Abstract
BACKGROUND
Vitamin D deficiency is highly prevalent in children with inflammatory bowel disease (IBD). This may contribute to an increased risk of poor bone health and may also influence the course of disease. An optimal treatment strategy of vitamin D therapy in children with IBD has not yet been established.
AIM
To analyse the published intervention studies of vitamin D therapy in children with IBD.
METHODS
A systematic review was conducted of clinical studies involving children with IBD (including Crohn disease or ulcerative colitis) who had received vitamin D therapy. Studies up to March 31st 2018 were identified through MEDLINE, PubMed, EMBASE and the Cochrane Library. Search terms included synonyms of the following terms: vitamin D, paediatric, supplementation, IBD. References of included articles based on abstract were searched for other relevant articles. All relevant articles were accessed and reviewed in full text. Studies fitting the set criteria were included and the remainder were excluded.
RESULTS
Two hundred and seventy-seven discrete articles were identified. Following assessment of these articles included in the initial search and application of inclusion and exclusion criteria, ten published studies were included in this review. The included studies showed a heterogeneity in study design, inclusion and exclusion criteria, baseline demographics and treatment strategies. Treatment regimens differed in length, supplemented form of vitamin D and factors based upon which dosage was adjusted. Each of the reports included in this review concluded their vitamin D regimens to be safe and well-tolerated. Few of the included studies reported secondary outcomes on the efficacy of vitamin D treatment upon the clinical course of disease or markers of inflammation. The majority of included trials were not sufficient in raising serum vitamin D levels to an adequate level (30 ng/mL) in children with IBD with vitamin D deficiency.
CONCLUSION
The included trials featured diverse treatment regimens that were predominantly insufficient in correcting vitamin D deficiency or maintaining adequate levels in children with IBD. Better treatment regimens are required for the management of vitamin D deficiency in children with IBD.
Keywords: Child, Inflammatory bowel diseases, Vitamin D, Therapeutics, Systemic review
Core tip: Vitamin D deficiency is commonly seen in children diagnosed with inflammatory bowel disease (IBD). Correction of deficiency and optimisation of vitamin D levels likely contribute to enhanced bone health and possibly to the underlying disease course. The optimal regimen and target for vitamin D therapy are not yet elucidated. This systematic analysis aimed to review the available reports of vitamin D therapy in children with IBD to assess efficacy and safety of this treatment, address the diversity of the available trials and provide recommendations for future trials in this field on the basis of the evidence reviewed.
INTRODUCTION
Vitamin D has a pivotal role in both calcium and immune homeostasis[1,2]. The significance of vitamin D in bone health is widely known: important relationships have been observed between vitamin D deficiency and rickets, osteopenia, osteoporosis, osteomalacia, muscle weakness and an increased risk of fracture[3,4]. Vitamin D has also been shown to have a key role in immune regulation, primarily with a role in the regulation of T cell development and function[5]. Recent studies have linked vitamin D deficiency with compromised immunity and an increased susceptibility to cardiovascular diseases, cancer and a variety of autoimmune diseases including rheumatoid arthritis, multiple sclerosis, type 1 diabetes mellitus, and inflammatory bowel disease (IBD)[6,7].
The IBD, which include Crohn disease (CD) and ulcerative colitis (UC), are characterized by chronic uncontrolled inflammation of the gastro-intestinal tract[8]. Around a quarter of patients diagnosed with IBD present before the age of 20 years[9]. Children with IBD typically have more extensive disease, higher rates of extra intestinal manifestations, and greater nutritional consequences than adults with these conditions. Furthermore, the incidence of paediatric IBD is increasing internationally[9-11].
Vitamin D deficiency is highly prevalent in children with IBD[12-14]. Some paediatric trials show that this may in part contribute to an increased risk of poor bone health[15,16], but contrasting conclusions have been shown[17]. Recent studies have also focused on the relationship between vitamin D deficiency and disease severity in children with IBD; while some limited data suggests an association of vitamin D deficiency with a more severe course of disease, other studies do not report such a relationship[18-20].
Although there are clearly a number of reasons to consider adequacy of vitamin D an important objective in children with IBD, the optimal regimen to achieve this has not yet been established. In recent years, several publications have assessed the outcomes of vitamin D therapy in children with IBD. While reviews of the reports evaluating vitamin D therapy in adults with IBD have been published, there has not yet been a systematic assessment of the published studies on vitamin D therapy in children with IBD. The aim of the present systematic review was to assess the safety and efficacy of vitamin D therapy in children with IBD, by reviewing the published studies that have evaluated the administration of vitamin D in this population. Secondary aims were to address the diversity of the available trials, to identify gaps in current knowledge and to identify aspects relevant to future trials of paediatric vitamin D therapy.
MATERIALS AND METHODS
Search strategy
Following establishment of a review protocol, a literature search was conducted of all published studies up to March 31st 2018 through MEDLINE, PubMed, EMBASE and the Cochrane Library. Search terms used included “vitamin D” OR “25-hydroxy vitamin D” OR “vitamin D2” OR “vitamin D3” OR “calcidiol” OR “cholecalciferol” OR “25(OH)D” OR “hydroxycholecalciferol” OR “ergocalciferol” OR “25-hydroxy vitamin D2” OR “dihydrotachysterol” OR “calcifediol” OR “dihydroxycholecalciferol” OR “calcitriol” OR “alfacalcidol” OR “alphacalcidol” OR “hydroxycholecalciferol”OR “colecalciferol” OR “cholecalciferol” in combination with “child” OR “childhood” OR “children” OR “paediatr*” OR “pediatr*” OR “adolescen*” in combination with “therap*” OR “treatment*” OR “regimen*” OR “supplementati*” OR “maintenance” OR “repleti*” in combination with “Crohn’s” OR “colitis” OR “inflammatory bowel disease” OR “inflammatory bowel diseases” OR “IBD”. References of included articles based on abstract were searched for other relevant articles. Duplicates were subsequently removed.
Eligibility criteria
Trials conducted on patients up to 18 years of age with IBD, CD or UC in which vitamin D therapy was given and where serum (s)-25OHD levels were reported at baseline and follow-up were included in this review. Only published manuscripts were included. Studies were excluded if (1) no vitamin D therapy was given; (2) they were conducted on patients with diseases other than IBD, CD or UC; (3) they were performed on adult patients; or (4) if they were not available in English language. Studies that included both children and adult patients and that did not report separate outcomes for paediatric patients were also excluded.
Data extraction
Key data was extracted independently from each selected article including: authors and date of publication; study design; location of the trial; sample size; condition of the study population (IBD/CD/UC); vitamin D deficiency and/or insufficiency cut-off points; dosage, length and cumulative dose of the vitamin D regimens; mean and standard deviation or interquartile range of s-25OHD levels at baseline and follow-up measurements, primary outcome of the study, and if available, any conclusions of the authors regarding the effect of vitamin D therapy administered in the trial. Vitamin D concentrations reported in nmol were adjusted to and reported in ng/mL, to allow for accurate comparisons across all studies.
RESULTS
Initial and final search findings
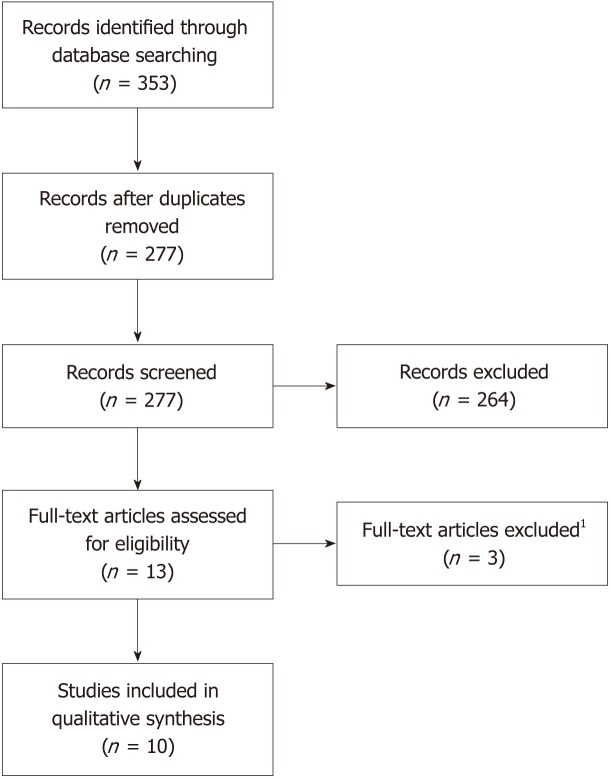
A total of 353 articles were identified following the specified searches. Seventy-six duplicates were removed. The remaining 277 article abstracts were analysed and included or rejected according to the protocol criteria. Of the 13 articles remaining, three were subsequently excluded after full-text analysis. A final total of 10 articles were included in this review (Figure 1).
Figure 1.
PRISMA flowchart outlining literature selection. 1These full-text articles were excluded because they were either cross-sectional studies with no follow-up s25OHD levels reported or vitamin D therapy was analysed as a dichotomous parameter with no information on dosage or length of regimen.
Five studies were designed to investigate the effect of vitamin D therapy on increasing vitamin D levels (Table 1), and four were designed to investigate the effect of therapies on parameters of bone health (Table 2). The final study was designed to investigate vitamin D therapy for maintaining sufficient vitamin D levels [21]. In the studies concerning bone health, vitamin D therapy was not the main intervention given; in one study vitamin D was administered to both the intervention and placebo group.
Table 1.
Characteristics of included studies: Evaluating vitamin D treatment in children with inflammatory bowel disease
| Study | N1 | Location | Design | Treatment regimen |
Vitamin D levels2 |
Treatment duration3 | Cumulative dose | Disease activity4 | |
| Before | After | ||||||||
| Wingate, 2014 | 83 (CD) | Canada | RCT | A: 400 IU/d D3; B: 2000 IU/d D3 | 24 ± 7.9; A: 26 ± 9.2; B: 22 ± 6.8 | A: 28.8 ± 9.2 (3 mo), 28 ± 8.6 (6 mo); B: 34 ± 10 (3 mo), 34.4 ± 10.4 (6 mo) | 26 (183) | A: 73200 IU; B: 366000 IU | A: < 10:83 B: < 10:88 |
| Pappa, 2012 | 71 (IBD) | United States | RCT | A: 2000 IU/d D2; B: 2000 IU/d D3; C: 50000 IU/wk D2 | A: 16.1 ± 1; B: 14.7 ± 0.8; C: 51.3 ± 1 | A:25.7 ± 2.2 (6 wk); B:31.5 ± 1.9 (6 wk); C: 40.8 ± 2.6 (6 wk) | 6 (42) | A: 84000 IU; B: 84000 IU; C: 300000 IU | A: <10:54; B: <10:54; C: <10:78 |
| Simek, 2016 | 32 (IBD) | United States | RCT | A: 5000 IU/10 kg/wk D3; B: 1000 IU/10 kg/wk D3 | A: 24 ± 7; B: 23.7 ± 8.5 | A: 41.5 ± 9.6 (8 wk), 30.8 ± 4.2 (12 wk); B: 49.2 ± 13.6 (8 wk), 35.1 ± 8.4 (12 wk) | 6 (42) | A: up to 150000 IU; B: up to 150000 IU | Unknown |
| Shepherd, 2015 | 76 (IBD) | Australia | Retrospective | < 3 yr: 200000 IU D3; 3-12 yr: 400000 IU D3; > 12 yr: 800000 IU D3 | 16.4 ± 3.2 | 58.4 ± 20.8 (1 mo); 34.8 ± 11.2 (3 mo); 27.6 ± 12.4 (6 mo) | Single dose | < 3 yr: 200000 IU D3; 3-12 yr: 400000 IU D3; > 12 yr: 800000 IU D3 | 10 (0-47.5) 0 mo; 2.5 (0-85) 6 mo |
| Santucci, 2014 | 61 (IBD) | United States | Retrospective | A: no treatment; B: 400 IU/d; C: 600-1200 IU/d; D: 2000 IU/d or 50000 IU/wk | 45% < 30 ng/mL (baseline); 50% < 30 ng/mL; 25% < 30 ng/mL; 88% < 30 ng/mL | Unknown | Unknown | Unknown | |
Number of patients recruited (type of disease);
Serum levels expressed as mean ± SD;
Treatment duration expressed as days (weeks);
Disease activity scores (Pediatric Crohn disease activity index or Pediatric ulcerative colitis disease activity index) at baseline unless otherwise stated. Expressed as median (range) or percentage of patients with score less than 10 (remission). IBD: Inflammatory bowel disease; CD: Crohn disease; UC: Ulcerative colitis.
Table 2.
Characteristics of included studies: Vitamin D treatment and evaluating bone health in children with inflammatory bowel disease
| Study | N1 | Location | Design | Treatment Regimen |
Vitamin D levels2 |
Main study outcome | Disease activity3 | |
| Baseline | Follow-up | |||||||
| Hradsky, 2017 | 55 (IBD) | Czech Republic | Cohort | 2000 IU/d D3 | 23.2 (17.2, 31.6) | 34 (24.4, 38.4) | Effect of vitamin D on trabecular BMD and muscle power | 5 (0-10) |
| Benchimol, 2007 | 72 (IBD) | North America | Prospective | A: no intervention; B: supplemental calcium or supplemental calcium & 50000 IU/m D2 | A: 27.6; B: 22.8 ± 8 or 24.4 ± 6 | B: 26.4 ± 6 or 30 ± 12 | Effect of vitamin D on lumbar spine BMD | A: 10.6 ± 10.6; B: 16.5 ± 15.7 |
| Leonard, 2016 | 121 (CD) | United States | RCT | A: 10 min LMMS/d; B: no LMMS (both 600 IU/d D3) | 31.5 ± 10.3 | 34.2 ± 12.4 (12 m); 37.7 ± 15.6 (24 m)4 | Effect of LMMS on BMD | < 10:52; 10-30:33; > 30:5 |
| Laakso, 2014 | 47 (IBD) | Finland | Cohort | Total vitamin D intake: 7.5 (1.6-19.5) μg/d (baseline); 12.6 (1.7-49.2) μg/d (follow-up) | 19.6 (8.6, 40.8) | 26.8 (10.4, 48) | BMD and BMC | < 10:72; 10-30:23; > 30:4 |
Number of patients recruited (type of disease);
Serum levels expressed as mean ± SD, mean and interquartile range, or mean;
Disease activity scores (Pediatric Crohn disease activity index or Pediatric ulcerative colitis disease activity index) at baseline expressed as mean (range), mean ± SD or percentage of patients with score less than 10, 10-30, or > 30;
Calculated in 105 patients who completed the trial. IBD: Inflammatory bowel disease; CD: Crohn disease; UC: Ulcerative colitis; BMD: Bone mineral density; LMMS: Low magnitude mechanical stimulation; BMC: Bone mineral content.
Findings from studies designed to examine the effect of vitamin D therapy used to treat deficiency
In a randomized trial by Wingate et al[22], 83 patients with quiescent CD aged 8-18 years were included. Patients taking corticosteroids (CS) within six weeks before enrolment and/or vitamin D supplements containing > 1000 IU daily were excluded. In this study, 2000 IU vitamin D3 was shown be superior to 400 IU vitamin D3 in raising mean s-25OHD concentrations during 6 months of supplementation. After 6 months of supplementation, 74% of subjects receiving a 2000 IU daily dose reached s-25OHD levels above 30 ng/mL, compared to 35% of subjects receiving 400 IU daily. During the trial, 2000 IU daily was not always significantly superior to 400 IU daily; however, the higher was never inferior to the lower dose in raising s-25OHD concentrations. There was no difference observed between groups in participants experiencing hypercalcemia, hyperphosphatemia, or exceeded safety cut-off level of urinary calcium to creatinine ratio. There was no observed difference in elevated C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR) concentrations between groups. Pediatric Crohn’s Disease Activity Index (PCDAI) scores were only defined at baseline. The authors did not report data concerning the occurrence of adverse events.
The study by Pappa et al[23] included 71 patients aged 5-21 years with a s-25OHD ≤ 20 ng/mL within 8 wk of enrolment. Exclusion criteria were liver or kidney failure, therapy with anticonvulsants metabolized through cytochrome P450, pregnancy, inability to take oral medication, attendance at a tanning salon once weekly or more, or receiving a treatment for vitamin D deficiency. This study showed superiority of both 2000 IU D3 daily and 50000 IU D2 weekly, over 2000 IU D2 daily in changing mean s-25OHD concentrations over a treatment period of 6 wk. Both 2000 IU D3 daily and 50000 IU D2 weekly raised s-25OHD concentrations above 20 ng/mL in 95% of the participants, while 75% of participants in the latter group achieved s-25OHD levels above 32 ng/mL (compared to 38% of the subjects who received 2000 IU D3 daily). The authors did not report whether the change in mean s-25OHD levels was significantly different between the 2000 IU D3 daily group and the 50000 IU D2 weekly group. One third of all participants experienced adverse events, but there was no difference in the frequency of adverse events between the three dosing regimens. None of the participants experienced hypercalcemia or hyperphosphatemia.
In a pilot study by Simek et al[24], a weight-based dosing schedule of vitamin D was used for a total of 6 wk. This study included 32 patients aged 8-21 years with a s-25OHD concentration below 30 ng/mL. Patients with a known history of renal or hepatobiliary disease, use of systemic CS within 60 d of enrolment or with an inability to swallow study drug capsules were excluded. A regimen of 10000 IU/10 kg vitamin D3 per week was compared with 5000 IU/10 kg vitamin D3 per week. At 8 and 12 wk, mean s-25OHD concentrations in the higher dosage group were maintained at a higher concentration than in the lower dosage group but did not differ significantly. The change in mean s-25OHD concentrations were not affected by participant weight. Calcium and parathyroid hormone (PTH) measurements were not significantly different between baseline and follow-up. In addition, no events of hypercalcemia or serious adverse events were observed. Measures of disease activity were not reported.
Shepherd et al[25] showed in their retrospective chart review that a single high age-adjusted dose of vitamin D (stoss therapy) increased s-25OHD concentrations significantly at 1, 3 and 6-mo follow-up in 76 vitamin D deficient children with IBD. Specific exclusion criteria for these participants were not reported. The s-25OHD concentration of all subjects increased to a level above 50 nmol (20 ng/mL) at 1 mo, remaining above 20 ng/mL in 96.6% of the subjects at 3 mo, and in 76.4% of the subjects at 6 months. A s-25OHD concentration above 75 nmol (30 ng/mL) was seen in 98% of the subjects at 1 mo and 63% of the subjects at 3 mo. At 6 mo, 17% of the subjects had not maintained a sufficient s-25OHD concentration of 20 ng/mL or above. The authors stated that there was no evidence of toxicity of vitamin D in any of the participants. Calcium, phosphate and PTH were measured at baseline and during follow-up, and showed no significant changes. Disease activity was reported at baseline.
Santucci et al[26] retrospectively reviewed vitamin and zinc deficiencies in a paediatric IBD cohort of 61 patients. Patients with coexisting medical conditions such as coeliac disease and cystic fibrosis that may affect vitamin D status were excluded. In total, the s-25OHD concentrations of 39 patients were assessed, of whom 21 participants received vitamin D treatment. This study showed that vitamin D supplementation with 2000 IU daily or 50000 IU weekly raised s-25OHD concentration to a level above 30 ng/mL in 88% children, compared to 50% in children receiving 400 IU daily and 25% in patients receiving 600-1200 IU daily. In comparison, 45% of participants normalised without any vitamin D supplementation. In total, 52% of the vitamin D deficient children in the cohort had normalisation of vitamin D levels after treatment with different vitamin D dosing regimens over a follow-up period of 6 months to 5 years. This review did not specify whether vitamin D2 or vitamin D3 was supplemented and the rates of adverse events in different dosing schedules were not reported.
Findings from the study designed to examine the effect of vitamin D therapy used to maintain sufficient vitamin D levels
Pappa et al[21] compared the efficacy and safety in maintaining s-25OHD above 32 ng/mL in two treatment arms; 400 IU vitamin D2 daily (arm A), 1000 IU vitamin D2 daily in summer and 2000 IU vitamin D2 daily in winter (arm B). Sixty-three patients with IBD aged 5-21 years with a s-25OHD above 20 ng/mL, were followed three-monthly for 12 mo. Exclusion criteria were coexisting liver or kidney disease, pregnancy, use of anticonvulsants metabolized through cytochrome P450 or inability to take oral medications. In both groups, less than 10% of all patients achieved the main outcome of s-25OHD ≥ 32 ng/mL at all follow-up visits; the proportion of patients achieving this cut-off was similar in the two groups. In addition, only 79% of patients in arm A and 73% of patients in arm B with s-25OHD measured at all four follow-up visits maintained a s-25OHD concentration above 20 ng/mL: meaning that respectively 21% and 27% of the subjects presented with a s-25OHD below 20 ng/mL in at least one follow-up visit. The mean s-25OHD of both groups decreased from baseline at every visit with borderline significance. This study found that longer outdoor exposure and higher oral vitamin D intake were independently associated with the achievement of s-25OHD concentration ≥ 32 ng/mL at all follow-up visits. There was no difference observed between groups of participants experiencing adverse events, hypercalcemia, hyperphosphatemia, or exceeding safety cut-off level of urinary calcium to creatinine ratio. Disease activity was measured at baseline, 80% of patients had a PCDAI score below 10 (remission), 16% were between 10 and 30 (mild) and 5% of patients were above 30 (moderate/severe).
Findings from studies designed to examine the effect of bone health related therapies (including vitamin D) in paediatric IBD patients
The cohort study of Hradsky et al[27] was designed to assess the effect of vitamin D3 therapy on bone health parameters and dynamic muscle function. In total, 55 patients aged 5-19 years with a diagnosis of IBD were included. Patients were excluded if they had another disease with known impact on the muscle-bone unit, if they were noncompliant to treatment, if they were pregnant, if they had undergone extensive small bowel surgery, if they were on total parenteral nutrition, or if they received growth hormone therapy. All patients received 2000 IU of vitamin D3 daily, for a median of 13.8 mo. Mean s-25OHD concentration at baseline was 58 nmol (23.2 ng/mL). This regimen resulted in 76% of the patients having s-25OHD concentration above 20 ng/mL throughout the study period. In addition, the vitamin D regimen in this cohort was associated with improvements in trabecular bone mineral density (BMD) at the tibia and muscle power. The majority of patients did not show increased PTH levels at follow-up. CRP levels were similar between baseline and follow-up visits. Similarly, PCDAI and the Paediatric Ulcerative Colitis Activity Index scores were not different between baseline and follow-up. The authors concluded that their regimen was safe.
Benchimol et al[28] investigated the effect of supplementation with 1000 mg calcium daily for 12 months to 1000 mg calcium daily for 12 mo along with 50000 IU vitamin D2 monthly for 6 months on BMD, compared to a control group who received no intervention. Seventy-two patients with IBD were included. Only subjects with an area lumbar spine BMD z score less than -1 were assigned to the intervention group. At baseline, children in the intervention group were shorter, thinner and experienced a greater bone age delay than subjects in the control group. The participants with CD in the intervention group trended towards a higher PCDAI score at baseline compared to controls. The mean s-25OHD of subjects receiving calcium alone and those receiving calcium and vitamin D supplementation together increased, with the latter group trending towards a level of 30 ng/mL, although the change from baseline was not statistically significant in either group. No serious adverse events were reported in any of the participants. No data on disease activity at follow-up or inflammatory markers were reported.
In a trial by Leonard et al[29], the effect of low-magnitude mechanical stimuli on several bone related measures including BMD was analysed in 121 patients. Patients with CD aged 8-21 years who had a tibial trabecular volumetric BMD below the 25th percentile were included. Exclusion criteria were weight > 250 pounds, pregnancy, a medical illness unrelated to CD with potential adverse effects on bone or body composition, plans to travel for more than 4 wk over the study period, a sibling or acquaintance enrolled in the trial, or developmental disorders prohibiting completion of study procedures. Both the control and intervention group were supplemented with 1000 mg calcium and 800 IU vitamin D3 daily for a period of 12 mo. Additional vitamin D intake in subjects was evaluated; if necessary the vitamin D supplementation was adjusted to prevent the average daily vitamin D intake from exceeding 2000 IU. Mean s-25OHD increased significantly but slightly among all participants. However, the proportion of patients with s-25OHD below 20 ng/mL did not change over the follow up period (7% at 12 mo and 8% at 24 mo). During the 12-month trial, there were 28 hospitalizations in the intervention group (n = 69) and 27 hospitalizations in the control group (n = 69), most of which were due to disease flares. In addition, 21 and 18 respectively reported symptoms at study visits.
A cohort study by Laakso et al[30] assessed lumbar spine areal BMD and height-adjusted whole body (less head) bone mineral content in 47 children and adolescents with IBD, with a median follow-up of 5 years. Vitamin D intake and s-25OHD were measured at baseline and follow-up. Patients aged 4-20 years with a diagnosis of IBD at least 3 mo before enrolment were included. Patients were excluded if they had other medical conditions unrelated to IBD that could affect growth or bone mass accrual. Total dietary vitamin D intake for all participants differed significantly between 7.5 μg (300 IU) [range 1.6 (64) - 19.5 (780) μg (IU)] at baseline and 12.6 μg (504 IU) [range 1.7 (68) - 49.2 (1968) μg (IU)] at follow-up. The proportion of patients using additional vitamin D supplements was 52% at baseline and 63% at follow-up. As this was a cohort study, vitamin D intake differed substantially between participants. This study found that patients with a low lumbar spine areal BMD had a greater total vitamin D intake than participants with a normal lumbar spine areal BMD, while their s-25OHD concentrations were not significantly higher. The authors suggested that this might possibly reflect impaired absorption of nutrients.
DISCUSSION
Safety
The authors of each of the reports included in this review concluded that their vitamin D regimens were safe and well-tolerated. One study mentioned an adverse event rate of 32%[23]; however, this rate was similar in all arms in their study and was therefore most likely not related solely to the vitamin D supplementation. Also, only patients with a s-25OHD concentration below 20 ng/mL were included in this study; the severe vitamin D deficiency at baseline may be related to the higher adverse event rate in this study group.
In general, few of the included studies provided quantitative data concerning adverse events. More detailed information on side effects and adverse events of vitamin D therapy is needed to compare safety between regimens and to define an optimal treatment dose. In future trials concerning regular or high dose vitamin D therapy, these markers should be measured strictly and possible side effects should be monitored.
Efficacy
Effects of vitamin D therapy on s-25OHD concentrations: Regarding an optimal cut-off value for vitamin D therapy, the Institute of Medicine has argued that s-25OHD levels above 30 ng/mL do not provide additional benefit[31]. However, other studies suggest that a level of at least 32 ng/mL is required for optimal intestinal calcium absorption[32,33]. A level of 30 ng/mL has also been mentioned as to be sufficient to reduce PTH activity[34]. Based on this evidence, a target s-25OHD level of at least 30 ng/mL for children with IBD should be considered with regards to optimal bone health.
In one of the included studies, 2000 IU D3 daily raised s-25OHD above 30 ng/mL in 74% of the participants after 6 months of treatment[22]. Another study showed a similar response after weekly dosing of 50,000 IU D3 for 6 wk[23]. In a third study, 2000 IU D3 daily was able to achieve a mean s-25OHD above 30 ng/mL in the study population throughout the course of treatment[27]. Both 5000 IU/10 kg vitamin D3 per week and 10000 IU/10 kg vitamin D3 per week for a period of 6 wk were able to raise s-25OHD above 30 ng/mL at week 8 in one of the trials; however, this effect was lost by week 12[24].
The stoss therapy seemed highly effective in raising s-25OHD levels above 30 ng/mL for at least 1 month and was more successful in maintaining concentrations above 30 ng/mL at 3 and 6 mo compared to other trials[25]. No other trials normalized mean s-25OHD from a deficient level to a concentration above 30 ng/mL. The trial of maintenance of vitamin D levels was not successful in raising s-25OHD levels greater than 20 ng/mL at baseline to a level above 32 ng/mL during a period of 12 months, as less than 10% of the study population achieved this level at each trimonthly visit[21].
Effects of vitamin D therapy on paediatric IBD: The influence of vitamin D therapy on disease severity is a further potential outcome of supplementation. This is relevant as the primary effects of vitamin D in this population (on factors such as BMD, bone resorption and calcium homeostasis) are inconsistent and the significance of hypovitaminosis D in children with IBD is still unclear[17]. In adults, more evidence of the role of vitamin D in treatment of IBD is available; small trials show promising results but remain conflicting[19,35]. Therefore, with the evidence for possible beneficial effects of vitamin D therapy on the clinical course of IBD in childhood being extremely limited, it would have been very useful if more of the included studies had reported disease severity related measures of the participants over time.
Only two of the included studies reported mean PCDAI scores at both baseline and follow-up[22,25]. However, neither included statistical data to support these findings. One study showed a nonsignificant difference in PCDAI scores between treatment groups[22]. They reported that the proportion of patients having a PCDAI ≤ 10 (quiescent disease) was smaller at follow-up than at baseline in both treatment arms. No statistical analyses comparing follow-up to baseline levels were reported. The other trial reported PCDAI scores varying from 0 to 47.5 (median 10) at baseline and from 0 to 85 (median 2.5) at 6 mo follow up[25].
Two included studies reported changes in anti-inflammatory markers subsequent to vitamin D treatment[21,22]. One reported lower levels of interleukin-6, CRP and ESR in participants taking higher doses of vitamin D[21]. The authors stated that the study was not designed to investigate the relationships between vitamin D intake and inflammatory markers and that these results should be interpreted with caution. In contrast, the second study reported no differences in CRP and ESR between treatment groups[22].
Both the limited data on this topic and their contrasting exploratory results call for prospective research that focuses on the effect of vitamin D therapy on disease severity of IBD, concerning clinical factors and anti-inflammatory effects.
Trial differences
While all included studies provide important data on vitamin D therapy in children with IBD, important study differences prevent direct comparisons of treatment regimens or conclusions as to an optimal dose of vitamin D. However, given the variability of disease and patient-related factors (see below), it may be more appropriate to consider personalized treatments rather than one regimen to suit all patients.
Study population: Regarding the studies designed to investigate efficacy of vitamin D therapy, two studies included only patients with a baseline s-25OHD concentration below 20 ng/mL[23,25]. One study included patients with s-25OHD concentration below 30 ng/mL[24]. The study focusing on maintenance therapy included only patients with a baseline s-25OHD level above 20 ng/mL[21]. Another study included only patients with quiescent CD[22].
Concerning exclusion criteria, patients taking corticosteroids shortly before enrolment were excluded in two studies[22,24]. Two trials excluded children taking additional vitamin D supplements[22,23]. Patients with a history of liver or kidney disease were excluded in three trials[21,23,24]. Two studies also excluded patients taking anticonvulsants metabolized through cytochrome P450[21,23].
Regarding the studies on bone health, one study excluded patients with coexisting diseases that might affect vitamin D status[26]. Another study excluded patients with plans to travel more than four weeks over the study interval[29]. Three studies excluded patients if they had a history of coexisting medical conditions unrelated to IBD that affected bone mass[27,29,30]. Patients who had undergone extensive small bowel surgery, patients on total parenteral nutrition and patients on growth hormone therapy were excluded in one study[27].
Due to these differences in inclusion and exclusion criteria, baseline differences in s-25OHD, co-medications, comorbidities, and disease severity exist between the various reports. Furthermore, via the principle of regression to the mean, patients with a lower baseline level of s-25OHD are more likely to achieve a greater mean change in s-25OHD than participants with a higher baseline level. This principle should be considered when comparing treatment regimens and when developing future dosing schedules.
Length of treatment regimen: Two studies both provided vitamin D therapy for 6 wk, whereas two other trials studied the effect of vitamin D supplementation over 6 mo[22-24,28]. One trial supplemented participants with vitamin D over 12 mo[29], two studies observed vitamin D levels after a median of respectively 13.8 mo and 5 years of supplementation[27,30], and one study reviewed the effect of a single dose of vitamin D with follow up for 6 months[25]. As suggested by the latter study, the duration of the effect of vitamin D could be dependent on the dosage, with higher dosages possibly having longer lasting effects[25].
Form of vitamin D used: While vitamin D is often still prescribed by the number of international units or IU, which is based on the presumption of an equal bioavailability between vitamin D2 and vitamin D3, a growing body of literature recognizes that vitamin D3 has a 2-3 times greater potency resulting in a higher s-25OHD with a longer duration of effect compared to vitamin D2[36,37]. Some trials treated patients using vitamin D2, while the majority supplemented with vitamin D3. One study showed superiority of vitamin D3 compared to the same dosage of vitamin D2 in raising s-25OHD levels in children with IBD [23]. These aspects of bioavailability should be considered in future trials, and a distinction in recommended dose for vitamin D2 and D3 separately is needed.
Dose adjustment: One study utilised an age-adjusted dosage regimen[25], while another used weight-adjusted dosing[24]. The remaining trials did not adjust the dosage for weight or age. One trial suggested that a weight-adjusted dosing regimen might be more suitable, as an increase in s-25OHD was inversely related to weight [23]. Furthermore, patients in this study who failed to achieve sufficient levels were heavier than average. This finding is in line with observations in healthy adults[38,39]. In contrast, the study using a weight-adjusted dosing schedule observed no effect of differing weight upon the change in s-25OHD[24]. One included study postulated that vitamin D is known to sequester in fat tissue and this could explain the variation in achieving sufficiency related to weight[23]. In contrast, recent literature did not observe a sequestration of vitamin D2 and vitamin D3 in fat[36].
Recent studies that describe an inverse relationship between an increase in mean s-25OHD level during therapy, and baseline s-25OHD concentration[38,39], would support a dosage regimen based on severity of vitamin D deficiency. Vitamin D may play a role in improving bone health in children with IBD[23,40]. In addition, one of the included studies mentioned studies showing a negative effect of glucocorticoids on BMD[27,41-44]. It seems logical to conclude that children with IBD receiving corticosteroids are likely to benefit from a higher dose of vitamin D. Currently, children with IBD receiving corticosteroids are often excluded from vitamin D trials or comprise a small proportion of study groups.
Taken together, these findings support the need for future studies that assess adjustment of vitamin D treatment based on weight and/or age, severity of vitamin D deficiency, treatment with corticosteroids and gender.
A different way to look at a personalized adjustment of vitamin D treatment is to identify the characteristics of patients failing to achieve sufficiency, such as heavier participants in one included study[23]. In addition, some patients are prescribed immunosuppressants that are associated with increased sun sensitivity and are therefore counselled to carefully use sunscreen, which negatively influences UV-B synthesis of vitamin D[45]; for these patients, a higher vitamin D dose might also be appropriate. Moreover, given that genetic variants in vitamin D hydroxylation are related to deficiency and a poor response to treatment[46], future studies might be needed to identify patients with these genetic variants in order to personalise their treatment. In general, future trials concerning vitamin D therapy in children with IBD should try to identify the characteristics of patients failing to achieve the target level of 30 ng/mL while bearing some of these possible explanations in mind.
Knowledge gaps
While the studies reviewed in this paper give important evidence concerning the efficacy and safety of vitamin D therapy in children with IBD, remaining questions need to be addressed in order to define optimal vitamin D treatment regimens for this patient group.
Safety threshold: Three included studies refer to a safety threshold of s-25OHD at 88 ng/mL, as vitamin D intoxication has not been reported below this level[21,23,27]. However, other authors mention this concentration was based on trials in healthy adults[47]; data concerning children and especially those with chronic disease is lacking. As some of the participants in the stoss study had increased levels that exceeded 88 ng/mL[25], without adverse events seen, continuing these single high-dose regimens might lead to new evidence concerning safety of vitamin D threshold. Participants achieving such high concentrations of s-25OHD should be carefully monitored, as potential risks include hypervitaminosis D with hypercalcaemia, hyperphosphatemia, hypercalciuria, nausea and renal calculi[48].
One included study referred to a daily upper tolerable intake of vitamin D of 3000 IU in children ages 4-8 years and 4000 IU in children 9 years and older, without reference to the form of vitamin D[22]. Due to the recent evidence that highlights the differences in bioavailability between vitamin D2 and vitamin D3, new studies on this topic should include different recommended intakes for vitamin D2 or D3, along with investigating if there is a difference in safety between vitamin D2 and to vitamin D3[37].
Efficacy: With the included trials, vitamin D was introduced as a new therapy for children with IBD. Possible beneficial effects of this treatment are related to bone health, anti-inflammatory markers and disease severity. While the included trials are beginning to establish accurate treatment regimens to achieve sufficient vitamin D levels, the relevance of vitamin D treatment in this population remains controversial[17]. Where four of the ten included studies in this review focused on bone health related measures as an outcome of therapies including vitamin D[27-30], only two studies reported disease severity related measures (PCDAI scores)[22,25], and two studies included anti-inflammatory markers as a secondary outcome[21,22].
As stated above, the effect of vitamin D on the course of disease in these children is still unclear. Large prospective trials are needed to establish the efficacy of vitamin D treatment in this population, with a focus on the clinical course of disease, bone health and anti-inflammatory related measures.
Absorption: The studies included in this review share different opinions on the absorption of vitamin D in children with IBD compared to healthy children or adults. One study allocated participants to intervention and control groups based on their BMD; the intervention group had a lower BMD than the control group[30]. The authors discussed that patients with a low lumbar spine areal BMD had a greater total vitamin D intake, while their s-25OHD levels were not significantly greater, reflecting a possible impaired absorption of vitamin D. In contrast, two other studies suggest that the absorption of vitamin D in their study population is likely not different from the healthy population[22,23]. However, all the participants in one of those trials had baseline s-25OHD levels below 20 ng/mL, and following the principle of regression to the mean these patients are more likely to achieve a greater change in s-25OHD with relatively lower vitamin D intake than patients or healthy children with a higher average s-25OHD concentration [23]. The participants in the second study all had quiescent CD: absorption in this group may differ to that in children with more active disease [22]. Future studies need to be carried out in order to validate the possibility of impaired absorption of vitamin D in children with IBD.
Adherence: Strict adherence is needed with a daily regimen in order to achieve optimal vitamin D levels. Lowering the treatment burden and improving adherence is likely to enhance s-25OHD status. A noncompliance rate around 30% was reported in both groups in one of the included studies[21]. In addition, another trial reported that 68% and 88% of participants who completed the study had adherence rates greater than 80% and 70%, respectively[22]. Furthermore, one trial stated that adherence in their trial was better with weekly than with either of the two daily regimens (89% vs 70% and 78%), therefore the authors recommend a weekly regimen[23]. On the other hand, the authors of the stoss study argued that a single dose treatment of vitamin D may minimise non-adherence to vitamin D treatment[25].
The purpose of the current study was to systematically review the available reports that had evaluated the efficacy and safety of vitamin D therapy in children with IBD. In addition to emphasizing the diversity between the included studies, this review has shown that the majority of included trials were not sufficient in raising s-25OHD concentrations of vitamin D deficient children with IBD to an acceptable level (30 ng/mL). Clearly adequate dosages are needed in order to achieve optimal levels above 30 ng/mL and to maintain these levels long term. The precise dosing regimen should consider various factors such as age, baseline s-25OHD concentration, weight, gender and disease activity. In addition, adherence with therapy is likely enhanced with weekly or single dosed regimens compared to daily regimens. Furthermore, the current data highlight the importance of applying dose adjustments and separate criteria for vitamin D2 and vitamin D3, measuring anti-inflammatory markers and further investigating safety in future trials on vitamin D therapy in paediatric patients with IBD.
This review has also emphasized that effects of vitamin D supplementation on disease severity related measures in paediatric IBD are not yet elucidated. As relationships between treatment with vitamin D and factors related to bone health, clinical course of disease and anti-inflammatory markers in paediatric IBD have not yet been clearly established, future trials should assess these topics prospectively.
Several limitations should be acknowledged. Firstly, only three published randomized controlled trials designed to investigate the efficacy of vitamin D therapy on s-25OHD concentrations were identified and included in this review. Because of the lack of comprehensive research on this topic, suggestions concerning treatment regimens in this review need to be confirmed in future trials. Second, given the diversity of included trials, the reviewed vitamin D regimens cannot be compared in a meta-analysis. Therefore, it is not possible to draw a conclusion about an optimal regimen; personalizing vitamin D treatment may be more appropriate. Notwithstanding these limitations, this first comprehensive assessment of vitamin D therapy in children with IBD provides important insights for future research in order to optimize the management of children with IBD.
ARTICLE HIGHLIGHTS
Research background
Vitamin D deficiency is commonly seen in children diagnosed with inflammatory bowel disease (IBD). While vitamin D is important for bone health, inadequate levels may also influence the course of disease.
Research motivation
To date, however, the optimal manner to correct and maintain adequate vitamin D levels in children with IBD have not been established.
Research objectives
The principal objective of this work was to systematically review the published literature focusing on vitamin D therapy in children with IBD. The further objective was to highlight opportunities for future work to augment and enhance the current understanding in this field.
Research methods
The published literature was reviewed systematically for publications that had focused on vitamin D therapy in children with IBD. The available data was then reviewed in detail.
Research conclusions
Ten published articles were identified and reviewed in detail. These reports included very different study designs with varied treatment regimens. Overall, there was no clear optimal regimen or approach that consistently corrected vitamin D deficiency and maintained levels over time.
Research perspectives
Vitamin D sufficiency is an important aspect of managing children with IBD. The available published literature does not provide a clear approach to the management of deficiency of vitamin D in this setting. Various aspects likely influence the response to vitamin D therapy: these are important factors that need to be taken into account in considering future work in this area.
ACKNOWLEDGEMENTS
The authors thank Dr Nanne de Boer for his helpful suggestions and assistance with this work.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: New Zealand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: All authors have no conflicts of interest to report.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Peer-review started: October 8, 2018
First decision: November 7, 2018
Article in press: January 10, 2019
P- Reviewer: Jamali R, Sebastian S S- Editor: Ma YJ L- Editor: A E- Editor: Bian YN
Contributor Information
Tarah Rigterink, Vrije Universiteit Amsterdam, Amsterdam 1081 HV, the Netherlands; Department of Paediatrics, University of Otago Christchurch, Christchurch 8041, New Zealand.
Laura Appleton, Department of Paediatrics, University of Otago Christchurch, Christchurch 8041, New Zealand.
Andrew S Day, Department of Paediatrics, University of Otago Christchurch, Christchurch 8041, New Zealand. andrew.day@otago.ac.nz.
References
- 1.Holick MF. Vitamin D and bone health. J Nutr. 1996;126:1159S–1164S. doi: 10.1093/jn/126.suppl_4.1159S. [DOI] [PubMed] [Google Scholar]
- 2.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, Fang M, Yazdi F, Garritty C, Sampson M, Barrowman N, Tsertsvadze A, Mamaladze V. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007;158:1–235. [PMC free article] [PubMed] [Google Scholar]
- 5.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92:60–64. doi: 10.1016/j.pbiomolbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 7.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelsen J, Baldassano RN. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008;14 Suppl 2:S9–11. doi: 10.1002/ibd.20560. [DOI] [PubMed] [Google Scholar]
- 10.Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 11.Malaty HM, Fan X, Opekun AR, Thibodeaux C, Ferry GD. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr. 2010;50:27–31. doi: 10.1097/MPG.0b013e3181b99baa. [DOI] [PubMed] [Google Scholar]
- 12.Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, Grand RJ. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118:1950–1961. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin AD, Wadhera V, Leach ST, Woodhead HJ, Lemberg DA, Mendoza-Cruz AC, Day AS. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. 2011;56:830–836. doi: 10.1007/s10620-010-1544-3. [DOI] [PubMed] [Google Scholar]
- 14.Sentongo TA, Semaeo EJ, Stettler N, Piccoli DA, Stallings VA, Zemel BS. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am J Clin Nutr. 2002;76:1077–1081. doi: 10.1093/ajcn/76.5.1077. [DOI] [PubMed] [Google Scholar]
- 15.Laakso S, Valta H, Verkasalo M, Toiviainen-Salo S, Viljakainen H, Mäkitie O. Impaired bone health in inflammatory bowel disease: a case-control study in 80 pediatric patients. Calcif Tissue Int. 2012;91:121–130. doi: 10.1007/s00223-012-9617-2. [DOI] [PubMed] [Google Scholar]
- 16.Harpavat M, Greenspan SL, O'Brien C, Chang CC, Bowen A, Keljo DJ. Altered bone mass in children at diagnosis of Crohn disease: a pilot study. J Pediatr Gastroenterol Nutr. 2005;40:295–300. doi: 10.1097/01.mpg.0000153278.98861.32. [DOI] [PubMed] [Google Scholar]
- 17.Pappa HM, Grand RJ, Gordon CM. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis. 2006;12:1162–1174. doi: 10.1097/01.mib.0000236929.74040.b0. [DOI] [PubMed] [Google Scholar]
- 18.Mouli VP, Ananthakrishnan AN. Review article: vitamin D and inflammatory bowel diseases. Aliment Pharmacol Ther. 2014;39:125–136. doi: 10.1111/apt.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholson I, Dalzell AM, El-Matary W. Vitamin D as a therapy for colitis: a systematic review. J Crohns Colitis. 2012;6:405–411. doi: 10.1016/j.crohns.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 20.El-Matary W, Sikora S, Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Dig Dis Sci. 2011;56:825–829. doi: 10.1007/s10620-010-1380-5. [DOI] [PubMed] [Google Scholar]
- 21.Pappa HM, Mitchell PD, Jiang H, Kassiff S, Filip-Dhima R, DiFabio D, Quinn N, Lawton RC, Bronzwaer ME, Koenen M, Gordon CM. Maintenance of optimal vitamin D status in children and adolescents with inflammatory bowel disease: a randomized clinical trial comparing two regimens. J Clin Endocrinol Metab. 2014;99:3408–3417. doi: 10.1210/jc.2013-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wingate KE, Jacobson K, Issenman R, Carroll M, Barker C, Israel D, Brill H, Weiler H, Barr SI, Li W, Lyon MR, Green TJ. 25-Hydroxyvitamin D concentrations in children with Crohn's disease supplemented with either 2000 or 400 IU daily for 6 months: a randomized controlled study. J Pediatr. 2014;164:860–865. doi: 10.1016/j.jpeds.2013.11.071. [DOI] [PubMed] [Google Scholar]
- 23.Pappa HM, Mitchell PD, Jiang H, Kassiff S, Filip-Dhima R, DiFabio D, Quinn N, Lawton RC, Varvaris M, Van Straaten S, Gordon CM. Treatment of vitamin D insufficiency in children and adolescents with inflammatory bowel disease: a randomized clinical trial comparing three regimens. J Clin Endocrinol Metab. 2012;97:2134–2142. doi: 10.1210/jc.2011-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simek RZ, Prince J, Syed S, Sauer CG, Martineau B, Hofmekler T, Freeman AJ, Kumar A, McElhanon BO, Schoen BT, Tenjarla G, McCracken C, Ziegler TR, Tangpricha V, Kugathasan S. Pilot Study Evaluating Efficacy of 2 Regimens for Hypovitaminosis D Repletion in Pediatric Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2016;62:252–258. doi: 10.1097/MPG.0000000000000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd D, Day AS, Leach ST, Lopez R, Messenger R, Woodhead HJ, Ledder O, Lemberg DA. Single High-Dose Oral Vitamin D3 Therapy (Stoss): A Solution to Vitamin D Deficiency in Children With Inflammatory Bowel Disease? J Pediatr Gastroenterol Nutr. 2015;61:411–414. doi: 10.1097/MPG.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 26.Santucci NR, Alkhouri RH, Baker RD, Baker SS. Vitamin and zinc status pretreatment and posttreatment in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;59:455–457. doi: 10.1097/MPG.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 27.Hradsky O, Soucek O, Maratova K, Matyskova J, Copova I, Zarubova K, Bronsky J, Sumnik Z. Supplementation with 2000 IU of Cholecalciferol Is Associated with Improvement of Trabecular Bone Mineral Density and Muscle Power in Pediatric Patients with IBD. Inflamm Bowel Dis. 2017;23:514–523. doi: 10.1097/MIB.0000000000001047. [DOI] [PubMed] [Google Scholar]
- 28.Benchimol EI, Ward LM, Gallagher JC, Rauch F, Barrowman N, Warren J, Beedle S, Mack DR. Effect of calcium and vitamin D supplementation on bone mineral density in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45:538–545. doi: 10.1097/MPG.0b013e3180dca0cc. [DOI] [PubMed] [Google Scholar]
- 29.Leonard MB, Shults J, Long J, Baldassano RN, Brown JK, Hommel K, Zemel BS, Mahboubi S, Howard Whitehead K, Herskovitz R, Lee D, Rausch J, Rubin CT. Effect of Low-Magnitude Mechanical Stimuli on Bone Density and Structure in Pediatric Crohn's Disease: A Randomized Placebo-Controlled Trial. J Bone Miner Res. 2016;31:1177–1188. doi: 10.1002/jbmr.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laakso S, Valta H, Verkasalo M, Toiviainen-Salo S, Mäkitie O. Compromised peak bone mass in patients with inflammatory bowel disease--a prospective study. J Pediatr. 2014;164:1436–43.e1. doi: 10.1016/j.jpeds.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 31.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: what dietetics practitioners need to know. J Am Diet Assoc. 2011;111:524–527. doi: 10.1016/j.jada.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 33.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112:659–662. doi: 10.1016/s0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 35.Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96:E447–E452. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 37.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 38.Blum M, Dallal GE, Dawson-Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J Am Coll Nutr. 2008;27:274–279. doi: 10.1080/07315724.2008.10719700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Groningen L, Opdenoordt S, van Sorge A, Telting D, Giesen A, de Boer H. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur J Endocrinol. 2010;162:805–811. doi: 10.1530/EJE-09-0932. [DOI] [PubMed] [Google Scholar]
- 40.Sylvester FA. IBD and skeletal health: children are not small adults! Inflamm Bowel Dis. 2005;11:1020–1023. doi: 10.1097/01.mib.0000188341.96726.15. [DOI] [PubMed] [Google Scholar]
- 41.Vihinen MK, Kolho KL, Ashorn M, Verkasalo M, Raivio T. Bone turnover and metabolism in paediatric patients with inflammatory bowel disease treated with systemic glucocorticoids. Eur J Endocrinol. 2008;159:693–698. doi: 10.1530/EJE-08-0429. [DOI] [PubMed] [Google Scholar]
- 42.Semeao EJ, Jawad AF, Zemel BS, Neiswender KM, Piccoli DA, Stallings VA. Bone mineral density in children and young adults with Crohn's disease. Inflamm Bowel Dis. 1999;5:161–166. doi: 10.1097/00054725-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Lopes LH, Sdepanian VL, Szejnfeld VL, de Morais MB, Fagundes-Neto U. Risk factors for low bone mineral density in children and adolescents with inflammatory bowel disease. Dig Dis Sci. 2008;53:2746–2753. doi: 10.1007/s10620-008-0223-0. [DOI] [PubMed] [Google Scholar]
- 44.Boot AM, Bouquet J, Krenning EP, de Muinck Keizer-Schrama SM. Bone mineral density and nutritional status in children with chronic inflammatory bowel disease. Gut. 1998;42:188–194. doi: 10.1136/gut.42.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raftery T, O'Sullivan M. Optimal vitamin D levels in Crohn's disease: a review. Proc Nutr Soc. 2015;74:56–66. doi: 10.1017/S0029665114001591. [DOI] [PubMed] [Google Scholar]
- 46.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 48.Diamond TH, Ho KW, Rohl PG, Meerkin M. Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: efficacy and safety data. Med J Aust. 2005;183:10–12. doi: 10.5694/j.1326-5377.2005.tb06879.x. [DOI] [PubMed] [Google Scholar]