Abstract
Aim:
The aim of this study was to investigate the expression of GKN1 and GKN2 genes as probable biomarkers for gastric cancer.
Background:
Gastric cancer is a multifactorial process characterized by the uncontrolled growth and dissemination of abnormal cells. Survival rates of gastric cancer tend to be poor, a plausible explanation is a combination of a late-stage diagnosis and limited access to treatment. In this regard, finding relevant and measurable biomarkers is urgently needed.
Methods:
27 samples of gastric cancer tissues were enrolled into this study, according to their pathological responses. The alteration of genes expression were evaluated by Real-Time PCR technique.
Results:
Our findings showed the significant reduction of Gastrokin-1 and Gastrokine-2 genes expression in the cancerous specimens in comparison with the normal tissues. (P = 0.008 and P = 0.004 respectively).
Conclusion:
Our findings showed the significant reduction of Gastrokin-1 and Gastrokine-2 genes expression in the cancerous specimens in comparison with the normal tissues. (P = 0.008 and P = 0.004 respectively).
Key Words: Gastric cancer, Gastrokine-1(GKN1), Gastrokine-2 (GKN2), Real-time PCR
Introduction
Gastric cancer (GC) is the second leading cause of death associated with cancer (1). In contrast with the reduction of the incidence of GC in the most developed nations, in some developing countries the high prevalence is observed. GC is a multi-factorial disease that caused by the presence of infectious agents, genetic and environmental subjects. Hence, extraterritorial studies are not probable to be cited in the other population (2). According to investigations, the incidence of GC in Iran is 1.49 in men and 25.9 in women per 100,000, so Iran is considered a high-risk country (3-5).
In the traditional category (Lauren), GC is divided into two categories: intestinal and diffuse types. Environmental factors and infection with Helicobacter pylori (H.pylori) may cause intestinal type, which is more benign, whereas genetic abnormalities are related more to diffuse type of disease, as the resistant type (6).
Due to the poor symptoms at the early stages of GC, most patients’ carcinogenesis is diagnosed with metastasis in the advanced stages, so the overall clinical outcome of GC patients remains unsatisfactory (7). In this regard, substantial studies have been done on the expression of genes associated with GC in order to improve early detection of this fatal disease. Gastrokine 1 (GKN1), and Gastrokine 2 (GKN2), are belonging to Gastrokine family, which are expressed noticeably in the normal tissue of the gastric epithelium and play a significant role in maintaining the integrity and homeostasis of gastric mucosa (8).
GKN1, also called antral mucosal protein (AMP)-18, CA11, FOVEOLIN, is a protein by molecular weight of 18 kDa, with 'cytokine-like' activity which is synthesized by the cells of the antral gastric mucosa (9). The level of GKN1 expression in the other sections of the gastrointestinal system and GC cell lines have been completely stopped. It is noteworthy that its expression in the inflammation zones caused by H.pylori is reduced as well (10). GKN1 reduces cell viability, proliferation, and colony formation by inhibiting cell cycle development and epigenetic modification by down-regulating the expression levels of DNMT1 and EZH2, and DNMT1 activity, and inducing apoptosis through the death receptor-dependent pathway. Thereby, GKN1 is involved in inhibition of development and progression of GC (11).
GKN family consists three members, GKN1, GKN2, and GKN3. Between them, GKN2, also known as, TFIZ1, GDD, and blottin, are mainly studied. GKN2 is a secretory protein of gastric epithelial cells and its expression is remarkably down regulated or absent in GC tumor tissues (12). In addition, over-expression of GKN2 significantly inhibits the JAK2/STAT3 signal pathway to further up-regulate Bax, and down-regulating Bcl-2, Cyclin D1, MMP2, and MMP9, therewith resulting in reduced proliferation and invasiveness, enhancement of apoptosis (13).
In the current study, we investigate GKN1 and GKN2 gene expression in Iranian population for the first time, in order to find their association with GC and achieve probable biomarkers.
Methods
Sampling
This study was performed on twenty-seven tissue samples from patients admitted to the Taleghani Hospital, Tehran. The samples were obtained from GC patients who had undergone surgery. None of the patients received preoperative treatment with chemotherapy, radiotherapy or immunotherapy. A part of each tissue sample was placed in 10% phosphate-buffered neutral formalin for pathologic diagnosis. The other part was immediately stored in RNA Later stabilizing solution (Qiagen, Germany ) at -70 °C for subsequent analysis.
RNA extraction and cDNA synthesis
Total RNA was isolated from normal and GC tissues using RNA Purification Mini Kit (FavorGen, Taiwan), according to the manufacturer's instructions. The concentration of RNA extract was measured with the Nanodrop (Thermo Fisher Scientific, USA) by absorbance at 260 nm. Total RNA was converted to cDNA, according to Yekta Tajhiz cDNA synthesis kit by using M-MLV RT enzyme and random hexamer primer. Quality and concentration of cDNA were determined by Nanodrop Device.
Quantitative real-time PCR
Initially, primers for the desired genes (GKN1 and GKN2) and internal control gene (GAPDH) were designed by Primer 3 software. To ensure the accuracy and specificity of the primers, Gene Runner (ver. 6.0.04) was used. Finally, primers were blasted at NCBI. The sequence of the primers is shown in Table 1. To carry out quantitative real-time PCR reaction, using qPCR Master Mix 2X (Yekta tajhiz azma, Taiwan) pursuant to the manufacturer's instructions. (The amount of 10µl SYBER Master Mix, 0.8 µl Forward primer, 0.8 µl Reverse primer,150 ng/µl cDNA and dH2O in total volume 20 µl were entered to Real-time PCR reaction.)
Table 1.
Primer sequence of target and reference genes
| Primer | Primer sequence | Gene bank ID |
|---|---|---|
| GKN1 Forward | 5’CCAAGGGCCTGATGTACTC 3’ | NM_019617.3 |
| GKN1 Reverse | 5’CTCTTGCATCTCCTCAGCC 3’ | |
| GKN2 Forward | 5’GGATCATGCTCTTCTACCAC3’ | NM_182536.2 |
| GKN2 Reverse | 5’GATTGTTCAGAGGAGGGATGTTC3’ | |
| GAPDH Forward | 5’GAAGGTGAAGGTCGGAGTCA3’ | NM_001256799.2 |
| GAPDH Reverse | 5’AATGAAGGGGTCATTGATCA3’ |
The alteration in mRNA expression’s level were compared with control group. Data were analyzed using using ABI 7500 and REST 2009 software version 2.0.13 and Prism software statistical package version 5 (GraphPad Software, USA). The t-test analsysis was used to determine alteration expression in GC samples compared with normal group. Significance was set as the difference P value is less than 0.01.
Results
In this study, 27 cancer specimens’ samples were selected to evaluate the altered expression of GKN1 and GKN2 genes. Pathological and clinical observation including age of patients, site and type of invasion was determined. The sample consisted of 22 male and 5 female patients between the ages of 28 and 81. The features of the studied sample are shown in table 2.
Table 2.
Features of the studied samples
| Feature | Frequency (%) | |
|---|---|---|
| Age | > 50 ≤ 50 |
20 (74%) 7 (36%) |
| Sex | Women Men |
5 (18%) 22 (81%) |
| Cancer site | Gastric cardia mass Gastric mucosa Gastric body Distal stomach |
7 (25.9%) 11 (40.8%) 6 (22.2%) 3 (11.1%) |
| Cancer type | Intestinal type Diffuse type |
18 (67%) 9 (33%) |
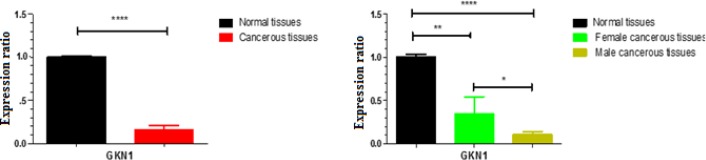
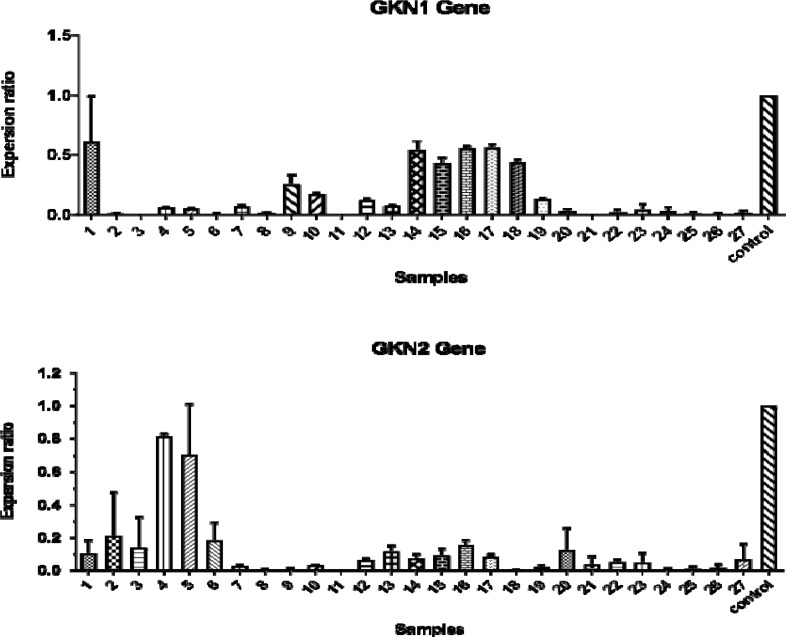
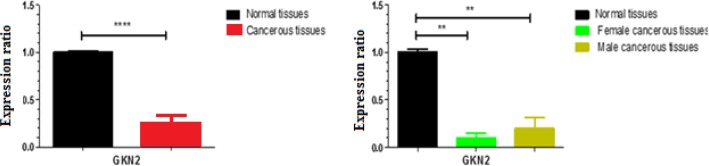
The decrease in expression of GKN1 and GKN2 genes in cancerous tissues were observed in all samples in comparison with normal tissue. The absence of GKN1 and GKN2 expression was confirmed at the mRNA level by real time PCR technique on 27 representative cancerous tissues (Figure 1-3).
Figure 1.
A: Alteration in the GKN1 gene expression in comparison with normal tissues. The expression was significantly down_regulated by mean factor(-0.8413 ± 0.04999) in the majority of 27 GC cases. GKN1 expression in sample group is different to control group. (P-value<0.0001). B: According to sexuality, GKN1 expression shows higher reduction in men
Figure 3.
Expression level of GKN1 and GKN2 in patients compared to normal individually
Discussion
GC is one of the leading types of cancer that has a high mortality rate. One of the main reasons for this high rate is the inability to diagnose the disease in the early stages. In fact, GC is the second leading cause of death associated with cancer (14). Althogh the statistical difference in prevalance of GC data among geographical distributions is critical, the investigation of national studies is more reliable. Recent studies have shown a significant reduction in the 5-year survival rate of GC patients in Iran (15). Therefore, a deeper understanding of the pathogenesis and biological features of GC is essential for enhancing early detection, treatment methods and patient's longevity. On the other hand, genetic and epigenetic changes play an important role in the GC process. Their effects are caused by altering protein expression levels or change their performance (16). The discovery of new biomarkers and their application, in conjunction with a traditional cancer diagnosis, staging, and prognosis, will help to improve early detection and patient’s care.
GKN1 and GKN2 genes are expressed extensively in the natural tissue of the gastric epithelium. Recent evidence illustrated that GKN1 and GKN2 may play an important role in the preservation of gastric mucosal homeostasis and function as a gastric individual tumor suppressor (8). The GKN1 gene mapped on chromosome 2 at 2p13.3 site, which resulted a protein with a molecular weight of 18 kDa. This protein plays an important role in stability and restoration process of mucus layer (17,18).
Figure 2.
A: The graph is associated with changes in GKN2 gene expression related to the normal tissues. Overall GKN2 expression was noticeably decreased by difference in mean factor (-0.8361 ± 0.08537). P-value is reported less than 0.0001.B: There is no significant difference in expression level of GKN2 in both genders
In 2003, Toback et al. found that GKN1 is responsible for repair of the gastric’s epithelialium after injuries and is also capable of inhibiting the proliferation of cancerous cells (9). In correlation with previous study, in 2011, JJ Yoon et al. examined the inactivation of the GKN1 gene in the progression of GC. They pointed out that inactivation of this gene plays an important role in the development of GC as a primary occurrence. They also reported a significant reduction level of the GKN1 expression in GC cells (19). In 2012, Mao et al., found that non-steroidal drugs and infection can lead to gastric disorders by reducing the expression of the GKN1 (20).
On the other hand, in contrast with GKN2, there are few investigations about the role of GKN2 in GC. The GKN2 gene, is located on chromosome 2 at 2p13.3, that encodes a 18.3-kDa protein (17). Based on the research conducted by Dai et al. GKN2 reduction or loss of expression was observed in GC cell lines BGC-823, SGC-7901 and AGS (8). Baus-Loncar and colleagues (2007) showed that GKN2 plays an important role in maintaining gastric mucosal homeostasis by regulating GKN1 activity (21). The relationship between expression of GKN1 and GKN2 was demonstrated by Am. A.et al. in 2015. They reported that there is an adjacent correlation between the expressions of these two genes. Moreover, they stated that there is a significant statistical connection between reduced levels of both GKN1 and GKN2 gene expression in GC tissue in comparison with normal tissues (22). Hence, GKN2 interacts with the GKN1 protein and regulates the amount of GKN1 that results in the formation of sustainability and homeostasis in the epithelial mucus layer.
In the current study, we examined GKN1 and GKN2 gene expression levels in an Iranian population with q-RT PCR method, for the first time. As our expectation, our data, in consistant with the other studies including KA et al. and also Rippa et al. both showed that the expression of GKN1 gene in the cancerous tissue was reduced (23,24). Another study had been done by Menheniott T.R et al. demonstreted the reuction expression in both gastrokines genes in gastric cancer tissues (25). Due to the important roles of GKN1 and GKN2 genes and their relationship, in the gastric mucosal defense mechanism and their gastric tumor suppressor activity, GKN1 and GKN2 might be reliable biomarkers for detecting GC in early stages. In this regard, comprehensive studies including investigation of the relationship between patient indices and changes in expression level of GKN1 and GKN2 are needed.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Norouzinia M, Asadzadeh H, Shalmani HM, Al Dulaimi D, Zali MR. Clinical and histological indicators of proximal and distal gastric cancer in eight provinces of Iran. Asian Pac J Cancer Prev. 2012;13:5677–9. doi: 10.7314/apjcp.2012.13.11.5677. [DOI] [PubMed] [Google Scholar]
- 2.Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. 2010;25:479–86. doi: 10.1111/j.1440-1746.2009.06188.x. [DOI] [PubMed] [Google Scholar]
- 3.Baghestani AR, Daneshva T, Pourhoseingholi MA, Asadzadeh H. Survival of Colorectal Cancer in the Presence of Competing- Risks - Modeling by Weibull Distribution. Asian Pac J Cancer Prev. 2016;17:1193–96. [PubMed] [Google Scholar]
- 4.Nazemalhosseini Mojarad E, Farahani RK, Haghighi MM, Aghdaei HA, Kuppen PJ, Zali MR. Clinical implications of BRAF mutation test in colorectal cancer. Gastroenterol Hepatol Bed Bench. 2013;6:6–13. [PMC free article] [PubMed] [Google Scholar]
- 5.Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z. Cancer incidence and mortality in Iran. Ann Oncol. 2009;20:556–63. doi: 10.1093/annonc/mdn642. [DOI] [PubMed] [Google Scholar]
- 6.Kashfi SM, Behboudi Farahbakhsh F, Nazemalhosseini Mojarad E, Mashayekhi K, Azimzadeh P, Romani S, et al. Interleukin-16 polymorphisms as new promising biomarkers for risk of gastric cancer. Tumour Biol. 2016;37:2119–26. doi: 10.1007/s13277-015-4013-y. [DOI] [PubMed] [Google Scholar]
- 7.Karbalaei R, Piran M, Rezaei-Tavirani M, Asadzadeh-Aghdaei H, Heidari MH. A systems biology analysis protein-protein interaction of NASH and IBD based on comprehensive gene information. Gastroenterol Hepatol Bed Bench. 2017;10:194–201. [PMC free article] [PubMed] [Google Scholar]
- 8.Dai J, Zhang N, Wang J, Chen M, Chen J. Gastrokine-2 is downregulated in gastric cancer and its restoration suppresses gastric tumorigenesis and cancer metastasis. Tumour Biol. 2014;35:4199–207. doi: 10.1007/s13277-013-1550-0. [DOI] [PubMed] [Google Scholar]
- 9.Toback FG, Walsh-Reitz MM, Musch MW, Chang EB, Del Valle J, Ren H, et al. Peptide fragments of AMP-18, a novel secreted gastric antrum mucosal protein, are mitogenic and motogenic. Am J Physiol Gastrointest Liver Physiol. 2003;285:G344–53. doi: 10.1152/ajpgi.00455.2002. [DOI] [PubMed] [Google Scholar]
- 10.Yoon JH, Seo HS, Choi SS, Chae HS, Choi WS, Kim O, et al. Gastrokine 1 inhibits the carcinogenic potentials ofn Helicobacter pylori CagA. Carcinogenesis. 2014;35:2619–29. doi: 10.1093/carcin/bgu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon JH, Choi YJ, Choi WS, Nam SW, Lee JY, Park WS. Functional analysis of the NH2-terminal hydrophobic region and BRICHOS domain of GKN1. Biochem Biophys Res Commun. 2013;440:689–95. doi: 10.1016/j.bbrc.2013.09.123. [DOI] [PubMed] [Google Scholar]
- 12.Fahlbusch FB, Ruebner M, Huebner H, Volkert G, Bartunik H, Winterfeld I, et al. Trophoblast expression dynamics of the tumor suppressor gene gastrokine 2. Histochem Cell Biol. 2015;144:281–91. doi: 10.1007/s00418-015-1336-0. [DOI] [PubMed] [Google Scholar]
- 13.Moss SF, Lee JW, Sabo E, Rubin AK, Rommel J, Westley BR, et al. Decreased expression of gastrokine 1 and the trefoil factor interacting protein TFIZ1/GKN2 in gastric cancer: influence of tumor histology and relationship to prognosis. Clin Cancer Res. 2008;14:4161–7. doi: 10.1158/1078-0432.CCR-07-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon JH, Kang YH, Choi YJ, Park IS, Nam SW, Lee JY, et al. Gastrokine 1 functions as a tumor suppressor by inhibition of epithelial-mesenchymal transition in gastric cancers. J Cancer Res Clin Oncol. 2011;137:1697–704. doi: 10.1007/s00432-011-1051-8. [DOI] [PubMed] [Google Scholar]
- 15.Norouzinia M, Asadzadeh H, Shalmani HM, Al Dulaimi D, Zali MR. Clinical and histological indicators of proximal and distal gastric cancer in eight provinces of Iran. Asian Pac J Cancer Prev. 2012;13:5677–79. doi: 10.7314/apjcp.2012.13.11.5677. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa T, Ishihara K. Protective effects of gastric mucus. In: Tonino P, editor. Gastritis and gastric cancer - new insights in gastroprotection, diagnosis and treatments. InechOpen; 2011. pp. 1–25. [Google Scholar]
- 17.Kim O, Yoon JH, Choi WS, Ashktorab H, Smoot DT, Nam SW, et al. GKN2 contributes to the homeostasis of gastric mucosa by inhibiting GKN1 activity. J Cell Physiol. 2014;229:762–71. doi: 10.1002/jcp.24496. [DOI] [PubMed] [Google Scholar]
- 18.Di Stadio CS, Altieri F, Minopoli G, Miselli G, Rippa E, Arcari P. Role of human GKN1 on APP processing in gastric cancer. Biochimie. 2017;135:149–53. doi: 10.1016/j.biochi.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Yoon JH, Kang YH, Choi YJ, Park IS, Nam SW, Lee JY, et al. Gastrokine 1 functions as a tumor suppressor by inhibition of epithelial-mesenchymal transition in gastric cancers. J Cancer Res Clin Oncol. 2011;137:1697–704. doi: 10.1007/s00432-011-1051-8. [DOI] [PubMed] [Google Scholar]
- 20.Mao W, Chen J, Peng TL, Yin XF, Chen LZ, Chen MH. Helicobacter pylori infection and administration of non-steroidal anti-inflammatory drugs down-regulate the expression of gastrokine-1 in gastric mucosa. Turk J Gastroenterol. 2012;23:212–9. doi: 10.4318/tjg.2012.0345. [DOI] [PubMed] [Google Scholar]
- 21.Baus-Loncar M, Lubka M, Pusch CM, Otto WR, Poulsom R, Blin N. Cytokine regulation of the trefoil factor family binding protein GKN2 (GDDR/TFIZ1/blottin) in human gastrointestinal epithelial cells. Cell Physiol Biochem. 2007;20:193–204. doi: 10.1159/000104166. [DOI] [PubMed] [Google Scholar]
- 22.Amer A Hasan, Mehri Igci, Ersin Borazan, Rozhgar A, Khailany , Emine Bayraktar, et al. Down-Regulated Gene Expression of GKN1 and GKN2 as Diagnostic Markers for Gastric Cancer. International Scholarly and Scientific Research & Innovation. 2015;9:532–5. [Google Scholar]
- 23.Oien KA, McGregor F, Butler S, Ferrier RK, Downie I, Bryce S, et al. Gastrokine 1 is abundantly and specifically expressed in superficialgastric epithelium, down-regulated in gastric carcinoma, and shows high evolutionary conservation. J Pathol. 2004;203:789–97. doi: 10.1002/path.1583. [DOI] [PubMed] [Google Scholar]
- 24.Rippa E, La Monica G, Allocca R, Romano MF, De Palma M, Arcari P. Overexpression of gastrokine 1 in gastric cancer cells induces Fas-mediated apoptosis. J Cell Physiol. 2011;226:2571–8. doi: 10.1002/jcp.22601. [DOI] [PubMed] [Google Scholar]
- 25.Menheniott TR, Kurklu B, Giraud AS. Gastrokines: stomach-specific proteins with putative homeostatic and tumor suppressor roles. Am J Physiol Gastrointest Liver Physiol. 2013;304:G109–21. doi: 10.1152/ajpgi.00374.2012. [DOI] [PubMed] [Google Scholar]