Abstract
Objectives
To investigate the association between benzodiazepine (BZD) use and the risk of chronic-onset poststroke pneumonia.
Design
Population-based propensity-matched retrospective cohort study.
Setting
Taiwan’s National Health Insurance Research Database.
Participants
Patients newly diagnosed with stroke between 2000 and 2012 were identified and, after propensity score matching, 7516 patients were enrolled. Among these, 3758 patients received BZDs after stroke while 3758 did not.
Outcome measures
HRs for developing pneumonia over 1 month after stroke according to BZD use were assessed using Cox proportional hazards regression models. Analyses according to cumulative defined daily doses (cDDDs) of BZDs and stratification for age and sex were also performed.
Results
During a mean follow-up time of 4.4 years, 1027 patients in the BZD cohort and 478 patients in the non-BZD cohort developed pneumonia over 1 month after stroke. Patients using BZDs after stroke had a higher pneumonia risk than did those not using BZDs (52.2vs32.6 per 1000 person-years, adjusted HR (aHR)=2.21, 95% CI (CI)=1.97 to 2.48, p<0.001). Analyses based on cumulative BZD dose revealed that all BZD user subgroups were associated with a higher risk of pneumonia. The aHRs for patients taking 1–90, 91–365 and >365 cDDDs of BZDs were 2.28 (95% CI=2.01 to 2.58; p<0.001), 2.09 (95% CI=1.77 to 2.47; p<0.001) and 2.08 (95% CI=1.72 to 2.52; p<0.001), respectively. The significant association between BZD use and increased pneumonia risk persisted even after stratifying subgroups by age and sex.
Conclusions
BZD use is associated with an increased risk of chronic-onset poststroke pneumonia.
Keywords: benzodiazepines, stroke, pneumonia, cohort study
Strengths and limitations of this study.
This retrospective cohort study was performed based on a nationwide population database, which contained one million subjects randomly sampled from Taiwan’s population.
This is the first large-scale study to demonstrate an association between benzodiazepine use and pneumonia risk in patients who had a stroke over a long-term follow-up period.
The claim-based data did not allow for the retrieval of certain clinical information (eg, patient lifestyle and physical, psychiatric or laboratory examination data), and thus we could not control or adjust for these potential confounders in our analyses.
Introduction
Pneumonia is one of the most common serious medical complications that occurs after stroke,1 affecting up to one-third of patients with stroke.2 3 Previous studies have indicated that poststroke pneumonia is independently associated with poor prognoses, including higher morbidity and mortality, and decreased functional outcomes.1 4–6 Poststroke pneumonia can be divided into two types according to the time of occurrence: acute-onset refers to pneumonia developed within 1 month of the stroke event, while chronic onset was referred as pneumonia developed after 1 month of stroke.7 Poststroke pneumonia is also a considerable financial burden to healthcare systems.8 Thus, developing strategies to prevent poststroke pneumonia is an important clinical issue.
As modulators of the γ-amino butyric acid type A receptor (GABAA), benzodiazepines (BZDs) are widely used for treating a variety of conditions, such as insomnia, anxiety, muscle spasm and epilepsy.9 Previous studies have postulated that BZDs may increase the risk of pneumonia, possibly on account of nocturnal and daytime sedation, an increased risk of aspiration and the possible depression of immune cells.9–13 However, the link between BZD use and increased risk of pneumonia has been disputed by other studies,14–18 and thus the association between these factors remains unclear.
A considerable proportion of stroke survivors are prescribed BZDs, owing to the high prevalence of poststroke psychological problems, such as insomnia, depression and anxiety.17 19 20 Although pneumonia is a serious medical complication that can lead to a poor prognosis, to our knowledge, only one prior study has addressed the association between BZD use and the risk of pneumonia development in patients poststroke; moreover, that study used a short follow-up period.17 Indeed, investigations into chronic-onset poststroke pneumonia over long-term follow-up periods in stroke survivors are still lacking. Therefore, we conducted a population-based retrospective cohort study to evaluate the association between BZD use and the risk of chronic-onset poststroke pneumonia in a large sample of stroke survivors over a long study period.
Methods
Data sources
We conducted a population-based retrospective cohort study by analysing claims data obtained from Taiwan’s National Health Insurance Research Database (NHIRD). The national health insurance (NHI) programme of Taiwan, which was launched in March 1995 and is administered by the government, is a mandatory single-payer NHI system that covers more than 99% of Taiwan’s residents and has contracts with 97% of the hospitals and clinics in Taiwan. Data in the current study were obtained from the Longitudinal Heath Insurance Database 2000 (LHID2000), a subset of the NHIRD that contains a representative database of one million people from NHI beneficiaries registered in Taiwan in the year 2000. For research purposes, the LHID2000 was systemically and randomly sampled by the National Health Research Institute from the Taiwanese population. The database included medical claims of all inpatient, outpatient, emergency department and home care services. Before releasing the database, information related to personal identification was encrypted to protect patient privacy and data security.21 22 National Health Research Institute approval was obtained prior to using the LHID2000 in this study. Diagnostic disease codes were derived using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). This coding is considered highly reliable in the NHIRD, as insurance claims have been investigated by medical reimbursement specialists and the coding system has undergone peer review.23
Study population
The same database used in our previous study was employed here.22 The database comprised adult patients (aged ≥20 years) with new-onset stroke occurring between 2000 and 2012; patients were identified by a primary discharge diagnosis of stroke (ICD-9-CM codes 430–437). The ‘index date’ was defined as the date of the new-onset stroke diagnosis, while ‘index hospitalisation’ was defined as concurrent hospitalisation for stroke. Exclusion criteria for this study were as follows: (1) history of stroke before the 2000–2012 study period, (2) history of pneumonia 1 year preceding the index date, (3) concurrent diagnosis of pneumonia during the index hospitalisation and/or (4) death during the index hospitalisation.
Exposure to BZDs
In Taiwan, BZDs are controlled by the Bureau of Controlled Drugs, and patients can only obtain such drugs through a doctor’s prescription. We identified BZD prescriptions written after the index hospitalisation using the prescription database in the LHID. The study population was then divided into BZD and non-BZD cohorts. The BZD cohort included patients who had used any BZDs after stroke during the follow-up period, while the non-BZD cohort comprised patients who were not prescribed BZDs poststroke. To evaluate the effects of different BZD doses, we divided the BZD cohort into three subgroups using defined daily dose (DDD) methodology—a WHO-recommended unit of measure employed to evaluate the prescribed amount of a drug for its main indication in an adult. Note that DDD methodology is widely used for the investigation of administrative pharmacy claim data.21 23 Using this approach, we calculated the cumulative DDD (cDDD) by determining the sum of the dispensed DDD of all BZDs. This value was then employed to quantify BZD use during the study follow-up period. As such, the BZD cohort was divided into 1–90, 91–365 and >365 cDDD subgroups.
Outcome measures and sensitivity analysis
The main outcome of this study was defined as the occurrence of chronic-onset poststroke pneumonia after index hospitalisation, as identified by a discharge diagnosis of pneumonia (ICD-9-CM codes of 480–486). In order to increase the accuracy of this outcome, only patients with pneumonia who required hospitalisation were considered. According to previous research, chronic-onset poststroke pneumonia is defined as pneumonia developing over 1 month after the incidence of stroke.7 As the present study evaluated chronic-onset poststroke pneumonia, cases of pneumonia diagnosed within 30 days of the stroke event were excluded. The identification of patients with pneumonia using ICD-9-CM coding in inpatient service has been previously validated and shows high accuracy.24 All subjects were followed up from the index date until the first occurrence of pneumonia, death or until 31 December 2013. Death was defined as a patient withdrawn from the NHI programme,25 as previous studies have indicated that this is an accurate and reliable proxy for date of death.26 27 Analyses stratified by age and sex were also performed.
In addition, to examine whether a diagnosis of pneumonia rendered in an outpatient service would influence our results, we performed a sensitivity analysis. For this analysis, pneumonia events were defined as those diagnosed during the follow-up period regardless of whether the diagnosis was rendered in an inpatient or outpatient service.
Covariates and propensity score matching
Baseline characteristics, such as demographic data, socioeconomic status, comorbidities and clinical conditions, were obtained using reimbursement claims data and ICD-9-CM and procedure codes. A pre-existing comorbidity was defined as a disease diagnosed at least one time during inpatient service or two times during outpatient visits within the year prior to the index date. The Charlson Comorbidity Index was calculated according to information related to pre-existing comorbidities.28 Baseline medication use was defined as any medication taken for at least 30 days over the course of the year preceding the index date. Information related to stroke severity proxies was obtained based on the indicated clinical condition during the index hospitalisation. Such information included diagnosis codes for hemiplegia and aphasia, operation/procedural codes for head surgery, mechanical ventilation and utilisation of the intensive care unit, as mentioned in our previous studies.22 29 In addition, estimated National Institutes of Health Stroke Scale (NIHSS) scores were calculated to represent the severity of neurological deficits, converting scores from a claims-based Stroke Severity Index (SSI)—a measure specifically developed for use with Taiwan’s NHIRD claims-based data—with a formula developed by Hsieh et al (estimated NIHSS=1.1722 × SSI − 0.7533).30 31 The SSI has been validated in previous studies and is highly correlated with the NIHSS and consequent functional outcomes after stroke.31–33 Additionally, some comorbidities may occur after stroke and also possibly cause a confounding effect. We therefore calculated an additional Charlson Comorbidity Index at the end point of follow-up, using the data on comorbidities from the year prior to the end-point date. Socioeconomic status was determined according to patient income and dwelling urbanisation levels. Income, which was accessed based on NHI premiums, was classified into four levels (New Taiwan dollars ≥40 000, 20 000–39 999, 1–19 999 and financially dependent). Urbanisation was classified into five levels, with level-1 corresponding to the most urbanised areas.34 Detailed descriptions of income and urbanisation levels have been described in our previous studies.22 29
In order to decrease the selection bias between groups, propensity score matching was performed to balance patient baseline characteristics, including age, sex, income level, urbanisation level, comorbidities, Charlson Comorbidity Index, stroke severity proxies and medication use (table 1). A logistic regression model was used to calculate a propensity score which estimated the probability of BZD use based on all baseline covariates for each BZD user and non-user. Using the method of nearest-neighbour matching without replacement (with a calliper width equal to 0.2 SD of the propensity score logit), we matched each BZD user with a non-BZD user.22 35 36
Table 1.
Baseline characteristics of patients poststroke in the BZD and non-BZD cohorts after propensity score matching
| BZD use | P value | ||||
| Yes (n=3758) | No (n=3758) | ||||
| n | % | n | % | ||
| Age (years) | 66.2±14.9 | 66.3±14.7 | 0.941 | ||
| Sex | 0.267 | ||||
| Male | 2455 | 65.3 | 2409 | 64.1 | |
| Female | 1303 | 34.7 | 1349 | 35.9 | |
| Income level (NTD) | 0.816 | ||||
| Financially dependent | 1123 | 29.9 | 1125 | 29.9 | |
| 1–19 999 | 1801 | 47.9 | 1826 | 48.6 | |
| 20 000–39 999 | 522 | 13.9 | 516 | 13.7 | |
| ≥40 000 | 312 | 8.3 | 291 | 7.7 | |
| Urbanisation level | 0.732 | ||||
| 1 (most urbanised) | 974 | 25.9 | 985 | 26.2 | |
| 2 | 993 | 26.4 | 1010 | 26.9 | |
| 3 | 791 | 21.0 | 744 | 19.8 | |
| 4 | 579 | 15.4 | 580 | 15.4 | |
| 5 (least urbanised) | 421 | 11.2 | 439 | 11.7 | |
| Comorbidities | |||||
| Charlson Comorbidity Index | 2.26±1.50 | 2.27±1.63 | 0.837 | ||
| Hypertension | 2666 | 70.9 | 2666 | 70.9 | 1.000 |
| Diabetes mellitus | 1263 | 33.6 | 1256 | 33.4 | 0.864 |
| COPD | 247 | 6.6 | 265 | 7.1 | 0.410 |
| Asthma | 114 | 3.0 | 112 | 3.0 | 0.893 |
| Chronic kidney disease | 180 | 4.8 | 198 | 5.3 | 0.342 |
| Cirrhosis | 200 | 5.3 | 207 | 5.5 | 0.721 |
| Coronary artery disease | 491 | 13.1 | 528 | 14.1 | 0.213 |
| Congestive heart failure | 192 | 5.1 | 202 | 5.4 | 0.605 |
| Pneumoconiosis | 6 | 0.2 | 7 | 0.2 | 0.781 |
| Hyperlipidaemia | 1096 | 29.2 | 1080 | 28.7 | 0.684 |
| Malignancy | 188 | 5.0 | 176 | 4.7 | 0.519 |
| Dementia | 207 | 5.5 | 208 | 5.5 | 0.960 |
| Depression | 34 | 0.9 | 50 | 1.3 | 0.079 |
| Parkinsonism | 107 | 2.8 | 106 | 2.8 | 0.945 |
| Epilepsy | 24 | 0.6 | 26 | 0.7 | 0.777 |
| Bipolar disorders | 3 | 0.1 | 3 | 0.1 | 1.000 |
| Alcohol-related disorders | 14 | 0.4 | 19 | 0.5 | 0.383 |
| Substance use disorders | 18 | 0.5 | 18 | 0.5 | 1.000 |
| Schizophrenia | 18 | 0.5 | 16 | 0.4 | 0.731 |
| Anxiety | 90 | 2.4 | 104 | 2.8 | 0.309 |
| Sleep disorders | 170 | 4.5 | 186 | 4.9 | 0.385 |
| Charlson Comorbidity Index at the end point of follow-up | 2.03±1.79 | 2.13±2.06 | 0.022 | ||
| Stroke severity proxies | |||||
| Estimated NIHSS | 8.0±6.0 | 7.7±5.8 | 0.061 | ||
| ICU utilisation | 937 | 24.9 | 871 | 23.2 | 0.075 |
| Mechanical ventilation | 308 | 8.2 | 299 | 8.0 | 0.703 |
| Hemiplegia | 562 | 15.0 | 530 | 14.1 | 0.295 |
| Aphasia | 69 | 1.8 | 63 | 1.7 | 0.598 |
| Neurosurgery | 202 | 5.4 | 210 | 5.6 | 0.685 |
| Use of medication | |||||
| Steroids | 109 | 2.9 | 116 | 3.1 | 0.636 |
| Antidiabetic agents | 795 | 21.2 | 821 | 21.8 | 0.465 |
| Antihypertensive agents | 1676 | 44.6 | 1715 | 45.6 | 0.366 |
| Statins | 319 | 8.5 | 332 | 8.8 | 0.594 |
| Proton pump inhibitors | 89 | 2.4 | 97 | 2.6 | 0.553 |
| Antiepileptics | 70 | 1.9 | 82 | 2.2 | 0.325 |
| Antiparkinsonian | 92 | 2.4 | 90 | 2.4 | 0.881 |
| Antipsychotics | 76 | 2.0 | 85 | 2.3 | 0.473 |
| Anxiolytics | 246 | 6.5 | 286 | 7.6 | 0.072 |
| Hypnotics and sedatives | 156 | 4.2 | 180 | 4.8 | 0.180 |
| Antidepressants | 79 | 2.1 | 97 | 2.6 | 0.170 |
Continuous data expressed as mean±SD and categorical data expressed as number and percentage.
BZD, benzodiazepine; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; NIHSS, National Institutes of Health Stroke Scale.; NTD, New Taiwan dollars.
Statistical analysis
Continuous variables between the BZD and non-BZD cohort were compared using independent t-tests, while categorical variables were compared using χ2 tests. The Kaplan-Meier method was performed to estimate the risk of developing pneumonia, and the log-rank test was used to compare differences between cumulative incidence curves. Univariate and multivariate Cox proportional hazards regression models were used to compute the HRs and corresponding 95% CIs for developing pneumonia after stroke; all baseline characteristics listed in table 1 were adjusted for when conducting the multivariate Cox proportional hazards regression models. To eliminate possible bias caused by competing mortality, modified Cox proportional hazards regression models were used with adjustment for competing risk events.25 37 Differences were considered statistically significant at a two-sided probability value of <0.05. All statistical analyses were performed using Stata V.13.
Patient and public involvement
Due to the present study having used deidentified secondary data, the patients and public were not directly involved in this study, and the need for consent was waived.
Results
Demographic characteristics
After propensity score matching according to the baseline characteristics listed in table 1, a total of 7516 patients with newly onset stroke were included in our study. Among these patients, 3758 received BZDs and were classified into the BZD cohort, while 3758 did not receive BZDs and were classified into the non-BZD cohort. Although most baseline characteristics were well-balanced after propensity score matching, significant differences were found regarding the baseline prevalence of sleep disorders and the proportion of patients using antihypertensive agents and anxiolytics; however, the actual between-group differences were minor (table 1).
Risk of chronic-onset poststroke pneumonia according to BZD use
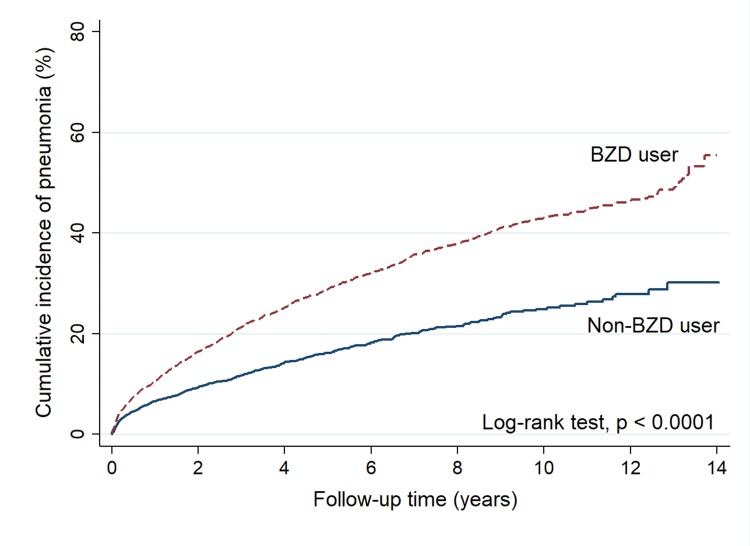
During a mean follow-up of 4.4 years, 1027 patients in the BZD cohort (52.2/1000 person-years), compared with 478 patients in the non-BZD cohort (32.6/1000 person-years), developed pneumonia. A Kaplan-Meier analysis revealed a significantly higher cumulative incidence of pneumonia in the BZD than in the non-BZD cohort (log-rank test, p<0.0001) (figure 1). Cox proportional hazards regression models revealed that BZD use after stroke was associated with an increased risk of pneumonia in both the univariate (crude HR=1.95, 95% CI=1.75 to 2.17, p<0.001) and multivariate models (adjusted HR (aHR)=2.21, 95% CI=1.97 to 2.48, p<0.001) (table 2).
Figure 1.
Kaplan-Meier curves showing cumulative incidences of pneumonia in patients with and without BZD use poststroke. BZD, benzodiazepine.
Table 2.
Risk of pneumonia after stroke among patients in the BZD and non-BZD cohorts
| BZD use | ||
| Yes | No | |
| Patient numbers | 3758 | 3758 |
| Pneumonia cases | 1027 | 478 |
| Person-years | 19 680.1 | 14 663.8 |
| Incidence rate* | 52.2 | 32.6 |
| Univariate model | ||
| Crude HR (95% CI) | 1.95 (1.75 to 2.17) | 1 (ref) |
| P value | <0.001 | |
| Multivariate model† | ||
| Adjusted HR (95% CI) | 2.21 (1.97 to 2.48) | 1 (ref.) |
| P value | <0.001 | |
*Per 1000 person-years.
†Multivariate Cox proportional hazard regression model, adjusting for all baseline characteristics (listed in table 1) and competing mortality.
BZD, benzodiazepine.
Analyses according to different cumulative BZD doses
Patients in all three cDDD subgroups had a higher risk of developing pneumonia than did the non-BZD cohort. The aHRs for the 1–90, 91–365 and >365 cDDD subgroups were 2.28 (95% CI=2.01 to 2.58; p<0.001), 2.09 (95% CI=1.77 to 2.47; p<0.001) and 2.08 (95% CI=1.72 to 2.52; p<0.001), respectively (table 3).
Table 3.
Risk of pneumonia after stroke according to different cumulative BZD doses
| Cumulative BZD doses | n | Pneumonia cases | Person- years | Incidence rate* | Univariate model | Multivariate model† | ||
| Crude HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |||||
| Non-user | 3758 | 478 | 14 663.8 | 32.6 | 1 (ref) | 1 (ref) | ||
| 1–90 cDDDs | 2507 | 702 | 11 760.2 | 59.7 | 2.10 (1.87 to 2.36) | <0.001 | 2.28 (2.01 to 2.58) | <0.001 |
| 91–365 cDDDs | 728 | 198 | 4216.6 | 47.0 | 1.84 (1.56 to 2.16) | <0.001 | 2.09 (1.77 to 2.47) | <0.001 |
| >365 cDDDs | 523 | 127 | 3703.4 | 34.3 | 1.47 (1.21 to 1.77) | <0.001 | 2.08 (1.72 to 2.52) | <0.001 |
*Per 1000 person-years.
†Multivariate Cox proportional hazard regression model, adjusting for all baseline characteristics (listed in table 1) and competing mortality.
BZD, benzodiazepine; cDDDs, cumulative defined daily doses.
Analyses after stratification for age and sex
The association between BZD use and the risk of pneumonia development in patients who had a stroke was further analysed after stratification for age and sex (table 4). Using this approach, we found a significantly greater risk of pneumonia in BZD users than in non-users in both age subgroups (<65 years old: aHR=2.42, 95% CI=1.84 to 3.17, p<0.001;≥65 years old: aHR=2.11, 95% CI=1.86 to 2.40, p<0.001). Additionally, BZD users had an increased risk of pneumonia in both male (aHR=2.46, 95% CI=2.13 to 2.84, p<0.001) and female (aHR=1.83, 95% CI=1.50 to 2.22, p<0.001) subgroups (table 4). Together, these results reveal that BZD use after stroke was associated with an increased risk of pneumonia regardless of age and sex.
Table 4.
Risk of pneumonia after stroke in the BZD and non-BZD cohorts after stratification for age and sex
| BZD use | Non-BZD use | Univariate model | Multivariate model* | |||||||||
| n | Pneumonia cases | Person-years | Incidence rate† | n | Pneumonia cases | Person-years | Incidence rate† | Crude HR(95% CI) | P value | Adjusted HR (95% CI) |
P value | |
| Age | ||||||||||||
| <65 | 1599 | 221 | 10 438.4 | 21.2 | 1626 | 77 | 7568.1 | 10.2 | 2.32 (1.79 to 3.00) | <0.001 | 2.42 (1.84 to 3.17) | <0.001 |
| ≥65 | 2159 | 806 | 9241.7 | 87.2 | 2132 | 401 | 7095.7 | 56.5 | 1.94 (1.72 to 2.18) | <0.001 | 2.11 (1.86 to 2.40) | <0.001 |
| Sex | ||||||||||||
| Male | 2455 | 721 | 12 709.5 | 56.7 | 2409 | 278 | 9945.3 | 28.0 | 2.35 (2.04 to 2.69) | <0.001 | 2.46 (2.13 to 2.84) | <0.001 |
| Female | 1303 | 306 | 6970.6 | 43.9 | 1349 | 200 | 4718.5 | 42.4 | 1.39 (1.16 to 1.66) | <0.001 | 1.83 (1.50 to 2.22) | <0.001 |
*Multivariate Cox proportional hazard regression model, adjusting for all baseline characteristics (listed in table 1) and competing mortality.
†Per 1000 person-years.
BZD, benzodiazepine.
Results of sensitivity analysis
In the sensitivity analysis, which considered the pneumonia event regardless of diagnosis in inpatient or outpatient service, BZD use was independently associated with increased risk of developing pneumonia (adjusted HR=1.94, 95% CI=1.77 to 2.14, p<0.001). The results for different cumulative doses of BZDs also revealed a pattern to our primary analyses. The detailed statistical values are shown in the online supplementary materials (online supplementary table S1).
bmjopen-2018-024180supp001.pdf (192.6KB, pdf)
Discussion
This population-based propensity-matched retrospective cohort study investigated the association between BZD use and the risk of chronic-onset poststroke pneumonia. We observed that patients prescribed BZDs poststroke had a risk of developing pneumonia that was 2.21 times higher than that for patients who had not been prescribed BZDs. To the best of our knowledge, this is the first large-scale study to demonstrate an association between BZD use and the risk of chronic-onset poststroke pneumonia over a long-term follow-up period.
Our results are consistent with those previously reported by studies that focused on the general population.9–11 For example, previous observational studies found a 54% to 176% increase in the risk of pneumonia development in BZD users when compared with non-BZD users (relative risk or OR: 1.54,9 1.8610 and 2.76.11 However, other observational studies have reported conflicting results, showing negative or non-significant relationships between BZD use and the risk of pneumonia.14 16–18 For instance, a previous case–control study on participants aged ≥65 years old did not find a significant association between BZD use and pneumonia risk; however, only 87 BZD users were found among all of the pneumonia cases included in that study.14 Another cross-sectional study using 30-day, 60-day and 90-day windows revealed protective effects of BZDs on the risk of pneumonia, but was conducted over a relatively short study period and focused on the general population.18
Pneumonia is a significant complication in stroke patients owing to the increased risk of apparent aspiration and dysphagia-associated microaspiration, as well as stroke-induced immunodepression.6 7 38 Acute-onset poststroke pneumonia, which develops within 1 month of the stroke event, is mainly associated with apparent aspiration and stroke-induced immunodepression.6 7 However, although apparent aspiration and immunodepression gradually attenuate following stroke recovery, the silent aspiration or microaspiration into the lower airways may still occur and cause chronic-onset poststroke pneumonia.7 39 BZDs are commonly used in stroke patients on account of the high prevalence of psychological problems in stroke patients17 19 20; previous studies have indicated that BZDs may be associated with the increased risk of aspiration and depression of immune cells.9–13 To the best of our knowledge, the only previous study to focus on the association between BZDs and pneumonia in stroke patients did not reveal a significant association between BZD use and the risk of pneumonia poststroke; however, in that study, the follow-up period for identifying pneumonia was only 90 days poststroke.17 In contrast, the current study, which specifically focused on the chronic phase of poststroke to evaluate pneumonia developed over 1 month after the stroke,7 included a large sample size and long-term follow-up period (mean follow-up time, 4.4 years). The present study revealed a significantly increased risk of chronic-onset pneumonia in stroke survivors using BZDs (aHR=2.21), even after carefully controlling for socioeconomic status, important comorbidities, stroke severity proxies and concomitant medications. In addition, the subgroup analyses, which were performed according to cumulative BZD doses and stratified for age and sex, also revealed a similar pattern of results, further strengthening our findings. As pneumonia is one of the most common serious medical complications following stroke and can cause not only poor functional outcomes but also high mortality and financial burdens, we believe that our study addresses a knowledge gap regarding the clinical management of patients after stroke.
Although the exact biological mechanisms underlying the influence of BZDs on pneumonia remain unclear, some hypotheses have been reported. One previous animal study suggested that BZDs may negatively influence immune function via the activation of GABAA receptors on immune cells, thus interfering with macrophages/monocytes and impairing cytokine release, phagocytosis and bacterial killing capabilities.12 In addition, BZDs may increase the risk of aspiration by decreasing lower oesophageal sphincter pressure, inducing the relaxation of muscles in the upper respiratory tract, and depressing the swallowing reflex, ultimately leading to pharyngeal dysfunction.11 13 40 Further research is necessary to explore these postulated mechanisms.
The strengths of the present study include its large sample size, use of a nationwide population database and the provision of sufficient power to evaluate the effect of BZDs on the risk of pneumonia development after stroke over a long-term follow-up period. The study design also provided better evidence in terms of epidemiology than previous studies that used case–control or cross-sectional designs. Moreover, performing propensity score matching before our analyses and then using multivariate Cox proportional hazard regression models with adjustment for competing mortality allowed us to rule out several important sources of bias and ensured that confounding factors were carefully controlled.
The following limitations of the present study should also be acknowledged. First, the claim-based database did not allow for the retrieval of certain clinical information (eg, patient lifestyle and physical, psychiatric or laboratory examination data). Although our study design ensured the control of several variables, unknown or unmeasured confounders could still exist. Second, by using deidentified claims, we could not obtain the patient details to analyse the medical history and identify exact mechanism of pneumonia; thus, we could not determine whether the pneumonia was caused by aspiration or not. Third, our analyses performed according to different cumulative BZD doses did not reveal an obvious dose–effect relationship. It is difficult to completely avoid an indication bias in observational studies that evaluate the effect of medication or intervention. Hence, the existence of unidentified residual confounders (ie, the effect of the underlying aetiologies needing BDZ prescription, other exposures, etc) cannot be completely ruled out from the present study. Thus, further prospective clinical trials are necessary to address these possible biases and to determine the cause-and-effect relationship between poststroke BZD use and the pneumonia risk. Finally, the accuracy of the diagnostic coding could not be directly confirmed due to the anonymity policy enforced in the NHIRD. However, only patients who were hospitalised under a primary diagnosis of stroke were included in our study population. The identification of stroke and pneumonia using ICD-9-CM coding in inpatient services has been previously validated and shows high accuracy.24 41 42 Additionally, the claims were routinely and randomly reviewed by the NHI Bureau to confirm the diagnostic accuracy. Given that hospitals and doctors in Taiwan are heavily fined in instances of misdiagnosis and coding errors, we feel confident in the validity of the criteria used for the inclusion of stroke and pneumonia cases in this study.
Conclusions
In summary, this population-based propensity-matched retrospective cohort study indicated an association between BZD use and an increased risk of chronic-onset poststroke pneumonia. However, further large-scale prospective studies are needed to determine possible cause–effect relationships.
Supplementary Material
Footnotes
Patient consent for publication: Not required.
H-KH and C-HL contributed equally.
Contributors: Study conception and design: S-ML, H-KH and C-HL. Acquisition of data: S-HY and H-KH. Analysis and interpretation of data: S-ML, S-HY, C-CL, H-KH and C-HL. Drafting of manuscript: S-ML and H-KH. Critical revision: S-ML, S-HY, C-CL, H-KH and C-HL.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: The study was approved by the Institutional Review Board of Tzu Chi Medical Center (REC No. IRB104-131C).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All relevant data are within the paper. No additional data are available.
References
- 1. Finlayson O, Kapral M, Hall R, et al. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology 2011;77:1338–45. 10.1212/WNL.0b013e31823152b1 [DOI] [PubMed] [Google Scholar]
- 2. Ding R, Logemann JA. Pneumonia in stroke patients: a retrospective study. Dysphagia 2000;15:51–7. 10.1007/s004550010001 [DOI] [PubMed] [Google Scholar]
- 3. Sellars C, Bowie L, Bagg J, et al. Risk factors for chest infection in acute stroke: a prospective cohort study. Stroke 2007;38:2284–91. 10.1161/STROKEAHA.106.478156 [DOI] [PubMed] [Google Scholar]
- 4. Johnston KC, Li JY, Lyden PD, et al. Medical and neurological complications of ischemic stroke: experience from the RANTTAS trial. RANTTAS Investigators. Stroke 1998;29:447–53. [DOI] [PubMed] [Google Scholar]
- 5. Vermeij FH, Scholte op Reimer WJ, de Man P, et al. Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: data from the Netherlands Stroke Survey. Cerebrovasc Dis 2009;27:465–71. 10.1159/000210093 [DOI] [PubMed] [Google Scholar]
- 6. Hannawi Y, Hannawi B, Rao CP, et al. Stroke-associated pneumonia: major advances and obstacles. Cerebrovasc Dis 2013;35:430–43. 10.1159/000350199 [DOI] [PubMed] [Google Scholar]
- 7. Teramoto S. Novel preventive and therapuetic strategy for post-stroke pneumonia. Expert Rev Neurother 2009;9:1187–200. 10.1586/ern.09.72 [DOI] [PubMed] [Google Scholar]
- 8. Katzan IL, Dawson NV, Thomas CL, et al. The cost of pneumonia after acute stroke. Neurology 2007;68:1938–43. 10.1212/01.wnl.0000263187.08969.45 [DOI] [PubMed] [Google Scholar]
- 9. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax 2013;68:163–70. 10.1136/thoraxjnl-2012-202374 [DOI] [PubMed] [Google Scholar]
- 10. Chen TY, Winkelman JW, Mao WC, et al. The use of benzodiazepine receptor agonists and the risk of hospitalization for pneumonia: a nationwide population-based nested case-control study. Chest 2018;153 10.1016/j.chest.2017.07.030 [DOI] [PubMed] [Google Scholar]
- 11. Salat D, Serra-Prat M, Palomera E, et al. Benzodiazepine Use and Risk of Community-Acquired Pneumonia in a Population-Based Cohort Study From North-Eastern Spain. Clinical Medicine Insights: Therapeutics 2017;9. [Google Scholar]
- 12. Sanders RD, Godlee A, Fujimori T, et al. Benzodiazepine augmented γ-amino-butyric acid signaling increases mortality from pneumonia in mice. Crit Care Med 2013;41:1627–36. 10.1097/CCM.0b013e31827c0c8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hårdemark Cedborg AI, Sundman E, Bodén K, et al. Effects of morphine and midazolam on pharyngeal function, airway protection, and coordination of breathing and swallowing in healthy adults. Anesthesiology 2015;122:1253–67. 10.1097/ALN.0000000000000657 [DOI] [PubMed] [Google Scholar]
- 14. Dublin S, Walker RL, Jackson ML, et al. Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case-control study. J Am Geriatr Soc 2011;59:1899–907. 10.1111/j.1532-5415.2011.03586.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Isbister GK, Downes F, Sibbritt D, et al. Aspiration pneumonitis in an overdose population: frequency, predictors, and outcomes. Crit Care Med 2004;32:88–93. 10.1097/01.CCM.0000104207.42729.E4 [DOI] [PubMed] [Google Scholar]
- 16. Almirall J, Serra-Prat M, Baron F, et al. The use of benzodiazepines could be a protective factor for community-acquired pneumonia (CAP) in ≤ 60-year-old subjects. Thorax 2013;68:964–5. 10.1136/thoraxjnl-2013-203634 [DOI] [PubMed] [Google Scholar]
- 17. Frank B, Fulton RL, Lees KR, et al. Impact of benzodiazepines on functional outcome and occurrence of pneumonia in stroke: evidence from VISTA. Int J Stroke 2014;9:890–4. 10.1111/ijs.12148 [DOI] [PubMed] [Google Scholar]
- 18. Iqbal U, Syed-Abdul S, Nguyen PA, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax 2013;68:591–2. 10.1136/thoraxjnl-2013-203211 [DOI] [PubMed] [Google Scholar]
- 19. Leppävuori A, Pohjasvaara T, Vataja R, et al. Insomnia in ischemic stroke patients. Cerebrovasc Dis 2002;14:90–7. 10.1159/000064737 [DOI] [PubMed] [Google Scholar]
- 20. Schöttke H, Giabbiconi CM. Post-stroke depression and post-stroke anxiety: prevalence and predictors. Int Psychogeriatr 2015;27:1805–12. 10.1017/S1041610215000988 [DOI] [PubMed] [Google Scholar]
- 21. Tsan YT, Lee CH, Wang JD, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol 2012;30:623–30. 10.1200/JCO.2011.36.0917 [DOI] [PubMed] [Google Scholar]
- 22. Lin SM, Yang SH, Liang CC, et al. Proton pump inhibitor use and the risk of osteoporosis and fracture in stroke patients: a population-based cohort study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2017. [DOI] [PubMed]
- 23. Methodology WCCfDSa. ATC/DDD Index 2018: WHO. 2018. https://www.whocc.no/atc_ddd_index/ (Accessed 10 Mar 2018).
- 24. Su VY, Liu CJ, Wang HK, et al. Sleep apnea and risk of pneumonia: a nationwide population-based study. CMAJ 2014;186:415–21. 10.1503/cmaj.131547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu CY, Chen YJ, Ho HJ, Cy W, Hj H, et al. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA 2012;308:1906–14. [DOI] [PubMed] [Google Scholar]
- 26. Lien HM, Chou SY, Liu JT. Hospital ownership and performance: evidence from stroke and cardiac treatment in Taiwan. J Health Econ 2008;27:1208–23. 10.1016/j.jhealeco.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 27. Cheng CL, Chien HC, Lee CH, et al. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int J Cardiol 2015;201:96–101. 10.1016/j.ijcard.2015.07.075 [DOI] [PubMed] [Google Scholar]
- 28. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 29. Lin SM, Yang SH, Cheng HY, et al. Thiazide diuretics and the risk of hip fracture after stroke: a population-based propensity-matched cohort study using Taiwan’s National Health Insurance Research Database. BMJ Open 2017;7:e016992 10.1136/bmjopen-2017-016992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsieh CY, Huang HC, Dp W, et al. Impact of rehabilitation intensity on mortality risk after stroke. Arch Phys Med Rehabil 2017;99:1042–1048 e6. [DOI] [PubMed] [Google Scholar]
- 31. Sung SF, Hsieh CY, Kao Yang YH, et al. Developing a stroke severity index based on administrative data was feasible using data mining techniques. J Clin Epidemiol 2015;68:1292–300. 10.1016/j.jclinepi.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 32. Sung SF, Hsieh CY, Lin HJ, et al. Validity of a stroke severity index for administrative claims data research: a retrospective cohort study. BMC Health Serv Res 2016;16:509 10.1186/s12913-016-1769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hung LC, Sung SF, Hsieh CY, et al. Validation of a novel claims-based stroke severity index in patients with intracerebral hemorrhage. J Epidemiol 2017;27:24–9. 10.1016/j.je.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu CY HY, Chuang YL, Chen YJ, et al. Liu JS Liang KY. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey (in Chinese). J Health Manage 2006;4:1–22. [Google Scholar]
- 35. Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. 2003. http://ideas.repec.org/c/boc/bocode/s432001.html (Accessed 30 Dec2016).
- 36. Chang CC, Chen YT, Hsu CY, et al. Dipeptidyl Peptidase-4 Inhibitors, Peripheral Arterial Disease, and Lower Extremity Amputation Risk in Diabetic Patients. Am J Med 2017;130:348–55. 10.1016/j.amjmed.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 37. Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94:496–509. [Google Scholar]
- 38. Smith CJ, Kishore AK, Vail A, et al. Diagnosis of Stroke-Associated Pneumonia: Recommendations From the Pneumonia in Stroke Consensus Group. Stroke 2015;46:2335–40. 10.1161/STROKEAHA.115.009617 [DOI] [PubMed] [Google Scholar]
- 39. Teramoto S, Fukuchi Y, Sasaki H, et al. High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: a multicenter, prospective study in Japan. J Am Geriatr Soc 2008;56:577–9. 10.1111/j.1532-5415.2008.01597.x [DOI] [PubMed] [Google Scholar]
- 40. Rushnak MJ, Leevy CM. Effect of diazepam on the lower esophageal sphincter. A double-blind controlled study. Am J Gastroenterol 1980;73:127–30. [PubMed] [Google Scholar]
- 41. Hsieh CY, Chen CH, Li CY, Cy L, et al. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc 2015;114:254–9. 10.1016/j.jfma.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 42. Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011;20:236–42. 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-024180supp001.pdf (192.6KB, pdf)