Abstract
Background
Tobacco abuse is a frequent issue in general practitioners' (GPs') offices, with doctors playing a key role in promoting smoking cessation to their patients. However, not all smokers are ready and willing to give up smoking. Thus, a GP focusing on smoking cessation alone might waste the opportunity to improve his patient’s health by supporting a change in another harmful behaviour pattern. The aim of this study is to determine whether multi-thematic coaching will lead to higher overall health benefits without resulting in a reduced rate of successful smoking cessations, compared with a monothematic smoking cessation approach.
Methods
The study is designed as a two-armed, double-blinded, cluster-randomised trial. GPs will be randomly assigned to the intervention or control group. In the intervention group, GPs will undergo training in patient-centred coaching, shared decision-making and motivational interviewing. The control group will be trained in a state-of-the-art smoking cessation algorithm.
GPs will approach adult cigarette-smoking patients and advise those included according to the GP’s group affiliation. The primary outcome is the between-group difference in the proportion of participants who achieve a beneficial change in at least one of seven different health-related behavioural dimensions, 12 months post baseline. Secondary outcomes include smoking cessation rates and the patients’ self-perceived smoking-related motivation, self-efficacy and planning behaviour. Additionally, covariates describing both GPs and patients will be collected before the start of the intervention, and process outcome measures in compliance with the RE-AIM (Reach Effectiveness Adoption Implementation Maintenance) framework will be recorded during the ongoing study.
Discussion
Tobacco consumption is still highly prevalent in the general population and often goes hand in hand with other behaviour patterns with adverse health effects. This study will add to the literature regarding effective strategies available to GPs to address unhealthy behaviour among their smoking patients beyond mere smoking cessation counselling. The study will also establish a basis for decisions about further promotion and dissemination of the coaching under study.
Trial registration
ISRCTN, ISRCTN38129107. Registered on 2 October 2017.
Electronic supplementary material
The online version of this article (10.1186/s13063-018-3071-z) contains supplementary material, which is available to authorized users.
Keywords: Smoking cessation, Health behaviour, Health promotion, Counselling, Motivational interviewing, Shared decision-making, Patient-centredness, Primary care
Background
Tobacco use is a frequent issue in general practitioners' (GPs') offices, with doctors playing a key role in promoting smoking cessation to their smoking patients [1]. For this purpose, standardised brief interventions involving both counselling and supporting drug therapy have been established and evaluated for the primary care setting over the last years [2, 3].
However, not all smokers are ready and willing to give up smoking. Therefore, up-to-date stop-smoking programmes are based on the transtheoretical model of behaviour change [4] and thus take the patients’ readiness to stop smoking [3, 5] into account.
The patient-centred “Health Coaching” programme [6], developed by the Swiss College for Primary Care Medicine [7], is based on similar principles. Remarkably, this programme does not focus on smoking cessation or any other single health behaviour alone but includes several health-related behavioural dimensions in parallel. Particular emphasis is put on the patient-driven choice of which topic to address. The Health Coaching programme has proved its efficiency and practicability in a clinical trial carried out within the setting of primary care practitioners in eastern Switzerland [8].
Combining state-of-the-art smoking cessation counselling with the Health Coaching approach, exploring patients’ motivation for smoking cessation can be expanded into a more comprehensive exploration of their readiness to improve their health in any prioritised topic, hopefully leading to additional benefits beyond those gained from tobacco abstinence. We assume that training the GPs’ communication skills and incorporating elements of shared decision-making and motivational interviewing into the counselling process will lead to higher overall health benefits for smokers. Importantly, this broader perspective of health behaviour changes should not be paid for in lower smoking cessation rates in comparison with patients exposed to an intervention tailored to smoking cessation alone.
Study hypothesis
We hypothesise that multidimensional and patients’ motivation-driven brief interventions by GPs improve several relevant health outcomes of smokers. We assume that these improvements surpass the beneficial effects of monothematic state-of-the-art smoking cessation counselling, and we further assume that the smoking cessation rates achievable through the novel interventions are not inferior to those known from state-of-the-art smoking cessation counselling.
To verify these hypotheses, we plan to train the GPs in the intervention group in how to coach smokers according to the Health Coaching programme [6], while the control group GPs will undergo training in monothematic smoking cessation counselling [5].
Methods/design
Study design and setting
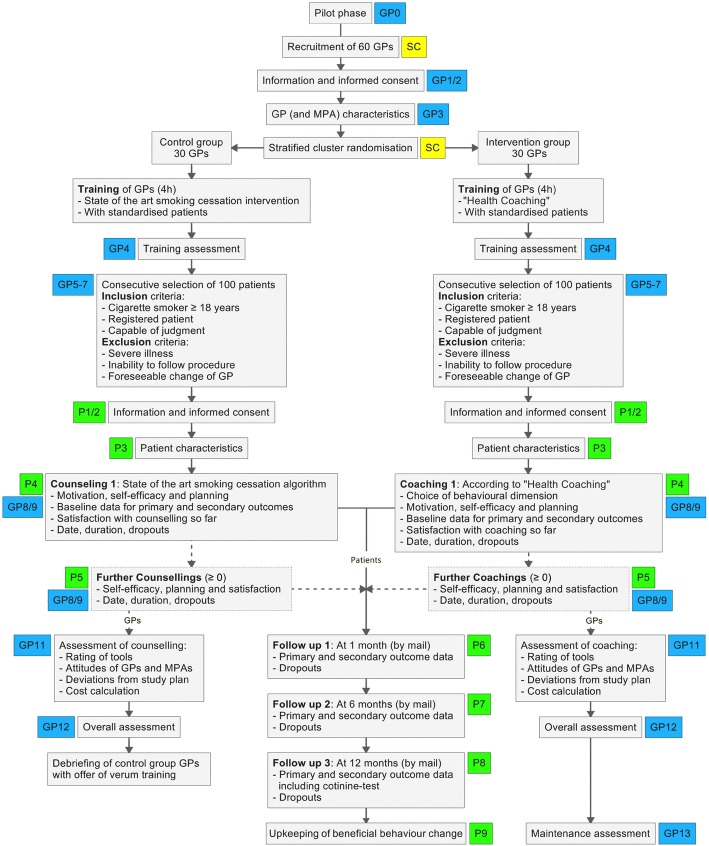
A cluster randomised controlled trial (randomisation on the GP level) will be conducted with 60 German-speaking GPs from the northern part of Switzerland (see Fig. 1 for the study flow chart). The design and methodology of the study are partly based on experiences gained from a previous trial carried out by the last author in eastern Switzerland [8]. The SPIRIT checklist for this protocol can be found in Additional file 1.
Fig. 1.
Study flow chart. Small coloured rectangles represent log files and case report forms used to collect data by the study centre (SC) and from general practitioners (GP) and patients (P). MPA medical assistant (medizinische Praxisassistentin)
Eligibility and recruitment of general practitioners
All interested GPs working in outpatient primary care in the German-speaking part of Switzerland are eligible to take part in the planned study, irrespective of their contract status, their experience in primary care, their age, sex or other specifics or affiliations. Included GPs must not have had any previous training in the Health Coaching programme.
In a first step, all members of an interested network of GPs will be invited through a formal letter from the Institute of Primary Care of the University of Zurich. In case of insufficient response, email remainders will be sent out before other doctors’ networks will be included in the recruitment process, and addresses of potential participants from existing in-house and freely accessible federal databases of primary care providers will be considered.
Cluster randomisation
Participating GPs will be listed chronologically, and GP characteristics collected prior to their allocation to either intervention or control arm. Until their required number is reached (according to the sample size calculation), the GPs will be allocated to the two equally sized study arms in blocks of 14–20 GPs each, by means of a tailored covariate constrained randomisation procedure [9] with an additional constraining criterion regarding potential contamination. In some more detail, a set of sufficiently balanced allocations with regard to the GPs' sex, contract status and practice type will be constructed and then purged from contamination-prone allocations (i.e. from those which put GPs belonging to the same group practice into different study arms), before randomly drawing the final allocation to be used in the study. All GPs from the same group practice will be allocated within the same block.
The block size was chosen to meet recommendations by Carter and Hood [10] and also with a view to training group sizes that can well be handled within the study centre’s resources. To generate the set of balanced allocations, we will use the web-based Shiny Balancer [11], and the additional reduction step will be carried out manually using Excel’s sorting and filtering capabilities. The final random draw will be carried out by a study nurse not involved in the later data analysis.
Patient recruitment and allocation
All GPs will be asked to approach, consecutively and regardless of the reason for the consultation, current cigarette smokers at least 18 years old. By insisting on consecutive targeting of all smokers, we aim to eliminate selection bias very likely to arise if GPs were allowed to select patients whom they deem suitable. In order to include a total of 200 patients, each of the 60 GPs is expected to recruit three to four patients on average.
Patient inclusion criteria:
Current cigarette smoker.
Male or female over 18 years of age.
Registered as patient in the recruiting GP’s patient base.
Capable of judgement with regard to participation in the study.
Signed informed consent after being informed.
Patient exclusion criteria:
Severe general or psychiatric illness (e.g. malignancy, major depressive episode, dementia, etc.).
Inability of the participant to follow the procedures of the study due to other reasons (e.g. language problems).
Foreseeable change of general practitioner within 1 year (e.g. due to planned relocation).
Any participating patient will be assigned to the trial arm of his GP without involving further randomisation, and subsequently a set of baseline characteristics (biological and social traits and particulars of their smoking habits) will be collected from every patient included. Since not all GPs might recruit the exact same number of patients and due to the deterministic allocation of patients, we expect to record—and document—some inevitable imbalance between the trial arms with regard to patient numbers and (patient) baseline characteristics. On the other hand, randomness in the GPs' appointment schedules together with consecutive patient inclusion and coupled with the patients’ blinding with regard to the intervention (see next subsection) will translate into largely randomly composed study clusters. This will prevent extremely imbalanced trial arms.
Blinding
Considering the nature of the interventions, full blinding of GPs and their patients in the strictest sense is not possible. However, in order to achieve the highest possible degree of double-blinding, both GPs and patients will merely be informed that the intervention is aimed at promoting beneficial health-related behaviour changes among smokers, but neither GPs nor patients will be told which of the two interventions (study or control) they provide or receive. Before consenting to participate, the patients will not be offered detailed information about the intervention they will receive, nor will they know details about the intervention in the respective other trial arm during the ongoing study. For those GPs or patients familiar with clinical studies, a minimum amount of related information will be available from registry databases and from the published protocol, which we consider inevitable.
At the end of the study, GPs will be debriefed and those in the control arm will be offered training in Health Coaching too. Ethical approval has been granted for the blinding procedures.
Intervention
Both the study and the control intervention consist of a communication training session for the participating GPs, identical in duration and organisation but different in content. Training sessions will take 4 h and involve standardised patients (i.e. actors posing as patients) and case vignettes tailored to health-related behaviour counselling [12]. The 60 GPs will be split into groups of 7–10, and each group will then be trained within a short interval of time.
The 30 control group GPs will receive training in smoking cessation counselling as put forward by Frei von Tabak [5].
The training of the 30 GPs in the study arm will cover the key elements of the Health Coaching programme, namely patient-centred counselling, shared decision-making, motivational interviewing and the use of validated tools from existing health promotion programmes such as PAPRICA—Physical Activity Promotion in Primary Care [13], Brief Interventions in Patients with Risky Alcohol Consumption [14] and aTavola (a short intervention programme for healthy eating) [15]. Like their counterparts in the control group, the GPs in the intervention group will also be trained in smoking cessation counselling according to Frei von Tabak [5].
The intervention on the patient level will consist of either activating and coaching patients in achieving a beneficial change of their self-selected unhealthy behaviour (study intervention group) or of smoking cessation counselling (control group) in up to three (or occasionally, if necessary, more) sessions. The number of sessions will depend on progress and the needs of the patients and will be decided on by both patients and their physicians. Coaching or counselling sessions can be carried out by medical assistants or nurses provided that they take part in a training course as already described.
Outcome measures
Primary outcome
The primary outcome is the difference between intervention and control group in the proportion of patients with any relevant health-promoting change (as defined in Table 1) in either smoking behaviour, body weight, physical activity, alcohol consumption, stress level, eating habits or another self-chosen health-related behavioural dimension. The primary outcome will be collected (including confirmatory cotinine testing) 12 months after baseline, and also as secondary outcomes at 1 and 6 months.
Table 1.
Definitions of health-relevant behavioural changes
| Behaviour dimension: relevance criterion | Measuring method | References |
|---|---|---|
| Smoking: abstinence or reduction of daily number of cigarettes by ≥ 50% from a baseline of ≥ 15 cigarettes | Self-declaration, confirmatory saliva cotinine test at 12 months for quitters | [18, 27] |
| Body weight: reduction by ≥ 5% if baseline-BMI ≥ 25 kg/m2 | Standardised home measurements | [28–30, 45, 46] |
| Physical activity: increase of MVPA by ≥ 90 min per week or increase of LIPA by ≥ 200 min per week, compared to baseline | Recollection-based self-declaration in questionnaire | [31–33] |
| Alcohol: reduction in number of standard drinks (10 g) per week by ≥ 7 drinks from a baseline of ≥ 14 drinks/week | Recollection-based self-declaration in questionnaire | [35, 36, 47] |
| Stress: reduction in score of the Perceived Stress Scale (PSS-10, German version) by ≥ 5, compared to baseline | Recollection-based self-declaration in validated questionnaire | [38–41] |
| Diet: increase by ≥ 10 in score of adapted MedDietScore questionnaire, compared to baseline | Recollection-based self-declaration in validated questionnaire | [44, 48] |
| Participant’s choice: increase by ≥ 2 levels on a 5 level Likert-type scale (−/0/+/++/+++) | Self-declaration in questionnaire | – |
BMI body mass index, LIPA light-intensity physical activity, MVPA moderate- to vigorous-intensity physical activity
The chosen criteria each reflect the lowest level (cut-off point) of a behavioural change with a proven health benefit. For rationales behind the choice of these levels and a justification for choosing “any change” as the binary primary outcome, see the Discussion section.
Secondary outcomes
Additional secondary outcomes are presented in Table 2. All secondary outcomes will be compiled at 1, 6 and 12 months after baseline from the same data already collected for the primary outcome, with the exception of self-efficacy as well as action and coping planning which are collected during the ongoing counselling on Likert-type scales with five levels.
Table 2.
Secondary outcomes
| Secondary outcome | |
|---|---|
| Smoking cessation rates in intervention and control groups among patients with high intrinsic motivation to stop smoking | |
| Smoking cessation rates in intervention and control groups among all participants | |
| Reduction in number of cigarettes per day, compared to baseline | |
| Weight loss in units of 1 kg, compared to baseline | |
| Increase in physical activity time per week in units of 5 min, compared to baseline | |
| Reduction in number of standard drinks per week, compared to baseline, and number of alcohol-free days per week | |
| Reduction in score of the Perceived Stress Scale (PSS-10, German version), compared to baseline | |
| Increase in score of translated MedDietScore questionnaire, compared to baseline | |
| Patients’ degrees of motivation and, if applicable, confidence to achieve and maintain a change in behaviour (self-efficacy) | |
| If applicable: availability of a plan on when and how to take action (action planning) and existence of a relapse plan (coping planning) |
Additionally, the covariates presented in Table 3 are collected, if applicable.
Table 3.
Covariates
| Covariate | Source |
|---|---|
| Biopsychosocial data of general practitioners (age, gender, experience, type of doctor’s practice, data of medical assistants/nurses) | GP |
| Biopsychosocial data of patients (age, gender, marital status, educational level, smoking status of partner) | Patient |
| Characterisation of smoking behaviour (age at onset of smoking, pack-years, number of cigarettes per day, time to first cigarette after wake up, number of previous attempts to stop smoking) | Patient |
| Participants’ choice of behavioural dimension | GP |
| Number and duration of coaching/counselling sessions (per patient and in total) | GP |
| Person conducting the coaching/counselling (doctor or specifically trained medical assistant) | GP |
| Type of smoking cessation intervention used | GP |
| Dispense of decision aids | GP |
| Involvement of partner or peers into the coaching/counselling process | GP |
| Perceived partner or peer support | Patient |
| Perceived willingness of partner or peers to achieve the same change in behaviour | Patient |
GP general practitioner
Process outcomes
In parallel to the ongoing study, additional data will be collected regarding the implementation, effectiveness and cost–benefit ratio of the intervention with the aim of forming a solid basis for decision about further promotion and dissemination of the Health Coaching programme. The results of the evaluation are expected to lead to more profound insights into the methodology of the intervention, in how to optimally train the multiplicators and into factors influencing the acceptance of the intervention by the target population. Furthermore, the process evaluation comprises an analysis of the costs generated by the intervention.
We chose the RE-AIM framework [16] by Glasgow et al. [17] as a comprehensive concept within which to address these objectives.
Process outcomes (Table 4) will be evaluated based on case report and log files used within the actual trial and also using additional methodology specific to the respective questions: semi-structured interviews, questionnaires, telephone interviews and focus group sessions.
Table 4.
Process outcomes
| Process evaluation outcome | Source |
|---|---|
| Reach: proportion and representativeness of individuals receiving the intervention (individual level) | |
| Rate of smokers among the GP's patients | GP |
| Rate of smokers invited to participate | GP |
| Rate of refusals, with reasons | GP |
| Patient dropout rate, with reasons | GP |
| Patients’ characteristics and representativeness | See covariates |
| Efficacy: success rates of the intervention (including satisfaction outcomes) under study conditions (individual level) | |
| Behaviour change rates | See outcomes |
| Changes in self-efficacy and planning | See outcomes |
| Patients’ current overall satisfaction with coaching programme | Patient |
| Adoption: proportion and representativeness of organisations willing to adopt the intervention, with consideration of enablers and barriers to adoption (organisational level) | |
| Rate of GPs invited to participate | SC |
| Rate of refusals, with reasons | SC |
| GP dropout rate, with reasons | SC |
| GPs' characteristics and representativeness | See covariates |
| Involvement of medical assistants/nurses | See covariates |
| Assistants'/nurses' characteristics | See covariates |
| Assessment of GPs' precognitions, understanding of coaching/counselling concept and increase in knowledge and skills, evaluation/rating of coaching/counselling concept and structure of training, recommendation to colleagues | GP |
| Time required for coaching/counselling | See covariates |
| Rating of coaching/counselling tools in matters of usefulness and manageability, enabling factors and barriers in coaching/counselling as perceived from practical experience | GP |
| Overall assessment: benefit for daily practical work, most crucial aspects (success factors and pitfalls), suggestions for improvement, overall satisfaction | GP |
| Implementation: extent to which the intervention is implemented under real-world conditions—patient adherence (individual level) and adherence of staff to study protocol (organisational level) | |
| Number of coaching/counselling sessions per patient | See covariates |
| GPs'/assistants'/nurses' attitudes, competences and (mental) barriers towards/in/to delivering the interventions | GP |
| Changes in contents or duration of coaching/counselling elements during the ongoing trial | GP |
| Completeness/integrity of data reported by GPs | SC |
| Costs arising from expenditure of coaching time | SC |
| Maintenance: sustainability of intervention over time—relapse rates (individual level) and integration of intervention into institutional routine (organisational level) | |
| Upholding of beneficial behaviour at 12 months after end of follow up | Patient |
| Number of GPs trained in HC until 12 months after end of follow up | HC registry |
| Number/frequency and quality/intensity of coaching over a period of 12 months beyond follow up | GP |
| Response among experts and media coverage | Experts, media |
| Maintenance costs | GP |
GP general practitioner, HC Health Coaching programme, SC study centre
Safety outcomes
Adverse events are defined as any untoward medical occurrences in patients after the intervention and do not necessarily have a causal relationship with study activities. In particular, the following will be considered as adverse events:
Complication of an existing disease.
Onset of new acute illness.
Hospitalisation or death.
Adverse events will have to be recorded by the GPs in the patients’ case report files and reported to the study centre. The study investigators will decide together with the respective GP whether there is a plausible connection between intervention and adverse event.
Follow up
At 1, 6 and 12 months post baseline the patients will report in mail form about their health behaviour status (regarding smoking, weight, activity, alcohol, diet, stress and—if applicable—self-chosen behaviour dimension). Patients claiming to have achieved tobacco abstinence at 12 months after baseline will also be asked to provide a saliva sample for confirmatory cotinine testing.
In addition to a base compensation, the GPs will be rewarded with financial compensation for every patient providing full follow-up data.
Sample size calculations
For sample size calculations with respect to the superiority hypothesis, we assume success rates of 40% in the intervention and 15% in the control group. The 40% assumption is based on results from the preceding study [8] in which one out of two participants declared subjective improvements in their self-chosen behavioural dimension after coaching. The success rate of 15% in the control group is the sum of a 10% smoking cessation rate achievable by state-of-the-art smoking cessation counselling according to Comuz et al. [18] plus an estimated 5% of “spontaneous” effects of smoking cessation counselling on one of the other behavioural dimensions. Using α = 5% (two-sided) and β = 10% we calculated a sample size of 62 participants per equally sized study arm [19] or 2 · 62 / (1 – 0.25) = 166 participating smokers in total after factoring in a dropout rate of 25%. To reflect the correlation structure of the clustered design, an intra-cluster correlation coefficient (ICC) of 4% was assumed, which is slightly higher than corresponding values suggested by Parker et al. [20], in order to account for rare cases of GPs from the same group practice. This assumption is also backed by (unpublished) calculations from the previous study [8] with similar cluster structure and outcome. Correction for the cluster effect increases the sample size to 166 · [1 + (4 – 1) · 0.04] = 186 or roughly 200 patients, assuming that a general practitioner is able to recruit four patients. Finally, allowing for a dropout rate of 15% among general practitioners, 186 / 4 / (1 – 0.15) = 56 (rounded to the next higher even number) or roughly 60 general practitioners must be recruited to detect the expected difference in the primary outcome between the two study arms.
Sample size calculations with respect to the non-inferiority hypothesis require additional assumptions. In the earlier study [8], 12% of smoking or non-smoking GP patients chose smoking cessation as their primary goal. For the present study with smoking participants only, we cautiously assume that in both study arms 25% (i.e. roughly double this number) are highly motivated to give up smoking. Based on Lindson-Hawley et al. [21], we further assume a 40% success rate among the highly motivated. Using a tentative and rather large non-inferiority margin of 10% as in [21] and β = 20%, this results in an uncorrected sample size of 297 highly motivated participants per study arm [22] or 2 · 297 / (1 – 0.25) · [1 + (4 – 1) · 0.04] · 4 = 3550 participants in total after correction for clustering and dropouts. Sample size calculations with regard to smoking cessation rates among all participants lead to even larger samples, not feasible with available resources. Accordingly, we chose “any relevant behaviour change” as the primary outcome and will consider smoking cessation efficacy among the secondary outcomes.
Data collection procedures
Patients will receive detailed written information on the aim of the study. After obtaining their written informed consent, data will then be collected by means of paper case report forms (CRFs). For every patient, a dossier with the different CRFs will be created. The forms will be encoded and the codes stored at each GP’s practice. Decoding will be possible if case-tracking is needed (in case of adverse events). Data transfer from paper to electronic form will be carried out and double-checked independently by different research associates.
Statistical analysis
Baseline characteristics (qualitative and quantitative variables) of GPs and patients will be calculated after recruitment is completed for each arm of the study, with corresponding 95% confidence intervals where applicable.
Crude success rates (i.e. rates of any relevant health-promoting behavioural change and smoking cessation rates) will be compared between intervention and control groups using χ2-tests after completion of follow up. As the main analysis, adjustment for cluster effects will then be performed by (multivariate) hierarchical logistic regression with individuals as the unit of analysis, grouping by GP as the random effect and covariates (including the balancing variables) as independent fixed effects.
Changes in outcome measures that do not meet the criteria for clinical relevance defined in Table 1 will be described and presented graphically (density plots, histograms), compared across study arms using χ2-tests or t-tests and adjusted by random-effects regression models analogous to those used for success rate comparisons, but with the respective behavioural change measures as dependent variables.
All analyses will be conducted following an intention-to-treat (ITT) approach (i.e. outcome data will be obtained from all participants and analysed “as randomised”). Missing values will be replaced by standard multiple imputation (MI) as recommended by Ma et al. [23] for clustered designs with variance inflation factors < 3. Non-responder imputation (NRI; i.e. conservatively treating all missing values as failures) will be used within the scope of sensitivity analyses.
Qualitative information from the process evaluation will be analysed using common coding techniques for qualitative data. A coding tree will be established deductively based on pre-determined domains of interest and refined by inductively adding codes where appropriate. Results will be reported in percentages or categories. Proportions or mean values of different subgroups will be compared using χ2-tests or t-tests.
Timeframe
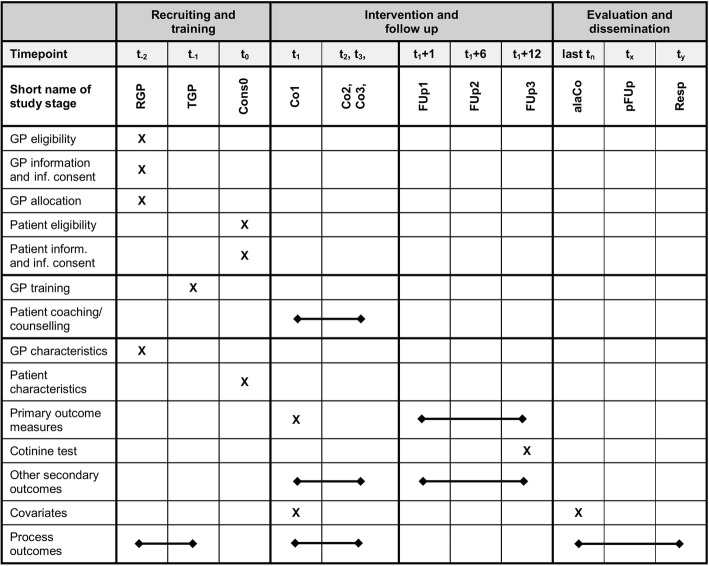
Fig. 2 shows for the SPIRIT diagram. The first GP was recruited in mid-2018. Patients’ eligibility screening and patient inclusion started in the fourth quarter of 2018 with the desired goal of including an average of about one patient per month per GP. Follow up will last for 12 months for each patient.
Fig. 2.
SPIRIT diagram for trial stages of enrolment, intervention and outcome assessment. GP general practitioner, RGP recruiting of GPs, TGP training of GPs, Cons0 consultation 0, Co1–3 coaching/counselling 1–3, FUp1–3 follow up 1–3, alaCo after coaching/counselling of last patient, pFUp post follow up, Resp expert response
Patient informed consent
Previous to study participation, the patients will receive written or verbal information about the content and extent of the planned trial from their individual GPs. In the case of acceptance, they will sign the informed consent form.
Data security/disclosure of original documents
The patient names and all other confidential information fall under medical confidentiality rules and will be treated according to appropriate Federal Data Security Laws. For contact maintenance and case tracking (e.g. in the case of adverse events), the patients’ identities will be known to a study nurse not involved in the analysis of the patient data. The patient names will not be accessible to the scientific study staff.
Discussion
Justification of the binary primary outcome
Any change
To our knowledge, the suggested composite outcome has not been used or validated before. It is largely unknown how—in terms of health benefits—a change in some behaviour dimension A compares with a change in another dimension B, and we know of no standardisation procedure to make sizes of changes comparable over different dimensions. To make matters worse, different behaviour changes are measured on different scales, and health benefits have been associated with absolute changes in some measures but with relative changes in others. We believe that, given these imponderabilities, the binary outcome “any relevant change in any component dimension” keeps the utmost possible intrinsic validity, since, firstly, the validity of the individual relevance criteria has been demonstrated and, secondly, no further assumptions on interaction or comparability of different behaviour changes are needed. Moreover, while using a dichotomous primary outcome measure inevitably incurs some loss of information, the study findings will be all the more meaningful for clinical care, provided that a statistical difference will be found [24]. The loss of information will be compensated for by analysing secondary outcomes.
Time aspects
Evaluating primary and secondary outcomes at 1, 6 and 12 months after baseline will allow capturing both easy-to-achieve early and hard-to-achieve late behavioural changes. The first two points of time were chosen in accordance with Lindson-Hawley et al. [21] and the last two correspond to the Russel Standard [25] for smoking cessation trials. Data collection at three different times will also provide information on the sustainability of beneficial behaviour changes at the level of the study collective, although not at the individual level. Duration of upkeep of beneficial behaviour patterns was not included among the primary or secondary outcomes because we do not know whether, for example, one sustained change is preferable over two different changes of short duration. However, we will assess the persistence of individual successes beyond follow up as part of the process evaluation.
Rationales for the choice of relevance criteria
Smoking
Within the present study, we consider halving the daily number of cigarettes as sufficiently associated with health benefits. We realise that the evidence supporting this criterion is not overwhelming, and there might even be no benefit in terms of mortality at all [26]. On the other hand, the rate of smoking-related cancers or lung cancers was considerably lower even in Tverdal and Bjartveit’s study [26], although not significantly so as in Godtfredsen et al. [27]. The two studies are somewhat contradicting in terms of size and significance of the effect, which suggests that a reduction by 50% might represent the limit of detection of health benefits from smoking reduction.
Body weight
A pro-rata weight loss of 5% is widely accepted as “clinically relevant” or “clinically significant”, see for example Stevens et al. [28], and numerous studies have been carried out using this criterion [29, 30]. Swift et al. [29], for example, demonstrated a significant beneficial effect on insulin levels whereas other cardiovascular risk factors showed short of significant changes in the desirable directions after clinically significant weight loss due to a weight reduction intervention.
Physical activity
Current guidelines recommend a minimum of 150 min of physical activity per week of at least moderate intensity (corresponding to ≥ 7.5 MET∙h). Beneficial health effects have been satisfactorily demonstrated for roughly 60% of this amount, for example by Wen et al. [31]. See also the recommendations of the US Physical Activity Guidelines Advisory Committee [32]. According to both sources, additional health effects have been shown for further activity increases by the same amount. Equivalent amounts or equivalent additional amounts of physical activity can be achieved with similarly beneficial results at lower intensities but with longer duration [33].
The obvious and tempting idea of using tracking devices had to be discarded on the grounds of practicability (distribution and maintenance of the devices), methodological considerations (recording of baseline activity before the first coaching or counselling session) and due to the sheer costs of providing appropriate sensors to all 200 participants. Out of consideration for the participants (who need to document their health behaviour in all seven dimensions) we also decided against relying on diaries to capture the amount of physical activity. This decision is backed by Timperio et al. [34], who point out that the use of physical activity logbooks does not increase estimates of validity of 7-day recall physical activity questionnaires.
Alcohol consumption
The cardioprotective role of alcohol consumption has most probably been overestimated in the past. Recent research, for example, by Rehm et al. [35] suggests a dose–risk relationship with a less pronounced J-shape compared to older results. According to Rehm et al., health risks exceed benefits for any amount of alcohol consumed beyond 10 g per day, irrespective of sex and age. The 2000 WHO guidelines [36] show increasing all-cause mortality estimates for intake classes starting from 10 g/day with class widths of 10 g/day. Our choice of criterion reflects both the cut-off point as well as this magnitude of change, but is based on a weekly assessment as used in most current guidelines on alcohol consumption.
We decided against extending the criterion to cover drink-free days because their benefit is still highly debatable and probably dependent on the total amount consumed [37]. In accordance with measuring physical activity and because the effort was again not deemed reasonable for the patients, we do not intend to use diaries or logbooks that would have to be filled in over several days.
Stress level
The Perceived Stress Scale (PSS-10) [38] was chosen for its widespread recognition and frequent use in psychometric research, for the availability of a free and validated German translation, and for its briefness and practicability. Furthermore, the PSS-10 is one of only few scales for which we were able to find satisfactory data describing quantitatively the score changes after stress relief interventions or the association between changes of the score and health outcomes.
Two relatively small studies by Wiegand et al. [39] and Kirby et al. [40], both carried out in non-clinical community settings and with baseline PSS-10 scores of approximately 24 (scale ranges from 0 to 40), found mean decreases in the total PSS-10 scores by about 6.3–9 after stress management interventions of 14 weeks or 10 days, respectively, and about 4.1–5 in the control groups. Based on these data we consider a score reduction by at least 5 as a relevant change. This choice gets some empirical support from results of validation studies that established standard deviations in the order of our criterion [41] and showed that our criterion roughly corresponds to the mean difference between psychiatric outpatients and the general population [42].
Eating habits
Concerning food intake there is a vast number of short dietary assessment instruments and screeners available [43] but none of them has prevailed as a generally recognised standard to capture overall diet quality in the primary care setting. The ideal tool was expected to cover the patient’s diet as a whole and without including food items unusual in a typical “local” diet. Furthermore, it was required that laypeople would be able to complete it within reasonable time and without expert knowledge, for example, about different nutritional components of various foods. Lastly, and if at all possible, it had to be validated with respect to some quantifiable morbidity or mortality outcome. The MedDietScore [44] meets these requirements best.
Again, we do not intend to use food diaries for similar reasons as mentioned earlier with respect to physical activity and alcohol consumption. Moreover, we will not try and assess eating habits beyond the mere composition of the diet because we do not know of any validated tools to cover both composition and additional aspects.
Strengths and limitations
The pragmatic study design allows for recognising beneficial changes in any health-related behaviour. However, there are some important limitations. The necessity to collect data regarding seven different behaviour dimensions requires a significant effort on the part of the participants. Overstraining their cooperation would most likely result in losses to follow up and poor data quality. In the context of the present study, it is therefore not possible to capture all behaviour changes with the highest desirable objectivity. Even though validated measuring instruments will be used as far as possible, recall and desirability bias as well as interpretation bias cannot fully be ruled out. Moreover, some of the chosen cut-off levels for clinical relevance are somewhat debatable. Lastly, we will not be able to completely avoid the selection of suitable cases either on the level of GPs or on the level of patients, even though the consecutive recruitment of patients will prevent the selection of “good risks” by their GPs up to a certain extent.
Desired impact
The knowledge gained from this study is likely to influence the way GPs deal with harmful behaviour patterns of their patients who smoke. It has the potential to increase care provision for morbidity and mortality risk behaviour beyond mere smoking cessation support. If shown to be effective and feasible, a coaching strategy as set out in the Health Coaching programme and adopted in this study, focusing on shared decision-making and the promotion of the patients’ intrinsic motivation to tackle their individual health problems, could ultimately reduce the high prevalence of risk behaviours in the smoking population.
Trial status
Patient recruitment had not yet started at the time of the first submission in February 2018 and is planned to take part from late 2018 until mid-2019.
Additional file
SPIRIT checklist: recommended items to address in a clinical trial protocol. (PDF 57 KB)
Acknowledgements
The authors would like to thank Georg Bauer, David Fäh and Isabelle Jacot Sadowski for helpful comments regarding the choice of primary outcome criteria.
Funding
The study is funded by the Tobacco-prevention fund TPF of the Swiss Federal Office of Public Health FOPH (Grant order no 17.003152, 7 March 2017). Additional funding is provided by the University and University Hospital Zurich.
Availability of data and materials
Data sharing is not applicable to this article as no datasets have yet been generated or analysed during the current study.
Abbreviations
- CRF
Case report form
- GP
General practitioner
- ICC
Intra-cluster correlation coefficient
Authors’ contributions
TG co-authored the design of the study, obtained ethical approval and drafted the manuscript. OS and TR participated in the design of the study and helped to draft the manuscript. AF, JC and EM-D participated in the design of the study and critically reviewed the manuscript. SN-J is the trial sponsor, conceived of the study, raised funding and helped to finalise the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study protocol has been approved by the Ethics Committee of the Canton of Zurich (KEK-ZH Ref no. 2017–02043, 23 January 2018). Informed consent will be sought from all participating GPs and patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thomas Grischott, Email: thomas.grischott@usz.ch.
Oliver Senn, Email: oliver.senn@usz.ch.
Thomas Rosemann, Email: thomas.rosemann@usz.ch.
Anja Frei, Email: anja.frei@uzh.ch.
Jacques Cornuz, Email: jacques.cornuz@chuv.ch.
Eva Martin-Diener, Email: eva.martin-diener@uzh.ch.
Stefan Neuner-Jehle, Email: stefan.neuner-jehle@usz.ch.
References
- 1.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53(9):1217–1222. [PubMed] [Google Scholar]
- 2.Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;(5):CD000165. [DOI] [PMC free article] [PubMed]
- 3.Humair JP, Cornuz J. A new curriculum using active learning methods and standardized patients to train residents in smoking cessation. J Gen Intern Med. 2003;18(12):1023–1027. doi: 10.1111/j.1525-1497.2003.20732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–395. doi: 10.1037/0022-006X.51.3.390. [DOI] [PubMed] [Google Scholar]
- 5.Frei von Tabak—ärztliche Beratung zum Rauchstopp. https://www.freivontabak.ch. Accessed 3 Oct 2017.
- 6.Gesundheitscoaching-KHM—Patient und Arzt gemeinsam unterwegs. http://www.gesundheitscoaching-khm.ch. Accessed 3 Oct 2017.
- 7.Kollegium für Hausarztmedizin. http://www.kollegium.ch. Accessed 3 Oct 2017.
- 8.Neuner-Jehle S, Schmid M, Grüninger U. The “Health Coaching” programme: a new patient-centred and visually supported approach for health behaviour change in primary care. BMC Fam Pract. 2013;14:100. doi: 10.1186/1471-2296-14-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulton LH. Covariate-based constrained randomization of group-randomized trials. Clin Trials. 2004;1(3):297–305. doi: 10.1191/1740774504cn024oa. [DOI] [PubMed] [Google Scholar]
- 10.Carter BR, Hood K. Balance algorithm for cluster randomized trials. BMC Med Res Methodol. 2008;8:65. doi: 10.1186/1471-2288-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grischott T. The Shiny Balancer—software and imbalance criteria for optimally balanced treatment allocation in small RCTs and cRCTs. BMC Med Res Methodol. 10.1186/s12874-018-0551-5 (forthcoming). [DOI] [PMC free article] [PubMed]
- 12.Neuner-Jehle S, Grüninger U, Schmid M. Efficacy of a communication skill training fostering health promotion in primary care: a mixed method analysis. J Community Med Health. 2016;6:413. [Google Scholar]
- 13.PAPRICA—Physical Activity Promotion in Primary Care. http://www.paprica.ch. Accessed 3 Oct 2017.
- 14.FOSUMOS—Forum Suchtmedizin Ostschweiz. https://www.fosumos.ch. Accessed 3 Oct 2017.
- 15.Martin B, Neuner-Jehle S, Martin-Diener E, Grüninger U, Bize R, Weil B, et al. Gesundheitsberatung in der medizinischen Grundversorgung, Teil 1. Schweiz Med Forum. 2016;16(43):916–920. [Google Scholar]
- 16.RE-AIM—Reach Effectiveness Adoption Implementation Maintenance. http://re-aim.org. Accessed 3 Oct 2017.
- 17.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. doi: 10.2105/AJPH.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornuz J, Jacot Sadowski I, Humair J-P. Ärztliche Rauchstoppberatung. Die Dokumentation für die Praxis. 3. Bern: Projekt FREI VON TABAK; 2015. [Google Scholar]
- 19.Power and Sample Size.com. http://powerandsamplesize.com/Calculators/Compare-2-Proportions/2-Sample-Equality. Accessed 20 June 2018.
- 20.Parker DR, Evangelou E, Eaton CB. Intraclass correlation coefficients for cluster randomized trials in primary care: the cholesterol education and research trial (CEART) Contemp Clin Trials. 2005;26(2):260–267. doi: 10.1016/j.cct.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Lindson-Hawley N, Banting M, West R, Michie S, Shinkins B, Aveyard P. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164(9):585–592. doi: 10.7326/M14-2805. [DOI] [PubMed] [Google Scholar]
- 22.Power and Sample Size.com. http://powerandsamplesize.com/Calculators/Compare-2-Proportions/2-Sample-Non-Inferiority-or-Superiority. Accessed 20 June 2018.
- 23.Ma J, Raina P, Beyene J, Thabane L. Comparison of population-averaged and cluster-specific models for the analysis of cluster randomized trials with missing binary outcomes: a simulation study. BMC Med Res Methodol. 2013;13:9. doi: 10.1186/1471-2288-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Govani SM, Higgins PD. How to read a clinical trial paper: a lesson in basic trial statistics. Gastroenterol Hepatol (N Y) 2012;8(4):241–248. [PMC free article] [PubMed] [Google Scholar]
- 25.West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100(3):299–303. doi: 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- 26.Tverdal A, Bjartveit K. Health consequences of reduced daily cigarette consumption. Tob Control. 2006;15(6):472–480. doi: 10.1136/tc.2006.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. JAMA. 2005;294(12):1505–1510. doi: 10.1001/jama.294.12.1505. [DOI] [PubMed] [Google Scholar]
- 28.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes. 2006;30(3):391–399. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 29.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Blair SN, Church TS. Effects of clinically significant weight loss with exercise training on insulin resistance and cardiometabolic adaptations. Obesity (Silver Spring) 2016;24(4):812–819. doi: 10.1002/oby.21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365(21):1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 32.Physical Activity Guidelines Advisory Commitee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington; 2008.
- 33.Beddhu S, Wei G, Marcus RL, Chonchol M, Greene T. Light-intensity physical activities and mortality in the United States general population and CKD subpopulation. Clin J Am Soc Nephrol. 2015;10(7):1145–1153. doi: 10.2215/CJN.08410814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timperio A, Salmon J, Rosenberg M, Bull FC. Do logbooks influence recall of physical activity in validation studies? Med Sci Sports Exerc. 2004;36(7):1181–1186. doi: 10.1249/01.MSS.0000132268.74992.D8. [DOI] [PubMed] [Google Scholar]
- 35.Rehm J, Zatonksi W, Taylor B, Anderson P. Epidemiology and alcohol policy in Europe. Addiction. 2011;106(Suppl 1):11–19. doi: 10.1111/j.1360-0443.2010.03326.x. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization (WHO) International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva: World Health Organization; 2000. Dept. of Mental Health and Substance Dependence. [Google Scholar]
- 37.Miller P, Plant M. Spreading out or concentrating weekly consumption: alcohol problems and other consequences within a UK population sample. Alcohol. 2005;40(5):461–468. doi: 10.1093/alcalc/agh169. [DOI] [PubMed] [Google Scholar]
- 38.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Newbury Park: Sage; 1988. [Google Scholar]
- 39.Wiegand B, Luedtke K, Friscia D, Nair M, Aleles M, McCloskey R. Efficacy of a comprehensive program for reducing stress in women: a prospective, randomized trial. Curr Med Res Opin. 2010;26(4):991–1002. doi: 10.1185/03007991003688193. [DOI] [PubMed] [Google Scholar]
- 40.Kirby ED, Williams VP, Hocking MC, Lane JD, Williams RB. Psychosocial benefits of three formats of a standardized behavioral stress management program. Psychosom Med. 2006;68(6):816–823. doi: 10.1097/01.psy.0000238452.81926.d3. [DOI] [PubMed] [Google Scholar]
- 41.Klein EM, Brähler E, Dreier M, Reinecke L, Müller KW, Schmutzer G, et al. The German version of the Perceived Stress Scale—psychometric characteristics in a representative German community sample. BMC Psychiatry. 2016;16:159. doi: 10.1186/s12888-016-0875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jovanović V, Gavrilov-Jerković V. More than a (negative) feeling: validity of the Perceived Stress Scale in Serbian clinical and non-clinical samples. Psihologija. 2015;48(1):5–18. doi: 10.2298/PSI1501005J. [DOI] [Google Scholar]
- 43.NIH Registry of Validated Short Dietary Assessment Instruments. https://epi.grants.cancer.gov/diet/shortreg/register.php. Accessed 3 Oct 2017.
- 44.Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. 2007;44(4):335–340. doi: 10.1016/j.ypmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Kritchevsky SB, Beavers KM, Miller ME, Shea MK, Houston DK, Kitzman DW, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS One. 2015;10(3):e0121993. doi: 10.1371/journal.pone.0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulrow CD, Chiquette E, Angel L, Cornell J, Summerbell C, Anagnostelis B, et al. Dieting to reduce body weight for controlling hypertension in adults. Cochrane Database Syst Rev. 1998;4:CD000484. doi: 10.1002/14651858.CD000484. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention (CDC). Alcohol and Public Health: U.S. Department of Health & Human Services; updated April 14, 2017. Available from: http://www.cdc.gov/alcohol.
- 48.World Health Organization (WHO) Diet, nutrition and the prevention of chronic diseases. Geneva: Report of a Joint WHO/FAO Expert Consultation; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SPIRIT checklist: recommended items to address in a clinical trial protocol. (PDF 57 KB)
Data Availability Statement
Data sharing is not applicable to this article as no datasets have yet been generated or analysed during the current study.