Abstract
Introduction
The built environment defines opportunities for healthy eating and physical activity and may thus be related to blood lipids. The aim of this study is to systematically analyse the scientific evidence on associations between built-environment characteristics and blood lipid levels in adults.
Methods
PubMed, EMBASE and Web of Science were searched for peer-reviewed papers on population-based studies up to 9 October 2017. We included studies that reported on built-environment characteristics and blood lipid levels in adult populations (≥18 years). Two reviewers independently screened titles/abstracts and full-texts of papers and appraised the risk of bias of included studies using an adapted version of the Quality Assessment Tool for Quantitative Studies. We performed meta-analyses when five or more studies had sufficient homogeneity in determinant and outcome.
Results
After screening 6902 titles/abstracts and 141 potentially relevant full-text articles, we included 50 studies. Forty-seven studies explored associations between urban versus rural areas with blood lipid levels. Meta-analyses on urban versus rural areas included 133 966 subjects from 36 studies in total. Total cholesterol levels were significantly and consistently higher in urban areas as compared with rural areas (mean difference 0.37 mmol/L, 95% CI 0.27 to 0.48). Urban/rural differences in high density lipoprotein cholesterol were inconsistent across studies and the pooled estimate showed no difference (0.00 mmol/L 95% CI −0.03 to 0.04). Low density lipoprotein (LDL) cholesterol and triglyceride levels were higher in urban than in rural areas (mean difference 0.28, 95% CI 0.17 to 0.39 and 0.09, 95% CI 0.03 to 0.14, respectively).
Conclusions
Total and LDL cholesterol levels and triglycerides were consistently higher in residents of urban areas than those of rural areas. These results indicate that residents of urban areas generally have less favourable lipid profiles as compared with residents of rural areas.
Prospero registration number
CRD42016043226.
Keywords: built environment, cholesterol, triglycerides, blood lipids, lifestyle behaviours
Key questions.
What is already known?
Built-environment characteristics are known to influence lifestyle behaviours such as physical activity and dietary behaviour.
These lifestyle behaviours are established determinants of blood lipid levels.
What are the new findings?
This systematic review and meta-analysis shows that low density lipoprotein and total cholesterol and triglyceride levels are consistently less favourable in urban areas as compared to rural areas.
No overall differences in high density lipoprotein cholesterol were found between urban and rural areas.
What do the new findings imply?
The ongoing urbanisation worldwide may have health consequences related to blood lipid levels.
Further research is needed to better understand in which way urbanisation may affect blood lipid levels.
Introduction
Elevated blood lipid levels are an established risk factor for cardiovascular diseases and contribute in a meaningful way to the global burden of disease. Globally, high total cholesterol (TC) levels are estimated to account for 4.5% of the total deaths.1–3 Physical activity and low consumption of food high in saturated fat and dietary cholesterol, and high intake of food high in unsaturated fatty acids, especially omega-3 fatty acids, are associated with more favourable blood lipid profiles.4–6 In particular, the favourable effects of physical activity on high density lipoprotein (HDL) cholesterol and triglycerides is well documented.7 Dietary and physical activity behaviour is, in turn, influenced by built-environment characteristics that directly and indirectly facilitate or inhibit the maintenance of a healthy lifestyle.8 9 For example, the availability, accessibility and affordability of food and fast-food outlets have been found to be associated with dietary behaviour,10 and the availability and proximity of opportunities to be physically active have been linked to leisure time physical activity.11 12 Hence, in their capacity to affect lifestyle behaviour, built-environment characteristics may be 'upstream' determinants of blood lipid levels.13–20
A common focus of the many studies that have investigated built-environment characteristics and blood lipid levels is the difference between residents of urban and rural areas. Urban-rural differences in blood lipid levels may be prevalent due to several aspects: urban areas may generally score higher on walkability as compared with rural areas, thereby facilitating light physical activity.21 22 This could have beneficial effects in terms of reducing blood lipid levels for those living in more rural areas. Also, it may be that adults living in exposure to unhealthy food (outlets) may differ across urban and rural areas, which may influence blood lipid levels via dietary intake. Systematic reviews that examined urban-rural differences in relation to other health outcomes reported that rural residence is associated with higher bodyweight18 and urban residence with higher risk/prevalence of type 2 diabetes,23 and, in India, with higher prevalence of hypertension.24 A cross-country study with 17 countries reported the rate of major cardiovascular events (myocardial infarction, stroke and heart failure) was higher in rural compared with rural areas in low-income and middle-income countries (LMIC).25 Interestingly, urban communities had higher risk factor scores. For policy makers, gaining insight into the health effects of urbanisation is highly relevant, as the United Nations projects that by 2050, 70% of the global population will reside in urban areas.26 27
In spite of it being a widely studied topic, a comprehensive overview of the relationship between built-environment characteristics and blood lipids is lacking. Therefore, we aimed to systematically review and meta-analyse the scientific evidence on associations between built-environment characteristics potentially related to physical activity, sedentary behaviour, dietary habits and blood lipid levels in adults.
Methods
We conducted a systematic review and meta-analysis of studies seeking to assess the association between the built environment and total, HDL and low density lipoprotein (LDL) cholesterol; HDL/LDL cholesterol ratio and/or triglyceride levels. The structure of this review conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)-statement. The protocol of this systematic review was published and registered in PROSPERO in advance (www.crd.york.ac.uk/prospero, ID:CRD42016043226).
Literature search strategy
To identify all relevant publications, we performed systematic searches in the bibliographic databases PubMed, EMBASE.com and the Web of Science Core Collection up to 9 October 2017 (LS, RdG). Search terms included indexed terms from MeSH in PubMed, EMtree in EMBASE as well as free texts in titles and abstracts. Search terms related to ‘cholesterol’ or ‘triglycerides’ were used in combination with search terms including ‘built environment’. Full-text, peer-reviewed articles in English, French and Dutch were included. Duplicate articles were excluded. The full search strategy for all databases can be found in online supplementary appendix A. In addition, reference lists of the full-text articles included were searched for potentially eligible articles (ie, backward screening) and a citation search (ie, forward screening (RdG)).
bmjgh-2018-001017supp001.pdf (200KB, pdf)
Screening and eligibility criteria
Study designs that sought to assess associations between the built environment and TC, HDL and/or LDL cholesterol and/or triglycerides were considered eligible for systematic review. Two authors (RdG and JL) independently screened all potentially relevant titles and abstracts. Subsequently, full-texts were screened for eligibility using prespecified inclusion and exclusion criteria. Studies were included if they: (1) reported on adults (aged >18 years or mixed age groups, thus drawing separate conclusions/results for adults); (2) were population-based; (3) were peer-reviewed, published, full-texts; (4) reported on the association between built and/or physical-environment characteristics and total, HDL and LDL cholesterol; HDL/LDL cholesterol ratio and/or triglyceride levels; (5) included objectively or subjectively measured built-environment characteristics; (6) and were written in Dutch, French or English. Studies were excluded if they reported on the same population as another study that was included (of these, only the most relevant article was included). There were no restrictions with regard to ethnicity or nationality of study populations. Studies were eligible for meta-analyses if descriptive statistics (mean, SD or SE and number of participants) were available as these are necessary to construct mean differences. Differences in judgement were resolved by reaching consensus (RdG and JL) and by consultation with a third author (KvdH) if disagreements were not resolved. Meta-analyses were performed in the event that more than five studies on the same environmental characteristic were identified with sufficient similarity in determinant and outcome.
Data extraction and study outcomes
A data extraction form was developed and pilot-tested on five randomly selected included studies and refined accordingly. Data were extracted by one author (RdG) and 5% were randomly checked (JL). The extraction form included author(s), country of study, year of publication, journal reference, participant characteristics (age, sex, number of participants and inclusion criteria pertaining to age), study design, data collection methods, environment characteristics and definition of the exposure. Only two comparators were extracted: if multiple urbanisation levels—that is, urban, rural, semirural—were reported, these were pooled into two categories where possible, otherwise only urban and rural were extracted. For this study, data on urban and rural areas were extracted based on the categorisation as provided by the authors of the included studies. Hence, no uniform definition was used. As part of the quality assessment, an item regarding the reporting on the used definition was included (see Q16 of online supplementary appendix B). Furthermore, we extracted the unit of measurement of blood lipids, whether lipid measurements were taken while fasting or non-fasting, summary measures of the outcome(s) including type of analysis and, if applicable, regression coefficient, CIs, mean, SD and whether or not a statistical difference was found.
bmjgh-2018-001017supp002.pdf (465.6KB, pdf)
In the event that more clarification or additional information was required, the authors of the original studies were contacted up to five times. First, three attempts to contact the first author were made and, if unsuccessful, the second author and, subsequently, the last author were contacted. When contact details of any of these authors could not be found, attempts were made to contact any of the other authors until five attempts were made. We requested information from authors of 47 of the studies included and successfully contacted authors of 33 studies.
Quality assessment
To assess the quality of the studies included, we used an adapted version of the Quality Assessment Tool for Quantitative Studies (QATQS, online supplementary appendix B), used previously for similar purposes.14 23 The adjusted QATQS was pilot tested for clarity on five studies included and consisted of the following six domains: study design, selection bias, withdrawals and dropouts, confounders, data collection and reporting. Although our research question differed from the majority of the research questions of the studies included, we assessed the quality of these studies in relation to our research question that is, the association between environment characteristics and the outcome. Analysis or reporting of the results may, therefore, have been appropriate for the research question of the original paper, but not sufficient in light of the aim of this systematic review. Each domain was rated as strong, moderate, weak or not applicable, which resulted in an overall quality score. Studies with at least three strong domains and no weak domains were classified as strong. Moderate was assigned to studies with two weak domains or fewer than three strong domains. Studies with more than two weak domains were rated as weak.
Data synthesis and analysis
A narrative of the findings from the studies included was written, structured around the type of outcome, the built-environment characteristics under study and the quality (strong/moderate vs weak). The meta-analyses were performed using R Studio V.0.99.896 and the Metafor package, using a random effects model. The pooled estimates in the forest plots were presented as mean differences with 95% CIs between groups. The forest plots were grouped by study quality (moderate-strong and weak) and by sex. Heterogeneity in study outcomes was assessed using the I2 statistic. We assessed potential publication bias by evaluating the symmetry of funnel plots for each blood lipid under study. Since the included studies were published over a considerable time span 1980–2017, additional sensitivity analyses were performed in which we meta-analysed studies stratified by three time periods: from 1980 to 1999, 2000–2009 and from 2010 to 2017.
Results
Study selection
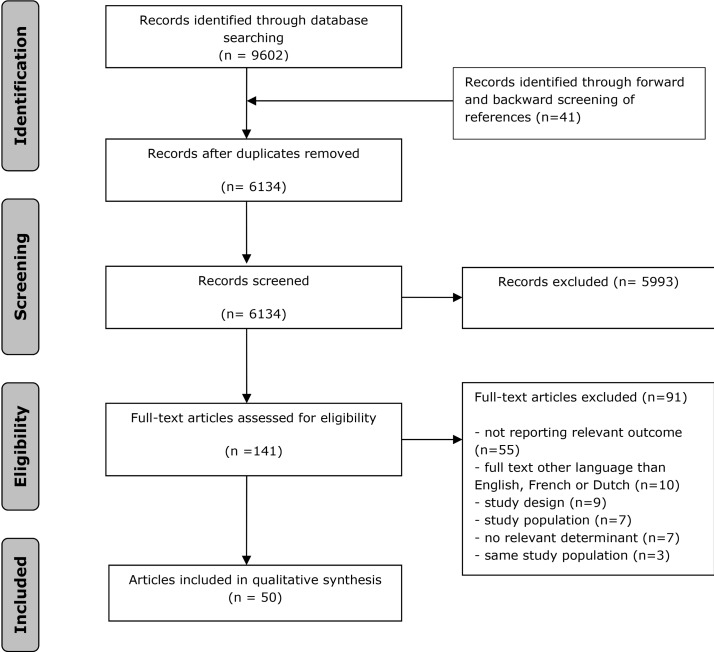
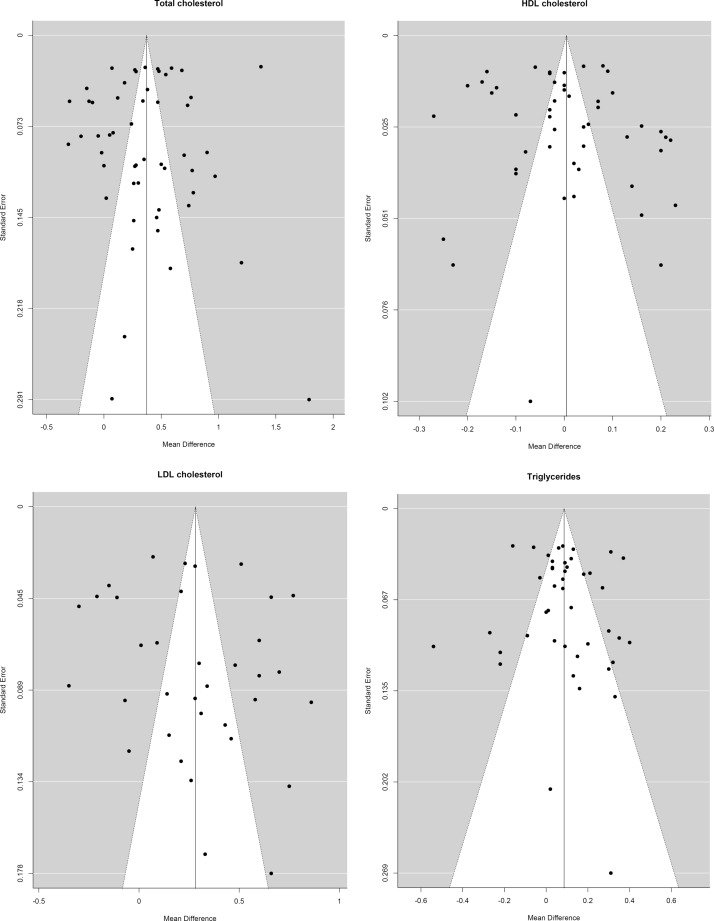
The search generated a total of 9602 articles, of which 3509 were duplicates, leaving 6134 unique articles, (see figure 1). We excluded 5993 articles after screening the titles and abstracts and reviewed the remaining 141 full-texts. Of those 141 full-texts, (1) 54 did not report on a relevant outcome; (2) 10 were in a language other than English, French or Dutch; (3) 10 studies were excluded because of study design; (4) 7 studies were excluded because of the study population; (5) 7 studies were excluded because no relevant built-environment determinants were studied and (6) 5 studies reported on 2 of the same study populations, therefore 3 of these studies were excluded. As a result, a total of 50 studies met the eligibility criteria and were included. Evidence of heterogeneity across studies included in the meta-analysis was observed, I2 ranged from 90.4% to 98.1%. The symmetry of the funnel plots (figure 2) suggests the absence of publication bias. The plots also show some dispersion on top, indicating heterogeneity in outcomes between studies, which is in line with the observed I2 statistic values.
Figure 1.
Flowchart.
Figure 2.
Funnel plots. HDL, high density lipoprotein; LDL, low density lipoprotein.
Study characteristics
The majority of the studies included (47) reported on differences in blood lipids between urban and rural environments. The characteristics of these studies are summarised in table 1. Most of these studies were conducted in Asia (30, of which 11 in India and 10 in China) and Africa (10). With the exception of two studies28 29 that had a longitudinal observational design, all urban/rural studies had a cross-sectional design and were published between 1980 and 2017, the median year of publication being 2009 (IQR: 2001–2015. With the exception of one study published in French,30 all studies were published in English. Seven studies provided a reference for their operationalisation of urban and rural areas, most often citing a national statistics bureau (see online supplementary appendix C). The majority of the studies (30) only stated which cities and villages were considered to be urban and rural, the remainder of the studies10 reported no information on their definitions. Thirty-three studies reported blood lipid levels for men and women separately, 12 studies for men and women combined, 3 exclusively for women and 2 for men. Of the 47 studies that investigated urban-rural environment differences, 2 investigated differences between people who lived in rural areas and those who migrated to an urban area.31 32 The remaining studies included in this review focused on accessibility of markets/parks (1) community-based interventions (1) and walkability (1).
Table 1.
Characteristics of included ‘urban-rural’ built environment characteristic studies
| Study | Country | Study design |
Number of participants | Blood lipid | Mean±SD mmol/L per blood lipid | ||||||||||
| Urban | Rural | Urban | Rural | ||||||||||||
| ♀♂ | ♀ | ♂ | ♀♂ | ♀ | ♂ | ♀♂ | ♀ | ♂ | ♀♂ | ♀ | ♂ | ||||
| Abdul-Rahim, Husseini46 | Palestine | CS | 492 | 500 | TC | 5.23±1.33 | 5.15±1.11 | ||||||||
| HDL | 0.90±0.22 | 1.17±0.45 | |||||||||||||
| LDL | 3.75±1.10 | 3.27±1.32 | |||||||||||||
| TG | 1.86±2.66 | 1.53±1.57 | |||||||||||||
| Aguilar-Salinas, Lerman-Garber47 | Mexico | CS | 167 | 81 | 56 | 40 | TC | 5.75±1.12 | 5.6±0.95 | 5.5±1.1 | 5.02±0.97 | ||||
| HDL | 1.26±0.33 | 1.08±0.26 | 1.26±0.28 | 1.31±0.36 | |||||||||||
| LDL | 3.93±1.08 | 3.86±0.93 | 3.6±1.1 | 3.2±0.92 | |||||||||||
| TG | 1.68±0.42 | 1.96±1.38 | 1.56±0.49 | 1.65±1.4 | |||||||||||
| Al-Nuaim48 | Saudi Arabia | CS | 864 | 875 | 584 | 601 | TC | 4.35±1.5 | 4.2±1.4 | 4.4±1.5 | 4.4±1.6 | ||||
| HDL | 1.2±0.5 | 1.3±0.6 | 1.3±0.8 | 1.4±0.8 | |||||||||||
| LDL | 3.3±1.3 | 3.1±1.5 | 3.0±1.5 | 2.5±1.0 | |||||||||||
| Cai, Zhang49 | China | CS | TC | 5.14±1.04 | 5.00±0.98 | 4.96±0.99 | 5.09±1.09 | ||||||||
| HDL | 1.60±0.48 | 1.37±0.40 | 1.57±0.41 | 1.37±0.48 | |||||||||||
| LDL | 3.32±1.00 | 3.23±0.95 | 3.14±0.96 | 3.37±1.11 | |||||||||||
| TG | 1.35±1.09 | 1.69±1.51 | 1.32±1.29 | 1.89±2.37 | |||||||||||
| Campos, Bailey50 | Costa Rica | CS | 86 | 99 | 88 | 103 | TC | 4.89±0.85 | 4.73±0.80 | 4.63±1.09 | 4.47±0.88 | ||||
| HDL | 1.19±0.26 | 1.01±0.23 | 1.16±0.23 | 1.09±0.23 | |||||||||||
| LDL | 3.05±0.75 | 2.87±0.78 | 2.84±0.88 | 2.72±0.80 | |||||||||||
| TG | 1.41±0.70 | 1.82±0.89 | 1.32±0.64 | 1.50±0.71 | |||||||||||
| Das, Pal51 | India | CS | 102 | 122 | 89 | 135 | TC | 4.97±0.60 | 5.12±0.76 | 5.28±0.60 | 5.14±0.74 | ||||
| HDL | 1.15±0.12 | 1.18±0.12 | 1.17±0.13 | 1.17±0.15 | |||||||||||
| LDL | 3.07±0.59 | 3.19±0.75 | 3.42±0.61 | 3.26±0.76 | |||||||||||
| TG | 1.63±0.26 | 1.64±0.32 | 1.51±0.25 | 1.55±0.32 | |||||||||||
| Delisle, Ntandou-Bouzitou33 | Benin | CS | 100 | 100 | 85 | 85 | HDL | 1.37±0.40 | 1.22±0.20 | 1.62±0.37 | 1.29±0.92 | ||||
| Du, Su52 | China | CS | 2879 | 918 | TC | 4.34 (3.04 to 5.15) | 4.20 (2.76 to 5.05) | ||||||||
| HDL | 1.15 (0.95 to 1.36) | 1.03 (0.83 to 1.26) | |||||||||||||
| LDL | 2.77 (2.33 to 3.32) | 2.78 (2.26 to 3.32) | |||||||||||||
| TG | 1.85 (1.13 to 3.94) | 2.05 (1.27 to 4.13) | |||||||||||||
| Gharbi, Belhani30 | Tunisia | CS | 201 | 168 | 155 | 146 | TC | 4.75±1.50 | 4.51±1.27 | 4.27±1.13 | 4.05±1.30 | ||||
| HDL | 1.07±0.39 | 0.90±0.13 | 1.05±0.44 | 0.80±0.15 | |||||||||||
| TG | 1.41±1.12 | 1.50±1.42 | 1.06±0.67 | 1.34±0.91 | |||||||||||
| Glew, Conn53 | Nigeria | CS | 77 | 55 | 79 | 42 | TC | 4.40±0.77 | 4.09±0.75 | 3.62±0.80 | 3.62±0.77 | ||||
| HDL | 1.29±0.29 | 1.16±0.27 | 1.06±0.30 | 0.96±0.34 | |||||||||||
| LDL | 2.40±0.70 | 2.17±0.65 | 1.94±0.71 | 1.91±0.65 | |||||||||||
| TG | 1.52±0.74 | 1.67±1.00 | 1.22±0.74 | 1.65±1.02 | |||||||||||
| Gregory, Dai54 | Guatemala | CS | 155 | 119 | 372 | 241 | TC | 4.26±0.85 | 4.35±0.91 | 4.21±0.79 | 4.00±0.83 | ||||
| HDL | 1.04±0.26 | 0.86±0.23 | 1.00±0.28 | 0.88±0.24 | |||||||||||
| LDL | 2.42±0.71 | 2.58±0.82 | 2.33±0.65 | 2.24±0.69 | |||||||||||
| TG | 1.82±1.01 | 2.06±1.15 | 1.91±0.91 | 1.93±1.00 | |||||||||||
| Gu, Reynolds55 | China | CS | 4163 | 3730 | 3851 | 3796 | TC | 5.08±1.23 | 5.00±1.22 | 4.81±1.24 | 4.72±1.29 | ||||
| HDL | 1.36±0.62 | 1.21±0.61 | 1.36±0.62 | 1.35±0.65 | |||||||||||
| LDL | 3.05±1.23 | 3.03±1.22 | 2.82±1.24 | 2.75±1.29 | |||||||||||
| TG | 1.50±1.23 | 1.75±1.83 | 1.42±1.24 | 1.38±1.29 | |||||||||||
| Gupta, Prakash56 | India | CS | 199 | 202 | TC | 4.55±1.11 | 4.27±0.96 | ||||||||
| HDL | 1.11±0.31 | 1.14±0.31 | |||||||||||||
| LDL | 2.78±1.01 | 2.50±0.85 | |||||||||||||
| TG | 1.42±0.62 | 1.38±0.52 | |||||||||||||
| He, Gu57* | China | CS | 4163 | 3730 | 3851 | 3796 | TC | 5.08 | 4.99 | 4.8 | 4.71 | ||||
| HDL | 1.35 | 1.21 | 1.36 | 1.34 | |||||||||||
| LDL | 3.06 | 3.02 | 2.81 | 2.75 | |||||||||||
| TG | 1.50 | 1.75 | 1.42 | 1.38 | |||||||||||
| Htet, Bjertness58 | Myanmar | CS | 379 | 376 | 362 | 369 | TC | 5.4±0.95 | 5.5±0.97 | 5.4±1.75 | 5.0±1.73 | ||||
| HDL | 1.1±0.19 | 1.2±0.19 | 1.3±0.19 | 1.3±0.38 | |||||||||||
| TG | 1.6±0.76 | 1.9±1.36 | 1.4±1.75 | 1.5±1.34 | |||||||||||
| Huang, Wu59 | China | CS | 2361 | 2552 | 2341 | 1631 | TC | 4.85±1.00 | 4.73±0.90 | 4.17±0.92 | 4.25±0.91 | ||||
| HDL | 1.46±0.36 | 1.33±0.32 | 1.37±0.32 | 1.33±0.33 | |||||||||||
| Joshi, Anjana60 | India | CS | 590 | 1452 | TC | 4.32±1.11 | 3.98±0.98 | ||||||||
| HDL | 1.01±0.31 | 1.01±0.31 | |||||||||||||
| LDL | 2.51±0.85 | 2.3±0.83 | |||||||||||||
| Kodaman, Aldrich61 | Ghana | CS | 1293 | 972 | 583 | 469 | TC | 4.70±1.09 | 4.41±1.1 | 3.94±0.95 | 3.68±0.94 | ||||
| HDL | 1.27±0.38 | 1.12±0.34 | 1.20±0.41 | 1.15±0.38 | |||||||||||
| LDL | 2.95±0.97 | 2.75±0.88 | 2.29±0.84 | 1.98±0.71 | |||||||||||
| TG | 0.94±0.64 | 0.87±0.53 | 0.93±0.59 | 0.93±0.60 | |||||||||||
| Lim, Jang28 | South Korea | LT | 2497 | 2523 | 2784 | 2240 | TC | 5.19±0.97 | 5.33±0.92 | 5.12±0.93 | 4.86±0.93 | ||||
| HDL | 1.34±0.31 | 1.21±0.27 | 1.30±0.31 | 1.27±0.33 | |||||||||||
| LDL | 3.21±0.89 | 3.37±0.93 | 3.14±0.88 | 2.86±0.99 | |||||||||||
| TG | 1.47±0.93 | 1.92±1.27 | 1.89±1.40 | 1.63±1.07 | |||||||||||
| Mbalilaki, Hellènius62 | Tanzania | CS | 225 | 259 | 256 | 245 | TC | 4.5±1.0 | 4.5±1.1 | 3.8±1.1 | 3.6±1.0 | ||||
| HDL | 1.2±0.3 | 1.1±0.3 | 1.0±0.4 | 0.9±0.3 | |||||||||||
| LDL | 2.7±0.9 | 2.7±1.0 | 2.1±0.9 | 2.0±0.8 | |||||||||||
| TG | 1.4±1.0 | 1.8±1.2 | 1.4±0.6 | 1.5±0.8 | |||||||||||
| Miranda, Gilman32 † | Peru | CS | 199 | 201 | TC | 5.04±1.03 | 4.03±0.86 | ||||||||
| HDL | 1.15±0.28 | 1.14±0.34 | |||||||||||||
| LDL | 3.10±0.88 | 2.21±0.70 | |||||||||||||
| TG | 1.52±1.23 | 1.28±0.80 | |||||||||||||
| Mohan, Gupta63 | India | CS | 2229 | 2616 | TC | 4.90±1.00 | 4.31±0.79 | ||||||||
| Mollentze, Moore64 | Orange Free State | CS | 468 | 290 | 574 | 279 | TC | 5.09±1.15 | 4.99±1.19 | 4.85±1.12 | 4.72±1.30 | ||||
| HDL | 1.36±0.45 | 1.38±0.51 | 1.20±0.34 | 1.24±0.49 | |||||||||||
| LDL | 3.16±1.06 | 2.94±1.16 | 3.15±1.11 | 2.80±1.01 | |||||||||||
| TG | 1.21±0.93 | 1.52±1.29 | 1.24±0.66 | 1.48±1.03 | |||||||||||
| Ntandou, Delisle65 | Benin | CS | 100 | 100 | 85 | 85 | TG | 0.75±0.3 | 0.89±0.4 | 0.72±0.3 | 0.81±0.4 | ||||
| Obirikorang, Osakunor66 | Ghana | CS | 312 | 360 | TC | 5.00 (4.65 to 5.50) | 4.80 (4.55 to 5.20) | ||||||||
| HDL | 1.00 (0.80 to 1.20) | 1.00 (0.80–1.20) | |||||||||||||
| LDL | 3.40 (3.05 to 3.80) | 3.10 (2.70 to 3.60) | |||||||||||||
| TG | 1.20 (0.80 to 1.40) | 1.35 (1.10 to 1.70) | |||||||||||||
| Oommen, Abraham67 | India | CS | 1341 | 1058 | 2132 | 1667 | TC | 4.7±1.08 | 4.91±1.05 | 4.52±1.10 | 4.53±1.18 | ||||
| HDL | 0.87±0.28 | 0.79±0.34 | 1.03±0.30 | 0.96±0.31 | |||||||||||
| TG | 1.40±0.89 | 1.73±1.20 | 1.27±0.81 | 1.55±1.28 | |||||||||||
| Pandey et al 2013 | India | CS | 2008 | 2616 | TC | 4.67±0.81 | 4.31±0.93 | ||||||||
| Patel, Woodward68 | Thailand | CS | 2002 | 1130 | 1210 | 963 | TC | 5.71±3.58 | 5.54±3.70 | 5.18±2.43 | 4.80±2.48 | ||||
| HDL | 1.34±0.89 | 1.19±0.67 | 1.13±0.67 | 1.06±0.62 | |||||||||||
| LDL | 3.71±3.13 | 3.61±3.70 | 3.28±2.78 | 2.86±2.48 | |||||||||||
| TG | 1.51±3.13 | 1.88±2.17 | 1.73±2.78 | 2.15±2.02 | |||||||||||
| Pongchaiyakul, Hongsprabhas69 | Thailand | CS | 290 | 305 | 187 | 134 | TC | 5.28±1.04 | 5.35±1.10 | 4.98±1.38 | 4.38±1.08 | ||||
| HDL | 1.53±0.34 | 1.36±0.29 | 1.31±0.29 | 1.32±0.30 | |||||||||||
| LDL | 3.16±0.94 | 3.1±1.00 | 2.85±1.15 | 2.24±0.88 | |||||||||||
| TG | 1.26±0.88 | 1.94±1.24 | 1.80±1.20 | 1.79±0.96 | |||||||||||
| Prabhakaran, Roy70 ‡ | India | LT | 9504 | TC | 4.98±0.97 | 4.96±1.14 | 4.35±1.01 | 4.93±1.09 | |||||||
| Reddy, Ramachandraiah71 | India | CS | 190 | 190 | TC | 4.62±1.17 | 3.85±0.92 | ||||||||
| HDL | 1.15±0.31 | 1.13±0.38 | |||||||||||||
| LDL | 2.67±1.04 | 2.09±0.77 | |||||||||||||
| TG | 1.70±0.64 | 1.43±0.49 | |||||||||||||
| Richter, Baumgartner72 | South-Africa | CS | 591 | 393 | 633 | 333 | TC | 5.02 (4.18 to 0.09) | 4.68 (3.84–5.71) | 4.85 (4.11 to 5.95) | 4.50 (3.81 to 5.53) | ||||
| HDL | 1.36 (1.03 to 1.78) | 1.50 (1.12 to 2.04) | 1.39 (1.09 to 1.84) | 1.45 (1.02 to 1.94) | |||||||||||
| LDL | 3.35 (2.55 to 4.12) | 2.86 (2.17 to 3.73) | 3.15 (2.50 to 4.09) | 2.85 (2.9 to 3.62) | |||||||||||
| TG | 1.18 (0.87 to 1.78) | 1.00 (0.78 to 1.46) | 1.09 (0.81 to 1.49) | 0.97 (0.76 to 1.35) | |||||||||||
| Russell-Jones, Hoskins73 | Fijian Mela-nesian | CS | 71 | 35 | 109 | 87 | TC | 5.2±1.3 | 5.6±1.6 | 4.0±1.0 | 3.81±1.0 | ||||
| Sarrafzadegan, Talaei74 | Iran | CS | 4572 | 1751 | TC | 5.64±1.35 | 5.33±1.34 | 5.74±1.38 | 5.46±1.29 | ||||||
| HDL | 1.24±0.27 | 1.16±0.26 | 1.27±0.27 | 1.19±0.25 | |||||||||||
| LDL | 3.41±1.10 | 3.13±1.11 | 3.52±1.14 | 3.34±1.07 | |||||||||||
| TG | 2.15±1.14 | 2.25±1.23 | 2.05±1.09 | 2.04±1.16 | |||||||||||
| Seck, Dia75 | Senegal | CS | 557 | 469 | TC | 6.75±0.52 | 5.38±0.26 | ||||||||
| Silambuselvi and Murugu Valavan76 | India | CS | TC | 5.44 | 5.11 | ||||||||||
| HDL | 1.16 | 1.39 | |||||||||||||
| LDL | 3.56 | 3.42 | |||||||||||||
| TG | 1.84 | 1.80 | |||||||||||||
| Singh, Rastogi77 | India | CS | 139 | 172 | 115 | 140 | HDL | 1.27±0.24 | 1.25±0.20 | 1.22±0.15 | 1.18±0.12 | ||||
| Snehalatha and Ramachandran78 | India | CS | 1521 | 2145 | TC | 4.47±0.94 | 3.93±0.93 | ||||||||
| HDL | 1.17±0.26 | 1.09±0.24 | |||||||||||||
| TG | 1.39±0.85 | 1.33±0.89 | |||||||||||||
| Song, Li29‡ | China | CS | 19 841 | 20 029 | TC | 4.05±6.24 | 4.66±4.23 | 3.79±5.21 | 4.54±5.66 | ||||||
| Tatsukawa, Sawayama79 | Japan | CS | 703 | 375 | 1688 | 676 | TC | 5.37±0.92 | 5.03±0.77 | 5.52±1.00 | 5.33±0.90 | ||||
| HDL | 1.52±0.35 | 1.43±0.34 | 1.67±0.37 | 1.46±0.37 | |||||||||||
| LDL | 3.22±0.84 | 2.92±0.72 | 3.37±0.89 | 3.22±0.81 | |||||||||||
| TG | 1.38±0.75 | 1.49±0.82 | 1.07±0.61 | 1.41±0.79 | |||||||||||
| Tazi, Abir-Khalil80 | Morocco | CS | 755 | 1047 | TC | 4.89±1.16 | 4.42±1.06 | ||||||||
| Vrdoljak, Marković81 | Croatia | CS | 1824 | 642 | TC | 5.90 | 5.72 | ||||||||
| HDL | 1.57 | 1.60 | |||||||||||||
| LDL | 3.53 | 3.52 | |||||||||||||
| TG | 1.80 | 1.94 | |||||||||||||
| Wang, Wei31 | China | CS | 547 | 763 | 862 | 676 | TC | 4.32±0.90 | 4.64±0.98 | 4.10±0.89 | 4.03±0.94 | ||||
| 1.20±0.30 | 1.05±0.28 | 1.18±0.31 | 1.09±0.32 | ||||||||||||
| 2.52±0.75 | 2.58±0.95 | 2.43±0.70 | 2.38±0.78 | ||||||||||||
| 1.31±1.00 | 2.28±2.35 | 1.07±0.73 | 1.24±1.27 | ||||||||||||
| Weng, Liu82 § | China | CS | 80 | 81 | 191 | 177 | TC | 4.45±0.72 | 4.22±0.72 | 3.39±1.11 | 3.39±1.73 | ||||
| HDL | 1.42±0.54 | 1.09±0.18 | 1.20±0.41 | 1.22±0.40 | |||||||||||
| LDL | 2.73±0.89 | 2.73±0.72 | 1.99±0.83 | 1.98±1.60 | |||||||||||
| TG | 1.04±0.80 | 1.52±0.99 | 0.78±0.41 | 0.65±0.67 | |||||||||||
| Woo, Chook83 | China | CS | 116 | 116 | TC | 5.16±0.96 | 5.14±1.02 | ||||||||
| HDL | 1.49±0.36 | 1.33±0.40 | |||||||||||||
| LDL | 3.16±0.87 | 3.21±0.94 | |||||||||||||
| TG | 1.08±0.67 | 1.30±1.04 | |||||||||||||
| Wyatt, Griew84 | Papua New Guinea | CS | 23 | 86 | 49 | 22 | TC | 3.96±0.88 | 3.64±0.86 | 3.78±1.09 | 3.57±1.29 | ||||
| TG | 0.61±0.25 | 0.63±0.19 | 0.60±0.38 | 0.60±0.18 | |||||||||||
| Xu, Ming85 | China | CS | 1467 | 890 | HDL | 1.30±0.30 | 1.32±0.31 | ||||||||
| TG | 1.58±1.18 | 1.49±1.03 | |||||||||||||
*Only SEM reported but no information on number of participants per group.
†Migration study. Only data on urban and rural levels are provided in this table. Aguilar-Salinas, Lerman-Garber.47
‡Longitudinal study. Total number of participants of first and second wave reported. Urban levels of first wave are provided in the urban male cell. Urban levels of second wave are depicted in the urban female cell. Accordingly, the rural levels of the first and second wave are provided in the cells for rural males and females, respectively.
§Migration study. Rural represents data of ‘Yi farmers’. Urban represents ‘Yi migrants’.
♀, females; ♂, males; ♀♂, males and females combined; CS, cross-sectional; HDL, high density lipoprotein; LDL, low density lipoprotein;NR, not reported; TC, total cholesterol;TG, triglycerides.
bmjgh-2018-001017supp003.pdf (187.5KB, pdf)
Quality assessment
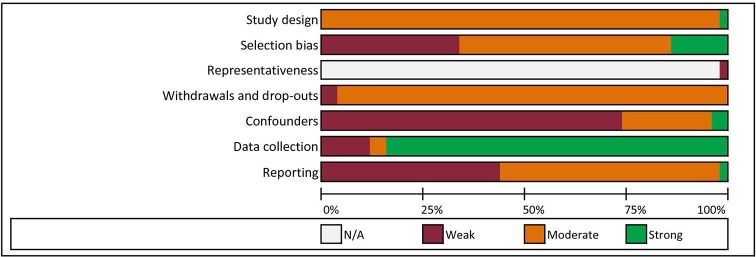
The overall rating of 12 studies (24%) was weak, 37 moderate (74%) and 1 strong (2%).33 A summary of the quality assessment scores of the studies included is shown in figure 3. The domain reporting was rated as weak in 22 studies (44%). The selection bias domain was assessed as strong in 7 studies (14%), as moderate in 26 studies (52%) and weak in 17 studies (34%). The ratings per domain per study are provided in online supplementary appendix D.
Figure 3.
Quality assessment overview.
bmjgh-2018-001017supp004.pdf (55.7KB, pdf)
Environmental characteristics
Urban-rural
Total cholesterol
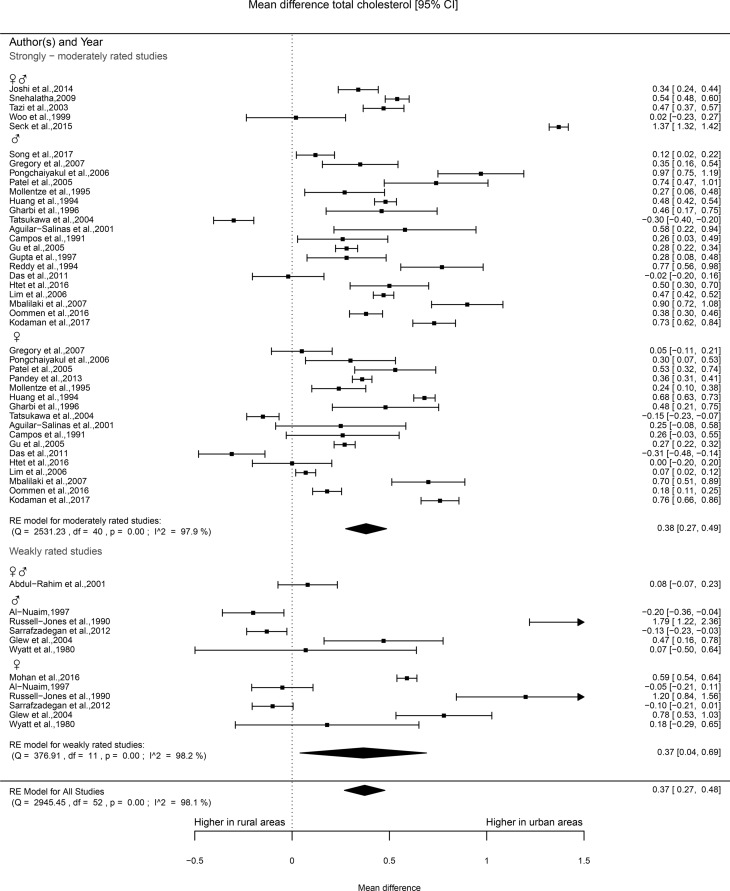
Forty studies provided information on TC levels, of which 30 were rated as being moderate in quality and 10 as weak. The majority of the studies of moderate quality reported TC levels in urban areas to be significantly higher compared with rural areas. Of the studies that reported results for men and women combined (10), 63% found significantly higher TC levels in urban areas. Of the studies that were stratified by sex (25) in general, higher TC values were reported for women (65%) and men (81%) who lived in urban areas as compared with rural areas. More heterogeneous results were found for the studies classified as weak. The percentage of these studies that reported higher levels of TC in urban areas ranged from 33% to 50%. Of the 32 studies that were eligible for meta-analysis, 25 were rated as moderate and 7 as weak. The meta-analysis of the studies of moderate quality showed significantly higher TC levels in urban areas as compared with rural areas (mean difference 0.37, 95% CI 0.27 to 0.48). Although the CI of point estimates of the studies classified as weak was wider than the CI of the moderate studies, the point estimate was still significantly higher for those residing in urban areas (mean difference 0.37 mmol/L, 0.04–0.69; see figure 4).
Figure 4.
Forest plot total cholesterol.
HDL cholesterol
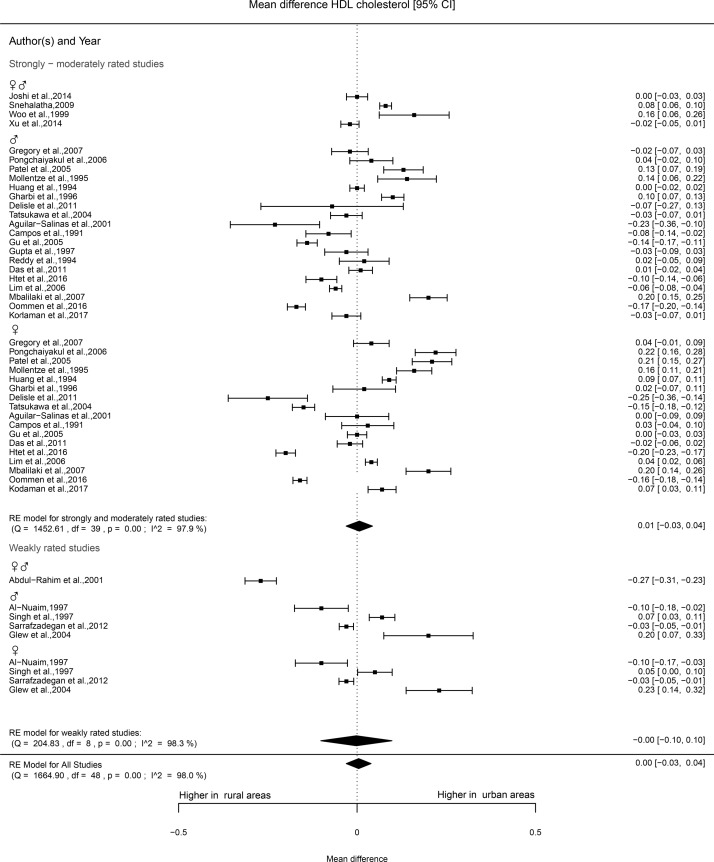
HDL cholesterol levels were reported in 36 studies. One such study was rated as strong,33 27 as moderate and 8 as weak. No clear pattern could be found in the results of the studies of moderate quality. The studies rated as of moderate and strong quality showed higher levels of HDL cholesterol in urban areas for women (47%), whereas for men, more studies reported higher HDL cholesterol levels in rural areas (41%). Most studies rated as weak (five out of eight) found no statistically significant difference. The meta-analysis included 28 studies in total of which 1 was rated as strong, 22 as moderate and 5 as weak. No differences in HDL cholesterol levels according to urban-rural were observed (0.00 mmol/L, −0.03 to 0.04) (figure 5).
Figure 5.
Forest plot HDL cholesterol. HDL, high density lipoprotein.
LDL cholesterol
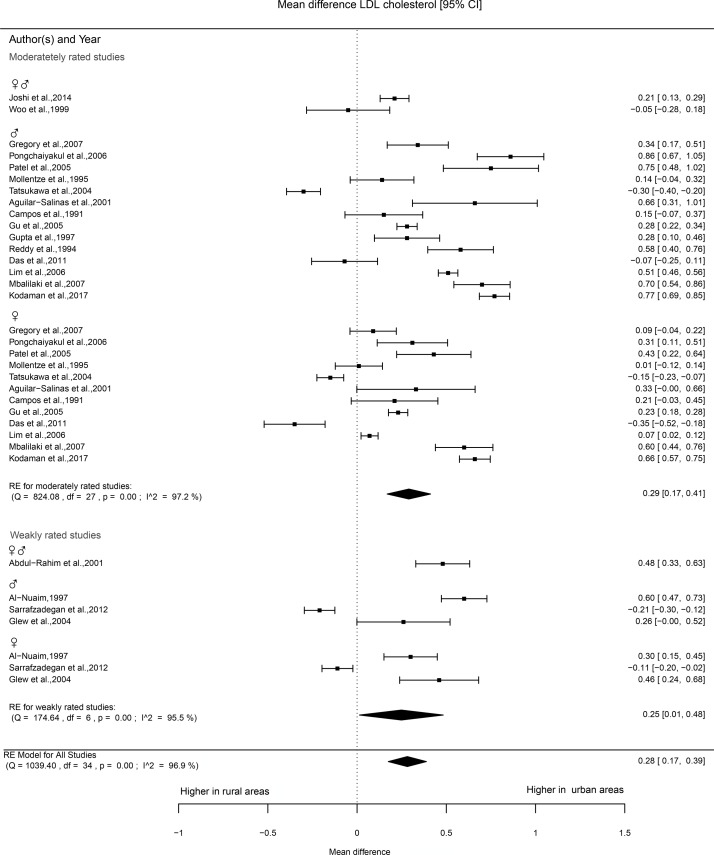
Information on LDL cholesterol levels was provided in 28 studies. Of these, 21 studies were rated as moderate and the remaining 7 as weak. In about 60% of the studies of moderate quality, significantly higher LDL cholesterol was reported in urban areas. The number of studies that were classified as weak was low (7) and comparisons made in those studies generally showed no statistically significant difference between urban and rural areas. Twenty studies were eligible for meta-analysis, of which 16 were rated as moderate and 4 as weak. The mean difference in the studies of moderate quality was 0.29 mmol/L (0.17 to 0.41), see figure 6, with higher levels in urban areas. Figure 4 shows that the patterns of the point estimates are similar across studies that reported on men and women separately or combined. The studies included that were rated as weak showed a mean difference between urban and rural areas of 0.25 mmol/L (0.01 to 0.48).
Figure 6.
Forest plot LDL cholesterol. LDL, low density lipoprotein.
Triglycerides
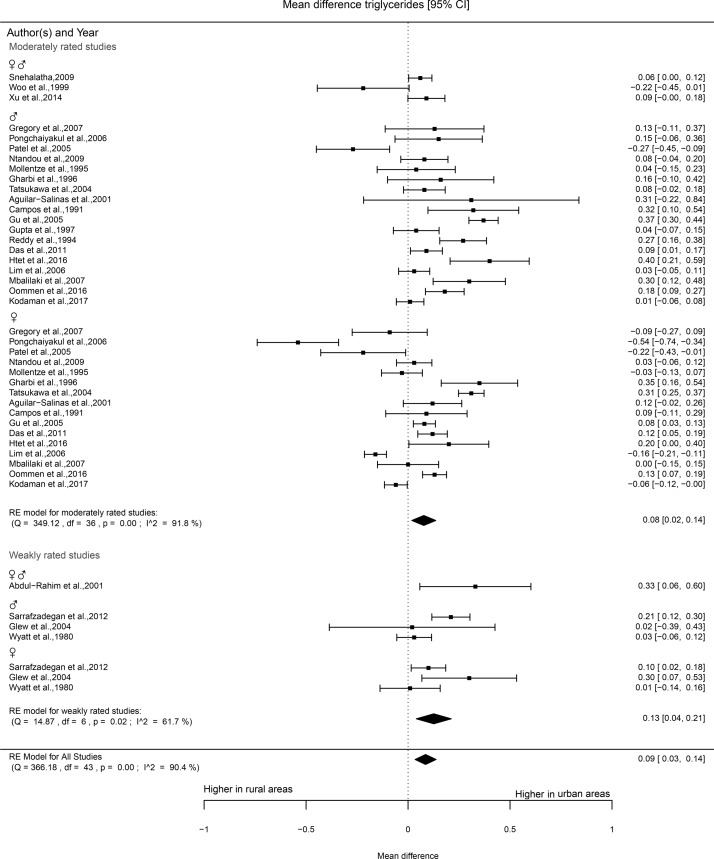
Of the 33 studies that reported triglyceride levels, 26 were rated as moderate and 7 as weak. Mixed results were found for studies that were rated as moderate and reported separately for women. In 30% of the comparisons (6) higher levels of triglycerides were found in urban areas; however, 38% reported no differences. Comparisons made by studies that reported triglycerides of men found 48% higher levels in urban areas. More than half of the comparisons (6 or 55%) of the studies of weak quality reported higher levels in urban areas. Three out of four studies that made separate comparisons for men did not show statistically significant differences between urban and rural areas. The meta-analysis included 25 studies, of which 21 were rated as moderate and 4 as weak. The forest plot of the moderately rated studies shows significantly higher triglyceride levels in urban areas as compared with rural areas (mean difference 0.08 mmol/L, 0.02 to 0.14, figure 7). The studies that were rated as weak showed higher triglyceride levels in urban areas (mean difference 0.13 mmol/L, 0.04 to 0.21).
Figure 7.
Forest plot triglycerides.
Sensitivity analyses with time periods
Studies performed in different time periods were quite consistent, apart from some small non-significant differences (online supplementary appendixes E1–4).
Migration studies
Two studies focused on migration to urban areas.31 32 In their investigation, Miranda et al 32 categorised three groups; urban residents, rural residents and those who migrated to urban areas at least 5 years ago. They found that total and LDL cholesterol, and triglyceride levels were similar in urban and migrant residents, but both were significantly higher than rural areas. The HDL cholesterol levels were approximately 1.44 mmol/L across all resident groups. In the other migration studies, similar patterns were reported, with the exception of HDL cholesterol levels in men, which were significantly lower in urban residents.32
Miscellaneous
We identified three studies investigating accessibility to parks, the impact of community-based interventions and walkability and blood lipid levels. The study investigating accessibility of parks and markets reported a positive association between distance to markets and HDL cholesterol.34 The community-based obesity and chronic disease prevention intervention study initiated various interventions on the physical, economic, social and political environments depending on the needs of the community. Slight improvement in blood lipid levels was reported after a 3-year follow-up.35 Increased walkability scores were unexpectedly found to be associated with increased triglyceride levels in the Multi-Ethnic Study of Atherosclerosis.36
Discussion
The studies on built-environment characteristics and blood lipid levels that are available to date focus predominantly on urban-rural differences. The current review reveals that LDL and TC and triglyceride levels are consistently less favourable in urban areas as compared with rural areas. No overall differences in HDL-cholesterol were found between urban and rural areas.
In the studies meta-analysed here, the pooled mean urban-rural differences in LDL, TC and triglyceride levels were 0.28 (0.17 to 0.39), 0.37 (0.27 to 0.47) and 0.09 (0.03 to 0.14) mmol/L. Guidelines from the National Cholesterol Education Program (NCEP) classify LDL cholesterol levels of <2.59 mmol/L as optimal, the range of LDL cholesterol levels of the included studies in the meta-analysis ranged from 1.06 to 3.93 mmol/L.37 TC levels below 5.18 mmol/L are considered desirable and triglyceride levels below 1.69 mmol/L are classified as normal by the NCEP guidelines. The range of TC and triglyceride levels of studies included in the meta-analysis ranged from 3.57 to 6.75 mmol/L and from 0.60 to 2.15 mmol/L respectively. On an individual level, the pooled mean differences may be considered small, but at a population level and from a public health policy perspective, this can be regarded as relevant.38 Although quantification in terms of the population attributable risk is difficult to estimate for our study population, a previous meta-analysis investigating the effect of statin use to reduce blood lipid levels identified a decrease of 1.00 mmol/L in LDL cholesterol to reduce the risk of ischaemic heart disease events by 11%.39 In addition, Rodger et al state that although associations of TC levels and risk of cardiovascular diseases attenuate with age, they remain strong and positive in the oldest age groups; 1 mmol/L lower cholesterol is associated with 15%–20% lower stroke risk and 20%–25% lower ischaemic heart disease.40 Anyway, differences in urban and rural areas are likely to become even more relevant as it is projected that 70% of the world’s population will reside in urban areas by 2050.26 27
Potential explanations for the urban-rural differences in blood lipids include differences in socioeconomic status, diet as well as occupational activities.10 26 41 42 To date, most of the studies on this topic have been carried out in LMIC, in which there is a stark contrast between the socioeconomic position of various inhabitants. In LMIC, living in certain urban areas—often referred to as slums—poses grave health risks due to the poor living conditions in such neighbourhoods and may negatively impact individuals’ lifestyles.43 In addition, urban areas, in general, are characterised by a relatively high availability of (fast-)food outlets and are conducive to the adoption of more western diets, rich in salt, sugar and saturated fat, potentially contributing to the unfavourable blood lipids observed.10 42 44 Another possible explanation is that in urban areas, occupations often involve office work that generally requires less physical activity as compared with labour in rural, agricultural settings.45 Some of the studies included selected very remote places as research contexts, where traditional dietary habits and frequent occupation-related physical activity (due to agriculture) are more prevalent. This may have introduced some selection bias that increased the contrast between urban and rural areas. Also, less heterogeneity might exist between urban and rural areas in non-LMIC at the level of occupation-related physical activity, food availability and dietary habits and social-economic status in comparison with LMIC. However, only few studies from high-income countries were included in this review.
This systematic literature review and meta-analysis provides strong evidence of an association between the built environment and lipid levels on the basis of a meta-analysis of 36 studies and 133 966 subjects. The findings contribute to our understanding of the relationship between urban versus rural areas, as a characteristic of the built environment and blood lipid levels. Our study also has certain limitations: the majority of the studies included were cross-sectional, preventing us from drawing causal inferences. The available studies to date, in general, do not allow for adjustment for potential confounding variables such as age, sex and socioeconomic position. Reliance on the quality as well as the reporting, of the original studies is, however, an inherent aspect of any systematic review. The large heterogeneity of settings and variation in quality of included studies made pooling of the results and synthesis challenging. However, reporting separately for studies rated as of weak and moderate/high quality provides at least some quantitative assessment of the overall association. Moreover, the findings were quite consistent, even across different time periods. The distribution curve for population blood lipid levels likely changed in the timespan that the included studies were published in 1980–2017. However, as we investigate associations of urban versus rural areas with these blood lipid levels, changes in population levels over time may not have a large impact. Another potential limitation is that there is no generally accepted definition of urban and rural. The majority of the included studies merely provided names of places and abstained from providing any definition of concepts or explaining why certain places were considered to be either rural or urban. Even when studies referred to census data, these data were not comparable between studies. It is, therefore, unclear as to whether relative rurality in a certain country is linearly associated with blood lipid levels or if there is a more absolute threshold level.
This comprehensive review shows a consistent association between LDL and TC and triglyceride levels and urban areas. The current focus of research on built-environment characteristics and blood lipids is largely on urban and rural differences, especially in LMIC. The lack of evidence on the association between urbanisation and blood lipid levels in more high-income countries needs to be addressed. Further study of the way in which urbanisation affects blood lipid levels is warranted in order to better inform and guide policy makers and urban planners to help diminish unfavourable blood lipid levels and, in doing so, combat associated non-communicable disease.
bmjgh-2018-001017supp005.pdf (4.8MB, pdf)
bmjgh-2018-001017supp006.pdf (4.4MB, pdf)
bmjgh-2018-001017supp007.pdf (3.8MB, pdf)
bmjgh-2018-001017supp008.pdf (4.4MB, pdf)
Footnotes
Handling editor: Seye Abimbola
Contributors: RdG, KvdH, WLAMdK, JB and JL conceived and designed the study. RdG, LJS and JL developed the search strategy. RdG and JL screened and performed the assessment of bias. RdG extracted the data. RdG, KvdH, WLAMdK, JB and JL interpreted the data. All authors gave final approval of the version to be published and have contributed to the manuscript. RdG is the guarantor.
Funding: This study was financially supported by a Product and Process Development Grant (PPOC-14-028) from Sanquin Blood Supply Foundation and by the VU University Medical Center.
Competing interests: None declared.
Patient consent for publication: Not required.
Data sharing statement: The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
- 1. Wilson PW, D'Agostino RB, Levy D, et al. . Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47. 10.1161/01.CIR.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 2. Lopez AD, Mathers CD, Ezzati M, et al. . Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747–57. 10.1016/S0140-6736(06)68770-9 [DOI] [PubMed] [Google Scholar]
- 3. WHO Global health risks: mortality and burden of disease attributable to selected major risks. Geneva, 2009. [Google Scholar]
- 4. Mannu GS, Zaman MJ, Gupta A, et al. . Evidence of lifestyle modification in the management of hypercholesterolemia. Curr Cardiol Rev 2013;9:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durstine JL, Grandjean PW, Davis PG, et al. . Blood lipid and lipoprotein adaptations to exercise: a quantitative analysis. Sports Med 2001;31:1033–62. 10.2165/00007256-200131150-00002 [DOI] [PubMed] [Google Scholar]
- 6. Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ 2006;174:801–9. 10.1503/cmaj.051351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trejo-Gutierrez JF, Fletcher G. Impact of exercise on blood lipids and lipoproteins. J Clin Lipidol 2007;1:175–81. 10.1016/j.jacl.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 8. Lakerveld J, Mackenbach J. The Upstream Determinants of Adult Obesity. Obes Facts 2017;10:216–22. 10.1159/000471489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCormack GR, Shiell A. In search of causality: a systematic review of the relationship between the built environment and physical activity among adults. Int J Behav Nutr Phys Act 2011;8:125 10.1186/1479-5868-8-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boone-Heinonen J, Gordon-Larsen P, Kiefe CI, et al. . Fast food restaurants and food stores: longitudinal associations with diet in young to middle-aged adults: the CARDIA study. Arch Intern Med 2011;171:1162–70. 10.1001/archinternmed.2011.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sallis JF, Floyd MF, Rodríguez DA, et al. . Role of built environments in physical activity, obesity, and cardiovascular disease. Circulation 2012;125:729–37. 10.1161/CIRCULATIONAHA.110.969022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Owen N, Leslie E, Salmon J, et al. . Environmental determinants of physical activity and sedentary behavior. Exerc Sport Sci Rev 2000;28:153–8. [PubMed] [Google Scholar]
- 13. Swinburn B, Egger G, Raza F. Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev Med 1999;29:563–70. 10.1006/pmed.1999.0585 [DOI] [PubMed] [Google Scholar]
- 14. Mackenbach JD, Rutter H, Compernolle S, et al. . Obesogenic environments: a systematic review of the association between the physical environment and adult weight status, the SPOTLIGHT project. BMC Public Health 2014;14:233 10.1186/1471-2458-14-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Renalds A, Smith TH, Hale PJ. A systematic review of built environment and health. Fam Community Health 2010;33:68–78. 10.1097/FCH.0b013e3181c4e2e5 [DOI] [PubMed] [Google Scholar]
- 16. Smith M, Hosking J, Woodward A, et al. . Systematic literature review of built environment effects on physical activity and active transport - an update and new findings on health equity. Int J Behav Nutr Phys Act 2017;14:158 10.1186/s12966-017-0613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dengel DR, Hearst MO, Harmon JH, et al. . Does the built environment relate to the metabolic syndrome in adolescents? Health Place 2009;15:946–51. 10.1016/j.healthplace.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leal C, Chaix B. The influence of geographic life environments on cardiometabolic risk factors: a systematic review, a methodological assessment and a research agenda. Obes Rev 2011;12:217–30. 10.1111/j.1467-789X.2010.00726.x [DOI] [PubMed] [Google Scholar]
- 19. Egger G, Swinburn B. An "ecological" approach to the obesity pandemic. BMJ 1997;315:477–80. 10.1136/bmj.315.7106.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sallis J, Owen N, Fisher E. Ecological Models of Health Behavior : Glanz K, Rimer B, Viswanath K, Health Behavior and Health Education: Theory, Research, and Practice. 4th edn United States: Jossey-Bass, 2008: 465–82. [Google Scholar]
- 21. Rodríguez DA, Evenson KR, Diez Roux AV, et al. . Land use, residential density, and walking. The multi-ethnic study of atherosclerosis. Am J Prev Med 2009;37:397–404. 10.1016/j.amepre.2009.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saelens BE, Handy SL. Built environment correlates of walking: a review. Med Sci Sports Exerc 2008;40(7 Suppl):S550–S566. 10.1249/MSS.0b013e31817c67a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. den Braver NR, Lakerveld J, Rutters F, et al. . Built environmental characteristics and diabetes: a systematic review and meta-analysis. BMC Med 2018;16:12 10.1186/s12916-017-0997-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anchala R, Kannuri NK, Pant H, et al. . Hypertension in India: a systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens 2014;32:1170–7. 10.1097/HJH.0000000000000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yusuf S, Rangarajan S, Teo K, et al. . Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med 2014;371:818–27. 10.1056/NEJMoa1311890 [DOI] [PubMed] [Google Scholar]
- 26. UN World Urbanization Prospects The 2014 Revision, 2015. [Google Scholar]
- 27. UNFPA The state of the world population 2007: unleashing the potential of urban growth, 2007. [PubMed] [Google Scholar]
- 28. Lim S, Jang HC, Lee HK, et al. . A rural-urban comparison of the characteristics of the metabolic syndrome by gender in Korea: the Korean Health and Genome Study (KHGS). J Endocrinol Invest 2006;29:313–9. 10.1007/BF03344102 [DOI] [PubMed] [Google Scholar]
- 29. Song PK, Li H, Man QQ, et al. . Trends in Determinants of Hypercholesterolemia among Chinese Adults between 2002 and 2012: Results from theNational Nutrition Survey. Nutrients 2017;9:279 10.3390/nu9030279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gharbi M, Belhani A, Aouidet A, et al. . [Level of cardiovascular risk factors in the urban and rural populations of Cap-Bon: Tunisia]. Rev Epidemiol Sante Publique 1996;44:125–32. [PubMed] [Google Scholar]
- 31. Wang B, Wei D, Wang C, et al. . Prevalence of dyslipidemia and associated factors in the Yi farmers and migrants of Southwestern China. Atherosclerosis 2012;223:512–8. 10.1016/j.atherosclerosis.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 32. Miranda JJ, Gilman RH, Smeeth L. Differences in cardiovascular risk factors in rural, urban and rural-to-urban migrants in Peru. Heart 2011;97:787–96. 10.1136/hrt.2010.218537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Delisle H, Ntandou-Bouzitou G, Agueh V, et al. . Urbanisation, nutrition transition and cardiometabolic risk: the Benin study. Br J Nutr 2012;107:1–11. 10.1017/S0007114511004661 [DOI] [PubMed] [Google Scholar]
- 34. Mena C, Fuentes E, Ormazábal Y, et al. . Role of access to parks and markets with anthropometric measurements, biological markers, and a healthy lifestyle. Int J Environ Health Res 2015;25:373–83. 10.1080/09603123.2014.958134 [DOI] [PubMed] [Google Scholar]
- 35. Raine KD, Plotnikoff R, Schopflocher D, et al. . Healthy Alberta communities: Impact of a three-year community-based obesity and chronic disease prevention intervention. Prev Med 2013;57:955–62. 10.1016/j.ypmed.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 36. Braun LM, Rodríguez DA, Evenson KR, et al. . Walkability and cardiometabolic risk factors: Cross-sectional and longitudinal associations from the Multi-Ethnic Study of Atherosclerosis. Health Place 2016;39:9–17. 10.1016/j.healthplace.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 38. Rose G. Strategy of prevention: lessons from cardiovascular disease. Br Med J 1981;282;::1847–51. (6279) 10.1136/bmj.282.6279.1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1423 10.1136/bmj.326.7404.1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rodgers A, Lawes CMM, Gaziano T, et al. . Chapter 45 The Growing Burden of Risk from High Blood Pressure, Cholesterol, and Bodyweight : Jamison D, Breman JG, Measham AR, et al., Disease Control Priorities in Developing Countries. 2nd edition New York: Oxford University Press, 2006. [Google Scholar]
- 41. Patil RR. Urbanization as a determinant of health: a socioepidemiological perspective. Soc Work Public Health 2014;29:335–41. 10.1080/19371918.2013.821360 [DOI] [PubMed] [Google Scholar]
- 42. Yusuf S, Reddy S, Ounpuu S, et al. . Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001;104:2746–53. [DOI] [PubMed] [Google Scholar]
- 43. United Nations The Millennium Development Goals Report 2014, 2014. [Google Scholar]
- 44. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med 2009;36:74–81. 10.1016/j.amepre.2008.09.025 [DOI] [PubMed] [Google Scholar]
- 45. Ng SW, Norton EC, Popkin BM. Why have physical activity levels declined among Chinese adults? Findings from the 1991-2006 China Health and Nutrition Surveys. Soc Sci Med 2009;68:1305–14. 10.1016/j.socscimed.2009.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abdul-Rahim HF, Husseini A, Bjertness E, et al. . The metabolic syndrome in the West Bank population: an urban-rural comparison. Diabetes Care 2001;24:275–9. 10.2337/diacare.24.2.275 [DOI] [PubMed] [Google Scholar]
- 47. Aguilar-Salinas CA, Lerman-Garber I, Pérez J, et al. . Lipids, apoprotein B, and associated coronary risk factors in urban and rural older Mexican populations. Metabolism 2001;50:311–8. 10.1053/meta.2001.20187 [DOI] [PubMed] [Google Scholar]
- 48. al-Nuaim AR. Serum total and fractionated cholesterol distribution and prevalence of hypercholesterolemia in urban and rural communities in Saudi Arabia. Int J Cardiol 1997;58:141–9. 10.1016/S0167-5273(96)02850-1 [DOI] [PubMed] [Google Scholar]
- 49. Cai L, Zhang L, Liu A, et al. . Prevalence, awareness, treatment, and control of dyslipidemia among adults in Beijing, China. J Atheroscler Thromb 2012;19:159–68. 10.5551/jat.10116 [DOI] [PubMed] [Google Scholar]
- 50. Campos H, Bailey SM, Gussak LS, et al. . Relations of body habitus, fitness level, and cardiovascular risk factors including lipoproteins and apolipoproteins in a rural and urban Costa Rican population. Arterioscler Thromb 1991;11:1077–88. 10.1161/01.ATV.11.4.1077 [DOI] [PubMed] [Google Scholar]
- 51. Das M, Pal S, Ghosh A. Prevalence of cardiovascular disease risk factors by habitat: a study on adult Asian Indians in West Bengal, India. Anthropol Anz 2011;68:253–64. 10.1127/0003-5548/2011/0099 [DOI] [PubMed] [Google Scholar]
- 52. Du GL, Su YX, Yao H, et al. . Metabolic risk factors of type 2 diabetes mellitus and correlated glycemic control/complications: a cross-sectional study between rural and urban uygur residents in xinjiang uygur autonomous region. PLoS One 2016;11:e0162611 10.1371/journal.pone.0162611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Glew RH, Conn CA, Vanderjagt TA, et al. . Risk factors for cardiovascular disease and diet of urban and rural dwellers in northern Nigeria. J Health Popul Nutr 2004;22:357–69. [PubMed] [Google Scholar]
- 54. Gregory CO, Dai J, Ramirez-Zea M, et al. . Occupation is more important than rural or urban residence in explaining the prevalence of metabolic and cardiovascular disease risk in Guatemalan adults. J Nutr 2007;137:1314–9. 10.1093/jn/137.5.1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gu D, Reynolds K, Wu X, et al. . Prevalence of the metabolic syndrome and overweight among adults in China. Lancet 2005;365:1398–405. 10.1016/S0140-6736(05)66375-1 [DOI] [PubMed] [Google Scholar]
- 56. Gupta R, Prakash H, Kaul V. Cholesterol lipoproteins, triglycerides, rural-urban differences and prevalence of dyslipidaemia among males in Rajasthan. J Assoc Physicians India 1997;45:275–9. [PubMed] [Google Scholar]
- 57. He J, Gu D, Reynolds K, et al. . Serum total and lipoprotein cholesterol levels and awareness, treatment, and control of hypercholesterolemia in China. Circulation 2004;110:405–11. 10.1161/01.CIR.0000136583.52681.0D [DOI] [PubMed] [Google Scholar]
- 58. Htet AS, Bjertness MB, Sherpa LY, et al. . Urban-rural differences in the prevalence of non-communicable diseases risk factors among 25-74 years old citizens in Yangon Region, Myanmar: a cross sectional study. BMC Public Health 2016;16:1225 10.1186/s12889-016-3882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang Z, Wu X, Stamler J, et al. . A north-south comparison of blood pressure and factors related to blood pressure in the People's Republic of China: a report from the PRC-USA Collaborative Study of Cardiovascular Epidemiology. J Hypertens 1994;12:1103–12. [PubMed] [Google Scholar]
- 60. Joshi SR, Anjana RM, Deepa M, et al. . Prevalence of dyslipidemia in urban and rural India: The ICMR–INDIAB study. PLoS One 2014;9:e96808 10.1371/journal.pone.0096808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kodaman N, Aldrich MC, Sobota R, et al. . Cardiovascular Disease Risk Factors in Ghana during the Rural-to-Urban Transition: A Cross-Sectional Study. PLoS One 2016;11:e0162753 10.1371/journal.pone.0162753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mbalilaki JA, Hellènius ML, Masesa Z, et al. . Physical activity and blood lipids in rural and urban Tanzanians. Nutr Metab Cardiovasc Dis 2007;17:344–8. 10.1016/j.numecd.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 63. Mohan I, Gupta R, Misra A, et al. . Disparities in prevalence of cardiometablic risk factors in rural, urban-poor, and urban-middle class women in India. PLoS One 2016;11:e0149437 10.1371/journal.pone.0149437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mollentze WF, Moore AJ, Steyn AF, et al. . Coronary heart disease risk factors in a rural and urban Orange Free State black population. S Afr Med J 1995;85:90–6. [PubMed] [Google Scholar]
- 65. Ntandou G, Delisle H, Agueh V, et al. . Abdominal obesity explains the positive rural-urban gradient in the prevalence of the metabolic syndrome in Benin, West Africa. Nutr Res 2009;29:180–9. 10.1016/j.nutres.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 66. Obirikorang C, Osakunor DNM, Anto EO, et al. . Obesity and cardio-metabolic risk factors in an urban and rural population in the Ashanti region-Ghana: A comparative cross-sectional study. PLoS One 2015;10:e0129494 10.1371/journal.pone.0129494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Oommen AM, Abraham VJ, George K, et al. . Prevalence of risk factors for non-communicable diseases in rural & urban Tamil Nadu. Indian J Med Res 2016;144:460–71. 10.4103/0971-5916.198668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Patel A, Woodward M, Stolk R, et al. . Serum lipid levels and the prevalence of dyslipidaemia among rural and urban Thai adults--are the NCEP III guidelines appropriate? J Med Assoc Thai 2005;88:1242–50. [PubMed] [Google Scholar]
- 69. Pongchaiyakul C, Hongsprabhas P, Pisprasert V, et al. . Rural-urban difference in lipid levels and prevalence of dyslipidemia: a population-based study in Khon Kaen province, Thailand. J Med Assoc Thai 2006;89:1835–44. [PubMed] [Google Scholar]
- 70. Prabhakaran D, Roy A, Praveen PA, et al. . 20-Year Trend of Cardiovascular Disease Risk Factors. Urban and Rural National Capital Region of Delhi, India. Global Heart 2016. [DOI] [PubMed] [Google Scholar]
- 71. Reddy KK, Ramachandraiah T, Reddanna P, et al. . Serum lipid peroxides and lipids in urban and rural Indian men. Arch Environ Health 1994;49:123–7. 10.1080/00039896.1994.9937465 [DOI] [PubMed] [Google Scholar]
- 72. Richter M, Baumgartner J, Wentzel-Viljoen E, et al. . Different dietary fatty acids are associated with blood lipids in healthy South African men and women: the PURE study. Int J Cardiol 2014;172:368–74. 10.1016/j.ijcard.2014.01.023 [DOI] [PubMed] [Google Scholar]
- 73. Russell-Jones DL, Hoskins P, Kearney E, et al. . Rural/urban differences of diabetes--impaired glucose tolerance, hypertension, obesity, glycosolated haemoglobin, nutritional proteins, fasting cholesterol and apolipoproteins in Fijian Melanesians over 40. Q J Med 1990;74:75–81. [PubMed] [Google Scholar]
- 74. Sarrafzadegan N, Talaei M, Kelishadi R, et al. . The influence of gender and place of residence on cardiovascular diseases and their risk factors. The Isfahan cohort study. Saudi Med J 2012;33:533–40. [PubMed] [Google Scholar]
- 75. Seck SM, Dia DG, Doupa D, et al. . Diabetes Burden in Urban and Rural Senegalese Populations: A Cross-Sectional Study in 2012. Int J Endocrinol 2015;2015:1–6. 10.1155/2015/163641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Silambuselvi K, Murugu Valavan V. Comparision on lipid profile level and prevalence of hypertension among rural and urban Post-Menopausal women. International Journal of Pharmaceutical and Clinical Research 2016;8:65–8. [Google Scholar]
- 77. Singh RB, Rastogi SS, Rastogi V, et al. . Blood pressure trends, plasma insulin levels and risk factors in rural and urban elderly populations of north India. Coron Artery Dis 1997;8:463–8. 10.1097/00019501-199707000-00009 [DOI] [PubMed] [Google Scholar]
- 78. Snehalatha C, Ramachandran A. Cardiovascular risk factors in the normoglycaemic Asian-Indian population--influence of urbanisation. Diabetologia 2009;52:596–9. 10.1007/s00125-009-1279-x [DOI] [PubMed] [Google Scholar]
- 79. Tatsukawa M, Sawayama Y, Maeda N, et al. . Carotid atherosclerosis and cardiovascular risk factors: a comparison of residents of a rural area of Okinawa with residents of a typical suburban area of Fukuoka, Japan. Atherosclerosis 2004;172:337–43. 10.1016/j.atherosclerosis.2003.10.007 [DOI] [PubMed] [Google Scholar]
- 80. Tazi MA, Abir-Khalil S, Chaouki N, et al. . Prevalence of the main cardiovascular risk factors in Morocco: results of a National Survey, 2000. J Hypertens 2003;21:897–903. 10.1097/01.hjh.0000059034.65882.83 [DOI] [PubMed] [Google Scholar]
- 81. Vrdoljak D, Bergman Marković B, Kranjčević K, et al. . How well do anthropometric indices correlate with cardiovascular risk factors? A cross-sectional study in Croati. Med Sci Monit 2012;18:PH6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weng X, Liu Y, Ma J, et al. . An urban-rural comparison of the prevalence of the metabolic syndrome in Eastern China. Public Health Nutr 2007;10:131–6. 10.1017/S1368980007226023 [DOI] [PubMed] [Google Scholar]
- 83. Woo KS, Chook P, Raitakari OT, et al. . Westernization of Chinese adults and increased subclinical atherosclerosis. Arterioscler Thromb Vasc Biol 1999;19:2487–93. 10.1161/01.ATV.19.10.2487 [DOI] [PubMed] [Google Scholar]
- 84. Wyatt GB, Griew AR, Martin FI, et al. . Plasma cholesterol, triglyceride and uric acid in urban and rural communities in Papua New Guinea. Aust N Z J Med 1980;10:491–5. 10.1111/j.1445-5994.1980.tb04964.x [DOI] [PubMed] [Google Scholar]
- 85. Xu S, Ming J, Yang C, et al. . Urban, semi-urban and rural difference in the prevalence of metabolic syndrome in Shaanxi province, northwestern China: a population-based survey. BMC Public Health 2014;14:104 10.1186/1471-2458-14-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2018-001017supp001.pdf (200KB, pdf)
bmjgh-2018-001017supp002.pdf (465.6KB, pdf)
bmjgh-2018-001017supp003.pdf (187.5KB, pdf)
bmjgh-2018-001017supp004.pdf (55.7KB, pdf)
bmjgh-2018-001017supp005.pdf (4.8MB, pdf)
bmjgh-2018-001017supp006.pdf (4.4MB, pdf)
bmjgh-2018-001017supp007.pdf (3.8MB, pdf)
bmjgh-2018-001017supp008.pdf (4.4MB, pdf)