of LT19. In general, long-term survival after LT was unusual, despite the achievement of close to 60% rate of complete remission, with or without incomplete count recovery19. The particular study revealed treatment-specified 3-year/5-year survival rates of 32%/10% for patients receiving allo-SCT, 19%/13% for patients achieving remissions following intensive chemotherapy but were not subsequently transplanted, and 1%/1% in the absence of both allo-SCT and chemotherapy-induced remission19. In other words, the survival benefit of allo-SCT in MPN20 might not extend to those with LT, which underscores the need to identify patients at risk, before they undergo LT.
Current prognostic models in PMF target OS and utilize both genetic and clinical risk factors: MIPSS70 (mutation-enhanced international prognostic scoring system for transplant-age patients)7, MIPSS70 + version 2.0 (karyotype-enhanced MIPSS70)4 and GIPSS (genetically-inspired prognostic scoring system)3. Both GIPSS and MIPSS70 + version 2.0 also predict LFS and the relevant risk factors in this regard included unfavorable karyotype, SRSF2 and ASXL1 mutations, platelet count < 100 × 109/l and circulating blasts ≥ 2%3,7. Other previously cited risk factors for LT in PMF include age > 65 years21, red blood cell transfusion need22, leukocyte count > 30 × 109/l23, platelet count < 50 × 109/l24, circulating blasts ≥ 3%25, increased levels of serum IL-8 and IL-2R26, C-reactive protein > 7 mg/l21 and bone marrow blasts ≥ 10%24. LT in PMF has also been associated with certain chromosomal abnormalities, including chromosome 17 aberrations24, monosomal karyotype27 and unfavorable karyotype including complex karyotype and those affecting chromosomes 5 or 728–30. More recent information suggests that patients with triple-negative driver mutational status2 and those who harbor ASXL1, SRSF2, IDH1 or IDH2 mutations14 were also at increased risk of LT.
The current study provides a highly discriminating LT predictive model for PMF, which was shown to be superior to both GIPSS and MIPSS70 + version 2.0 in its LT predictive accuracy (Fig. 2). However, it should be noted that almost all of the variables used in the new LT predictive model (i.e., IDH1, ASXL1, SRSF2 mutations, circulating blasts ≥ 3%, age > 70 years and moderate/severe anemia) were previously associated with shortened LFS (see above). What is different in the current study was i) the much larger sample size of informative cases; ii) the distinction between early events (logistic analysis of LT risk in the first 5 years of diagnosis) and overall risk (assessed by Cox analysis of LFS); iii) the combined analysis of mutations, cytogenetic abnormalities and clinical variables, in order to decipher inter-independent risk factors; and iv) development of a novel LT predictive model that includes both genetic and clinical risk factors. From a practical standpoint, the new LT risk model for PMF complements GIPSS and MIPSS70 + version 2.0 and should provide additional layer of prognostic information to assist with treatment decision-making, especially in terms of patient selection for allo-SCT. The current study also confirms the prognostic importance of specific mutations, sex-adjusted anemia and excess circulating blasts, for both OS and LFS, in PMF. Our observations require further validation, which might not be easy to accomplish, considering the difficulty in securing adequate number of informative cases.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arber DA, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507–2513. doi: 10.1182/blood-2014-05-579136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tefferi A, et al. GIPSS: genetically inspired prognostic scoring system for primary myelofibrosis. Leukemia. 2018;32:1631–1642. doi: 10.1038/s41375-018-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tefferi A, et al. MIPSS70+Version 2.0: mutation and karyotype-enhanced international prognostic scoring system for primary myelofibrosis. J. Clin. Oncol. 2018;36:1769–1770. doi: 10.1200/JCO.2018.78.9867. [DOI] [PubMed] [Google Scholar]
- 5.Samuelson Bannow BT, et al. Hematopoietic cell transplantation for myelofibrosis: the dynamic international prognostic scoring system plus risk predicts post-transplant outcomes. Biol. Blood Marrow Transplant. 2018;24:386–392. doi: 10.1016/j.bbmt.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tefferi, A. Primary myelofibrosis: 2019 update on diagnosis, risk-stratification and management. Am.J. Hematol. 93, 1551–1590 (2018). [DOI] [PubMed]
- 7.Guglielmelli P, et al. MIPSS70: mutation-enhanced international prognostic score system for transplantation-age patients with primary myelofibrosis. J. Clin. Oncol. 2018;36:310–318. doi: 10.1200/JCO.2017.76.4886. [DOI] [PubMed] [Google Scholar]
- 8.Tefferi A, et al. Targeted next-generation sequencing in myelodysplastic syndromes and prognostic interaction between mutations and IPSS-R. Am. J. Hematol. 2017;92:1311–1317. doi: 10.1002/ajh.24901. [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A, et al. Targeted deep sequencing in primary myelofibrosis. Blood Adv. 2016;1:105–111. doi: 10.1182/bloodadvances.2016000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaffer L. G., Slovak M. L., Campbell L. J., (editors). ISCN 2009: An International System for Human Cytogenetic Nomenclature (2009) (Karger; Basel, 2009).
- 11.Cervantes F, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 12.Tefferi A, et al. Revised cytogenetic risk stratification in primary myelofibrosis: analysis based on 1002 informative patients. Leukemia. 2018;32:1189–1199. doi: 10.1038/s41375-018-0018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolosi M, et al. Sex and degree of severity influence the prognostic impact of anemia in primary myelofibrosis: analysis based on 1109 consecutive patients. Leukemia. 2018;32:1254–1258. doi: 10.1038/s41375-018-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vannucchi AM, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861–1869. doi: 10.1038/leu.2013.119. [DOI] [PubMed] [Google Scholar]
- 15.Tefferi A, et al. U2AF1 mutation types in primary myelofibrosis: phenotypic and prognostic distinctions. Leukemia. 2018;32:2274–2278. doi: 10.1038/s41375-018-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbui T, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J. Clin. Oncol. 2011;29:3179–3184. doi: 10.1200/JCO.2010.34.5298. [DOI] [PubMed] [Google Scholar]
- 17.Cervantes F, et al. Acute transformation in nonleukemic chronic myeloproliferative disorders: actuarial probability and main characteristics in a series of 218 patients. Acta Haematol. 1991;85:124–127. doi: 10.1159/000204873. [DOI] [PubMed] [Google Scholar]
- 18.Tefferi A, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27:1874–1881. doi: 10.1038/leu.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tefferi A, et al. Blast phase myeloproliferative neoplasm: Mayo-AGIMM study of 410 patients from two separate cohorts. Leukemia. 2018;32:1200–1210. doi: 10.1038/s41375-018-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tefferi A, et al. Allogeneic hematopoietic stem cell transplant overcomes the adverse survival effect of very high risk and unfavorable karyotype in myelofibrosis. Am. J. Hematol. 2018;93:649–654. doi: 10.1002/ajh.25053. [DOI] [PubMed] [Google Scholar]
- 21.Barbui T, et al. Elevated C-reactive protein is associated with shortened leukemia-free survival in patients with myelofibrosis. Leukemia. 2013;27:2084. doi: 10.1038/leu.2013.207. [DOI] [PubMed] [Google Scholar]
- 22.Passamonti F, et al. Incidence of leukaemia in patients with primary myelofibrosis and RBC-transfusion-dependence. Br. J. Haematol. 2010;150:719–721. doi: 10.1111/j.1365-2141.2010.08275.x. [DOI] [PubMed] [Google Scholar]
- 23.Dupriez B, et al. Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system. Blood. 1996;88:1013–1018. [PubMed] [Google Scholar]
- 24.Tam CS, et al. Dynamic model for predicting death within 12 months in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. J. Clin. Oncol. 2009;27:5587–5593. doi: 10.1200/JCO.2009.22.8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, et al. Risk factors for leukemic transformation in patients with primary myelofibrosis. Cancer. 2008;112:2726–2732. doi: 10.1002/cncr.23505. [DOI] [PubMed] [Google Scholar]
- 26.Tefferi A, et al. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J. Clin. Oncol. 2011;29:1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 27.Vaidya R, et al. Monosomal karyotype in primary myelofibrosis is detrimental to both overall and leukemia-free survival. Blood. 2011;117:5612–5615. doi: 10.1182/blood-2010-11-320002. [DOI] [PubMed] [Google Scholar]
- 28.Gangat N, et al. DIPSS plus: A refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J. Clin. Oncol. 2011;29:392–397. doi: 10.1200/JCO.2010.32.2446. [DOI] [PubMed] [Google Scholar]
- 29.Caramazza D, et al. Refined cytogenetic-risk categorization for overall and leukemia-free survival in primary myelofibrosis: a single center study of 433 patients. Leukemia. 2011;25:82–88. doi: 10.1038/leu.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quintás-Cardama A, Kantarjian H, Pierce S, Cortes J, Verstovsek S. Prognostic model to identify patients with myelofibrosis at the highest risk of transformation to acute myeloid Leukemia. Clin. Lymphoma, Myeloma Leuk. 2013;13:315–8.e2. doi: 10.1016/j.clml.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]