Abstract
Objectives
This study aims to summarise the features and trends of thyroid carcinoma in the past two decades in China.
Design, setting and participants
Clinical data obtained from 10 798 patients treated by thyroidectomy from 1994 to 2015 at the Department of General Surgery of the People’s Liberation Army General Hospital, Beijing, China were retrospectively analysed.
Outcome measures
Incidence and histopathological features of thyroid cancer were compared and the risk factors for local lymph node metastasis analysed.
Results
Our data indicated a significant increase in the detection of thyroid cancer (from 16.8% to 69.8%, p<0.01). Among the 5235 thyroid cancer cases, papillary thyroid carcinoma (PTC) was the most common histotype, accounting for 95.1% of all malignancies over the 22-year period. Among the 4979 PTCs, micro-PTCs (mPTC) with the largest diameter ≤10 mm has gradually become the dominant form, and its percentage in PTCs has increased from 13.3% in the biennial period of 1994–1995 to 51.2% in 2010–2011. Furthermore, the size of the tumour has decreased significantly from 2.3±1.1 cm in 1994 to 1.2±0.9 cm in the largest diameter (p<0.01), while the average age at diagnosis and female dominance remained unchanged during the period. Logistic regression showed that tumour nodules>1 cm and male gender were the main risk factors for local lymph node metastasis (LNM), whereas patients over 45 years had lower risk.
Conclusions
During the 22-year period, an increased detection of thyroid cancer, particularly mPTC, was found while the occurrence of LNM decreased. Our results suggest that the current preoperative diagnosis and risk stratification are adequate, supporting the published guidelines for the diagnosis of thyroid cancer.
Keywords: thyroid cancer, papillary thyroid carcinoma, microptc
Strengths and limitations of this study.
The strength of this study lies in that >10 000 cases of thyroid cancer over a period of 22 years were retrospectively studied to summarise the features and trends of thyroid cancer in patients with thyroidectomies in Beijing, China.
The clinical practice was reviewed over this long period of time to evaluate the performance of the preoperative diagnosis and risk stratification in a major hospital in Beijing.
The retrospective nature of this study led to limited survival data, which should have been included to evaluate the improvement of clinical outcome during the 22-year period.
Introduction
Thyroid nodules occur in about 4%–7% of the population, and 8%–16% of these contain malignant elements.1 Thyroid cancer is the most common endocrine cancer,1 and approximately 90% of these are differentiated, including predominantly papillary thyroid carcinoma (PTC) and, to a less extent, follicular thyroid carcinoma.2 These subtypes are generally associated with a more favourable prognosis and higher cure rate,3 when compared with less common but more aggressive forms of thyroid cancer, such as medullary thyroid cancer, anaplastic thyroid cancer and thyroid sarcoma or lymphoma.4
The past few decades have witnessed an increase in the incidence of thyroid cancer, while the associated mortality remains unchanged.5–9 In addition, the number of newly diagnosed thyroid cancer cases with smaller tumours increased. Between 2008 and 2009, 39% of patients with thyroid cancer were diagnosed with tumours smaller than 1 cm, when compared with 25% of patients diagnosed between 1988 and 1989.10 This was due to the proactive screening of thyroid cancer, particularly through the application of sensitive surveillance modalities in recent years.11–15 Advanced imaging techniques, such as ultrasonography, CT, MRI and positron emission tomography, along with fine-needle aspiration biopsy have dramatically improved the detection of small thyroid nodules, and facilitated the early diagnosis of thyroid cancer.16–18
However, the increased incidence of thyroid cancer has also been considered to be associated with overdiagnosis18–22 and environmental factors.6 23 The screening of highly prevalent thyroid nodules for those with malignant potential remains challenging, although a number of guidelines have recently been proposed for the diagnosis and management of thyroid nodules.24 Identifying nodules with high risk of malignancy during thyroidectomy represents a cost-effective approach.25 26 Therefore, in this study, the features and trends of thyroid cancer in patients with thyroidectomies in China were retrospectively analysed to examine whether the decrease in diagnosable size of thyroid nodules is associated with misdiagnosis, providing further insight into its diagnosis and management.
Materials and methods
Written informed consent was obtained from all patients for retrospective studies.
Data collection
The clinical database for patients undergoing thyroidectomies from 1994 to 2015 at the Department of General Surgery of PLA General Hospital (Beijing, China) was revisited, and the thyroid pathology reports were screened and reviewed to analyse the correlation of the size of thyroid nodules with other pathological features.
The clinical parameters used in the analysis included front neck discomfort or pain, dysphagia, dysphonia or hoarseness, dyspnoea, sign of thyroid nodule and thyroid gland enlargement, as well as ultrasound features. These data were individually checked, and cases with inconsistent data were excluded before the statistical analysis. As a result, 10 798 eligible cases with thyroidectomies from 1994 to 2015 were recruited.
Data analysis and statistics
Benign or malignant thyroid nodules were diagnosed based on pathological features, and malignant nodules were reported as PTC, follicular (FTC), medullary (MTC) or anaplastic thyroid carcinoma (ATC). PTCs were further subclassified into clinically relevant PTCs (crPTCs) >10 mm in diameter and micro-PTCs (mPTCs) ≤10 mm in diameter (the largest diameter of a nodule). These subgroups were compared, when appropriate, in the analysis.
Patients were treated by surgery, often with lymph node dissection, depending on the clinical features and the number, location and size of the involved lymph node, which was assessed by preoperative imaging analysis and intraoperative frozen section pathological analysis. Tumours within 10 mm in size and had no involvement of lymph nodes were resected without the removal of lymph nodes. When the preoperative imaging revealed no abnormalities in the lymph nodes, but malignancy was confirmed by intraoperative frozen section analysis, ipsilateral central neck node dissection was performed. If the preoperative sonography or intraoperative examination revealed abnormal lymph nodes, and frozen section analysis confirmed the malignancy, improved lateral neck lymph node dissection was performed.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, V.17.0; IBM, Armonk, New York, USA). Data were presented as mean±SD or mean±SE, when appropriate; Χ2 test was used to compare different categories and generate the trend lines. Furthermore, t-test was used for comparisons between groups, and logistic regression was performed to calculate the risk factors for lymph node metastasis (LNM). The data analysis was adjusted for confounders, such as age, gender, nodule size and the presence of Hashimoto thyroiditis. A two-tailed p<0.05 was considered to be statistically significant.
Patient and public involvement
This retrospective study did not involve patients or the public in the development of research initiatives and outcome measures, as well as the design and implementation of the study. The study covered a period of 22 years, which ranged between 1994 and 2015. Therefore, there was no possibility or plan to disseminate the results among the participants.
Results
Malignancy rate increased from 1994 to 2015
The numbers of benign and malignant cases of thyroid cancer detected during thyroidectomy from 1994 to 2015 are summarised in table 1. As the number of thyroidectomy surgeries increased due to the improved diagnosis of thyroid cancer, the number of benign and malignant cases gradually increased. Noticeably, the malignancy rate of thyroid nodules dramatically increased from 16.8% during the biennial period of 1994–1995 to 69.8% during the period of 2014–2015 (p<0.05), while the benign rate decreased from 83.2% to 30.2% (p<0.05).
Table 1.
Detection rate of malignancy among patients undergoing thyroidectomy from 1994 to 2015
| Period | 94–95* | 96–97 | 98–99 | 00–01 | 02–03 | 04–05 | 06–07 | 08–09 | 10–11 | 12–13 | 14–15 | Total | P value |
| Benign, n
(%) |
114 (83.2) | 136 (86.1)†‡ | 169 (88.0)†‡ | 268 (83.0)† | 286 (80.1)†‡ | 495 (79.2)‡ | 535 (71.4)†‡ | 738 (64.6)†‡ | 901 (52.4)†‡ | 1033 (42.2)†‡ | 890 (30.2)†‡ | 5563 (51.5) | <0.001 |
| Cancer, n
(%) |
23 (16.8) | 22 (13.9)*† | 23 (12.0)*† | 55 (17.0)* | 71 (19.9)*† | 130 (20.8)† | 214 (28.6)*† | 405 (35.4)*† | 817 (47.6)*† | 1413 (57.8)*† | 2060 (69.8)*† | 5235 (48.5) | |
| Total | 137 | 158 | 192 | 323 | 357 | 625 | 749 | 1143 | 1718 | 2446 | 2950 | 10 798 |
*The last 2 digits of the years are displayed (hereafter).
†Percentages significantly different from the previous year.
‡Percentages significantly different within the period of 94–95.
The diagnosis rate of PTC increased from 1994 to 2015
Among the 10 798 cases recruited, 5235 cases were found to be malignant (table 2). Furthermore, a majority of patients (63.6%) were found with multiple cancers. Moreover, 48.1% of patients had unilateral cancer, and the remaining 51.9% of patients had bilateral cancer (table 2). In addition, most of the malignant cases were recorded as PTCs, which exhibited a significant increase from 65.3% during the period of 1994–1995 to 98.2% during the period of 2014–2015 (p<0.05, figure 1), while the diagnosis rates of other histotypes concurrently decreased (table 2 and figure 1).
Table 2.
Pathological classification of all thyroid malignancies from 1994 to 2015
| Period | 94–95 | 96–97 | 98–99 | 00–01 | 02–03 | 04–05 | 06–07 | 08–09 | 10–11 | 12–13 | 14–15 | P for difference | P for trend |
| PTC, n (%) | 15 (65.3) | 17 (77.3) | 17 (73.9) | 39 (70.9) | 50 (70.4) | 107 (82.3) | 185 (86.4) | 383 (94.6) | 768 (94.0) | 1376 (97.4) | 2023 (98.2) | <0.001 | <0.001 |
| FTC, n (%) | 3 (13.0) | 1 (4.5) | 4 (17.3) | 10 (18.1) | 9 (12.7) | 17 (13.1) | 18 (8.4) | 9 (2.2) | 25 (3.0) | 17 (1.2) | 20 (0.9) | <0.001 | <0.001 |
| MTC, n (%) | 1 (4.3) | 2 (9.1) | 1 (4.3) | 4 (7.3) | 2 (2.8) | 1 (0.8) | 2 (0.9) | 5 (1.2) | 14 (1.7) | 12 (0.8) | 12 (0.6) | <0.001 | <0.001 |
| ATC, n (%) | 0 (0) | 0 (0) | 1 (4.3) | 1 (1.8) | 3 (4.2) | 1 (0.8) | 1 (0.5) | 2 (0.5) | 1 (0.1) | 1 (0.1) | 2 (0.1) | <0.001 | <0.001 |
| Other, n (%) | 4 (17.4) | 2 (9.1) | 0 (0) | 1 (1.8) | 7 (9.9) | 4 (3.1) | 7 (3.3) | 6 (1.5) | 9 (1.1) | 7 (0.5) | 3 (0.1) | <0.001 | <0.001 |
| Unilateral | 14 (60.9) | 14 (63.6) | 13 (56.5) | 31 (56.4) | 38 (53.5) | 72 (55.4) | 122 (57.0) | 213 (52.6) | 389 (47.6) | 616 (43.6) | 991 (48.1) | 0.071 | <0.001 |
| Bilateral | 9 (39.1) | 8 (36.4) | 10 (43.5) | 24 (43.6) | 33 (46.5) | 58 (44.6) | 92 (43.0) | 192 (47.4) | 428 (52.4) | 797 (56.4) | 1069 (51.9) | ||
| Solitary | 7 (30.4) | 13 (59.1) | 11 (47.8) | 27 (49.1) | 28 (39.4) | 59 (45.4) | 105 (49.0) | 172 (42.5) | 292 (35.7) | 456 (32.3) | 749 (36.4) | <0.001 | <0.001 |
| Multiple | 16 (69.6) | 9 (40.9) | 12 (52.2) | 28 (50.9) | 43 (60.6) | 71 (54.6) | 109 (51.0) | 233 (57.5) | 525 (64.3) | 957 (67.7) | 1311 (63.6) | ||
| All cancer, n | 23 | 22 | 23 | 55 | 71 | 130 | 214 | 405 | 817 | 1413 | 2060 |
ATC, anaplastic thyroid carcinoma; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; other histotypes of carcinoma collectively refer to cancer other than PTC, FTC, MTC and ATC, including poorly differentiated thyroid cancer, squamous cell carcinomas, B cell lymphomas of thyroid, spindle cell carcinoma, adenoid cystic carcinoma, renal clear cell metastasis and Langerhans cell histocytosis of the thyroid gland; PTC, papillary thyroid carcinoma.
Figure 1.
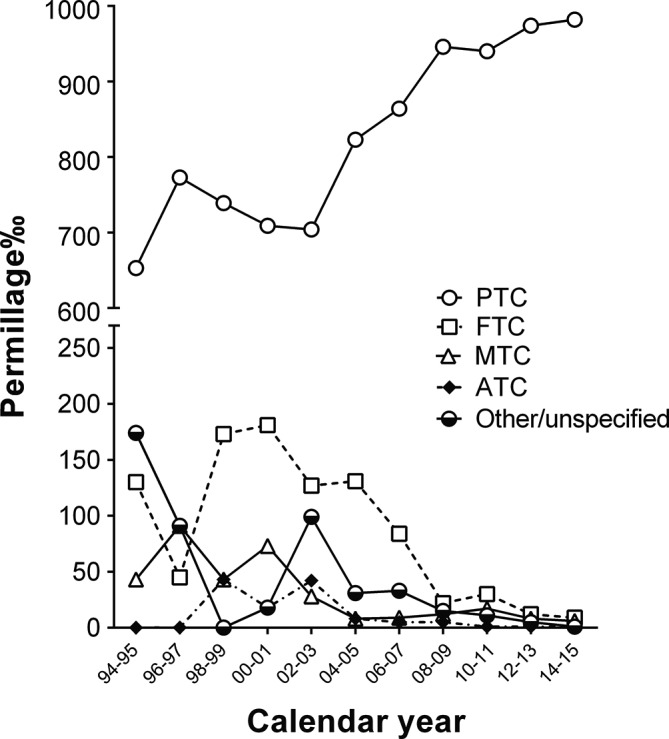
Trends in the diagnosis of various subtype thyroid malignancies, presented as permillage (rate per thousand) of thyroid cancer, from 1994 to 2015. ATC, anaplastic thyroid carcinoma; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; PTC, papillary thyroid carcinoma.
Detection size of thyroid nodules decreased from 1994 to 2015
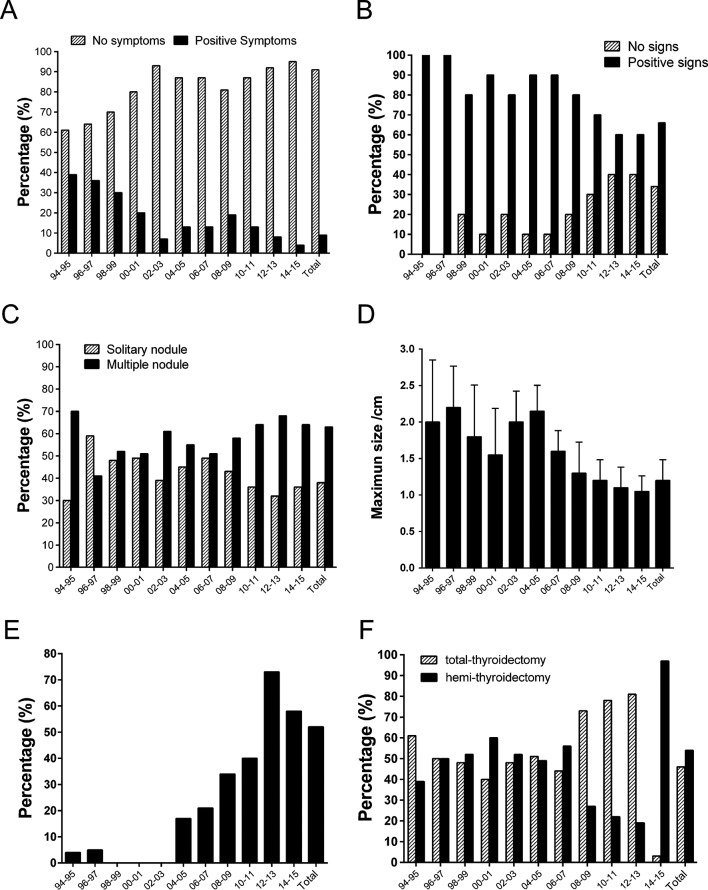
The review of the clinicopathological information of malignant cases suggested that most of these patients were free of clinically relevant symptoms and physical signs (figure 2A and B), and only 9% of patients exhibited different degrees of discomfort, such as pain and dysphagia. The enlargement of thyroid glands were often noticed, but not necessarily by health professionals, during routine physical examination. However, 34% of tumours were impalpable, which was likely due to the depth of the intrathyroidal microcarcinoma. By ultrasonography, 37% of patients were detected with single thyroid tumours, while the remaining patients had multiple lesions (figure 2C).
Figure 2.
Clinical, surgical and ultrasound features during the evolvement of thyroidectomy and the diagnosis of thyroid cancer from 1994 to 2015. Data on the symptoms (A), physical signs (B), nodule multiplicity (C), ultrasonographic sizes of nodules (D), fine-needle aspiration ratio (E) and thyroidectomy types (F) were summarised.
From 1994 to 2015, the size of thyroid nodules has been reported to significantly decrease, with the mean diameter reduced from 2.6±1.4 cm to 1.2±0.9 cm (figure 2D). This trend roughly correlates with the increase in the rate of preoperative fine-needle aspiration (FNA), which ranged from 15% in 2004 to 74% in 2013, although this rate regressed to 57% between 2014 and 2015 (figure 2E). In addition, during the same period, the total thyroidectomy rate remained unchanged from 1994 to 2007, but gradually increased from 2008 to 2013, and decreased sharply to 3% from 2014 to 2015 (figure 2F).
The size of the thyroid cancer at diagnosis and metastasis rate decreased from 1994 to 2015
Considering the high prevalence of PTCs, 4980 cases were compared with 255 cases of other subtypes all-together, and further data analysis was restricted to this type of thyroid cancer. As shown in table 3, the mean age of patients at diagnosis was 43.7±11.3 years, with no significant variation from 1994 to 2015. Furthermore, the incidence of PTC was considerably higher in women than in men, in which approximately two-thirds of these patients were female. It is noteworthy that the average diameter of PTCs at diagnosis decreased from 2.3±1.1 cm in 1994 to 1.2±0.9 cm in 2015 (p<0.05, table 3). Correlating with this trend was the increase in the percentage of mPTCs from 13.3% between 1994 and 1995 to 51.2% between 2010 and 2011 (p<0.05, table 3). This likely resulted from a more meticulous pathological examination. Interestingly, the detection rate of mPTCs decreased to 26.5 between 2014 and 2015.
Table 3.
Clinicopathological features of PTCs from 1994 to 2015
| Period | 94–95 | 96–97 | 98–99 | 00–01 | 02–03 | 04–05 | 06–07 | 08–09 | 10–11 | 12–13 | 14–15 | Total | P for difference | P for trend |
| Age, year | 46.9±14.3 | 43.1±10.7 | 41.1±12.9 | 44.9±14.8 | 44.6±14.5 | 43.8±12.4 | 44.6±12.4 | 42.7±12.4 | 43.9±11.2 | 43.9±11.1 | 43.6±10.9 | 43.7±11.3 | 0.643 | |
| Male, n
(%) |
5 (33.3) | 3 (17.6) | 6 (35.3) | 11 (28.2) | 18 (36) | 34 (31.8) | 60 (32.4) | 115 (30.0) | 227 (29.6) | 366 (26.6) | 616 (30.4) | 1461 (29.3) | 0.434 | 0.664 |
| Female, n
(%) |
10 (66.7) | 14 (82.4)*† | 11 (64.7.0)* | 28 (71.8)* | 32 (64)*† | 73 (68.2)† | 125 (67.6)*† | 268 (70.0)*† | 541 (70.4)*† | 1010 (73.4)* | 1407 (69.6)* | 3519 (70.7) | ||
| Size‡, cm | 2.3±1.1 | 2.6±1.9 | 2.1±1.3 | 2.2±1.6 | 2.2±1.4 | 2.2±1.6 | 1.7±1.1*† | 1.5±1.0b | 1.3±0.9*† | 1.2±0.8*† | 1.2±0.9† | 1.4±1.0 | <0.001 | |
| crPTC, n
(%) |
13 (86.7) | 15 (88.2) | 12 (70.6) | 32 (82.1) | 41 (82.0) | 87 (81.3) | 118 (63.8)* | 238 (62.1)† | 375 (48.8)*† | 696 (50.6)b | 1486 (73.5)* | 3113 (62.5) | <0.001 | 0.213 |
| mPTC, n
(%) |
2 (13.3) | 2 (11.8) | 5 (29.4) | 7 (17.9) | 9 (18.0) | 20 (18.7) | 67 (36.2) | 145 (37.9) | 393 (51.2) | 680 (49.4) | 537 (26.5) | 1866 (37.5) | ||
| LNM, n
(%) |
5 (33.3) | 5 (29.4) | 7 (41.2) | 22 (56.4) | 17 (34) | 27 (25.2) | 55 (29.7) | 96 (25.1) | 192 (25.0) | 372 (27.0) | 414 (20.5)* | 1212 (24.3) | <0.001 | <0.001 |
| No-LNM, n
(%) |
10 (66.7) | 12 (70.6) | 10 (58.8) | 17 (43.6) | 33 (66) | 80 (74.8) | 130 (70.3) | 287 (74.9) | 576 (75.0) | 1004 (73.0) | 1609 (79.5) | 3768 (75.7) | ||
| Total | 15 | 17 | 17 | 39 | 50 | 107 | 185 | 383 | 768 | 1376 | 2023 | <0.001 | <0.001 |
*Percentages significantly different from the previous year.
†Percentages significantly different within the period of 08–09.
‡Mean diameter.
crPTC, clinical relevant papillary thyroid carcinoma; LN, lymph node; LNM, lymph node metastasis; mPTC, micropapillary thyroid carcinoma.
Among the PTC cases studied, 1212 patients were found with LNM, but its occurrence significantly decreased with the reduction in tumour size (p<0.05, table 3). This was associated with the increased diagnosis rate of mPTCs, which shows an apparently lower LNM potential in comparison with crPTCs (table 4). Before 2007, the difference between the LNM rates of mPTC and crPTC were appreciable, but this not statistically significant most of time due to the limited size of the cohort. With the increase in PTC diagnosis after 2008, a significantly lower LNM occurrence was found for mPTCs, when compared with crPTCs (table 4).
Table 4.
Rate of local lymph node metastasis in patients with crPTC and mPTC from 2008 to 2013
| Period | 94–95 | 96–97 | 98–99 | 00–01 | 02–03 | 04–05 | 06–07 | 08–09 | 10–11 | 12–13 | 14–15 | Total | P for difference | P for trend |
| crPTC, n (%) | 3 (23.1) | 5 (33.3) | 6 (50.0) | 19 (59.4) | 17 (41.5) | 24 (27.6) | 35 (29.7) | 73 (30.7) | 130 (34.7) | 243 (34.9) | 330 (22.2) | 885 (28.4) | <0.001 | <0.001 |
| mPTC, n (%) | 2 (100) | 0 (0) | 1 (20.0) | 3 (42.9) | 0 (0) | 3 (15.0) | 20 (29.9) | 23 (16.0) | 62 (15.8) | 129 (19.0) | 84 (15.6) | 327 (17.5) | 0.005 | 0.087 |
| P value | 0.032 | 0.331 | 0.252 | 0.425 | 0.017 | 0.243 | 0.978 | 0.001 | 0.000 | 0.000 | 0.001 |
crPTC, clinical relevant papillary thyroid carcinoma; mPTC, micropapillary thyroid carcinoma.
Determinant factors of local lymph node metastasis in PTC, mPTC and crPTC
To identify the risk factors for local LNM in PTC, mPTC and crPTC, a logistic regression analysis was performed. The results support the correlation of LNM with nodules >1 cm (OR 2.480, 95% CI 0.428 to 0.613; p<0.001) and male gender (OR 1.627, 95% CI 1.376 to 1.923; p<0.001), and its inverse correlation with the age of ≥45 years (OR 0.512, 95% CI 2.040 to 2.902; p<0.001) after adjustment for other covariates in all PTCs (figure 3A, table 2). Hashimoto’s thyroiditis presented no risk for LNM (figure 3A). Consistent data were also obtained from patients with mPTC and crPTC (figure 3B and C; tables 3 and 4).
Figure 3.
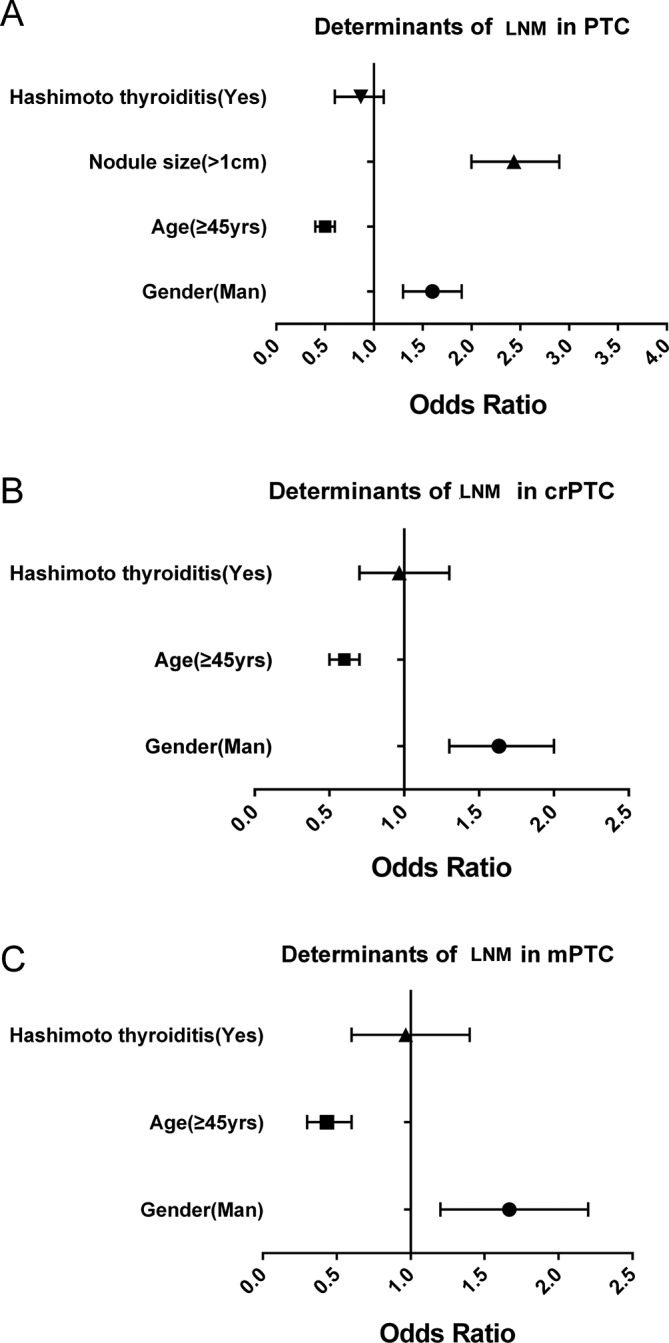
Determinant factors of the lymph node metastasis of papillary thyroid carcinoma (PTC) (A), micropapillary thyroid carcinoma (mPTC) (B) and clinically relevant papillary thyroid carcinoma (crPTC) (C). Logistic regression was performed to define factors associated with local lymph node metastasis (LNM).
Discussion
Thyroid cancer is diagnosed in nearly 300 000 people annually all over the world.27 Although these malignancies are mostly differentiated and generally associated with a good prognosis, the involvement of local lymph nodes is frequently observed. Proactive screening for malignancies from a substantial population of thyroid nodes and accurate histopathological stratification are crucial for the clinical decision and improvement of patient outcome. The present study reviewed 10 798 cases of thyroidectomy undertaken in a major hospital located in one of the most populated city in the world for a period of over 22 years, summarised the clinical features of 5235 cases of thyroid cancer, and analysed the trend of PTCs and risk factors for predicting LNM. Among these patients, about 20% were from Beijing, while the rest 80% were from other regions in China where the disease could not be diagnosed definitely in local hospitals. Therefore, our patient cohort generally represented all China even though bias towards the difficult cases might exist.
The present analysis unveiled a gradual but significant increase in thyroid malignancy detection rate between the period of 1994 and 2015, which ranged from 16.8% to 69.8%. These percentages are generally within the wide-range detection rate (6.7%–56%) of thyroid cancer worldwide,25 28 29 although data between 2014 and 2015 was relatively higher than earlier periods. Furthermore, the detection rates varied across different centres, which were likely caused by the different guidelines and algorithms followed by physicians and surgeons. The increase in detection rate from 1994 to 2015 reflects the improvement in diagnosis, through the advances in imaging technology and the increasing use of FNA, together with the shift of routine node dissection towards therapeutic dissection in the treatment. In addition, the appropriate preoperative risk stratification of thyroid nodules, which was a result of improved clinical evaluation, thyroid function test, ultrasonography and molecular diagnosis in recent years, has diverted a lot of patients from thyroidectomy, contributing to the increased diagnosis of thyroid cancer. These data support published guidelines30 31 and highlights the importance of combining imaging, FNA, cytology and molecular diagnosis. However, the contribution of social and environmental stresses could not be ruled out, which is beyond the scope of the present retrospective study. It is noteworthy that the present study covered a period during which China was undergoing the largest scale of industrialisation in modern history. This inevitably led to dramatic social and environmental changes.
Papillary carcinoma accounts for 95.1% of malignancies in the whole cohort, and reached as high as 98.2% among patients diagnosed between 2014 and 2015. These rates were comparable to the findings of another study performed in Nanjing, China,25 but were higher than those in other reports,32 which was likely due to the increase in PTCs, as previously suggested.33 The high detection rate for PTC was attributable to the increasing use of head and neck imaging, which detects subpalpable (yet potentially malignant) thyroid nodules, and ultrasound-guided FNA, which improves the preoperative detection of malignancy. With the use of neck ultrasonography and other diagnostic approaches, the average size of PTCs at diagnosis has significantly reduced from 2.3±1.1 cm in 1994 to 1.2±0.9 cm in 2015, and this was similar to the trends reported in previous studies.10 25 This progress allows for the early detection of thyroid cancer, which leads to the increasing trend of hemithyroidectomy, when compared with total thyroidectomy, and the intensified follow-up of patients with thyroid cancer in recent years in our practice following the American Thyroid Association (ATA) guidelines.30 31 33 However, the overdiagnosis and overtreatment of indolent diseases have been increasingly recognised. The most recent ATA guidelines suggest a more parsimonious evaluation of thyroid nodules and a more judicious use of thyroid cancer treatment, in order to minimise risk and maximise benefits to patients.33
The present data suggest that nodules >1 cm and the male gender predict a high risk of LNM, while the age of ≥45 years is associated with low LNM potentials, and this is consistent with a previous finding.25 Therefore, more attention is needed for these patients in future practice. However, in this single-centre study, factors taken into account for the logistic regression analysis were limited due to the retrospective nature of the study. Future studies are needed to characterise the association of other clinical and biochemical parameters with thyroid cancer metastasis and patient outcome. Characterising the contribution of social and environmental stresses to the occurrence of thyroid cancer would also be of significant social economical value.
In the present study, the clinical data of 10 798 patients treated by thyroidectomy was retrospectively analysed to determine the features and trends of thyroid cancer in the past 22 years. The present data suggested a significant increase in the detection rate of thyroid cancer, particularly mPTC, which has become the dominant form of thyroid cancer. As a result, the occurrence of LNM has been decreasing with time. The present results suggest the adequacy of present preoperative diagnosis and risk stratification, although overdiagnosis and overtreatment require future attention of physicians and surgeons in view of the rapid increase in the number of thyroidectomies.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the invaluable contribution of the doctors and nurses of Chinese PLA General Hospital and the patients involved in this study.
Footnotes
LZ and PP contributed equally.
Contributors: ZL designed the study, collected and analysed data and wrote the manuscript. PP contributed in writing the manuscript. ZL, LYK, WFL, YGQ, DJ and WXL assisted with the data presentation and manuscript writing. LZH, DJT and MYM contributed in the study design, data analysis and manuscript writing. All authors read and approved the final manuscript.
Funding: This study was supported by a grant from the Medical Science and Technology Foundation of the Military ’Twelve-Five' Programme (CWS11J063) to ZHL.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The Chinese People’s Liberation Army (PLA) General Hospital Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data will be available on request to the corresponding author.
References
- 1. Burman KD, Wartofsky L. Clinical practice. N Engl J Med Overseas Ed 2015;373:2347–56. [DOI] [PubMed] [Google Scholar]
- 2. Ron E, Schneider AB. Thyroid Cancer : Schottenfeld D, Cancer Epidemiology and Prevention. New York: Oxford University Press, 2006:975–94. [Google Scholar]
- 3. Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med 1998;338:297–306. 10.1056/NEJM199801293380506 [DOI] [PubMed] [Google Scholar]
- 4. Spielman DB, Badhey A, Kadakia S, et al. Rare Thyroid Malignancies: an Overview for the Oncologist. Clin Oncol 2017;29:298–306. 10.1016/j.clon.2017.01.041 [DOI] [PubMed] [Google Scholar]
- 5. Brito JP, Kim HJ, Han SJ, et al. Geographic distribution and evolution of thyroid cancer epidemic in South Korea. Thyroid 2016;26:864–5. 10.1089/thy.2016.0057 [DOI] [PubMed] [Google Scholar]
- 6. Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev 2009;18:784–91. 10.1158/1055-9965.EPI-08-0960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pandeya N, McLeod DS, Balasubramaniam K, et al. Increasing thyroid cancer incidence in Queensland, Australia 1982-2008 - true increase or overdiagnosis? Clin Endocrinol 2016;84 10.1111/cen.12724 [DOI] [PubMed] [Google Scholar]
- 8. Uhry Z, Colonna M, Remontet L, et al. Estimating infra-national and national thyroid cancer incidence in France from cancer registries data and national hospital discharge database. Eur J Epidemiol 2007;22:607–14. 10.1007/s10654-007-9158-6 [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Wang W. Increasing incidence of thyroid cancer in Shanghai, China, 1983-2007. Asia Pac J Public Health 2015;27:NP223–9. 10.1177/1010539512436874 [DOI] [PubMed] [Google Scholar]
- 10. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317–22. 10.1001/jamaoto.2014.1 [DOI] [PubMed] [Google Scholar]
- 11. Brito JP, Gionfriddo M, Morris JC, et al. Overdiagnosis of thyroid cancer and graves’ disease. Thyroid 2014;24:402–3. 10.1089/thy.2013.0425 [DOI] [PubMed] [Google Scholar]
- 12. Brito JP, Hay ID, Morris JC. Low risk papillary thyroid cancer. BMJ 2014;348:g3045 10.1136/bmj.g3045 [DOI] [PubMed] [Google Scholar]
- 13. Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ 2013;347:f4706 10.1136/bmj.f4706 [DOI] [PubMed] [Google Scholar]
- 14. Kilfoy BA, Zheng T, Holford TR, et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control 2009;20:525–31. 10.1007/s10552-008-9260-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trimboli P, Guglielmi R, Monti S, et al. Ultrasound sensitivity for thyroid malignancy is increased by real-time elastography: a prospective multicenter study. J Clin Endocrinol Metab 2012;97:4524–30. 10.1210/jc.2012-2951 [DOI] [PubMed] [Google Scholar]
- 16. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006;295:2164–7. 10.1001/jama.295.18.2164 [DOI] [PubMed] [Google Scholar]
- 17. Kent WD, Hall SF, Isotalo PA, et al. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ 2007;177:1357–61. 10.1503/cmaj.061730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sosa JA, Hanna JW, Robinson KA, et al. Increases in thyroid nodule fine-needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery 2013;154:1420–7. Discussion 6-7 10.1016/j.surg.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 19. Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”-- screening and overdiagnosis. N Engl J Med 2014;371:1765–7. 10.1056/NEJMp1409841 [DOI] [PubMed] [Google Scholar]
- 20. Brito JP, Davies L. Is there really an increased incidence of thyroid cancer? Curr Opin Endocrinol Diabetes Obes 2014;21:405–8. 10.1097/MED.0000000000000094 [DOI] [PubMed] [Google Scholar]
- 21. Franceschi S, Vaccarella S. Thyroid cancer: an epidemic of disease or an epidemic of diagnosis? Int J Cancer 2015;136:2738–9. 10.1002/ijc.29311 [DOI] [PubMed] [Google Scholar]
- 22. Morris LG, Tuttle RM, Davies L. Changing trends in the incidence of thyroid cancer in the United States. JAMA Otolaryngol Head Neck Surg 2016;142:709–11. 10.1001/jamaoto.2016.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aschebrook-Kilfoy B, Grogan RH. Re: Brito et al., overdiagnosis of thyroid cancer and Graves’ disease. Thyroid 2014;24:403–4. 10.1089/thy.2013.0584 [DOI] [PubMed] [Google Scholar]
- 24. Paschke R, Hegedüs L, Alexander E, et al. Thyroid nodule guidelines: agreement, disagreement and need for future research. Nat Rev Endocrinol 2011;7:354–61. 10.1038/nrendo.2011.1 [DOI] [PubMed] [Google Scholar]
- 25. Liu X, Zhu L, Wang Z, et al. Evolutionary features of thyroid cancer in patients with thyroidectomies from 2008 to 2013 in China. Sci Rep 2016;6:28414 10.1038/srep28414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu XY, Zhu LJ, Cui D, et al. Annual financial impact of thyroidectomies for nodular thyroid disease in China. Asian Pac J Cancer Prev 2014;15:5921–6. 10.7314/APJCP.2014.15.14.5921 [DOI] [PubMed] [Google Scholar]
- 27. La Vecchia C, Malvezzi M, Bosetti C, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer 2015;136:2187–95. 10.1002/ijc.29251 [DOI] [PubMed] [Google Scholar]
- 28. Wienhold R, Scholz M, Adler JR, et al. The management of thyroid nodules: a retrospective analysis of health insurance data. Dtsch Arztebl Int 2013;110:827–34. 10.3238/arztebl.2013.0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 2007;111:508–16. 10.1002/cncr.23116 [DOI] [PubMed] [Google Scholar]
- 30. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214. 10.1089/thy.2009.0110 [DOI] [PubMed] [Google Scholar]
- 31. Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2006;16:109–42. 10.1089/thy.2006.16.109 [DOI] [PubMed] [Google Scholar]
- 32. Othman NH, Omar E, Naing NN. Spectrum of thyroid lesions in hospital Universiti Sains Malaysia over 11years and a review of thyroid cancers in Malaysia. Asian Pac J Cancer Prev 2009;10:87–90. [PubMed] [Google Scholar]
- 33. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1–133. 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.