Significance
Specialized cells in the inner ear translate sound into electrical signals for hearing, which are transferred to the brain through the cochlear nerve. Many of the molecular components of the inner ear are currently unknown. This paper uses a genetic approach to identify GRAP as a gene mutated in human deafness. In mice, Grap is present at the neuronal fibers innervating the auditory hair cells. The Drosophila homolog of the human Grb2-related adaptor protein (GRAP), drk, localizes to the synaptic terminals of neurons, and its disruption leads to hearing organ abnormalities associated with defects in locomotor behavior. We conclude that GRAP/drk plays an indispensable role in hearing.
Keywords: adaptor proteins, drk, Drosophila, GRAP, nonsyndromic hearing loss
Abstract
We have identified a GRAP variant (c.311A>T; p.Gln104Leu) cosegregating with autosomal recessive nonsyndromic deafness in two unrelated families. GRAP encodes a member of the highly conserved growth factor receptor-bound protein 2 (GRB2)/Sem-5/drk family of proteins, which are involved in Ras signaling; however, the function of the growth factor receptor-bound protein 2 (GRB2)-related adaptor protein (GRAP) in the auditory system is not known. Here, we show that, in mouse, Grap is expressed in the inner ear and the protein localizes to the neuronal fibers innervating cochlear and utricular auditory hair cells. Downstream of receptor kinase (drk), the Drosophila homolog of human GRAP, is expressed in Johnston’s organ (JO), the fly hearing organ, and the loss of drk in JO causes scolopidium abnormalities. drk mutant flies present deficits in negative geotaxis behavior, which can be suppressed by human wild-type but not mutant GRAP. Furthermore, drk specifically colocalizes with synapsin at synapses, suggesting a potential role of such adaptor proteins in regulating actin cytoskeleton dynamics in the nervous system. Our findings establish a causative link between GRAP mutation and nonsyndromic deafness and suggest a function of GRAP/drk in hearing.
Hearing loss (HL) is the most common sensory disorder with an incidence of 1–3 in 1,000 births (1). Genetic factors show high heterogeneity and are responsible for more than half of the cases with congenital HL (2). It is estimated that 70% of genetic HL is nonsyndromic sensorineural HL and mostly shows an autosomal recessive inheritance pattern (1, 3). The identification of DNA variants causing HL is rapidly advancing due to technological developments in genome sequencing. However, delineating causality for a novel rare variant in a gene not previously associated with HL is difficult and often requires functional studies in model organisms.
The mouse has been shown to be an excellent model organism to study human deafness due to the anatomic and physiologic similarities of their auditory systems with those of humans (4). Recent studies of Drosophila’s hearing organ, Johnston’s organ (JO), highlight the molecular and genetic conservations of the mechanosensory transduction in flies and vertebrates (5, 6). Several key genes first identified in Drosophila, such as atonal, crinkled, and nanchung, that are required for the specification or function of auditory cell types in the JO are also important for the normal development or function of the vertebrate ear (5). In addition, 20% of Drosophila auditory-associated genes have human homologs and are implicated in hearing disorders (7).
In this paper, via exome and genome sequencing of over 60 consanguineous multiplex families with autosomal recessive nonsyndromic HL, which are negative for variants in known deafness genes (details of families are given in SI Appendix, Table S1), we have identified a GRAP variant in two unrelated Turkish families. We show that Grap is expressed in the inner ear of mice during the development and in adults. Moreover, Grap localizes in the neuronal fibers that innervate the auditory hair cells. To provide evidence for the causality of the variant, we established a loss-of-Drosophila ortholog (downstream of receptor kinase, drk) model, which recapitulates aspects of the human phenotype. In addition, our functional studies of growth factor receptor-bound protein 2 (Grb2)-related adaptor protein (GRAP) in vivo suggest a causal link between mutant GRAP and deafness.
Results
Affected Individuals Diagnosed with Nonsyndromic Profound Deafness.
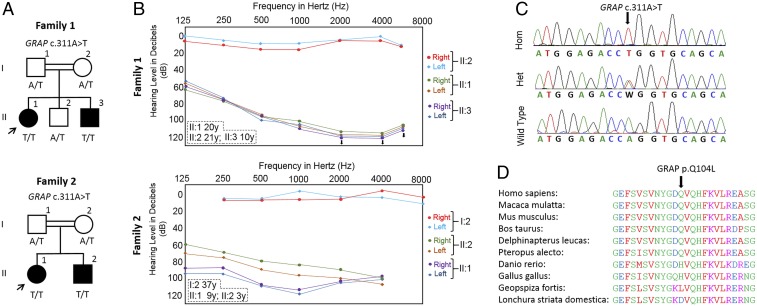
Four affected individuals from two consanguineous families of Turkish origin (Fig. 1A) showed congenital profound sensorineural hearing loss (Fig. 1B). Audiograms of one unaffected member from each family were normal (Fig. 1B). The remainder of the physical examinations were normal. In the affected individuals, there was no history of pre- or postnatal exposure to ototoxic medications, head or sound trauma, neonatal jaundice, premature birth, intrauterine infections, or perinatal hypoxia. They all had normal gross motor development without balance problems, vertigo, dizziness, or nystagmus. Tandem walking was normal, and the Romberg test was negative.
Fig. 1.
Nonsyndromic profound deafness is diagnosed in affected individuals who are homozygous for a GRAP variant. (A) Pedigrees and segregation of the GRAP c.311A>T variant in families 1 and 2. (B) Hearing thresholds obtained from pure tone audiograms of the affected individuals showing severe to profound HL. Unaffected individuals display normal hearing. Ages indicated are at the time of the audiograms. (C) Electropherograms showing the identified variant. The wild-type (WT) traces are from an unrelated individual. Hom, homozygous mutant, Het, heterozygous mutant. (D) Amino acid sequences of the partial SH2 domain of GRAP in different species. Amino acid Gln104 (Q104) is highly conserved in mammals.
Exome and Genome Sequencing Identifies a GRAP Variant.
The coverages of targeted regions in the exome data for the 10-fold read depth were 87% and 86%, and the average read depths were 50× and 52× in the probands of family 1 and family 2, respectively. Genome sequencing showed average sequencing read depths of 66× and 44×; coverages of, at least, 4× were 96.1% and 99.7% of the genome in the probands of family 1 and family 2, respectively. The analysis of data did not reveal a single nucleotide polymorphism, indel, or copy number variant in any of the previously recognized genes for nonsyndromic HL (https://hereditaryhearingloss.org) or syndromic HL (OMIM; www.omim.org). After applying the filtering criteria specified under the Materials and Methods section, we identified a variant mapping to a run of homozygosity (SI Appendix, Tables S2 and S3), chr17:18,927,685 (Hg19) and NM_006613.3: c.311A>T (p.Gln104Leu) in GRAP in both probands. Sanger sequencing showed that the variant cosegregates with deafness as an autosomal recessive trait in both families (Fig. 1 A and C). The variant has been seen in only 1 of 242,154 alleles in the gnomAD database (allele freq: 0.000004129) and is absent from over 500 Turkish exomes in our in-house database. Exons and intron–exon boundaries of homozygous runs in both samples are well covered with exome and genome sequencing (SI Appendix, Table S3). The variant affects a conserved residue (GERP: 4.45), and multiple in silico analysis tools predict it as damaging (SI Appendix, Table S4).
Screening of the variant in 690 unrelated Turkish probands with nonsyndromic HL did not show a positive sample. A two point logarithm of the odds score of 3.7364 was calculated for the two families between the identified variant and the phenotype assuming autozygosity. Shared ancestry of the variant encompassing ∼1.9 Mb was demonstrated via flanking single nucleotide variant genotypes (SI Appendix, Table S5).
Sanger sequencing of complementary DNA (cDNA) covering exon-exon boundaries obtained from a saliva sample of a proband did not show abnormal splicing caused by the GRAP c.311A>T variant (SI Appendix, Fig. S1; the primer sequences used are available in SI Appendix, Table S6).
MYO15A is a known nonsyndromic HL gene located ∼900 kb away from the GRAP c.311A>T variant. Whole genome sequencing did not identify a potentially causative variant within MYO15A (SI Appendix, Table S7).
The p.Gln104Leu Variant Most Likely Alters Interactions of GRAP with Its Cellular Partners.
GRAP encodes a protein consisting of a central Src homology 2 (SH2) domain flanked by two Src homology 3 (SH3) domains (8). The SH2 domain is required for interacting with phosphotyrosine-containing sites of the activated receptors and cytoplasmic proteins (8). The p.Gln104Leu variant is located within the SH2 domain of GRAP. We modeled the atomic structure of its SH2 domain in a complex with a tyrosine-phosphorylated (pY) peptide harboring the pYVNV sequence (SI Appendix, Fig. S2). The Gln104 and the preceding Asp103 residues are both located close to the peptide-binding pocket, although they do not interact directly with pY peptide residues. This suggests that p.Gln104Leu is unlikely to completely disrupt interactions of the SH2 domain of GRAP with its cellular partners and that it may be involved in attuning these interactions.
The conservation at GRAP residue 104 extends only through mammals (Fig. 1D). In many birds, such as finches, the AspGln dyad located at positions 103/104 in human GRAP is altered to residues, such as LysAsp (Bengalese finch; Lonchura striata domestica) and LysLeu (medium ground finch; Geospiza fortis). As anatomy and physiology (such as the audible sound frequency range) of the cochlea between mammals and finches are considerably different, it is quite conceivable that the p.Gln104Leu variant may lead to more serious consequences in the context of GRAP signaling in humans than its naturally occurring variants in finches.
Grap Is Expressed in the Mouse Inner Ear.
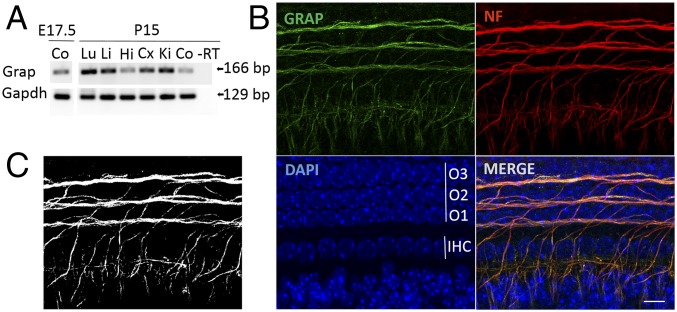
Human (NP_006604.1) and mouse (NP_082093.1) GRAP/Grap are composed of 217 amino acids sharing 92% identity. Total RNA was isolated from tissues of WT animals at E17.5 and P15. Reverse transcription polymerase chain reaction (RT-PCR) using specific primer pairs (SI Appendix, Table S6) produced a unique band of 166 bp corresponding to the WT Grap messenger RNA in all tested tissues. Gapdh expression was utilized as the control (Fig. 2A).
Fig. 2.
Grap is expressed in the mouse inner ear. (A) RT-PCR of Grap expression in different tissues at embryonic 17.5 and postnatal day 15, in different mouse tissues. Gapdh expression was used as the control. Co, cochlea; Cx, cortex; Hi, hippocampus; Ki, kidney; Li, liver; Lu, lung. (B) Representative z-stack projection of P0 whole mount cochlea stained for Grap (green). The red signals correspond to the neurofilament (NF) heavy chain that stains the spiral ganglion neuron (SGN) innervation of hair cells. Cell nuclei are counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Note the localization of Grap at the length of the nerves. (Scale bar: 10 µm.) (C) Black and white image indicates colocalization of Grap and NF as a result of the colocalization highlighter plugin (ImageJ).
Both the Shield (https://shield.hms.harvard.edu/) and gEAR databases (https://umgear.org/) show low levels of Grap expression in the inner and outer auditory hair cells. The gEAR database shows that Grap might be expressed also in the utricle. To evaluate the localization of Grap in the inner ear, we first validated an anti-GRAP antibody (SI Appendix, Fig. S3) and then utilized it for immunofluorescence performed in whole mount cochleae from P0 WT mice as well as a cross section of the inner ear (Fig. 2B and SI Appendix, Figs. S4 and S5). Immunostaining shows that Grap is localized in the SGN fibers innervating auditory hair cells, both inner and outer, as well as the utricular hair cells (Fig. 2B and SI Appendix, Figs. S4 and S5). An antibody recognizing the neurofilament heavy chain was utilized to counterstain hair cell innervation (Fig. 2 B and C).
drk, the Drosophila Homolog of GRAP, Is Expressed in the Drosophila Hearing Organ, JO.
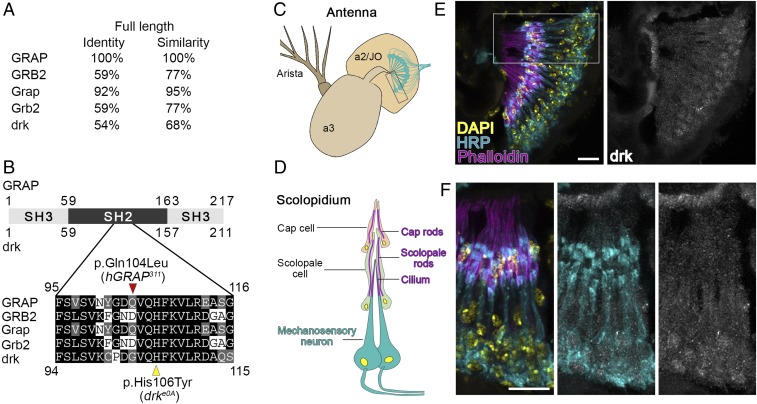
GRAP and GRB2, the mammalian homologs of Drosophila drk and Caenorhabditis elegans Sem-5, belong to the same protein family and share identical protein architectures (SH3-SH2-SH3) (9, 10). In humans, GRAP and GRB2 have distinct expression patterns and possibly functions (8, 11). Bioinformatics analysis of amino acid sequences suggests that human GRAP and Drosophila drk share 54% identity and 68% similarity (Fig. 3 A and B and SI Appendix, Fig. S6).
Fig. 3.
drk is the Drosophila homolog of human GRAP and is expressed in the scolopidia. (A) Homology of human, mouse, and Drosophila adaptor proteins GRAP/GRB2/drk scored by full-length amino acid identity and similarity. (B) Diagram illustrating the protein structure of GRAP and drk. Sequence alignment of a partial SH2 domain shows evolutionary conservation (the black background indicating identical amino acid and the gray background indicating a similar amino acid). The red arrow head indicates the mutation identified in humans with nonsyndromic HL. The yellow arrow head indicates a Drosophila mutant allele. (C) Schematic shows Drosophila antenna. Mechanosensory neurons (labeled with cyan) are suspended within the JO, which is the A2 segment of the fly antenna. (D) Schematic shows the organization of one scolopidium (boxed area in C). (E) Confocal micrographs show the expression pattern of drk in the JO. DAPI labels nuclei, horseradish peroxidase (HRP) labels neuronal membranes, and phalloidin labels cap rods, scolopale rods, and actin bundles in the cilium. (F) Confocal micrographs of the high magnification of scolopidia (boxed area in E). (Scale bars: E and F, 10 µm.)
JO is the component of the Drosophila auditory system required for sensing gravity, wind flow, and near-field sound (12, 13). To examine the function of the Drosophila homolog of GRAP in hearing, we first examined the distribution of drk in JO. There are around 200 scolopidia suspended within JO; these are the functional units of hearing and share evolutionarily conserved mechanosensory transduction mechanisms with vertebrate hair cells (7) (Fig. 3 C and D). Immunostaining of JO cryosections reveals the expression pattern of drk in scolopidia, including mechanosensory neurons, scolopale cells, and cap cells (Fig. 3 E and F).
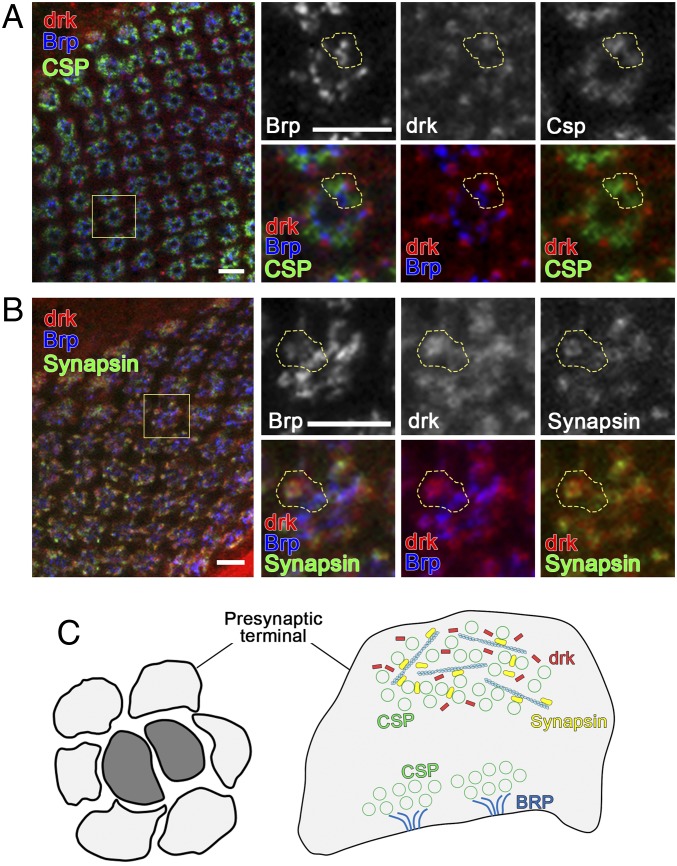
drk is distributed at the cell bodies and neurites of JO neurons (Fig. 3F); however, the localization of drk at the neuronal terminals has not been examined. drk was first identified through a genetic screen for modifiers of signaling mediated by the protein tyrosine kinase Sevenless, which is required for the proper development of the Drosophila compound eye (14). We thus examined the localization of drk at lamina synapses where photoreceptor cells and lamina neurons make synaptic contacts. The repetitive lamina cartridge has a well-defined structure that has been extensively exploited to characterize the cellular actions of proteins at synapses (15). Through the colabeling of several synaptic markers, we found that, at the presynaptic terminals, drk partially overlaps with the active zone associated cytoskeletal matrix protein Bruchpilot (Brp) and has a distinct expression pattern from synaptic vesicle protein cysteine string protein (CSP) (Fig. 4 A and C). Moreover, drk has a specific localization that highly overlaps with synapsin, a presynaptic phosphoprotein that associates with vesicles and the cytoskeleton, and regulates synaptic vesicle clustering and plasticity (16) (Fig. 4 B and C).
Fig. 4.
drk colocalizes with synapsin at lamina synapses. (A and B) Confocal micrographs show the localization of drk within lamina synapses. Brp labels the active zone structure. CSP labels synaptic vesicles (A), and synapsin (B) associates with vesicles and the cytoskeleton within presynaptic terminals. (Scale bars: 5 µm.) (C) Schematic shows the localization of synaptic proteins within lamina presynaptic terminals.
Mutations in drk Lead to Hearing Loss in Drosophila.
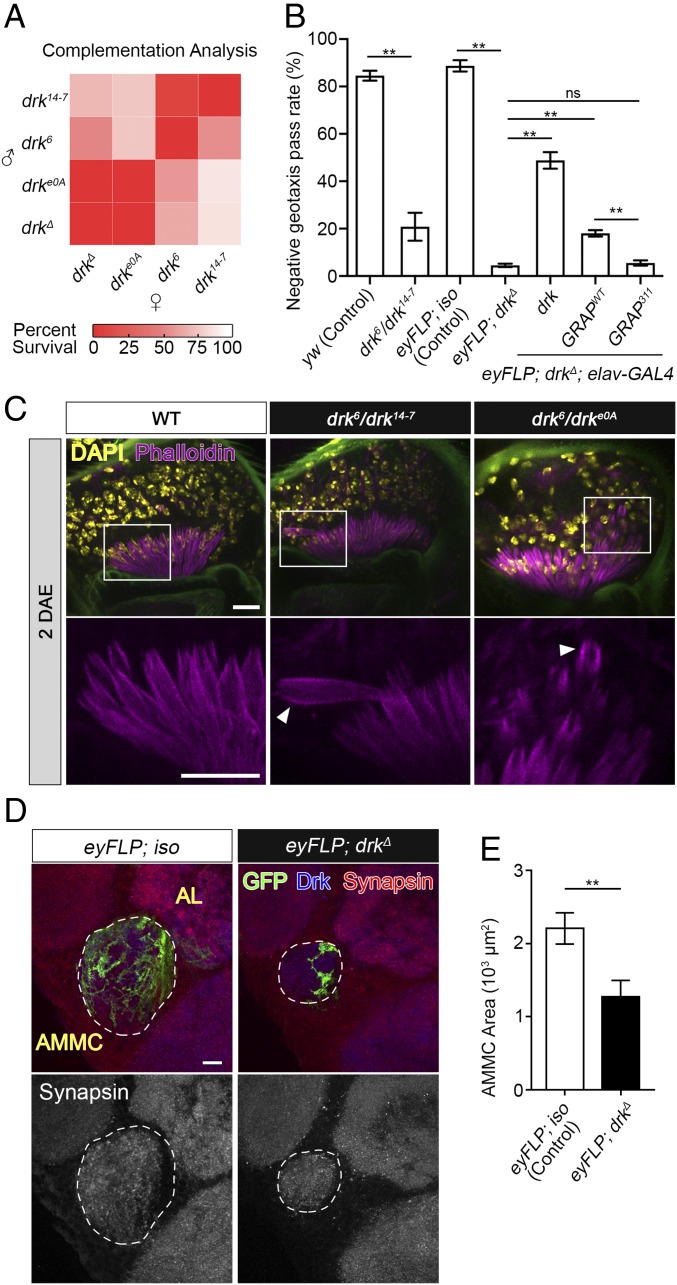
Several drk mutant alleles, including ethyl methanesulfonate (EMS)-induced point mutations (drke0A, drk6, and drk14−7) and deletion (drkΔ), have been previously identified (7). Specifically, the drke0A allele possesses a point mutation in the invariable residue (p.His106Tyr) in the SH2 domain, adjacent to the p.Gln104Leu mutation in GRAP, which affects both the phosphotyrosine binding and the localization of drk (10). Complementation analysis shows 100% adult lethality of the homozygous mutants and various survival defects of different compound heterozygous mutant animals (Fig. 5A), suggesting that drk is essential for development and survival. To examine the impact of drk mutations on the JO function, we first used negative geotaxis analysis to determine the level of gravity sensing, balance, and coordination (13, 17). Since compound heterozygous drk6/drk14−7 (♂/♀) have a 56% survival rate (Fig. 5A), we first tested the negative geotaxis performance in surviving drk6/drk14−7 adults and found severe locomotor deficits in these flies compared with WT flies, suggesting defects in gravity sensing and/or neuromuscular system dysfunction (Fig. 5B). To overcome the lethality of homozygous flies and to determine the function of drk in the JO, we generated mosaic animals (eyFLP; drkΔ) with a homozygous deletion of drk in the antenna and the eye but heterozygous in the rest of the body through flippase/flippase recognition target (Flp/FRT)-mediated mitotic recombination using the eyFLP system (18). We observed severe locomotor deficits in mosaic flies suggesting strong defects in gravity sensing as the main reason for the climbing defects because the JO is homozygous for drkΔ whereas the body (neuromuscular tissue) is heterozygous (Fig. 5B). Next, we examined the cellular morphology of scolopidia in two compound heterozygous flies including drk6/drk14−7 and drk6/drke0A as well as the mosaic flies eyFLP; drkΔ. We observed disorganized scolopidia in the mutant flies as revealed by phalloidin staining, which labels cap rods, scolopale rods, and the actin bundles within the cilia of the mechanosensory neurons (19) (Figs. 3D and 5C, and SI Appendix, Fig. S7). The JO mechanosensory neurons project their axons into the brain and largely innervate the antennal mechanosensory motor center (AMMC), the primary processing region for auditory input (20). To visualize the effects of the loss of drk in JO neurons, we used mosaic analysis with a repressible cell marker (MARCM) technique (21) to label the JO neuron clones that are homozygous for drk deletion (drkΔ/Δ). Compared with isogenized control, we observed significantly reduced AMMC area, indicating a significant loss of drkΔ/Δ sensory nerve terminals in the brain (Fig. 5 D and E). These results suggest that drk is required for functional and morphological integrities of the scolopidia, sensory neurons, and the AMMC brain neuropil.
Fig. 5.
drk mutations cause the morphological abnormalities of the scolopidia and locomotor deficits that can be suppressed by GRAPWT but not GRAP311 expression. (A) Heat map shows percent survival of drk compound heterozygous flies that survive to adults. drk14−7, drk6, and drke0A are EMS-induced mutant alleles and drkΔ is a deletion allele. (B) Negative geotaxis performance [mean ± standard error of the mean; each data point obtained from a group of 9 or 10 individuals, n =5 for drk6/drk14−7 group (due to low survival rate) and n = 10 for all of the rest of the groups (>100 flies per genotype tested)] of female flies at 2 days after eclosion (DAE). (C) Confocal micrographs show the morphology of actin bundles in the scolopidium. Both drk6/drk14−7 and drk6/drke0A flies have disorganized phalloidin staining patterns (the white arrowheads). (D) Mosaic analysis with a repressible cell marker analysis of AMMC. Green fluorescent protein (GFP) marks the WT patches in eyFLP; iso group, and drkΔ/Δ patches in eyFLP; drkΔ group. (E) Quantification of the AMMC area outlined in D (mean ± standard deviation; n = 3). (Scale bars: 10 µm.) One-way analysis of variance post hoc Tukey test; **P < 0.01. ns, not significant.
GRAP311 Mutant Protein Shows a Loss-of-GRAP Function When Expressed in Vivo.
To gain insight into the causative nature of the human mutant GRAP in vivo, we generated transgenic flies carrying WT (GRAPWT) and mutant (GRAP311) human GRAP: UAS-GRAPWT-HA or UAS-GRAP311-HA. GRAP was overexpressed using a pan-neuronal driver C155-GAL4, and the GRAP overexpression had no effects on the overall morphology of fly brains (SI Appendix, Fig. S8A). Surprisingly, we found an up-regulation of the endogenous drk when overexpressing GRAP in the nervous system (SI Appendix, Fig. S8A). Importantly, overexpression of GRAP311 induced a significantly higher level of drk compared with that of GRAPWT in both the mushroom body and the antenna lobe (SI Appendix, Fig. S8B). In addition, our negative geotaxis analysis suggests that both drk and GRAPWT overexpression significantly reduces the negative geotaxis performance of the flies, whereas overexpression of GRAP311 does not affect the negative geotaxis behavior (SI Appendix, Fig. S8C). These data suggest a potential genetic interaction between human GRAP and Drosophila drk, a tight regulation of drk/GRAP expression in neurons, and a difference in protein function between GRAPWT and GRAP311 in vivo.
Next, we expressed drk, GRAPWT, and GRAP311 in the mosaic animals using the pan-neuronal driver elav-GAL4 to test whether expressing drk/GRAP transgenes could rescue the negative geotaxis deficits. As shown in Fig. 5B, expressing drk or GRAPWT significantly improves the negative geotaxis performance of the mosaic flies (mean pass rate of 48.8% for the drk group and 18% for the GRAPWT group). However, GRAP311 expression has no effect on the negative geotaxis behavior of mosaic animals (mean pass rate of 5.5%). These data suggest a functional homology between drk and GRAPWT and the deleterious consequence of GRAP311.
Discussion
In this paper, we present a GRAP variant (c.311A>T; p.Gln104Leu; GRAP311) that is associated with nonsyndromic HL in humans. We further carried out functional studies of GRAP in vivo to provide evidence for its function in hearing. Studies in mice and Drosophila show that orthologs of GRAP are expressed in the hearing organ of each species. Furthermore, mutations in the Drosophila ortholog cause locomotor deficits and morphological abnormality of the scolopidia and sensory neurons in the JO, the Drosophila organ for hearing and balance. Importantly, Drosophila drk and human GRAP are functionally homologous as expressions of WT but not mutant human GRAP cDNA that can rescue the sensory phenotype in the drk mutant flies. These results establish a necessary role of GRAP and its orthologs in hearing and the deleterious nature of the GRAP311 variant.
GRAP was cloned as a cytoplasmic signaling protein that possesses the same structural arrangement as Grb2 (8). The SH2 domains directly recognize phosphotyrosine-containing sites on activated receptor protein kinases (RTKs) and cytoplasmic proteins. It has been suggested that the adaptor proteins Grb2/Sem-5/drk provide a functional link between RTKs and activation of Ras signaling (10, 17, 18). The SH3 domains, particularly those located on the amino-terminal side of Grb2/Sem-5/drk, bind the C-terminal tail of the Sos protein (8). Similarly, Grap binds to Sos through its amino-terminal SH3 domain (8). The stable association of Grb2/drk with the Ras guanine nucleotide exchange factor, Sos, and membrane localization of the Grb2/drk-Sos complex results in the activation of Ras (10, 14, 22–24). Grap is known to be expressed in lymphocytes and complexed with other proteins, including Sos, upon T-cell activation (8, 11). A mutant mouse model deficient of Grap shows an augmented mitogenic response of lymphocytes; ectopic expression of Grap leads to an interruption of signal transmission from the Ras-Erk pathway into the nucleus. This suggests a negative regulatory role of Grap in mediating mitogenic responses of lymphocytes (25). In human B cells, GRB2 and GRAP amplify signaling by the stimulated B-cell receptors and connect them to the activation of the Ras-controlled Erk-MAP kinase pathway (26).
An in vivo RNA interference screen has found a potential role for drk in actin filament organization; however, the underlying mechanisms remain unclear (17). Our data provide evidence for drk in actin organization, which may relate to the dysfunction of scolopidia in drk mutant JO. In addition, GRAP/drk may be involved in the neural development of the hearing organ. Neurotrophins play a critical role in neural development, regulating differentiation, neurite extension, target innervation, and survival (27). Brain derived neurotrophic factor (BDNF) is a neurotrophin known to influence neurons in the inner ear via high-affinity RTKs in the cochlea (28). Mice deficient in BDNF exhibit reduced cochlear neuronal populations especially in the apical turn (29–31). Mice null for TrkB, a RTK utilized by BDNF, are reported to lose 15–20% of spiral ganglion neurons (30, 31). Inhibition of Ras in spiral ganglion explants treated with BDNF eliminates the BDNF-induced increase in spiral ganglion neurite number (32). Given the role of GRAP connecting RTKs to Ras signaling, its expression in mice in the neurons innervating hair cells and disruption of mechanosensory neurons with reduced AMMC size in Drosophila mutants, it is possible that GRAP plays a role in the development of neural connections of the hearing organ through the modification of Ras signaling. Our observation of the drk and GRAP overexpression phenotypes suggests the tight regulation of this RTK-GRAP-Ras signaling pathway where too much or too little GRAP results in sensory dysfunction. To further support an important role of GRAP in hearing, mutations in the gene coding for SOS1, a protein that is known to interact with GRAP, as well as in other members of the Ras signaling lead to Noonan and related syndromes that include sensorineural HL as a finding (33–35).
In conclusion, our paper unveils a necessary role of GRAP in hearing. Its localization to the neuronal tissue of the hearing organ in multiple species suggests a conserved role in the transmission of electrical signals of hearing to the brain.
Materials and Methods
More details of Materials and Methods are in the SI Appendix.
Human Subjects.
This paper was approved by the University of Miami Institutional Review Board and the Ankara University Medical School Ethics Committee (Turkey). A signed informed-consent form was obtained from each participant or, in the case of a minor, from the parents. Detailed past medical histories and thorough physical examinations including otoscopy and ophthalmoscopy revealed no abnormalities, and electrocardiograms were unremarkable.
DNA Sequencing and Bioinformatics.
Exome and genome sequencing were performed on the probands of each family by using a previously published protocol (36, 37). Variants were filtered with minor allele frequency thresholds of 0.005 for recessive and 0.0005 for dominant variants as recommended (38). We used GERP > 2 for conservation (39). We also filtered variants by using the criteria of the combined annotation-dependent depletion (CADD) score > 20 (40), the deep annotation-dependent neural network (DANN) score > 0.95 (41), and they were damaging for Provean (provean.jcvi.org/index.php) and FATHMM (fathmm.biocompute.org.uk/) for missense variants. XHMM and Conifer were used for the copy number variant (CNV) detection with exome data (42); CNVs were called using a CNVnator with genome data (43). Sanger sequencing was used for confirmation and cosegregation of the variant. Homozygous runs were detected from exome data and were used during the filtering of variants (SI Appendix, Tables S2 and S3).
Mouse Studies.
WT C57BL/6 mice were bred and maintained at the University of Miami. All procedures were approved by the University of Miami Institutional Animal Care and followed the National Institutes of Health (NIH) Guidelines, “Using Animals in Intramural Research” (44). Cochlear expression of Grap was checked in embryos of 17.5 dpc and P15 via RT-PCR. Immunofluoresence was performed on P0 using a goat anti-GRAP polyclonal antibody (ab9703; Abcam) as the primary antibody.
Drosophila Studies.
The following fly strains were used in the studies: drk14−7 (27622), drk6 (27623), drke0A (5691), actin-GAL4, FRT42Diso, eyFLP; FRT42D, w+, and cl−, obtained from Bloomington Drosophila Stock Center; drkΔP24 (named as drkΔ in this paper) from E. Skoulakis’s laboratory. All drk alleles were normalized to the w− background and balanced with the CyO, KrGFP chromosome. For mosaic analysis, drkΔ was recombined with the FRT42Diso chromosome. Climbing behavior was measured as previously described (45). For immunofluorescence studies in Drosophila, the following primary and secondary antibodies were used in this paper: anti-drk (from E. Skoulakis’s laboratory), anti-Brp (DSHB, AB_2314866), anti-Csp (DSHB AB_528183), anti-synapsin (DSHB, AB_2313867), Cy5 conjugated anti-HRP (123175021; Jackson ImmunoLab), Alexa 546 conjugated phalloidin (A22283; ThermoFisher Scientific), and Cy5 conjugated anti-HRP and secondary antibodies conjugated to Alexa 488/568/647 (ThermoFisher Scientific).
Supplementary Material
Acknowledgments
This paper was supported by NIH Grants R01DC009645 and R01DC012836 (to M.T.), the Dr. John T. Macdonald Foundation (to C.L.), the Lois Pope LIFE Fellows Program (to C.L., Y.Z., and T.G.M.), NIH Grant R21GM119018 (to R.G.Z.), by Taishan Scholar Project (Shandong Province, People’s Republic of China) (to R.G.Z.), and by funds from the Sylvester Comprehensive Cancer Center (to A.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810951116/-/DCSupplemental.
References
- 1.Morton CC, Nance WE. Newborn hearing screening–A silent revolution. N Engl J Med. 2006;354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 2.Dror AA, Avraham KB. Hearing impairment: A panoply of genes and functions. Neuron. 2010;68:293–308. doi: 10.1016/j.neuron.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Korver AM, et al. Congenital hearing loss. Nat Rev Dis Primers. 2017;3:16094. doi: 10.1038/nrdp.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown SD, Hardisty-Hughes RE, Mburu P. Quiet as a mouse: Dissecting the molecular and genetic basis of hearing. Nat Rev Genet. 2008;9:277–290. doi: 10.1038/nrg2309. [DOI] [PubMed] [Google Scholar]
- 5.Boekhoff-Falk G. Hearing in Drosophila: Development of Johnston’s organ and emerging parallels to vertebrate ear development. Dev Dyn. 2005;232:550–558. doi: 10.1002/dvdy.20207. [DOI] [PubMed] [Google Scholar]
- 6.Lewis MA, Steel KP. A cornucopia of candidates for deafness. Cell. 2012;150:879–881. doi: 10.1016/j.cell.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Senthilan PR, et al. Drosophila auditory organ genes and genetic hearing defects. Cell. 2012;150:1042–1054. doi: 10.1016/j.cell.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 8.Feng GS, et al. Grap is a novel SH3-SH2-SH3 adaptor protein that couples tyrosine kinases to the Ras pathway. J Biol Chem. 1996;271:12129–12132. doi: 10.1074/jbc.271.21.12129. [DOI] [PubMed] [Google Scholar]
- 9.Clark SG, Stern MJ, Horvitz HR. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- 10.Olivier JP, et al. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993;73:179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- 11.Trüb T, Frantz JD, Miyazaki M, Band H, Shoelson SE. The role of a lymphoid-restricted, Grb2-like SH3-SH2-SH3 protein in T cell receptor signaling. J Biol Chem. 1997;272:894–902. doi: 10.1074/jbc.272.2.894. [DOI] [PubMed] [Google Scholar]
- 12.Yorozu S, et al. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458:201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamikouchi A, et al. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 14.Simon MA, Bowtell DD, Dodson GS, Laverty TR, Rubin GM. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991;67:701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- 15.Hamanaka Y, Meinertzhagen IA. Immunocytochemical localization of synaptic proteins to photoreceptor synapses of Drosophila melanogaster. J Comp Neurol. 2010;518:1133–1155. doi: 10.1002/cne.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasin A, et al. Synapsin regulates activity-dependent outgrowth of synaptic boutons at the Drosophila neuromuscular junction. J Neurosci. 2014;34:10554–10563. doi: 10.1523/JNEUROSCI.5074-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, et al. TRPA channels distinguish gravity sensing from hearing in Johnston’s organ. Proc Natl Acad Sci USA. 2009;106:13606–13611. doi: 10.1073/pnas.0906377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, et al. The E3 ligase Ubr3 regulates Usher syndrome and MYH9 disorder proteins in the auditory organs of Drosophila and mammals. eLife. 2016;5:e15258. doi: 10.7554/eLife.15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boekhoff-Falk G, Eberl DF. The Drosophila auditory system. Wiley Interdiscip Rev Dev Biol. 2014;3:179–191. doi: 10.1002/wdev.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy M. Unraveling the auditory system of Drosophila. Curr Opin Neurobiol. 2010;20:281–287. doi: 10.1016/j.conb.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- 22.Skolnik EY, et al. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993;260:1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- 23.Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 24.Simon MA, Dodson GS, Rubin GM. An SH3-SH2-SH3 protein is required for p21Ras1 activation and binds to sevenless and Sos proteins in vitro. Cell. 1993;73:169–177. doi: 10.1016/0092-8674(93)90169-q. [DOI] [PubMed] [Google Scholar]
- 25.Shen R, et al. Grap negatively regulates T-cell receptor-elicited lymphocyte proliferation and interleukin-2 induction. Mol Cell Biol. 2002;22:3230–3236. doi: 10.1128/MCB.22.10.3230-3236.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanshylla K, et al. Grb2 and GRAP connect the B cell antigen receptor to Erk MAP kinase activation in human B cells. Sci Rep. 2018;8:4244. doi: 10.1038/s41598-018-22544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bibel M, Barde YA. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 28.Pirvola U, et al. Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hear Res. 1994;75:131–144. doi: 10.1016/0378-5955(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 29.Bianchi LM, et al. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development. 1996;122:1965–1973. doi: 10.1242/dev.122.6.1965. [DOI] [PubMed] [Google Scholar]
- 30.Fritzsch B, Silos-Santiago I, Bianchi LM, Fariñas I. The role of neurotrophic factors in regulating the development of inner ear innervation. Trends Neurosci. 1997;20:159–164. doi: 10.1016/s0166-2236(96)01007-7. [DOI] [PubMed] [Google Scholar]
- 31.Fritzsch B, Silos-Santiago I, Bianchi LM, Farinas I. Effects of neurotrophin and neurotrophin receptor disruption on the afferent inner ear innervation. Semin Cell Dev Biol. 1997;8:277–284. [PubMed] [Google Scholar]
- 32.Mullen LM, et al. Ras/p38 and PI3K/Akt but not Mek/Erk signaling mediate BDNF-induced neurite formation on neonatal cochlear spiral ganglion explants. Brain Res. 2012;1430:25–34. doi: 10.1016/j.brainres.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bademci G, et al. Variations in multiple syndromic deafness genes mimic non-syndromic hearing loss. Sci Rep. 2016;6:31622. doi: 10.1038/srep31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarkozy A, Digilio MC, Dallapiccola B. Leopard syndrome. Orphanet J Rare Dis. 2008;3:13. doi: 10.1186/1750-1172-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Trier DC, et al. External ear anomalies and hearing impairment in Noonan Syndrome. Int J Pediatr Otorhinolaryngol. 2015;79:874–878. doi: 10.1016/j.ijporl.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Bademci G, et al. Comprehensive analysis via exome sequencing uncovers genetic etiology in autosomal recessive nonsyndromic deafness in a large multiethnic cohort. Genet Med. 2016;18:364–371. doi: 10.1038/gim.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowling KM, et al. Genomic diagnosis for children with intellectual disability and/or developmental delay. Genome Med. 2017;9:43. doi: 10.1186/s13073-017-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shearer AE, et al. Utilizing ethnic-specific differences in minor allele frequency to recategorize reported pathogenic deafness variants. Am J Hum Genet. 2014;95:445–453. doi: 10.1016/j.ajhg.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper GM, et al. NISC Comparative Sequencing Program Distribution and intensity of constraint in mammalian genomic sequence. Genome Res. 2005;15:901–913. doi: 10.1101/gr.3577405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kircher M, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quang D, Chen Y, Xie X. DANN: A deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics. 2015;31:761–763. doi: 10.1093/bioinformatics/btu703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bademci G, et al. Identification of copy number variants through whole-exome sequencing in autosomal recessive nonsyndromic hearing loss. Genet Test Mol Biomarkers. 2014;18:658–661. doi: 10.1089/gtmb.2014.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abyzov A, Urban AE, Snyder M, Gerstein M. CNVnator: An approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res. 2011;21:974–984. doi: 10.1101/gr.114876.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
- 45.Li C, et al. Spermine synthase deficiency causes lysosomal dysfunction and oxidative stress in models of Snyder-Robinson syndrome. Nat Commun. 2017;8:1257. doi: 10.1038/s41467-017-01289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.