Abstract
Objectives
The aim of this study was to compare the effect of intra-rectal administration of lidocaine gel alone versus lidocaine gel plus topical fentanyl on pain reduction in prostate biopsy.
Methods
In a double-blind randomized clinical trial, 96 patients who met the inclusion criteria were randomly assigned into two groups. 1) The treatment group: Lidocaine gel (2%) 50 g and 2) the intervention group: Lidocaine gel (2%) 50 g and fentanyl gel 50 µg. During the prostate biopsy, the VAS score was recorded. Blood pressure, heart rate, and patient level of consciousness were also analyzed.
Results
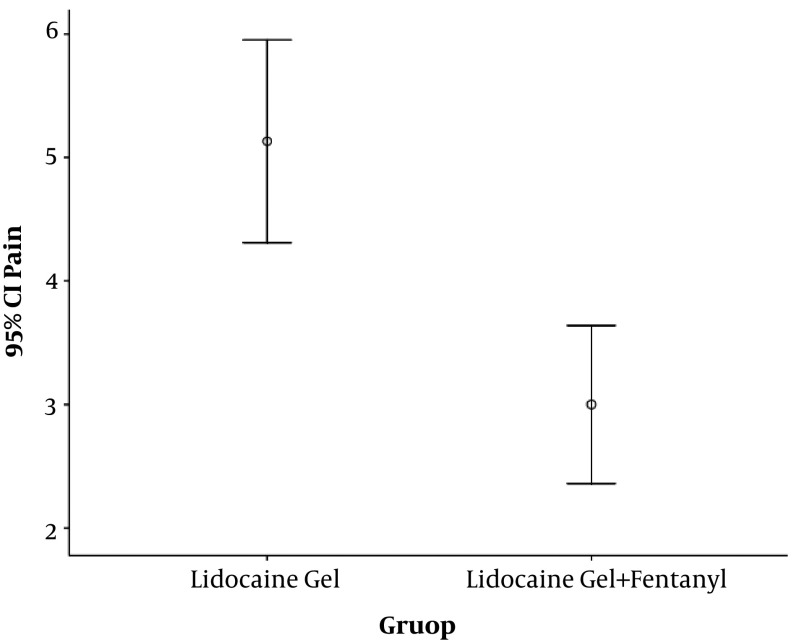
The mean VAS score was 5.1 ± 2 and 3.0 ± 2, which was lower in the intervention group (P value < 0.001). In terms of consciousness after biopsy, there was no difference between the two groups (P value = 0.358). There was no difference between the groups in terms of mean blood pressure and heart rate before and during the prostate biopsy. Finally, in terms of consciousness after the prostate biopsy, there was no difference between the current treatment and intervention groups.
Conclusions
The combination of lidocaine gel and fentanyl with a dose of 50 µg has a significant effect on reducing the pain associated with prostate biopsy in comparison with lidocaine gel alone. The antinociceptive effect of the above regimens is not associated with hemodynamic changes and changes in patients' consciousness.
Keywords: Analgesia, Fentanyl, Lidocaine, Pain, Prostate Biopsy
1. Background
Prostate cancer is the most common cancer in men and each year, 700,000 new patients are diagnosed but susceptibility is not well known (1). An algorithm, primarily based on PSA kinetics for practical use in the continuing care of these patients, has been presented by Wilkinson et al. (2). Early detection of this cancer is the most important step in treatment (3). PSA test is the first step in screening (4); however, the prostate biopsy (under the guidance of ultrasound through the rectum) is the most sensitive diagnostic procedure that urologists do in the next stage. During a biopsy, attention to the prevention of pain is important; so, three common methods are generally used: (1) General anesthesia, (2) topical anesthesia by injection, and (3) topical anesthesia by the gel. The first method requires the presence of anesthesiologists in hospitals. This group of patients may experience adequate analgesia (5, 6) but it is more costly for the patient. The second method has lower costs for the patients; however, it requires injection and thus, is an invasive procedure. The third method has the benefits of the second method, plus that there is no injection, and it is non-invasive and simple. However, patients often do not have enough analgesia. In the present study, to further reduce pain during and after biopsy, the third method was done using intrarectal lidocaine gel with and without fentanyl. Fentanyl is an opiate drug that has local absorption. In previous studies, the effect of lidocaine gel alone or in combination with some anesthetics, in the form of both cream and injection, has been evaluated and confirmed (7-9).
2. Objectives
So far, no study has been done to evaluate the synergistic effects of lidocaine gel with fentanyl or its pharmaceutical derivatives, which was the aim of this study.
3. Methods
This randomized controlled clinical trial was approved by the Ethics Board Committee of the Anesthesiology Department of Tehran University of Medical Sciences (TUMS) and registered at the Iranian Registry of Clinical Trials from 5/21/2016 to 02/19/2017. We obtained informed consent from the patients. The study population included patients with suspected prostate cancer who needed a prostate biopsy and had the inclusion criteria. The patients undergoing prostate biopsy (high PSA or abnormal rectal examination) and weighing at least 40 kg (88 pounds) were included in the study. The exclusion criteria were uncontrolled high blood pressure, contraindication of local anesthetic drugs, the risk of heart, kidney, and hepatic diseases, and addiction.
3.1. Procedure
In a double-blind randomized clinical trial, patients who had the inclusion criteria were randomly divided into two groups using permuted block (block size 4) randomization. For the treatment group, lidocaine gel (2%) 50 g and for the intervention group, lidocaine gel (2%) 50 g plus fentanyl 50 µg were applied. In the current treatment group, rectal lidocaine gel and in the intervention group, lidocaine gel and fentanyl were administered. In order to facilitate the entry of syringe administration, 5 mL of 2% lidocaine gel were applied around the sphincter in advance. In the intervention group, these drugs were administered separately. The purpose of administrating the drugs separately was to guarantee the absorption of fentanyl (which was applied as only one mL in 50 g lidocaine gel) to the mucus of the rectum. As a result, lidocaine gel and fentanyl were not mixed. If so, the amount of fentanyl absorbed by the mucus of the rectum would be unclear. After filling the syringe with lidocaine gel, the syringe was kept ahead, while 3 mL of the tip of the syringe was air. Then, 50 µg of fentanyl were added.
Since lidocaine was in the form of gel and fentanyl was liquid, these two drugs did not mix with each other and the position of liquid fentanyl was above the gel. While the patient was in the lateral position, the head of the syringe was turned to a horizontal state. The tip of the syringe was passed through the anus and when it entered the rectum, the administration of the contents of the syringe was done. As fentanyl was in the tip of the syringe, fentanyl entered the rectum first, followed by the lidocaine gel. We ensured that fentanyl was adjacent to the rectum mucosa because it was not mixed with the lidocaine gel. Since rectum mucosa, unlike the skin, has the ability to absorb fat-soluble liquids such as fentanyl and because the rectum mucosa is close to the prostate blood flow, fentanyl was absorbed to the prostate capsule rapidly. It should be noted that if analgesia was not sufficient during the biopsy, patients received IV analgesics. After the insertion, patients in both groups underwent biopsy. Prostate biopsy was done by the person who was unaware of the prescribed solution. During and after the biopsy, the VAS score, the mean blood pressure, and the heart rate were measured. After the biopsy, patients’ consciousness was measured and recorded via Modified Criteria for Determination of Discharge to home. In this criterion, “fully awake” scores two points and “arousable” scores one point.
During the biopsy, blood pressure and heart rate were recorded. During and 30 minutes after the biopsy, the VAS score was measured and recorded.
3.2. Data Analysis
In order to report the quantitative and qualitative variables, mean/standard deviation and percentage of classes were used, respectively. Following the confirmation of the normality of continuous variables using the Kolmogorov-Smirnov test, the t-test was used to compare the quantitative variables. The Chi-square test or Fisher exact test was used to determine the association between the qualitative variables. To analyze the data, SPSS version 20 software was used (statistical analysis was performed using the SPSS version 20 software). A P value ≤ 0.05 was considered statistically significant.
4. Results
After applying the exclusion criteria, 48 patients received lidocaine gel and 48 patients received lidocaine gel plus 50 µg of topical fentanyl. As demonstrated in Table 1, no significant difference was observed in baseline characteristics between the two groups.
Table 1. Baseline Characteristicsa.
| Variables | Age (Years) | Weight (kg) | Volume of the Prostate (G) | PSA (Ng/Dl) | Duration of the Procedureb |
|---|---|---|---|---|---|
| Lidocaine gel | 66.0 ± 9 | 73.6 ± 12 | 52.6 ± 12 | 22.2 ± 28 | 53.7 ± 22 |
| Lidocaine gel + topical fentanyl | 68.2 ± 10 | 71.0 ± 10 | 60.3 ± 23 | 20.8 ± 24 | 57.8 ± 28 |
| P value | 0.317 | 0.304 | 0.076 | 0.816 | 0.484 |
aValues are presented as mean (+SD).
bMean duration from gel insertion to the biopsy (min).
The mean (+SD) VAS score was 5.1 ± 2 in the lidocaine group and 3.0 ± 2 in the lidocaine plus fentanyl group (Figure 1). A significant difference was observed between the two groups (P value < 0.001). Before administration, the mean (+SD) systolic pressure was 153.26 ± 24.09 and 155.13 ± 27.90 mmHg, respectively (P value = 0.756). The mean (+SD) diastolic pressure was 90.16 ± 13.69 and 90.71 ± 16.45 mmHg, respectively (P value = 0.874). During biopsies, the mean (+SD) systolic pressure was 154.9 ± 28 and 149.9 ± 25 mmHg (P value = 0.425). The mean (+SD) diastolic pressure during biopsy was 93.1 ± 16 and 89.3 ± 15 mmHg (P value = 0.319). The mean (+SD) heart rate before administration was 75.4 ± 14 and 80.1 ± 13 beats/min (P value = 0.151) and during the prostate biopsy, it was 77.6 ± 15 and 81.2 ± 15 beats/min (P value = 0.309). Finally, in terms of consciousness after a prostate biopsy, there was no difference between the current treatment and intervention groups. The frequency of the patients with level one of consciousness was 10.5% and 2.6%, respectively, and the frequency with level two of consciousness was 89.5% and 97.4%, respectively (P value = 0.358).
Figure 1. The mean pain score (VAS).
5. Discussion
The main purpose of perioperative pain control is to provide an adequate comfort level and acceptable side effects for patients. Effective postoperative analgesia improves patients’ outcomes as observed by early ambulation, decreased side effects, and reduced incidence of postoperative chronic pain (10). There are several publications addressing that the successful use of systemic lidocaine (administered as a patch or intravenously) could prevent the development of some chronic post-surgical pain syndromes (7). In this study, we showed that lidocaine gel in combination with topical fentanyl was associated with more improvements in the pain from prostate biopsies. During prostate biopsies, our results showed no significant difference in hemodynamic variables.
Regarding the combination of an analgesic drug and lidocaine gel, few studies are available. Although various studies have been conducted on the effectiveness of lidocaine gel on pain reduction from biopsy, all emphasized the insufficiency of this method. It should be noted that the peripheral nerve block is more effective in pain reduction than the use of lidocaine gel or lidocaine gel combined with other drugs. The combination of gel and periprostatic nerve block provides better pain control than the two modalities alone (8, 9). However, due to the side effects in the peripheral nerve block, especially in cases of increased risk of local infection, the use of gel could be associated with high patient satisfaction. Recent studies have shown the effect of lidocaine on various forms of pain relief from the biopsy. Ates et al. reported that perianal intrarectal application of 10 mL 2% lidocaine gel improves the severity of biopsy pain (11). Imani et al. reported that the combination of three drugs, lidocaine gel, diltiazem, and meperidine, was associated with a significant reduction in pain during the prostate biopsy (12). In this study, the amount of lidocaine gel was 50 mL. Cormio reported that noninfiltrative anesthetics were safe, easy-to-administer, and well accepted by patients (11). In the study of Sataa et al., they reported intrarectal lidocaine application and apical periprostatic nerve block are the safe techniques that significantly reduce pain (6). As 50 µg fentanyl is not a high amount, especially in the form of trans-mucosal, we suggest administering 50 µg of fentanyl for future studies. A sensitive and specific method was developed for the quantitative analysis of topical fentanyl (13).
5.1. Conclusion
Lidocaine gel alone and in combination with topical fentanyl is associated with a reduction in the pain from prostate biopsies, but this reduction was higher in lidocaine combined with fentanyl.
Acknowledgments
We are indebted to the Research and Development Center of Sina Hospital for their support in statistics, and Mr. Mahdi Alijani, MA, for data collection. The authors would like to thank Mrs. Bita Pourmand (Urology Research Center) for editing the manuscript.
Footnotes
Ethical Considerations: IRCT registration number: IRCT201612013773N17.
Financial Disclosure: None declared.
Funding/Support: None declared.
Contributor Information
Farsad Imani, Email: imanifar@tums.ac.ir.
Mohammadreza Khajavi, Email: khajavim@tums.ac.ir.
Tayeb Gavili, Email: taiebegavli@gmail.com.
Pejman Pourfakhr, Email: pourfakhr@razi.tums.ac.ir.
Reza Shariat Moharari, Email: moharari@tums.ac.ir.
Farhad Etezadi, Email: etezadi@tums.ac.ir.
Seyed Reza Hosseini, Email: srhoseini@tums.ac.ir.
References
- 1.Fleshner N, Zlotta AR. Prostate cancer prevention: Past, present, and future. Cancer. 2007;110(9):1889–99. doi: 10.1002/cncr.23009. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson AN, Brundage MD, Siemens R. Approach to primary care follow-up of patients with prostate cancer. Can Fam Phys. 2008;54(2):204–10. [PMC free article] [PubMed] [Google Scholar]
- 3.Kalish LA, McDougal WS, McKinlay JB. Family history and the risk of prostate cancer. Urology. 2000;56(5):803–6. doi: 10.1016/S0090-4295(00)00780-9. [DOI] [PubMed] [Google Scholar]
- 4.Alacreu JMA, Rosales SN, Alba AB, Furio FE, Martinez FM, Cruz JFJ. PSA and hK2 in the diagnosis of prostate cancer. Actas Urol Esp. 2008;32(6):575–88. doi: 10.1016/S0210-4806(08)73891-9. [DOI] [PubMed] [Google Scholar]
- 5.Tobias-Machado M, Verotti MJ, Aragao AJ, Rodrigues AO, Borrelli M, Wroclawski ER. Prospective randomized controlled trial comparing three different ways of anesthesia in transrectal ultrasound-guided prostate biopsy. Int Braz J Urol. 2006;32(2):172–9. doi: 10.1590/s1677-55382006000200007. discussion 179-80. [DOI] [PubMed] [Google Scholar]
- 6.Sataa S, Ramzi M, Mohamed C, Imed BS, Sami BR, Ghassen H, et al. [Local anesthesia in transrectal ultrasound-guided prostate biopsy: Apical periprostatic nerve block versus endorectal lidocaine gel. A randomized controlled trial of 100 patients.]. Tunis Med. 2010;88(4):217–22. (Fre). [PubMed] [Google Scholar]
- 7.Yousefshahi F, Predescu O, Francisco Asenjo J. The efficacy of systemic lidocaine in the management of chronic pain: A literature review. Anesth Pain Med. 2017;7(3):e44732. doi: 10.5812/aapm.44732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannarini G, Autorino R, Valent F, Mogorovich A, Manassero F, De Maria M, et al. Combination of perianal-intrarectal lidocaine-prilocaine cream and periprostatic nerve block for pain control during transrectal ultrasound guided prostate biopsy: A randomized, controlled trial. J Urol. 2009;181(2):585–91. doi: 10.1016/j.juro.2008.10.002. discussion 591-3. [DOI] [PubMed] [Google Scholar]
- 9.Yun TJ, Lee HJ, Kim SH, Lee SE, Cho JY, Seong CK. Does the intrarectal instillation of lidocaine gel before periprostatic neurovascular bundle block during transrectal ultrasound guided prostate biopsies improve analgesic efficacy? A prospective, randomized trial. J Urol. 2007;178(1):103–6. doi: 10.1016/j.juro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 10.Imani F. Postoperative pain management. Anesth Pain Med. 2011;1(1):6–7. doi: 10.5812/kowsar.22287523.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ates F, Dursun F, Malkoc E, Yilmaz O, Soydan H, Sen H, et al. Comparison of two different doses of lidocaine on the pain sensation during transrectal ultrasound-guided prostate biopsy. Turk J Urol. 2016;42(3):145–9. doi: 10.5152/tud.2016.38107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imani F, Moghaddam Y, Shariat Moharari R, Etezadi F, Khajavi MR, Hosseini SR. Intrarectal lidocaine-diltiazem-meperidine gel for transrectal ultrasound guided prostate biopsy. Anesth Pain Med. 2015;5(3):e22568. doi: 10.5812/aapm.5(3)2015.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehdizadeh A, Toliyat T, Rouini MR, Kobarfard F. Introducing a full validated analytical procedure as an official compendial method for fentanyl transdermal patches. DARU J Pharm Sci. 2005;13(2):46–51. [Google Scholar]