Abstract
Aim:
In this study, the transcriptome profile of Barrett's esophagus (BE) was examined for identification potential related biomarkers in view of interacting charactering.
Background:
Since BE is known as a precursor of esophageal cancer, the molecular studies of this condition could be essential.
Methods:
Gene expression data of BE in comparison with normal cases, GSE34619 was retrieved from Gene Expression Omnibus. Differentially expressed genes (DEGs) were determined applying GEO2R online software. The DEGs then were analyzed in terms of centrality properties via constructing an interaction network.
Results:
The data indicate that there are two sets of hub-bottlenecks panels with distinguishable values in BE. The first group shows that BE is very susceptible to develop cancer, and the second one implied on central characteristic of some DEGs as previously were also reported for BE pathogenicity. In addition, these genes are also implicated in cancer shift from certain conditions.
Conclusion:
On the whole, taking together these findings explain and support the cancerous origin of BE and introduced a panel of nominated biomarkers that could be more specific for BE rather than other types of esophageal problems. However, a complementary study to support this claim is suggested.
Key Words: Barrett's esophagus, Transcriptome, Protein interaction maps, Cancer development
Introduction
Barrett's esophagus (BE) occurs in the distal esophagus in which the normal tissue is replaced by metaplastic columnar epithelium (1). About 1%–2% of the general population are affected by this condition that is progressed from gastro-esophageal reflux (GER). In addition, the incident of this condition is developing in western countries (2). Risk factors related to BE are anatomical (hiatus hernia), genetic, and lifestyle (smoking, consumption of alcoholic beverages, and hyperacidity (3, 4). One of the vital concerns related to BE is its capability to develop to Esophageal adenocarcinoma (EAC) as one of the important gastrointestinal cancers (5) with the chance of conversion of about 0.3% per year (3). While this chance is lower in BE without dysplasia, BE cases with dysplasia have the higher chance of developing EAC. Moreover, as the grade of dysplasia increases, the risk of developing to EAC grows as well which could be up to 10% yearly (1). As these sequence modification could be concluded to cancer state, it is important to establish novel detection methods for different types of BE with high sensitivity and specificity. For this aim, molecular studies are encouraged for identifying biomarkers with diagnosis and screening features (1). These investigations could be more beneficial if be studied in a high throughput format such as omics approaches. In this way, a set of identified biomarkers could be applicable for a disease surveillance and treatment approaches. Bioinformatics on the other hand, could provide more knowledge that is substantial in this regard. In a way that, introduced biomarkers by studies such as genomics, proteomics, and metabolomics can be further validated through bioinformatics (6). Those significant biomarkers in the disease state that have also central properties in an interaction network pattern of proteins, could be more promising relative to other ones (7). Therefore, protein-protein interaction (PPI) network analysis as a relatively new discipline may present additional concept of possible contributing markers of that specific disease (8). Here, putative biomarkers of BE are examined via analyzing and screening protein interaction maps and the related tactics to better understand the disease mechanisms and consequently applicable for intervention and treatment goals.
Methods
Microarray data series GSE34619 were obtained from GEO database which were included 10 differential gene expression profiles of BE and 8 normal squamous esophagus (NE) samples. Extracted RNAs from endoscopic samples were analyzed via GPL6244 platform. Data are published by di pietro M and coauthors (2012) entitled evidence for a functional role of epigenetically regulated midcluster HOXB genes in the development of Barrett's esophagus.
The profile samples were compared by boxplot analysis and to be considered as matched data via GEO2R analysis and the top 250 DEGs based on p-value were selected. Using p-value less than 0.05, 2≤ fold change (FC) ≤ 0.5, and excluding the uncharacterized individuals, the identified DEGs were included in PPI network analysis. The query genes were interacted by Cytoscape software (9) via STRING plugin. The network was analyzed by Network Analyzer application of Cytoscape software. Two central parameters including degree and betweenness centrality were considered to screen nodes of the network. The nodes with degree value above mean + 2 standard deviation were determined as hubs and the top 5% nodes based on betweenness were identified as bottlenecks (10). Common hubs and bottlenecks were introduced as hub-bottlenecks (central nodes).
Results
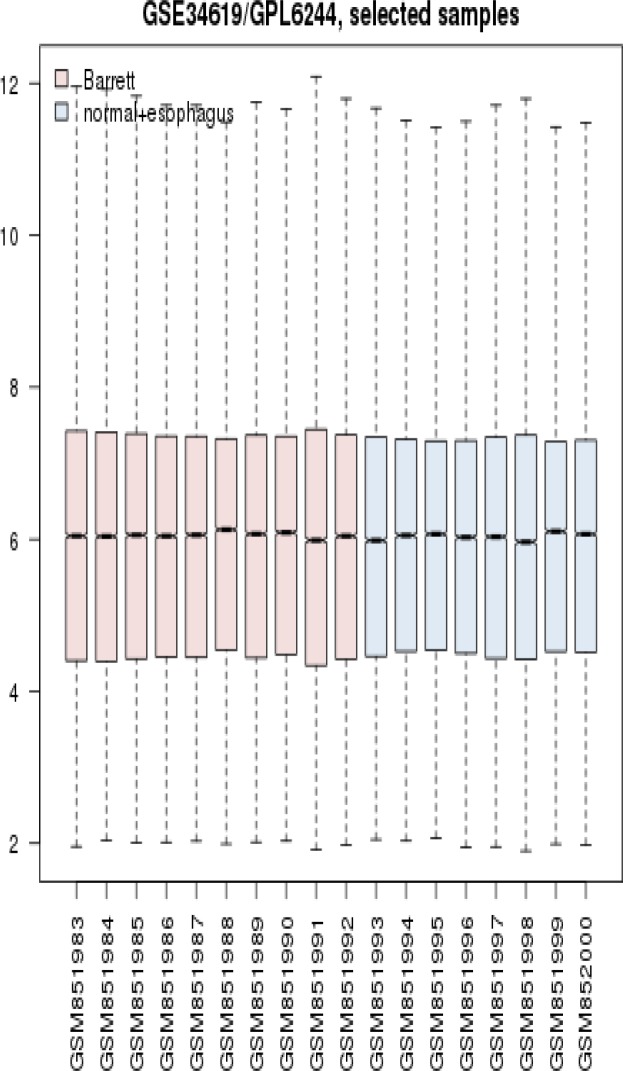
Since boxplot analysis is a suitable method which can be used to determine comparable samples to match profiles. As it is shown in figure 1 the samples are statistically comparable. Normalized distribution of gene expression profiles of Barrett patients and normal-esophageal individuals show similar pattern; however, include different DEGs.
Figure 1.
boxplot analysis of gene expression profiles 10 Barrett patients (red colored ones) and 8 normal-esophageal samples (blue colored samples) are presented. Vertical axe refers to normalized amount of gene expression change amounts and horizontal axe corresponds to samples
Numbers of 232 DEGs were included in PPI network analysis which 212 of them were recognized. The network was constructed and 128 connected components were identified (see figure 2). The network included 110 isolated nodes and 1 main connected component that was characterized by 82 nodes and 96 edges. As it is represented in the figure 2 the nodes of the constructed network do not have potent affinity to interact to each other’s. Numbers of 100 relevant genes were added to the query genes which led to construction of a main connected component including 247 nodes and 3584 edges. Degree value distribution equation (y=12.233x-0.492; correlation=0.852; R-squared=0.340 (which is computed on logarithmized values)) refers to scale free network. Numbers of eight central nodes were determined that are tabulated in the table 1. Since none of the query genes are not included in the central nodes, the eight hub-bottleneck genes including the query genes were introduced (see table 2).
Figure 2.
PPI network of 232 query DEGs of BE in comparison with NE is constructed by Cytoscape software via STRING database. 20 genes were not recognized ant the network includes: 110 isolated node, 11 double nodes, 4 triple, 1 tetrad, 1 8-nodes component, and a main connected component including 82 nodes and 96 edges
Table 1.
Numbers of eight central nodes of Barrett network are presented. The nodes are sorted by BC value
| R | name | description | Degree | BC |
|---|---|---|---|---|
| 1 | AKT1 | v-akt murine thymoma viral oncogene homolog 1 | 110 | 0.0668 |
| 2 | RHOA | ras homolog family member A | 94 | 0.0482 |
| 3 | PRDM10 | PR domain containing 10 | 83 | 0.0461 |
| 4 | SRC | v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (avian) | 85 | 0.0439 |
| 5 | EGFR | epidermal growth factor receptor | 82 | 0.0417 |
| 6 | TP53 | tumor protein p53 | 74 | 0.0403 |
| 7 | HRAS | v-Ha-ras Harvey rat sarcoma viral oncogene homolog | 77 | 0.0410 |
| 8 | EGF | epidermal growth factor | 77 | 0.0327 |
Table 2.
. Numbers of eight central nodes of Barrett network (merely including the query genes) are presented. The nodes are sorted by BC value
| R | name | description | Degree | BC |
|---|---|---|---|---|
| 1 | CFTR | cystic fibrosis transmembrane conductance regulator (ATP-binding cassette sub-family C, member 7) | 51 | 0.02 |
| 2 | PRKCA | protein kinase C, alpha | 83 | 0.02 |
| 3 | PPARG | peroxisome proliferator-activated receptor gamma | 41 | 0.01 |
| 4 | SH3GL1 | SH3-domain GRB2-like 1 | 27 | 0.01 |
| 5 | CXCR2 | chemokine (C-X-C motif) receptor 2 | 53 | 0.01 |
| 6 | LPAR3 | lysophosphatidic acid receptor 3 | 49 | 0.01 |
| 7 | GNA15 | guanine nucleotide binding protein (G protein), alpha 15 (Gq class) | 56 | 0.01 |
| 8 | CCL28 | chemokine (C-C motif) ligand 28 | 43 | 0.01 |
Discussion
The molecular study of BE has been in great attention recently. One of which is transcriptome profiling of normal subjects versus Barrett’s patients which is based on t-Test statistical analysis and evaluating the fold change of expression difference of genes. In this study the DEGs between these two conditions has been examined in terms of interaction properties. In the basic differential expression profiling, genes are introduced just by their importance in the expression changes; however, in a network analysis approach, the genes are screened and the most critical individuals can be recognized as the key genes of that disease. To get an informative examination of a network, some genes are required to participate as highly interacting elements. Otherwise, the network could not be considered as a scale-free network. In our study, at the first estimation of DEGs network construction, genes were not able to communicate with each other densely as it is represented in figure 2. Nevertheless, after adding neighbor genes to this constructed network of query genes, an informative pattern of an interacting network obtained that was valuable for continuing further analysis. Based on this finding, central nodes were identified as AKT1, RHOA, PRDM10, SRC, EGFR, TP53, HRAS, and EGF. Which all of them were from the neighbor genes known as added ones. In the other words, these genes can be characterized as elements that were essential in the network construction and foundation as well as integrity and strength of the network. Although these query DEGs are important based on significantly expression modification, still it is also important to examine their possible central role in the network. For this reason, on spite of considering the first 8 hub-bottlenecks that were from added genes we studied the next first 8 hub-bottlenecks that were among the query genes. These genes are CFTR, PRKCA, PPARG, SH3GL1, CXCR2, LPAR3, GNA15, and CCL28. Therefore, two panels of 8 elements associated with BE were introduced that in the first panel only centrality properties are considered but in the second one has the both centrality and expression values. In the first panel, genes are mostly relate to cancer and cellular cycle, cell proliferation, and cell signaling processes (11, 12). Two interpretations can be concluded from this group of identified central genes: first, these genes are mostly common among different malignancies. Second, these genes cannot be introduced as specific potential biomarkers of BE. Thus, the first concept can be interpreted as the relationship between BE and cancer. This indicated that BE could be regards as an Esophagus cancer risk factor as reported by some studies (13, 14). Hence, this is another support for these previous studies.
Considering the second panel the top central DEGs is CFTR that has been previously reported to have some associations with BE and oesophageal adenocarcinoma (15). The second critical gene is PRKCA that is also involved in BE, esophagitis, esophageal squamous cell carcinoma, and adenocarcinoma of esophagus. It is also reported that PRKCA expression is associated with PLCE1(16). PPARG, the other element of this panel in addition to participate in Barrett's adenocarcinoma pathogenicity, plays crucial role in cell proliferation and apoptosis in different tumors as well(17).
Moreover, there was no identified study related to involvement of SH3GL1, CXCR2, LPAR3, and GNA15in BE. Yet, CXCR genes are recognized by one study that is responsible for cancer development from esophagitis to a cancerous condition (18). Besides, LPAR and GNA15 genes showed some roles in cancer signaling for endometrial adenocarcinoma and esophagus tumor, respectively (19, 20). The latest gene, CCL28 demonstrated significant linkage in the progression from BE to the adenocarcinoma of esophagus (21). On the whole, all of the nodes of the panel 2, are linked to cancer development and consequently their altered expression may correspond to this incident. This fact may implies on the cancer oriented nature of BE and showing that which genes may have fundamental roles in this processes. Furthermore, the second panel in addition to its prominent feasible properties in cancer risk, could also be introduced as an exclusive set for BE (22).
It can be concluded that there are some genes in BE network with possible responsibility to cancer condition transition. These genes are important to analysis and validate in large samples of interest for diagnosis and treatment goals.
Acknowledgment
This research was supported by Shahid Beheshti University of Medical Sciences.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Larki P, Gharib E, Yaghoob Taleghani M, Khorshidi F, Nazemalhosseini-Mojarad E, Asadzadeh Aghdaei H. Coexistence of KRAS and BRAF Mutations in Colorectal Cancer: A Case Report Supporting The Concept of Tumoral Heterogeneity. Cell J. 2017;19:113–117. doi: 10.22074/cellj.2017.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bus P, Kestens C, Ten Kate FJ, Peters W, Drenth JP, Roodhart JM, et al. Profiling of circulating microRNAs in patients with Barrett's esophagus and esophageal adenocarcinoma. J Gastroenterol. 2016;51:560–70. doi: 10.1007/s00535-015-1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contino G, Vaughan TL, Whiteman D, Fitzgerald RC. The Evolving Genomic Landscape of Barrett's Esophagus and Esophageal Adenocarcinoma. Gastroenterology. 2017;153:657–73. doi: 10.1053/j.gastro.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crous-Bou M, Feskanich D, Stampfer MJ, Fuchs CS, De Vivo I, Jacobson BC. Gene-environment interactions and the risk of Barrett's esophagus in three US cohorts. Genetic Epidemiology. 2013;37:643–56. [Google Scholar]
- 5.Rostami Nejad M, Nazemalhosseini Mojarad E, Nochi Z, Fasihi Harandi M, Cheraghipour K, Mowlavi GR, et al. Echinococcus granulosus strain differentiation in Iran based on sequence heterogeneity in the mitochondrial 12S rRNA gene. J Helminthol. 2008;82:343–47. doi: 10.1017/S0022149X0804594X. [DOI] [PubMed] [Google Scholar]
- 6.Mansouri V, Rezaei Tavirani S, Zadeh-Esmaeel MM, Rostami-Nejad M, Rezaei-Tavirani M. Comparative study of gastric cancer and chronic gastritis via network analysis. Gastroenterol Hepatol Bed Bench. 2018;11:343–51. [PMC free article] [PubMed] [Google Scholar]
- 7.Dashatan NA, Tavirani MR, Zali H, Koushki M, Ahmadi N. Prediction of Leishmania Major Key Proteins via Topological Analysis of Protein-Protein Interaction Network. GMJ. 2018:7. doi: 10.22086/gmj.v0i0.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamanian Azodi M, Peyvandi H, Rostami-Nejad M, Safaei A, Rostami K, Vafaee R, et al. Protein-protein interaction network of celiac disease. Gastroenterol Hepatol Bed Bench. 2016;9:268–277. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Yang H, Gao C. Potential biomarkers for heart failure. J Cell Physiol. 2018 doi: 10.1002/jcp.27632. [DOI] [PubMed] [Google Scholar]
- 10.Safari-Alighiarloo N, Taghizadeh M, Seyyed Mohammad T, Shahsavari S, Namaki S, Khodakarim S, et al. Topological analysis of blood differentially expressed genes in protein-protein interaction network in type 1 diabetes. Koomesh . 2016:86–94. [Google Scholar]
- 11.Deng W, Gu L, Li X, Zheng J, Zhang Y, Duan B, et al. CD24 associates with EGFR and supports EGF/EGFR signaling via RhoA in gastric cancer cells. J Transl Med. 2016;14:32. doi: 10.1186/s12967-016-0787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valizadeh R, Bahadorimonfared A, Rezaei-Tavirani M, Norouzinia M, Ehsani Ardakani MI. Evaluation of involved proteins in colon adenocarcinoma: an interactome analysis. Gastroenterol Hepatol Bed Bench. 2017;10:S129–38. [PMC free article] [PubMed] [Google Scholar]
- 13.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett's esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669–76. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazemalhosseini Mojarad E, Farahani RK, Haghighi MM, Aghdaei HA, Kuppen PJ, Zali MR. Clinical implications of BRAF mutation test in colorectal cancer. Gastroenterol Hepatol Bed Bench. 2013;6:6–13. [PMC free article] [PubMed] [Google Scholar]
- 15.Gharahkhani P, Fitzgerald RC, Vaughan TL, Palles C, Gockel I, Tomlinson I, et al. Genome-wide association studies in oesophageal adenocarcinoma and Barrett's oesophagus: a large-scale meta-analysis. Lancet Oncol. 2016 Oct;17:1363–73. doi: 10.1016/S1470-2045(16)30240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Bao Y, Ma M, Zhang S, Zhang Y, Yuan M, et al. Clinical significance of the correlation between PLCE 1and PRKCA in esophageal inflammation and esophageal carcinoma. Oncotarget. 2017 May;8:33285–99. doi: 10.18632/oncotarget.16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Taie OH, Graf T, Illert B, Katzenberger T, Mörk H, Kraus MR, et al. Differential effects of PPARgamma activation by the oral antidiabetic agent pioglitazone in Barrett's carcinoma in vitro and in vivo. J Gastroenterol. 2009;44:919–29. doi: 10.1007/s00535-009-0086-y. [DOI] [PubMed] [Google Scholar]
- 18.Verbeke H, Geboes K, Van Damme J, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta. 2012;1825:117–29. doi: 10.1016/j.bbcan.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Sharifian A, Pourhoseingholi MA, Emadedin M, Rostami Nejad M, Ashtari S, Hajizadeh N, et al. Burden of Breast Cancer in Iranian Women is Increasing. Asian Pac J Cancer Prev. 2015;16:5049–52. doi: 10.7314/apjcp.2015.16.12.5049. [DOI] [PubMed] [Google Scholar]
- 20.Wasniewski T, Woclawek-Potocka I, Boruszewska D, Kowalczyk-Zieba I, Sinderewicz E, Grycmacher K. The significance of the altered expression of lysophosphatidic acid receptors, autotaxin and phospholipase A2 as the potential biomarkers in type 1 endometrial cancer biology. Oncol Rep. 2015;34:2760–7. doi: 10.3892/or.2015.4216. [DOI] [PubMed] [Google Scholar]
- 21.Picardo S, Sommerville G, Maher S, Reynolds J. the Ccl28 chemokine is differentially expressed in response to bile acid across the Barrett's oesophagus to adenocarcinoma sequence: o11. BJS. 2011;98 [Google Scholar]
- 22.Rostami Nejad M, Rostami K, Cheraghipour K, Nazemalhosseini Mojarad E, Volta U, Al Dulaimi D, et al. Celiac disease increases the risk of Toxoplasma gondii infection in a large cohort of pregnant women. Am J Gastroenterol. 2011;106:548–49. doi: 10.1038/ajg.2010.425. [DOI] [PubMed] [Google Scholar]