Abstract
Background
Osteoarthritis is a progressive multifactorial condition of the musculoskeletal system with major symptoms including pain, loss of function, damage of articular cartilage and other tissues in the affected area. Knee osteoarthritis imposes major individual and social burden, especially with the cost and complexity of surgical interventions. Mesenchymal stem/stromal cells have been indicated as a treatment for degenerative musculoskeletal conditions given their capacity to differentiate into tissues of the musculoskeletal system.
Methods
A systematic search will be conducted in Medline, Embase, Cochrane Library, Scopus and relevant trial databases of English, Japanese, Korean, German, French, Italian, Spanish and Portuguese language papers published or in press to June 2018, with no restrictions on publication year applied. References will be screened and assessed for eligibility by two independent reviewers as per PRISMA guidelines. Cohort, cross-sectional or case controlled studies will be included for the analysis. Data extraction will be conducted using a predefined template and quality of evidence assessed. Statistical summaries and meta-analyses will be performed as necessary.
Discussion
Results will be published in relevant peer-reviewed scientific journals and presented at national or international conferences by the investigators.
Trial registration
The protocol was registered on the PROSPERO international prospective register of systematic reviews prior to commencement, CRD42018091763.
Electronic supplementary material
The online version of this article (10.1186/s13018-019-1070-8) contains supplementary material, which is available to authorized users.
Background
Osteoarthritis (OA) is a progressive condition affecting the articular cartilage and underlying subchondral bone, leading to significant pain and limitations in movement [1]. Knee OA is the most prevalent form of arthritis worldwide and is one of the leading causes of disease and disability amongst aging populations [2]. Recommended treatments for Knee OA can improve symptoms in many patients [3] but do not modify the underlying degeneration of the articular cartilage and alterations in architecture of the surrounding tissue. Emerging treatments derived from cellular products including platelet-rich plasma, bone marrow aspirate and mesenchymal stem/stromal cells (MSCs) have been proposed as minimally invasive alternatives to conventional therapies [4]. In particular, MSCs have been indicated as a promising treatment for degenerative musculoskeletal conditions given their anti-inflammatory properties and capacity to differentiate into osteochondral tissues [4–7].
MSCs can be obtained from the stroma of various tissues, including bone marrow, umbilical cord blood, adipose tissue, peripheral blood and synovium, and expanded in culture to increase yield and enhance desired functional properties [8]. The optimal choice of tissue source is based on considerations of patient safety, ease of access, yield and indications of functional improvements in preclinical and early clinical studies [7]. Evidence obtained in vitro and in animal models indicates that MSCs from different tissue sources differ regarding their cell surface protein expression and capacity to differentiate into specific cell types [9–13]. Thus, it is not currently clear whether the source of cells has a substantial impact on functional or structural outcomes following injection into osteoarthritic knees.
There are a large number of preclinical studies reporting a beneficial effect of MSCs on cartilage degeneration and injury, ranging from mouse [14, 15], rabbit [16–18], guinea pig [19], horse [20], goat [21], to pig models of OA [22, 23]. However, the degree of methodological heterogeneity and limitations in translational relevance for particular animal models of arthritis have complicated interpretations of results [24–26]. Nonetheless, a growing number of clinical studies indicate that mesenchymal stromal cells have the potential to reduce pain; increase joint mobility, walking ability and cartilage/meniscus growth and repair tissue extension over the subchondral bone [5, 27]. In addition, a number of studies have reported no serious adverse events as a result of MSC treatment [5, 28]. However, it is not clear whether these outcomes have been examined consistently across studies.
Considering the aforementioned lack of clarity regarding cell source, methodological factors, clinical translation and outcome measurement, a systematic review is required to synthesize and evaluate the quality of the available evidence regarding the safety and efficacy of mesenchymal stem/stromal cells for knee OA. The primary objective of this review is to establish in patients or animal models of knee osteoarthritis treated with culture-expanded mesenchymal stem/stromal cells from adipose tissue, bone marrow or synovium, with or without adjunct nonoperative therapies, the clinical, structural and functional outcomes of treatment, as well as the incidence and severity of adverse events. The secondary objective of this review is to identify study, measurement and other methodological characteristics associated with treatment outcomes.
Methods
The protocol was registered on the PROSPERO international prospective register of systematic reviews, registration number CRD42018091763. The systematic review follows the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement [29] and protocol (PRISMA-P) guidelines [30].
Eligibility criteria
Relevant characteristics for included studies were determined using the PICOS (Population, Intervention, Comparison, Outcomes, Study Design) framework for formulating the research question and defining eligibility criteria for the literature search [31]. Characteristics for preclinical and clinical studies are presented separately as follows:
Population (inclusion/exclusion criteria)
All animal models of knee osteoarthritis will be considered for review, without exclusions relative to specimen sex, activity level or age. Studies will be excluded where knee (‘stifle joint’) osteoarthritis is secondary to another condition under examination (e.g. joint instability, fracture or other condition). Clinical studies involving patients diagnosed with radiographic evidenced osteoarthritis will be considered for review, without exclusions relative to sex or activity level. Articles will be excluded from analysis if they include paediatric cases (aged under 18 years at diagnosis).
Intervention
Studies will be included if they involve the use of culture-expanded, mesenchymal stem/stromal cells from any source delivered by intra-articular injection. Studies will be excluded if they report the delivery of cells during surgical procedures or include other cell populations in the injected concentrate.
Comparators
Comparators considered will include placebos, conventional non-operative therapies including steroid injections, exercise and NSAIDs and cases unaffected by knee osteoarthritis.
Outcomes
For preclinical research, studies including outcomes relevant to human osteoarthritis including histological appearance of cartilage and bone, results of noninvasive imaging and measurements of pain and function will be included. Biochemical analyses with unclear relevance to human OA will not be included in the review. For clinical research, studies reporting any outcomes relevant to the efficacy and safety of MSC injection will be included in the analysis. Particular attention will be paid to validated measures of patient-reported outcomes.
Study designs
Observational studies (cohort, cross-sectional and case-controlled prospective or retrospective studies) or randomized controlled trials (RCTs) comparing outcomes of culture-expanded MSC treatment with other modalities at any follow up period will be included. Systematic reviews will be used to source additional primary materials but will not be included in the analysis. The results of meta-analyses will be included as a study in the analysis if they meet the remaining inclusion criteria. English, Japanese, Korean, German, French, Spanish, Italian and Portuguese language papers in publication will be included, with no restrictions on publication year.
Information sources
A systematic search will be conducted in Pubmed, Medline, Embase via Ovid SP, Cochrane Library and Scopus via EBSCO and relevant clinical trials databases of English language papers in publication as of June 2018, with no restrictions on publication year applied. (EBSCO, AMED, CINAHL, EMBASE, Cochrane, LILACS, MEDLINE, PEDro, Scielo, Scopus & Web of Knowledge.) Secondary searching of reference lists of key articles and grey literature will be undertaken in order to identify any additional studies potentially missed in electronic search. Active researchers in the field will be contacted to ensure relevant references have been captured.
Search strategy
To permit the search to return other primary studies that were not included to the published reviews, medical subject headings (MeSH) terms and keywords such as systematic review, review and meta-analysis will be excluded. The following are the main key domains: (1) anatomical region, (2) pathology and (3) intervention (Fig. 1). Keywords within concept areas will be mutually inclusive (via ‘OR’ operator) and will be combined with the other key areas using an ‘AND’ operator. The search will be comprised of the following components, which will be performed individually prior to filtering for duplicate records and preliminary analysis:
Anatomical region: knee OR tibia OR femur OR patella OR tibiofemoral OR patellofemoral
Pathology: osteoarthrit* OR arthros* OR gonarthrosis OR arthrit* OR degenerat*
Intervention: (mesenchym* OR stem OR strom*) AND (adipos* OR ‘bone marrow’ OR umbilic* OR MSC) OR allogen* OR autologous AND (cultur* OR ‘culture expanded’)
Fig. 1.
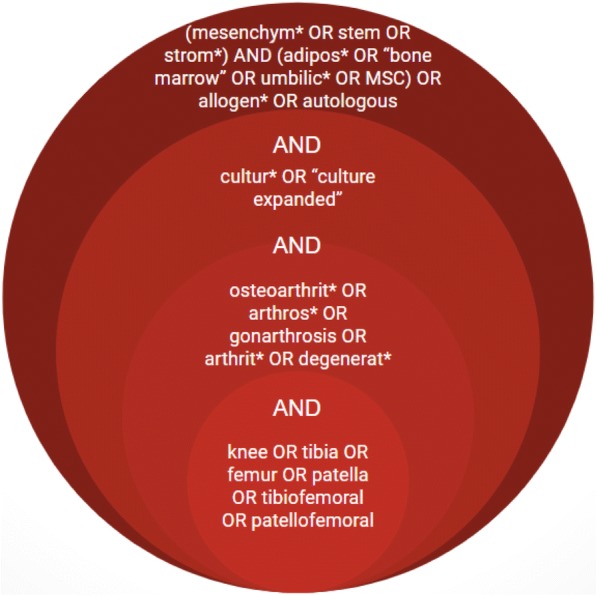
Search strategy for the systematic review
The search strategy will be adjusted for application to other databases as appropriate. Search results will be supplemented by drawing relevant articles from the following:
Reference lists from included studies, prioritizing systematic reviews and meta-analyses
Clinical trial reports from Cochrane Central Register of Controlled Trials, Australia and New Zealand Clinical Trials Register, Clinicaltrials.gov, World Health Organization International Clinical Trials Registry
Study records
The study search and selection process will be based on the four-phase PRISMA flow process [32] for identification, screening, assessment of eligibility and inclusion of studies for the systematic review. A web-based bibliographic software package (Paperpile LLC, Vienna, Austria) will be used for data management. Citations and abstracts identified during the study search will be imported to the bibliographic software and duplicates removed. The study selection process will be performed independently by two reviewers. Title and abstract screening will be performed and full text files will be retrieved and uploaded to the reference software. Eligible studies will be identified for inclusion in the review. Data extracted and synthesized by the two independent reviewers will be the following: author names, publication years, design of the included primary studies, inclusion criteria for primary studies, group intervention and comparison of the primary studies, tools used for outcomes assessment, the outcomes of interest and references of the primary studies. Customized forms will be used for assessment of eligibility during the selection process and extraction of data. Consensus for inclusion and data extraction will be established amongst co-authors prior to review commencement, with study eligibility and data extraction forms piloted by each reviewer prior to use. Where agreement for study inclusion or data extraction is unable to be reached by the two reviewers, a third reviewer from the study team will be consulted.
Data items
Study parameters, population characteristics, treatment factors and outcomes will be extracted from included animal and human studies corresponding to the relevant items in Additional file 1.
Outcomes
For preclinical studies, outcomes considered will include clinically relevant outcomes such as mortality, morbidity and adverse events. Structural outcomes considered include results of histological analyses, including grading of pathology according to the Osteoarthritis Research Society International (OARSI) histopathology initiative guidelines for specific animal models [33–37], and other commonly used measures such as the Grading of Recommendations Assessment, Development and Evaluation (HHGS)/Mankin score and its modifications, the O’Driscoll and Pineda scores [38]. Outcomes of noninvasive imaging including cartilage thickness [39], presence of osteosclerotic lesions or intraosseous cysts [40] visible on MRI will also be included for analysis. Functional outcomes considered for analysis will include behavioural and mechanical measures of nociception and gait analysis such as hind paw weight as appropriate to specific species [25].
For clinical studies, outcomes considered for analysis will include clinically relevant outcomes, such as mortality, morbidity and adverse events, classified as per the US Department of Health and Human Services Common Terminology Criteria for Adverse Events [41]. Structural outcomes will include results of arthroscopic evaluation, specifically ratings of severity such as the International Cartilage Repair Society (ICRS) clinical cartilage injury classification system [42, 43], and Oswestry Arthroscopic Score (OAS) [44]. Also considered will be the results of medical imaging, including ratings of x-rays such as the Kellgren-Lawrence (KL) Classification of Osteoarthritis [45] and ratings of pathology via magnetic resonance imaging such as the OMERACT Knee Inflammation MRI Scoring System (KIMRISS) [46], the Boston Leeds Osteoarthritis Knee Score (BLOKS) [47], the MRI Osteoarthritis Knee Score (MOAKS) [48] and the Whole-Organ Magnetic Resonance Imaging Score (WORMS) [49]. Results of histological analyses considered for analysis include grading systems such as the HHGS [50] and the OARSI Cartilage Histopathology Assessment System [51]. Patient-reported outcomes considered for review include validated measures of treatment response [52, 53], including measures of knee function, pain, quality of life and patient satisfaction, such as the Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC) [54], the Knee injury and Osteoarthritis Outcome Score (KOOS) [55], Knee Pain Scale (KPS) [56] and visual analogue scales (VAS). Objective functional outcomes including strength, range of motion, locomotion, gait and proprioception will also be examined if reported in included studies.
Risk of bias
The Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias tool will be applied to pre-clinical (animal) studies [57]. This is an assessment tool adapted from the Cochrane risk of bias tool for randomized controlled trials with human participants [58] and the two tools display significant overlap. Independent scoring of risk of bias for included studies will be performed by two reviewers, with consensus reached by discussion. The ROBINS-I (‘Risk of Bias In Non-randomized Studies - of Interventions’) tool [59] will be used to assess the observational studies eligible for inclusion. Potential risks will be assessed over seven bias domains: baseline confounding, participant selection, classification of intervention, deviations from intended intervention, missing data, outcomes measurement and reporting [59, 60]. For any randomized trials, the RoB2.0 tool will be used to rate risk of bias [61]. An overall risk of bias judgement will be determined as either low, moderate, serious or critical risk of bias or no information for each specified outcome. Where more than one outcome of an included study is to be assessed, the risk of bias across the seven domains will be repeated for each key outcome, and a risk of bias judgement will be reported for all outcomes.
Data synthesis and meta-analysis
Data synthesis and meta-analysis will be performed separately for clinical and pre-clinical studies, following the guidelines published by Shamseer et al. [30] and Hooijmans et al. [62], respectively. Where the same outcome has been reported across a sufficient number of studies, a quantitative synthesis will be conducted. Data from included studies will be loaded into Review Manager (v5.3) and heterogeneity index (I-squared) will be calculated. Given anticipated heterogeneity amongst studies, a random-effects meta-analysis followed by subgroup analyses will be performed if deemed appropriate. Subgroups chosen for analysis will include the different tissue sources of MSCs (specifically bone marrow vs adipose vs peripheral blood vs synovium) and autologous vs allogeneic cells. Results of meta-analyses will be presented graphically via forest plots, and summary effects will be presented. Publication bias will be assessed using funnel plots with standard error. Where required, mirroring of low sample studies will be used to enable visualization. Where quantitative synthesis is not appropriate, the extracted data will be summarized in tables and narrative interpretation provided, with particular emphasis on methodological heterogeneity and outcome measures.
Confidence in cumulative evidence
The revised and validated methodological index for non-randomized studies (MINORS) criteria [63] will be used to assess the strength of non-randomized studies included for the review. The MINORS tool applies a scoring system across 12 items to assess the methodological and scientific value of studies, with the first 8 items relating to non-comparative studies and all 12 items relevant for comparative studies. Each item will be scored from 0 to 2, with 0 indicating a lack of reporting of the item, 1 indicating inadequate reporting and 2 indicating adequate reporting of the item in the evaluated study with maximum scores for non-comparative and comparative studies of 16 and 24, respectively. The MINORS score for non-randomized studies will be categorized as per 0 < MINORS score < 6 to indicate a very low quality evidence, 6 ≤ MINORS score < 10 to indicate low quality of evidence, 10 ≤ MINORS score < 14 to indicate fair quality of evidence and MINORS score > 15 to indicate good quality of evidence. Where randomized controlled trials are included, in the context of a primary comparison between alternative interventions with respect to the review outcomes, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system will be utilized to assess study quality [58]. For preclinical evidence, the methods proposed by Hooijmans et al. [64] will be used to rate the quality of evidence against the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for animal research [65].
Discussion
The results of this review will be published in relevant scientific journals or presented at national or international conferences (‘publications’) by the Investigators.
Documenting protocol amendments
Protocol amendments and updates will be documented via PROSPERO online register. The nature of the changes made will be recorded, dated and accessible along with the most recent version within the record audit trail under the systematic review protocol registration number CRD42018091763.
Additional file
Screening and core dataset (CDS) template for Systematic Review. (XLSX 466 kb)
Acknowledgments
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study will be made available from the corresponding author on reasonable request upon publication of the systematic review in a peer-reviewed journal.
Abbreviations
- ARRIVE
Animal Research: Reporting of In Vivo Experiments
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HHGS
Histological-Histochemical Grading system
- ICRS
International Cartilage Regeneration and Joint Preservation Society
- KIMRISS
Knee Inflammation MRI Scoring System
- KL
Kellgren-Lawrence
- KOOS
Knee injury and Osteoarthritis Outcome Score
- KPS
Knee Pain Scale
- MeSH
Medical subject headings
- MINORS
Methodological index for non-randomized studies
- MOAKS
MRI Osteoarthritis Knee Score
- MRI
Magnetic resonance imaging
- MSC
Mesenchymal stem/stromal cell
- OA
Osteoarthritis
- OARSI
Osteoarthritis Research Society International
- OAS
Oswestry Arthroscopic Score
- PICOS
Population, Intervention, Comparator, Outcomes, Study design
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International prospective register of systematic reviews
- RCT
Randomized clinical trial
- ROBINS
Risk Of Bias In Non-randomised Studies - of Interventions
- SYRCLE
Systematic Review Centre for Laboratory Animal Experimentation
- VAS
Visual analogue scale
- WOMAC
Western Ontario and McMasters Universities Osteoarthritis Index
- WORMS
Whole-Organ Magnetic Resonance Imaging Score
Authors’ contributions
MHB, CS and WDM contributed to the review concept and study design. FH, NM and WDM provided clinical input for the review. MHB and CS provided input to the development of the search strategies and methodologies for the literature review. CS contributed to the risk assessment and data synthesis strategy and provided statistical expertise. MHB, CS, KH, MM, JL and WDM drafted the review protocol. All authors provided feedback and approved the final protocol. WDM is the guarantor of the review.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Meredith Harrison-Brown, Email: mharrisonbrown@ebma.com.au.
Corey Scholes, Email: cscholes@ebma.com.au.
Kholoud Hafsi, Email: kholoud.hafsi2@gmail.com.
Maimuna Marenah, Email: mcm645@gmail.com.
Jinjie Li, Email: jinjie.li99@gmail.com.
Fadi Hassan, Email: fadi.hassan@nhs.net.
Nicola Maffulli, Email: n.maffulli@qmul.ac.uk.
William D. Murrell, Email: doctormurrell@gmail.com
References
- 1.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42:1–9. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 2.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 3.AAOS. Treatment of osteoarthritis of the knee: evidence-based guideline: American Academy of Orthopaedic Surgeons; 2013. Available from: https://www.aaos.org/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf
- 4.Anz AW, Bapat A, Murrell WD. Concepts in regenerative medicine: past, present, and future in articular cartilage treatment. J Clin Orthop Trauma. 2016;7:137–144. doi: 10.1016/j.jcot.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong L, Zheng L-Z, Qin L, Ho KKW. Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Translat. 2017;9:89–103. doi: 10.1016/j.jot.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorgensen C, Djouad F, Bouffi C, Mrugala D, Noël D. Multipotent mesenchymal stromal cells in articular diseases. Best Pract Res Clin Rheumatol. 2008;22:269–284. doi: 10.1016/j.berh.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Ruetze M, Richter W. Adipose-derived stromal cells for osteoarticular repair: trophic function versus stem cell activity. Expert Rev Mol Med. 2014;16:e9. doi: 10.1017/erm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellows CR, Matta C, Zakany R, Khan IM, Mobasheri A. Adipose, bone marrow and synovial joint-derived mesenchymal stem cells for cartilage repair. Front Genet. 2016;7:213. doi: 10.3389/fgene.2016.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C-Y, Wu X-Y, Tong J-B, Yang X-X, Zhao J-L, Zheng Q-F, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6:55. doi: 10.1186/s13287-015-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, et al. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. 2018;9:168. doi: 10.1186/s13287-018-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, et al. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 13.Reinisch A, Etchart N, Thomas D, Hofmann NA, Fruehwirth M, Sinha S, et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood. 2015;125:249–260. doi: 10.1182/blood-2014-04-572255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64:3604–3613. doi: 10.1002/art.34626. [DOI] [PubMed] [Google Scholar]
- 15.Diekman BO, Wu C-L, Louer CR, Furman BD, Huebner JL, Kraus VB, et al. Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents posttraumatic arthritis. Cell Transplant. 2013;22:1395–1408. doi: 10.3727/096368912X653264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Horie M, Driscoll MD, Sampson HW, Sekiya I, Caroom CT, Prockop DJ, et al. Implantation of allogenic synovial stem cells promotes meniscal regeneration in a rabbit meniscal defect model. J Bone Joint Surg Am. 2012;94:701–712. doi: 10.2106/JBJS.K.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toghraie F, Razmkhah M, Gholipour MA, Faghih Z, Chenari N, Torabi Nezhad S, et al. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med. 2012;15:495–499. [PubMed] [Google Scholar]
- 19.Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, Watanabe S, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14:R31. doi: 10.1186/ar3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27:1675–1680. doi: 10.1002/jor.20933. [DOI] [PubMed] [Google Scholar]
- 21.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 22.Lee KBL, Hui JHP, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cells. 2007;25:2964–2971. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 23.Dutton AQ, Choong PF, Goh JC-H, Lee EH, Hui JHP. Enhancement of meniscal repair in the avascular zone using mesenchymal stem cells in a porcine model. J Bone Joint Surg Br. 2010;92:169–175. doi: 10.1302/0301-620X.92B1.22629. [DOI] [PubMed] [Google Scholar]
- 24.Gregory MH, Capito N, Kuroki K, Stoker AM, Cook JL, Sherman SL. A review of translational animal models for knee osteoarthritis. Arthritis. 2012;2012:764621. doi: 10.1155/2012/764621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little CB, Zaki S. What constitutes an “animal model of osteoarthritis” – the need for consensus? Osteoarthr Cartil. 2012;20:261–267. doi: 10.1016/j.joca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. 2016;11:19. doi: 10.1186/s13018-016-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bansal H, Comella K, Leon J, Verma P, Agrawal D, Koka P, et al. Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med. 2017;15:141. doi: 10.1186/s12967-017-1242-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop Springer. 2016;40:1755–1765. doi: 10.1007/s00264-016-3162-y. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor D, Green S, Higgins J. Defining the review question and developing criteria for including studies. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Chichester, West Sussex: Wiley; 2008. p. 81–94. ISBN: 978-0-470-51845-8.
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook JL, Kuroki K, Visco D, Pelletier J-P, Schulz L, Lafeber FPJG. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the dog. Osteoarthr Cartil. 2010;18(Suppl 3):S66–S79. doi: 10.1016/j.joca.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Laverty S, Girard CA, Williams JM, Hunziker EB, Pritzker KPH. The OARSI histopathology initiative–recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthr Cartil. 2010;18(Suppl 3):S53–S65. doi: 10.1016/j.joca.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Kraus VB, Huebner JL, DeGroot J, Bendele A. The OARSI histopathology initiative–recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthr Cartil. 2010;18(Suppl 3):S35–S52. doi: 10.1016/j.joca.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative–recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr Cartil. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 37.McIlwraith CW, Frisbie DD, Kawcak CE, Fuller CJ, Hurtig M, Cruz A. The OARSI histopathology initiative–recommendations for histological assessments of osteoarthritis in the horse. Osteoarthr Cartil. 2010;18(Suppl 3):S93–105. doi: 10.1016/j.joca.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 38.Rutgers M, van Pelt MJP, Dhert WJA, Creemers LB, Saris DBF. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthr Cartil. 2010;18:12–23. doi: 10.1016/j.joca.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Watson PJ, Carpenter TA, Hall LD, Tyler JA. Cartilage swelling and loss in a spontaneous model of osteoarthritis visualized by magnetic resonance imaging. Osteoarthr Cartil. 1996;4:197–207. doi: 10.1016/S1063-4584(96)80016-1. [DOI] [PubMed] [Google Scholar]
- 40.Nolte-Ernsting CC, Adam G, Bühne M, Prescher A, Günther RW. MRI of degenerative bone marrow lesions in experimental osteoarthritis of canine knee joints. Skeletal Radiol. 1996;25:413–420. doi: 10.1007/s002560050108. [DOI] [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Services . Common terminology criteria for adverse events (CTCAE) 2017. [Google Scholar]
- 42.Brittenberg M, Peterson L. Introduction of an articular cartilage classification. ICRS Newsl. 1998;1:5–8. [Google Scholar]
- 43.Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212–34. [DOI] [PubMed]
- 44.Smith GD, Taylor J, Almqvist KF, Erggelet C, Knutsen G, Garcia Portabella M, et al. Arthroscopic assessment of cartilage repair: a validation study of 2 scoring systems. Arthroscopy. 2005;21:1462–1467. doi: 10.1016/j.arthro.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474:1886–1893. doi: 10.1007/s11999-016-4732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaremko JL, Jeffery D, Buller M, Wichuk S, McDougall D, Lambert RG, et al. Preliminary validation of the knee inflammation MRI scoring system (KIMRISS) for grading bone marrow lesions in osteoarthritis of the knee: data from the osteoarthritis initiative. RMD Open. 2017;3:e000355. doi: 10.1136/rmdopen-2016-000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67:206–211. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 48.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthr Cartil. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. doi: 10.2106/00004623-197153030-00009. [DOI] [PubMed] [Google Scholar]
- 51.Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier J-P, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 52.Escobar A, Gonzalez M, Quintana JM, Vrotsou K, Bilbao A, Herrera-Espiñeira C, et al. Patient acceptable symptom state and OMERACT-OARSI set of responder criteria in joint replacement. Identification of cut-off values. Osteoarthr Cartil. 2012;20:87–92. doi: 10.1016/j.joca.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Pham T, van der Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT-OARSI initiative: osteoarthritis research society international set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr Cartil. 2004;12:389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 55.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and osteoarthritis outcome score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 56.Rejeski WJ, Ettinger WH, Jr, Shumaker S, Heuser MD, James P, Monu J, et al. The evaluation of pain in patients with knee osteoarthritis: the knee pain scale. J Rheumatol. 1995;22:1124–1129. [PubMed] [Google Scholar]
- 57.Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43. [DOI] [PMC free article] [PubMed]
- 58.Ghogomu EAT, Maxwell LJ, Buchbinder R, Rader T, Pardo Pardo J, Johnston RV, et al. Updated method guidelines for cochrane musculoskeletal group systematic reviews and metaanalyses. J Rheumatol. 2014;41:194–205. doi: 10.3899/jrheum.121306. [DOI] [PubMed] [Google Scholar]
- 59.Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sterne J, Higgins J, Elbers R, Reeves B, Development group for ROBINS-I. Risk of bias in non-randomized studies of interventions (ROBINS-I): detailed guidance. 2016. Available from http://www.riskofbias.info. Accessed 12 June 2018.
- 61.Higgins JP, Sterne JA, Savović J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, McKenzie J, Boutron I, Welch V, et al., editors. Cochrane methods. 2016. [Google Scholar]
- 62.Hooijmans CR, IntHout J, Ritskes-Hoitinga M, Rovers MM. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J. 2014;55:418–426. doi: 10.1093/ilar/ilu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slim K, Nini E, Forestier D, Kwiatkowski F. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 64.Hooijmans CR, de Vries RBM, Ritskes-Hoitinga M, Rovers MM, Leeflang MM, IntHout J, et al. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS One. 2018;13:e0187271. doi: 10.1371/journal.pone.0187271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Screening and core dataset (CDS) template for Systematic Review. (XLSX 466 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study will be made available from the corresponding author on reasonable request upon publication of the systematic review in a peer-reviewed journal.


