Abstract
Patent blue V dye (PBV) is frequently used as a perioperative drug for lymphangiography, as well as a food additive. Hypersensitivity to PBV is poorly documented in adults and had not been previously described in children. The diagnosis of PBV allergy depends on corroboration of history consistent with an IgE-mediated reaction and confirmatory skin tests. We present in this paper a paediatric case of PBV anaphylaxis and of biphasic reaction that exemplifies the challenges involved in diagnosing and managing this rare but potentially life-threatening allergic reaction.
Keywords: paediatrics (drugs and medicines), drug therapy related to surgery, immunology, medical management, unwanted effects / adverse reactions
Background
Patent blue V dye (PBV) is part of the triarylmethane dye group.1–5 It is also known as E131, acid blue 3 or disulfine blue. It is used as a food additive, cosmetics, textile dye and component of medicine products like laxatives.2 3 5–9 It is also commonly used as a perioperative drug to visualise lymph nodes in sentinel node biopsy (in the case of breast cancer and malignant melanoma) or in lymphadectomy,1–9 as well as in varicocelectomy.10 11 It is selectively absorbed by lymphatics, binds to albumin at a rate of 50%, and is secreted mainly in the bile and in the urine.3 6 8 9 12
Hypersensitivity reactions to PBV are immediate: they usually develop up to 1 hour after exposure. Although it seems to be IgE-mediated, the exact mechanism and immunogenic portion of PBV for allergies have not been identified.2 7 8 The incidence of all kinds of PBV reactions in adults undergoing sentinel lymph node marking is estimated about 0.1%–2.8%.1–4 9 12
The diagnosis of anaphylaxis is established when IgE-mediated reactions affect more than two organ systems, a drop of blood pressure greater than 30% from the patient’s baseline or a systolic blood pressure lower than 90 mm Hg.13 The three most common causes of anaphylaxis are food, venom and medications.14 15 Drug-induced anaphylaxis represents about 1/4000 emergency visits. In children, the most common drug allergies are non-antibiotics (62.7%), more specifically non-steroidal anti-inflammatory drugs (NSAIDs) (21.6%). In adults, the major culprit is antibiotics (57.8%), mainly beta-lactams (28.1%).16
Biphasic reactions14 17–20 are defined by an asymptomatic period, followed by a second hypersensitivity reaction spontaneously occurring from 1 to 72 hours after the resolution of the initial reaction.4 6 14 17 19 In contrast, protracted anaphylactic reactions do not have an asymptomatic period and last for about 5 hours.21 Biphasic reactions comprise 0.6% to 4.6% of anaphylactic reactions.17 19 20 and have been previously described for PBV allergy.3 4 6 9
We present in this paper a case of biphasic allergic reaction to PBV in a teenager that underlines challenges in the diagnosis and management of patients with this hypersensitivity.
Case presentation
A 17-year-old boy with a history of intermittent exercise-induced asthma, and known allergy to cats and dogs, presented with suspected allergic reaction during a left laparoscopic varicocelectomy. The patient has had no known drug allergies and reported that he tolerates NSAIDs, such as ibuprofen. About 50 min after the intrascrotal injection of 2 mL of PBV (25 mg/mL) and the administration of ketorolac, an NSAID, he developed facial erythema and urticaria on both hands that rapidly spread on his arms, chest and legs. (figure 1) No respiratory or gastrointestinal symptoms were observed during the initial phase of the reaction. He was transferred to the postanesthesia care unit. As the patient was still under sedation, intravenous antihistamines (Benadryl 50 mg), H2 antagonist (Zantac 50 mg) and intravenous glucocorticoids (methylprednisolone 75 mg) were administered. Symptoms resolved completely within a few minutes.
Figure 1.
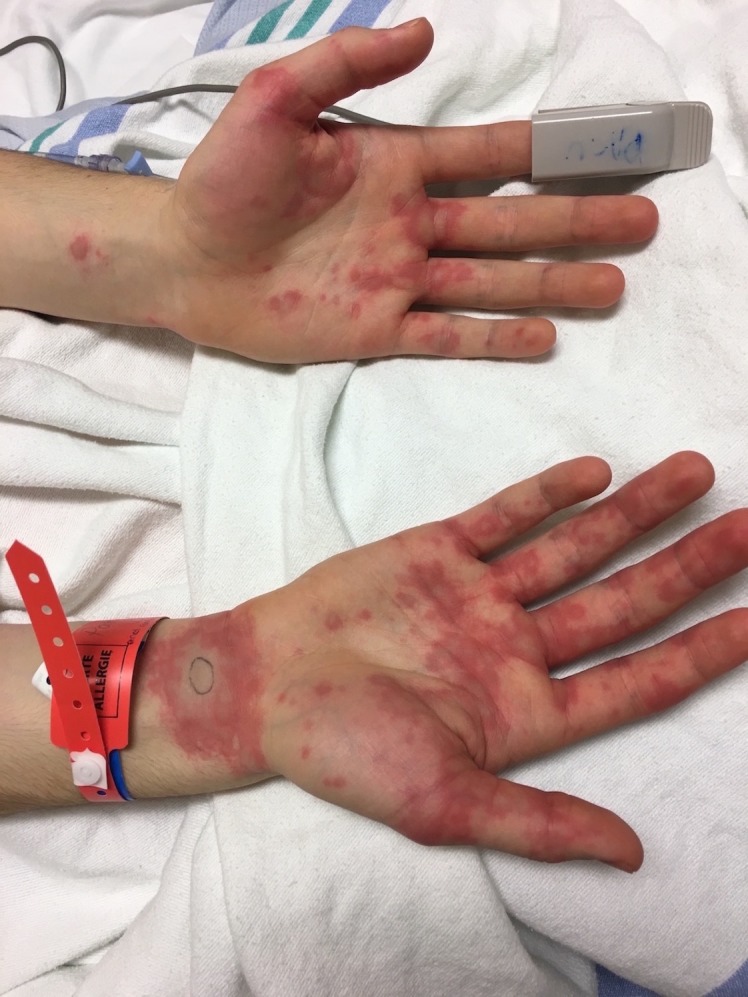
A 17-year-old boy during a laparoscopic varicocelectomy presents with rapidly progressing urticaria.
Two hours later, the patient started to experience neck and back pruritus and erythema, as well as abdominal cramps. Vital signs were stable and normal. Despite oral antihistamine (Reactine 10 mg) that was immediately given, his pruritus persisted and he reported throat tightness. He developed difficulty breathing and urticaria. Given that his symptoms were consistent with anaphylaxis, he was promptly given supplemental oxygen (5 L/min) and administered intramuscular epinephrine (1:1000, 0.5 mL) with gradual resolution of symptoms 75 min later.
The tryptase level, used to exclude mastocytosis, was assessed 16 hours after the second hypersensitivity reaction and was normal at 1.2 ug/L. Twenty hours after his second reaction, the patient was discharged. Assessment with the allergy clinic was scheduled in the following month.
Investigations
Skin prick allergy tests to undiluted PBV (50 mg) and ketorolac (30 mg/mL) were performed, as they were the only medications administered within 1 hour prior to the reaction. Therefore, they were the most likely medications to have induced both allergic reactions. For the skin prick allergy tests, as per guidelines, histamine (10 mg/mL) was the positive reference and for the negative reference, a saline solution of 0.9% NaCl was used.22 Skin prick allergy tests to PBV and ketorolac were negative. Afterwards, due to the previous tolerance of NSAIDs, only an intradermal hypersensitivity test to PBV was performed, with an intention to proceed to intradermal allergy tests for ketorolac, in case of a negative result to PBV. The dilutions were from 1:100 to 1:10. The control test was done with a saline solution (NaCl 0.9%). It was also used to dilute PBV. It revealed a clear weal and a flare positive reaction for the 1:10 dilution (figure 2).
Figure 2.
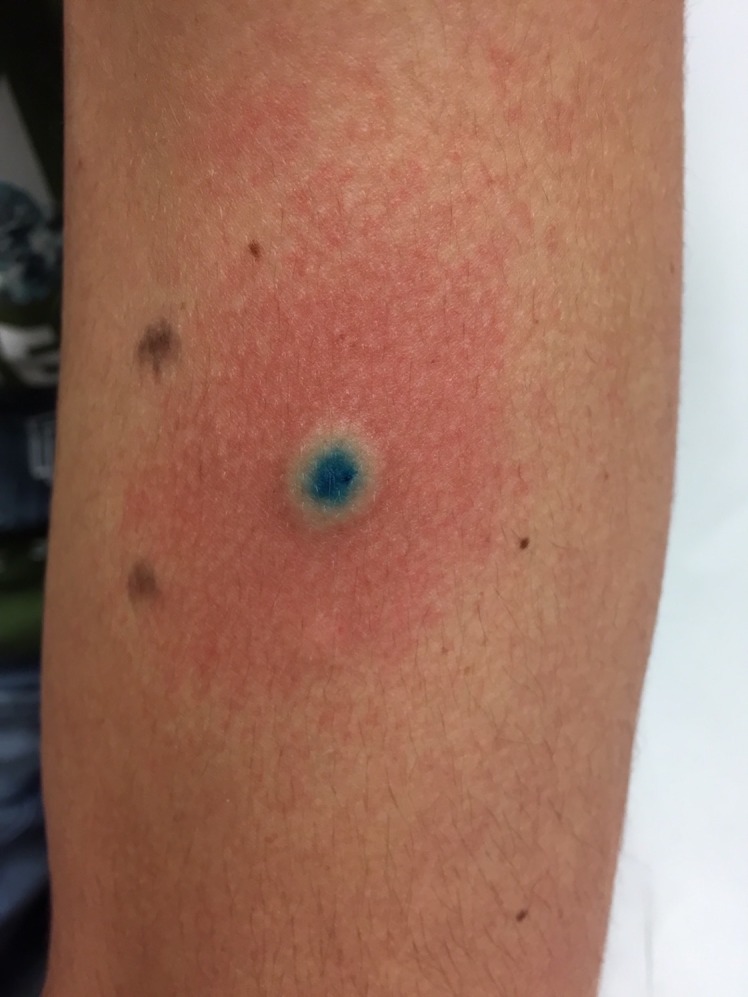
Intradermal allergy test to patent blue dye with a clear weal and positive reaction.
Serial tryptase measurements (usually 1–2 hours after the onset of symptoms and at baseline) are indicated to confirm the diagnosis of anaphylaxis, especially when it is suspected to be drug-triggered, like in our case. It is also recommended when it is venom-related or idiopathic. According to British guidelines, serum tryptase levels should be continuously measured.23 Unfortunately, in our case, the tryptase level was assessed only 16 hours after initial symptoms. Although the normal single measurement is not in line with guidelines to confirm the diagnosis of anaphylaxis, it rules out mastocytosis. In this case, the history is clearly compatible with the diagnosis of anaphylaxis.13
Differential diagnosis
The patient presented with a rapid generalised flushing and urticaria within 1 hour after the exposure to a potential allergen. Additionally, 2 hours after the resolution of his first reaction, he presented with cutaneous symptoms (urticaria), gastrointestinal symptoms (abdominal cramps) and respiratory symptoms (throat tightness and dyspnoea), establishing the diagnosis of anaphylaxis.13 Therefore, this presentation is consistent with the diagnosis of a biphasic type one hypersensitivity with anaphylaxis.
The three most common triggers of IgE-mediated reactions and anaphylaxis are food, venom and medications. In children, 84.5% of anaphylactic shocks are due to food, most commonly peanut, tree nuts and eggs.14 15 However, in this patient’s case, since the patient underwent a surgery with no exposure to food in the hours prior to the reaction, food allergen was unlikely. Given the temporal association with medications given during the operation, mainly PBV and ketorolac, drug-induced anaphylaxis is the most likely diagnosis. Common triggers of drug-induced allergic reactions during surgery are neuromuscular blocking agents, latex, antibiotics, local anaesthetics, anaesthetic induction, antiseptics, opioids, NSAIDs and colloids. Chlorhexidine, a topical antiseptic, is a common culprit in perioperative anaphylaxis. However, it is improbable in our case, given that it was used after the patient’s reaction, with no symptoms. Latex can also be a potential trigger. However, it is unlikely in our case, as healthcare professionals in our hospital use latex-free gloves.1 2 8 24 In our case, the most likely culprits are the medications given within 1 hour prior to the initial symptoms. Given that PBV and ketorolac are the only administered medications within this time frame, they are the most probable allergens and other agents were dismissed. Given the negative skin prick allergy tests and prior tolerance to NSAIDs, ketorolac hypersensitivity is less likely. Therefore, intradermal hypersensitivity tests were only performed for PBV. The results were positive, making PBV hypersensitivity the most likely.
Regarding eruptive skin rashes in children, they can be associated to viral exanthema. However, it is improbable in this case, given the clear temporal association with the specific drug culprit, the rapid resolution after the use of epinephrine and the lack of any allergic reactions after avoidance of PBV.
Treatment
Avoidance of PBV and any possible sources of it was recommended. A medical note was added to his medical file for his allergy to PBV and he was advised to obtain a MedicAlert bracelet.
In the case that viewing lymphatic vessels is required in the future, methylene blue can be an alternative: it has a lower risk of inducing an allergic reaction. However, it is less readily absorbed by lymphatics and can cause necrosis and capsular contraction.1 2 4 7 In the case of an inevitable use of PBV, the injection of a lower volume has been reported to decrease the risk of allergic reactions.4
Outcome and follow-up
A PBV challenge was not performed, since there are no published protocols or any data on their sensitivity and specificity. A challenge was also considered unethical, given the high risk of anaphylaxis. The patient did not experience any allergic events in the following 6 months.
Discussion
This case exemplifies the diagnostic and management challenges for patients with PBV allergy. Presentations of allergic reactions to PBV greatly vary from one individual to another, and the mechanism underlying it is still unresolved. Johansson et al 8 hypothesised that IgE-sensitised patients do not react to PBV itself, but rather to a special combination of hapten with an unidentified serum carrier. Regardless of the validity of this hypothesis, allergic reactions to PBV are considered IgE-mediated.2 7 8
The history of reaction is consistent with previous reports on the clinical presentation of PBV allergy. However, all of the previously reported cases were adults. Compared with other intravenous drugs inducing anaphylaxis, PBV allergy tends to have a more delayed onset and symptoms usually appear, on average, 30 min after exposure.2–7 9 12
It is reported that PBV allergy presents with symptoms limited to the skin in 69%–87% of cases (eg, urticaria, pruritus, generalised rash). A key diagnostic tool is the blue colouration of the cutaneous plaques.1 3–5 7 9 12 However, in our case, this blue colouration was not present. There are no data on the proportion of patients lacking this blue hue. It is hypothesised that, in some cases, smaller amounts of dye reaching the systemic circulation are not sufficient to stain the urticarial plaques.6 7 There is currently no validated explanation for this non-colouration.
An interesting characteristic of our case is the biphasic reaction.14 17 18 20 It is reported that biphasic reactions account for 0.6%–4.6% of anaphylactic reactions.17 19 20 It represents about 25% of fatal or near-fatal food-induced allergies, and 23% of drug-induced allergies.14 Biphasic reactions to PBV have been previously described in adults.3 4 6 9 To our knowledge, it is the first case reporting biphasic reactions to PBV in a teenager. It is hypothesised that these reactions are due to the delayed release of inflammatory mediators, such as prostaglandins, nitric oxide and leukotrienes.9 Other potential explanations relate to the long half-life of PBV and the slow release of PBV from the parenchymal tissue that could trigger a second reaction.4 9 Unlike most biphasic reactions,19 the second reaction was more severe than the initial one. The mechanism underlying this phenomenon remains unclear. It is possible that this reaction developed due to the fact that epinephrine was not used promptly to treat the reaction.19 Therefore, it is recommended to promptly administer epinephrine as stipulated in the guidelines on anaphylaxis management, regardless of severity.13
Our case supports the use of skin tests to confirm the diagnosis of PBV allergy. It is recommended to perform PBV skin prick allergy tests first, with a dilution of up to 1:1000.2 4 6 7 25 In the case of a negative result or to confirm a positive response, intradermal hypersensitivity tests are required and reported to have 100% sensitivity.24
This paper has some limitations. Given that tryptase levels were drawn 16 hours after the event, its levels could not be used to establish the presence of anaphylaxis, since we do not have the tryptase level during the reaction. In anaphylaxis, tryptase levels peak between 2–5 hours after symptom initiation.14 However, the history is clearly compatible with the diagnosis of anaphylaxis.13
The diagnosis of PBV allergy is challenging due to the uncertainty of its mechanism, to the potential absence of the characteristic blue urticaria, to the variability in clinical presentation, and to its poor documentation, especially in children. Hypersensitivity to PBV should always be considered in the differential diagnosis of allergic reactions occurring postsurgery when PBV is used, even in the absence blue colouration of the cutaneous plaques. Educational programmes contributing to prompt epinephrine use are required in order to appropriately manage these patients.
Learning points.
Consider patent blue V dye (PBV) as a trigger for anaphylaxis postsurgically in children and adolescents.
Reactions to PBV can happen without a cutaneous blue discolouration.
Epinephrine should be administered rapidly to prevent a biphasic reaction.
Footnotes
Patient consent for publication: Parental/guardian consent obtained.
Contributors: ML was responsible for collecting the data, writing the case report, completing the documents related to the submission, revising and submitting the manuscript. CM was the physician who assessed the patient during his allergic reaction, made the diagnosis, reviewed the documents regarding the case report and revised the manuscript. MB-S was responsible for the investigations of the patient’s allergy, for reviewing the documents related to the case report, to help for logistics and revising the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Barthelmes L, Goyal A, Newcombe RG, et al. Adverse reactions to patent blue V dye - the NEW START and ALMANAC experience. Eur J Surg Oncol 2010;36:399–403. 10.1016/j.ejso.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 2. Haque RA, Wagner A, Whisken JA, et al. Anaphylaxis to patent blue V: a case series and proposed diagnostic protocol. Allergy 2010;65:396–400. 10.1111/j.1398-9995.2009.02248.x [DOI] [PubMed] [Google Scholar]
- 3. Maranhão MV, da Nóbrega DK, Anunciação CE, et al. Allergic reaction to patent blue dye in breast surgery - case report. Braz J Anesthesiol 2016;66:433–6. 10.1016/j.bjane.2014.02.018 [DOI] [PubMed] [Google Scholar]
- 4. Mertes PM, Malinovsky JM, Mouton-Faivre C, et al. Anaphylaxis to dyes during the perioperative period: reports of 14 clinical cases. J Allergy Clin Immunol 2008;122:348–52. 10.1016/j.jaci.2008.04.040 [DOI] [PubMed] [Google Scholar]
- 5. Wong A, Spillane A. Breast Surgeons of Australia and New Zealand Incorporated (BreastSurgANZ). Patent Blue V dye anaphylaxis: experience of Australian and New Zealand surgeons. ANZ J Surg 2014;84:37–41. 10.1111/j.1445-2197.2012.06277.x [DOI] [PubMed] [Google Scholar]
- 6. Howard JD, Moo V, Sivalingam P. Anaphylaxis and other adverse reactions to blue dyes: a case series. Anaesth Intensive Care 2011;39:287–93. [DOI] [PubMed] [Google Scholar]
- 7. Hunting AS, Nopp A, Johansson SG, et al. Anaphylaxis to Patent Blue V. I. Clinical aspects. Allergy 2010;65:117–23. 10.1111/j.1398-9995.2009.02192.x [DOI] [PubMed] [Google Scholar]
- 8. Johansson SG, Nopp A, Oman H, et al. Anaphylaxis to Patent Blue V. II. A unique IgE-mediated reaction. Allergy 2010;65:124–9. 10.1111/j.1398-9995.2009.02191.x [DOI] [PubMed] [Google Scholar]
- 9. Liang MI, Carson WE. Biphasic anaphylactic reaction to blue dye during sentinel lymph node biopsy. World J Surg Oncol 2008;6:79 10.1186/1477-7819-6-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Capolicchio JP, El-Sherbiny M, Brzezinski A, et al. Dye-assisted lymphatic-sparing laparoscopic varicocelectomy in children. J Pediatr Urol 2013;9:33–7. 10.1016/j.jpurol.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 11. Golebiewski A, Krolak M, Komasara L, et al. Dye-assisted lymph vessels sparing laparoscopic varicocelectomy. J Laparoendosc Adv Surg Tech A 2007;17:360–3. 10.1089/lap.2006.0072 [DOI] [PubMed] [Google Scholar]
- 12. Tripathy S, Nair PV. Adverse drug reaction, patent blue V dye and anaesthesia. Indian J Anaesth 2012;56:563 10.4103/0019-5049.104576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report–second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med 2006;47:373–80. 10.1016/j.annemergmed.2006.01.018 [DOI] [PubMed] [Google Scholar]
- 14. Ben-Shoshan M, Clarke AE. Anaphylaxis: past, present and future. Allergy 2011;66:1–14. 10.1111/j.1398-9995.2010.02422.x [DOI] [PubMed] [Google Scholar]
- 15. Soller L, Ben-Shoshan M, Harrington DW, et al. Adjusting for nonresponse bias corrects overestimates of food allergy prevalence. J Allergy Clin Immunol Pract 2015;3:291–3. 10.1016/j.jaip.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 16. Gabrielli S, Clarke AE, Eisman H, et al. Disparities in rate, triggers, and management in pediatric and adult cases of suspected drug-induced anaphylaxis in Canada. Immun Inflamm Dis 2018;6:3–12. 10.1002/iid3.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee S, Bellolio MF, Hess EP, et al. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2015;3:408–16. 10.1016/j.jaip.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 18. Lee JM, Greenes DS. Biphasic anaphylactic reactions in pediatrics. Pediatrics 2000;106:762–6. 10.1542/peds.106.4.762 [DOI] [PubMed] [Google Scholar]
- 19. Lieberman PL, Feldweg A, Simons F. Biphasic and protracted anaphylaxis. https://www.uptodate.com/contents/biphasic-and-protracted-anaphylaxis (Accessed 1 Jun 2018).
- 20. Penney K, Balram B, Trevisonno J, et al. Incidence of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol 2015;135:AB204 10.1016/j.jaci.2014.12.1602 [DOI] [PubMed] [Google Scholar]
- 21. Kim TH, Yoon SH, Lee SY, et al. Biphasic and protracted anaphylaxis to iodinated contrast media. Eur Radiol 2018;28:1242–52. 10.1007/s00330-017-5052-0 [DOI] [PubMed] [Google Scholar]
- 22. Kowal K, DuBuske L, Wood RA. Overview of skin testing for allergic disease. https://www.uptodate.com/contents/overview-of-skin-testing-for-allergic-disease (Accessed 1 Nov 2018).
- 23. Buka RJ, Knibb RC, Crossman RJ, et al. Anaphylaxis and clinical utility of real-world measurement of acute serum tryptase in UK Emergency Departments. J Allergy Clin Immunol Pract 2017;5:1280–7. 10.1016/j.jaip.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 24. Platt P, Roberts L. Anaphylaxis to patent blue dye–misadventure or misdemeanour? Anaesth Intensive Care 2011;39:166–8. [DOI] [PubMed] [Google Scholar]
- 25. Brockow K, Garvey LH, Aberer W, et al. Skin test concentrations for systemically administered drugs–an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2013;68:702–12. 10.1111/all.12142 [DOI] [PubMed] [Google Scholar]


