Abstract
The use of Translocator Protein 18 kDa (TSPO) as a clinical neuroimaging biomarker of brain injury and neuroinflammation has increased exponentially in the last decade. There has been a furious pace in the development of new radiotracers for TSPO positron emission tomography (PET) imaging and its use has now been extensively described in many neurological and mental disorders. This fast pace of research and the ever-increasing number of new laboratories entering the field often times lack an appreciation of the historical perspective of the field and introduce dogmatic, but unproven facts, related to the underlying neurobiology of the TSPO response to brain injury and neuroinflammation. Paradoxically, while in neurodegenerative disorders and in all types of CNS pathologies brain TSPO levels increase, a new observation in psychiatric disorders such as schizophrenia is decreased brain levels of TSPO measured by PET. The neurobiological bases for this new finding is currently not known, but rigorous experimental design using multiple experimental approaches and careful interpretation of results is critically important to provide the methodological and/or biological underpinnings to this new observation. This review provides a perspective of the early history of validating TSPO as a biomarker of brain injury and neuroinflammation and a critical analysis of controversial topics in the literature related to the cellular sources of the TSPO response. The latter is important in order to provide the correct interpretation of PET studies in neurodegenerative and psychiatric disorders. Furthermore, this review proposes some yet to be explored explanations to new findings in psychiatric disorders and new approaches to quantitatively assess the glial sources of the TSPO response in order to move the field forward.
Keywords: translocator protein 18 kDa (TSPO), peripheral benzodiazepine receptor (PBR), neuroinflammation, neurodegenerative disease, Positron Emission Tomography (PET), biomarker, schizophrenia, microglia, astrocytes
A). The peripheral benzodiazepine receptor: early history of validation as a biomarker of brain injury and neuroinflammation
1. From PBR to TSPO and beyond:
The peripheral benzodiazepine receptor (PBR) was first described as a binding site for diazepam (valium) in the quest to identify the endogenous receptor for benzodiazepines in the brain [Baestrup and Squires, 1977]. Studies using tritium-labeled diazepam discovered that brain tissue expressed [3H]-diazepam specific binding sites, that could be displaced by the benzodiazepine clonazepam [Baestrup and Squires, 1977]. It was also discovered that kidney, liver, and lungs also expressed a specific binding site for [3H]-diazepam that was not displaced by clonazepam but was sensitive to Ro5–4864, a benzodiazepine derivative of diazepam that lacks affinity for the GABAa receptor [Baestrup and Squires, 1977]. Furthermore, [3H]diazepam binding in kidney was associated with the mitochondrial fraction, while in the brain it was associated with the plasma membrane fraction [Baestrup and Squires, 1977]. These findings lead to the identification of the peripheral benzodiazepine receptor binding site (also previously known as mitochondria benzodiazepine receptor, and omega-3 receptor), because it bound the benzodiazepine [3H]-diazepam and it was highly expressed in peripheral tissue; thus, the original name peripheral benzodiazepine receptor. This was a fortuitous finding because diazepam is a drug that has high binding affinity for both the central benzodiazepine receptor (CBR) associated with the GABAa receptor, and the PBR [Maragos et al., 1982]. Subsequently, detailed studies showed that in fact the CBR and PBR were distinct proteins that differed in tissue distribution, pharmacology, and cellular and subcellular localization [Patel and Maragos, 1982; Benavides et al., 1983; Anholt et al., 1986] with CBR primarily expressed in neurons and PBR in glial cells [Schoemaker et al., 1982; Starosta-Rubinstein et al., 1987; Junck et al., 1989].
The use of the non-benzodiazepine isoquinoline carboxamide PK11195 (initial studies used the R,S-racemic form and later studies used the active R-enantiomer) and the GABAa receptor nonactive derivative of diazepam Ro5–4864 (4’-chlorodiazepam) as selective ligands for PBR with no affinity for the CBR facilitated the characterization of brain PBR binding sites. Early studies using different models of brain injury and neurodegeneration indicated that PBR levels measured by [3H]-PK11195 and [3H]-Ro5–4864 specific binding increased markedly at primary and secondary sites of brain injury [Gehlert et a., 1985; Doble et al., 1987; Benavides et al., 1987; Dubois et al., 1988; Benavides et al., 1988; Benavides et al., 1990; Diorio et al., 1991; Miyazawa et al., 1995; Guilarte et al., 1995] and these studies provided the first description of PBR’s association with gliosis in animal models of brain injury [Dubois et al., 1988; Benavides et al., 1990; Diorio et al., 1991; Miyazawa et al., 1995; Guilarte et al., 1995]. Despite the fact that the name PBR was used for many years, it was misleading since it was not an exclusively “peripheral” organ protein nor was it a receptor in the classical sense of neurotransmitter receptors. At the time, PBR was proposed to “transport” or “translocate” cholesterol from the inner to the outer mitochondria membrane for pregnenolone synthesis; the rate-limiting step in the production of neurosteroid [Krueger and Papadopoulos, 1990]. It is ironic that while the name PBR was changed to translocator protein 18 kDa (TSPO) in 2006 based on its proposed function and molecular weight [Papadopoulos et al., 2006], recent studies using conditional and global TSPO knockout mice have questioned the cholesterol “translocator” function of TSPO [Morohaku et al., 2014; Tu et al., 2014; 2015].
2. Early validation of TSPO as a biomarker of gliosis:
The ability to image and quantitatively measure TSPO levels in the living human brain using Positron Emission Tomography (PET) has significant advantages over other methodologies because it provides a direct dynamic view of glial responses to ongoing brain injury and inflammation. TSPO can also be used ex vivo to assess regional brain injury with exquisite spatial resolution and sensitivity using quantitative receptor autoradiography and more recently immunofluorescence confocal imaging. Many studies during the last three decades have demonstrated that TSPO is a sensitive biomarker that is able to detect neuronal injury ranging from subtle neuronal terminal damage [Guilarte et al., 2003; Chen and Guilarte, 2008], to frank neuronal loss [Guilarte et al., 1995; Kuhlmann and Guilarte 1997, 1999, 2000], demyelination [Chen et al., 2004; Chen and Guilarte, 2006], virus-induced inflammation [Cagnin et al., 2001a; Mankowski et al., 2003; Hammoud et al., 2005; Coughlin et al., 2014], and neurodegeneration in animal models [Ji et al., 2008; Domene et al., 2016; Loth et al., 2016] and in humans with neurological disease [Banati et al., 2000; Turner et al., 2004; Coughlin et al., 2015; 2017]. The cellular basis of TSPO as a biomarker of brain injury is due to the fact that the hallmark response of the brain to injury is the activation of microglia and astrocytes, the glial cell types that express and upregulate TSPO [Chen and Guilarte, 2008]. In the normal brain neuropil, TSPO is expressed at very low levels, but TSPO levels increase rapidly as a result of brain injury anywhere in the brain and from any etiology [Venneti et al., 2006; Chen and Guilarte, 2008]. TSPO levels increase in glial cells prior to behavioral manifestation of disease or histological detection of neurodegeneration, making it an early biomarker of brain injury and inflammation [Kuhlmann and Guilarte, 1997; Loth et al., 2016; Domene et al., 2016]. TSPO levels not only increase at primary sites of brain injury and inflammation, but also at secondary sites [Venneti et al., 2006; Cagnin et al., 2001a; Kulhmann and Guilarte 1999; Turner et al., 2004]. These characteristics make TSPO an exceptional biomarker that can be used to assess recovery from injury [Chen and Guilarte, 2006] and monitor the effectiveness of therapeutic strategies [Doorduin et al., 2008].
The early studies of TSPO as a biomarker of brain injury and inflammation was primarily driven by the use of radiolabeled ligand binding studies using receptor binding techniques (“grind and bind filtration assay”) or quantitative receptor autoradiography in ex vivo brain tissue. As noted above, these studies provided early evidence that TSPO levels measured by radioligand binding were associated with gliosis in areas of the brain undergoing neurodegeneration. However, the cellular sources of the TSPO response in injured brain tissue was not clearly defined, although some of the early studies described TSPO as being associated with microglia activation and thus, neuroinflammation [Myers et al., 1991; Stephenson et al., 1995; Banati et al., 1997; Vowinckel et al., 1997; Conway et al., 1998]. The paucity of information on the cellular sources of the TSPO response was largely due to the lack of molecular and cellular biology tools including the lack of well-characterized and validated TSPO antibodies. Furthermore, despite significant efforts from several laboratories including our own, to develop in situ hybridization techniques for mapping the spatial distribution of TSPO gene expression in healthy and diseased brain tissue, no such method was ever developed or validated.
B). A critical analysis of the glial sources of the TSPO response
1. TSPO in microglia and/or astrocytes?-a controversy that remains embedded in the literature today:
Early studies from a number of laboratories using quantitative receptor autoradiography demonstrated that the levels of radioligand binding to TSPO was very low in the normal brain neuropil but increased dramatically as a result of different types of brain pathology [Benavides et al., 1983, 1987, 1988, 1990; Gehlert et al.,1985; Dubois et al., 1988; Myers et al., 1991; Stephenson et al., 1995; Banati et al., 1997; Vowinckel et al., 1997; Conway et al., 1998; Guilarte et al., 1995; Kuhlmann and Guilarte 1997, 1999, 2000; Chen et al., 2004; Chen and Guilarte, 2006]. At this time, autoradiography (film and/or emulsion) and receptor binding studies were the only methods available to identify and assess the cellular sources of the TSPO response. Film autoradiography has tissue level resolution while emulsion autoradiography has resolution at the cellular level. Using [3H]PK11195 quantitative receptor autoradiography and emulsion autoradiography, several laboratories including our own examined the TSPO response to brain injury using a variety of different animal models of CNS pathologies.
The approach taken in our laboratory was to use well-characterized neurotoxicant-based animal models of neurodegeneration that would target different brain regions as primary sites of injury and would produce different degrees of injuries to examine the sensitivity of TSPO in detecting a signal from the injured brain. We used neurotoxicants that had not been used previously in the TSPO literature in order to complement other models being used at the time. Combined, these different models would provide face-validity to TSPO as a general biomarker of brain injury and inflammation based on the glial response [Chen and Guilarte, 2008]. The first neurotoxicant used in our laboratory was trimethyltin (TMT) which has a well-characterized regional and temporal pattern of neuropathology in the limbic system [Guilarte et al., 1995]. In the initial study, we found that increased levels of [3H]PK11195 specific binding to TSPO faithfully tracked the regional and temporal pattern of TMT-induced neuropathology. Furthermore, the increased temporal pattern of [3H]PK11195 binding in the hippocampus followed the levels of glial cell activation based on immunostaining and astrocytes based on glial fibrillary acidic protein (GFAP) ELISA [Guilarte et al., 1995]. However, these studies were not as methodologically advanced as to what is possible and available today [see section C.4 below].
At approximately the same time, other laboratories using transient global forebrain ischemia, facial nerve axotomy, and experimental autoimmune encephalomyelitis, attributed the TSPO response to brain injury exclusively to microglia and/or macrophage infiltration at the sites of injury with no apparent association to astrocytes [Myers et al., 1991; Stephenson et al., 1995; Banati et al., 1997; Vowinckel et al., 1997; Conway et al., 1998]. However, careful examination of these early (and later) studies that attributed the cellular sources of the TSPO response exclusively to microglia reveals several limitations including: 1) visual inspection of the spatial pattern of TSPO autoradiography and immunohistochemistry of glial markers was only done at one time point in the progression of the injured brain [Stephenson et al., 1995; Mirzaei et al., 2016]; 2) astrocyte staining was noted as being done but images were not provided [Banati et al., 1997]; 3) immunohistochemistry for astrocytes was not performed at all [Vowinckel et al., 1997; Kannan et al., 2007; Arlicot et al., 2008; Toyama et al., 2008; Miller et al., 2013; Harhausen et al., 2013]; 4) microglia was noted in the title as being the source of the TSPO signal, but microglia staining was not performed [Yankam Njiwa et al., 2016]; or 5) despite the fact that the spatial pattern of gene expression of the astrocytic marker GFAP was similar to the spatial distribution of [3H]PK11195 binding (autoradiography) at later time points in the progression of the injured brain in a global forebrain ischemia model, the authors conclude that because the early temporal expression of the GFAP mRNA and [3H]PK11195 binding were different, it supported the proposed localization of TSPO in activated microglia [Conway et al., 1998]. This conclusion was provided despite the fact that the spatial distribution of the GFAP mRNA and [3H]-PK11195 binding closely overlapped (by visual inspection) at a later time point (8 days after the ischemia event-see Figure 2 panels F and I in Conway et al., 1998) and microglia immunohistochemistry was not performed.
These initial studies were taken as experimental evidence of an exclusive localization of TSPO in microglia with no association with astrocytes. They formed the foundation for the current disregard of the significance of the astrocyte contribution to elevated TSPO levels in many neurological and neurodegenerative conditions. Another significant methodological pitfall of the early studies was that associations of the spatial pattern of [3H]PK11195 binding using film or emulsion autoradiography as compared to the pattern of immunostaining of microglia and astrocyte markers were performed by visual inspection and could be influenced by experimenter interpretation. To this point, it is relevant to note that when publications using the same approach and animal model of brain injury were compared, significantly different interpretations were provided. For example, Banati et al., [1997] examining the TSPO response in the facial nucleus at 1, 4, and 21 days after facial nerve axotomy indicated that [3H]PK11195 emulsion autoradiography was restricted to microglia (based on immunostaining; no astrocyte staining was provided). However, another group using the same facial nerve axotomy model noted that [3H]PK11195 binding was associated with the staining of both microglia and astrocytes during a similar time period following axotomy, i.e., 5–10 days [Gehlert et al., 1997]. They indicate that following facial nerve transaction, elevated levels of TSPO measured by increased [3H]PK11195 binding colocalized based on visual inspection with the immunostaining of both microglia and astrocytes [Gehlert et al., 1997].
2. TSPO radioligand binding and correlation with glial cell number and immunofluorescent staining of glial markers:
Another approach used in the early studies to assess the cellular sources of the TSPO response was to correlate the level of [3H]PK11195 binding to TSPO with the number of cells immunostained with a microglia/macrophage and/or an astrocyte marker. In an animal model of traumatic brain injury (TBI), increased [3H]PK11195 binding was correlated with the number of ED-1-positive cells for microglia/macrophages or GFAP-positive astrocytes [Rao et al., 2000]. The number of ED-1 or GFAP positive cells were both shown to significantly correlate with [3H]PK11195 binding but it was noted that there was a “better” correlation with ED-1 than with GFAP. The authors seem to disregard the astrocytic TSPO response with the statement that “microglia/macrophages are the major cell type associated with the increased TSPO expression following TBI” while considering that “the possibility of some astroglial contribution to the increased TSPO expression following TBI cannot be ruled out” [Rao et al., 2000]. Regardless of their interpretation of the results, one experimental limitation of this study is that cell counting did not use unbiased stereological cell counting methods nor was a description of the morphological or cell size criteria used to count cell number was provided. The latter is important with ED-1 positive microglia/macrophages since in the injured brain there is clustering of microglia/macrophages (microglial nodule) and loss of ramifications with microglia activation (or with entry of peripheral macrophages) making it difficult to determine the actual number of cells in a cluster.
Another study of a macaque model of neuroAIDS, [11C]PK11195 PET imaging was performed in 11 simian immunodeficiency (SIV) infected macaques and 2 non-infected controls [Venneti et al., 2004]. The animals used had a wide range of age and the length of infection varied significantly from 56 to 1,622 days. This PET study showed that [11C]PK11195 brain uptake was increased in 6 animals that developed SIV encephalopathy (SIVE) compared to those that did not. Postmortem brain analysis of the 6 SIVE animals using [3H]PK11195 autoradiography confirmed the increased TSPO levels in SIVE relative to SIV animals and controls. Immunofluorescence for a macrophage marker (CD68), astrocytes (GFAP), or neurons (MAP2) was performed in the same regions. The authors then correlated the levels of [11C]R-PK11195 specific binding in vivo with the levels of astrocytes, macrophages, and neurons based on the immunofluorescent signal. It was noted that TSPO levels using [3H]PK11195 binding in postmortem tissue or in vivo [11C]PK11195 was correlated with the CD68 signal but not with the GFAP or MAP2 signal using confocal laser microscopy. In this study, the authors used an average pixel fluorescence for a particular cellular phenotype using confocal imaging and the latter determined the cellular phenotype that was associated with the ex vivo [3H]PK11195 binding [Venneti et al., 2004]. The study concludes that the increased TSPO in the brain of SIVE animals correlated with the abundance of microglia/macrophages and not astrocytes. However, these two different endpoints, that is receptor binding (postmortem) or TSPO radioligand uptake (in vivo) and fluorescent imaging cannot be associated unless it is demonstrated that both signals are originating in the same cell which is not possible based on the approach used.
In a later study, the same authors examined the glial sources of the TSPO response in postmortem tissue from a variety of human neurological conditions such as cerebral infarct, multiple sclerosis, frontotemporal dementia, amyotrophic lateral sclerosis, and Alzheimer’s disease [Venneti et al., 2008]. Despite the fact that analysis of CD68 (microglia/macrophage) and GFAP (astrocytes) were elevated in all neurological conditions, the authors conclude that the TSPO signal arises from microglia based on correlation of autoradiography area with immunofluorescence area. Further, in a rat model of TBI, a similar conclusion was provided despite significant astrocyte activation [Venneti et al., 2007]. Lastly, in a study using postmortem brain tissue from Alzheimer’s disease subjects, in the abstract and title the authors note that PK11195 binding correlates with the extent of microglia activation despite the fact that an association was also found between [3H]PK11195 binding and GFAP immunostaining [Venneti et al., 2009]. The latter study is indicative of an investigator-based selective bias to an association of the TSPO signal with microglia and disregard the association with astrocytes. A common error in the TSPO literature.
While these early studies were important contributions to the TSPO literature and used the best available methods at the time, they had significant methodological limitations that could lead to biased interpretations based on the fact that: 1) the cellular sources of the TSPO response were determined by examining the spatial pattern of TSPO radioligand binding using film or emulsion autoradiography with the pattern of immunostaining of microglia/macrophage or astrocyte markers which required visual inspection and potential experimenter bias, or 2) were based on correlation of the number of microglia/macrophage- or astrocyte-labeled cells or immunofluorescent signal intensity with [3H]PK11195 binding. However, the counting of labeled cells did not use state-of-the-art unbiased stereological cell counting methods [see Kanaan et al., 2010; Lemus et al., 2015] and were often based on gross analysis of immunofluorescence signal intensity. Therefore, there was a great need to use more rigorous experimental approaches in order to begin to provide a more quantitative, accurate, and direct assessment of the glial sources of the TSPO signal in the injured brain.
3. TSPO immunohistochemistry-A new era:
As noted previously, one of the limiting factors of the early studies examining the cellular sources of the TSPO response was the lack of commercially available, validated TSPO-specific antibodies that could be used for experiments in which double label of TSPO with specific glial markers could be performed. In 2000, using the same TMT animal model that we first described in 1995 (single i.p. injection of 8 mg/kg TMT in rats), we performed studies on the cellular sources of the TSPO response [Kuhlmann and Guilarte, 2000]. The temporal TSPO response to TMT-induced neurodegeneration was performed at 2 days, 14 days, and 6 weeks following a single 8.0 mg/kg TMT injection. Besides the typical methods used at this time (film and emulsion autoradiography), we also performed the first TSPO immunohistochemistry with a validated TSPO antibody provided by Dr. V. Papadopoulos. The results showed that the levels of [3H]PK11195 binding in various limbic brain regions, including the hippocampus, increased significantly as a function of time following TMT injection. Furthermore, [3H]PK11195 binding to TSPO was sustained in brain regions undergoing neurodegeneration. We also showed that the cellular sources of the TSPO response was driven by an early microglia response that resolved by 6 weeks after injury while the astrocytic response appeared at 14 days and remained present at 6 weeks following TMT administration. TSPO immunohistochemistry provided the first evidence that TSPO-labeled cells in the TMT-treated hippocampus, with active neurodegeneration based on silver staining, expressed morphology consistent with both activated microglia and astrocytes. Importantly, the presence of TSPO in both glial cell types was confirmed with the first-ever double-label immunostaining of TSPO with either Griffonia simplicifolia isolectin B4 for microglia or GFAP for astrocytes [Kuhlmann and Guilarte, 2000]. This study provided the first direct evidence based on double-label immunohistochemistry of a distinct TSPO colocalization with both microglia and astrocytes. However, it did not perform analysis of TSPO signal cellular colocalization with glial markers as a function of brain region or time after brain injury [see sections C.4 below]. Nevertheless, it was the first study to use a TSPO-specific antibody and showed that TSPO colocalization occurred with both microglia and astrocytes.
C). Longitudinal studies demonstrate different temporal patterns of TSPO expression in activated microglia and astrocytes:
Glial cells have a dynamic response to different brain pathologies in their morphology and function. Therefore, performing studies at a single point in time does not provide an accurate representation of the dynamic nature of the glial response in the time-continuum of brain disease or injury [McNeela et al., 2017]. To address this important aspect of the cellular sources of the TSPO response, we used another model of neurotoxicant-induced brain injury using cuprizone. Cuprizone is a copper chelator that damages oligodendrocytes and produces widespread demyelination of the brain [Praet et al., 2014]. This neurotoxicant-induced animal model of demyelination was selected because cuprizone-induced demyelination exhibits a recovery phase with extensive remyelination once the animal is removed from the cuprizone exposure [Praet et al., 2014]. Using this model, we assessed the glia cell TSPO response in a demyelination-remyelination continuum [Chen et al., 2004; Chen and Guilarte, 2006]. This was an excellent animal model to examine two important questions in the early validation of TSPO as a biomarker of brain injury and inflammation: 1) Is TSPO able to track not only brain injury but also resolution of injury? and 2) what are the dynamics of the glial cell TSPO response during the demyelination-remyelination continuum?
Demyelination phase: The results of the demyelination study showed a dose-response relationship between cuprizone dose and [3H]R-PK11195 specific binding to TSPO based on autoradiography analysis of brain regions undergoing demyelination. Demyelinating brain regions were confirmed by myelin staining using Black Gold histochemistry [Chen et al., 2004; Chen and Guilarte, 2006]. Examination of the temporal response of [3H]R-PK11195 binding, microglia staining with Mac-1 and astrocyte staining with GFAP, found that increased [3H]RPK11195 binding at 2 weeks of cuprizone exposure was primarily driven by microglia since there was limited or no staining of astrocytes at this time point in brain regions exhibiting increased [3H]R-PK11195 binding to TSPO. However, at 3 and 4 weeks of cuprizone exposure when [3H]R-PK11195 increased dramatically compared to non-exposed control animals, there was markedly increased staining of both microglia and astrocytes supporting a role of both glial cell types in the TSPO response but with different temporal profiles. That is, an early microglia response followed by a later activation of astrocytes. [3H]R-PK11195 emulsion microautoradiography with glial marker immunostaining confirmed the colocalization of the TSPO signal with both microglia and astrocytes. These studies showed the importance of examining the course of brain injury using a longitudinal design because if we had only performed the study at 2 weeks after cuprizone exposure our conclusion would have been that the TSPO response is exclusively from microglia. However, if would fail to demonstrate that in this animal model of brain injury, astrocytes are also activated at later time points in the same brain regions undergoing demyelination. Furthermore, [3H]R-PK11195 emulsion autoradiography demonstrated TSPO cellular colocalization with both microglia and astrocytes at 3 and 4 weeks of exposure [Chen et al., 2004].
Remyelination phase: We also examined the remyelination phase of the cuprizone model using a similar approach of [3H]R-PK11195 quantitative autoradiography and immunostaining for glial markers and Black Gold histochemistry for myelin in the corpus callosum [Chen and Guilarte, 2006]. The corpus callosum is a major white matter fiber track connecting the cerebral hemispheres. In this study, we also performed [11C]R-PK11195 microPET in similarly treated animals. We found that as previously shown [Chen et al., 2004], 3 weeks of cuprizone exposure of the animals resulted in approximately a 5-fold increase in [3H]R-PK11195 binding to TSPO in the corpus callosum relative to non-treated controls. The increase in [3H]R-PK11195 binding measured at 3 weeks of cuprizone-induced demyelination decreased significantly at 3 and 6 weeks after removing animals from the cuprizone exposure and it was inversely associated with increased levels of myelin staining imaged by Black Gold histochemistry [Chen and Guilarte, 2006]. By 6 weeks of remyelination there was approximately a 50% decrease in the maximal [3H]-R-PK11195 specific binding measured at 3 weeks of cuprizone-induced demyelination. This decrease in [3H]R-PK11195 binding to TSPO during the remyelination phase was associated with increased myelin staining in the corpus callosum and with little or no labeled Mac-1 positive microglia by 6 weeks of remyelination. On the other hand, GFAP-labeled astrocytes also decreased but there were more labeled astrocytes in the same brain region remaining at 6 weeks of remyelination [Chen and Guilarte, 2006]. Considering that [3H]RPK11195 was still significantly elevated to approximately 50% of the maximal demyelination response at the 6 weeks remyelination time point, strongly suggests that astrocytes provide a significant, if not the majority of the TSPO signal at 6 weeks of remyelination. Finally, [11C]RPK11195 microPET confirmed a significantly increased TSPO signal during the demyelination phase with the TSPO signal achieving complete recovery to control levels by 10 weeks following removal from the cuprizone exposure [Chen and Guilarte, 2006].
Collectively, these two longitudinal studies demonstrated that the TSPO response can track not only disease onset and progression but also resolution of injury. Furthermore, it demonstrates a critical point on the importance of examining the TSPO response in a longitudinal fashion in the context of the glial sources. That is, if one examines the glial sources of the TSPO response at an early time point (2 weeks) after initiation of cuprizone-induced demyelination, one would conclude that the TSPO response is exclusively from microglia since no astrocyte staining was present at this time. Similarly, if one only examined the 6-week time point after removing the animals from the cuprizone exposure during the remyelination phase, one would conclude that the TSPO signal is exclusively from astrocytes since at 6 weeks there was no or minimal microglia staining. In both cases, the conclusions when utilizing a single time point would have been correct for those specific time points but would have been completely inaccurate when taken into consideration the progression of the disease as there is contribution of both microglia and astrocytes during the different phases of the demyelination-remyelination continuum.
1. Studies support an astrocyte contribution to the TSPO response in diverse pathologies:
Following our TMT and cuprizone studies [Kulhmann and Guilarte, 2000; Chen et al., 2004; Chen and Guilarte, 2006] and due to the biological importance of understanding the glial sources of the TSPO response to brain injury for proper interpretation of PET studies and for potential therapeutic approaches, other laboratories interrogated this question using TSPO immunohistochemistry. They confirmed that the TSPO signal is from both microglia and astrocytes using a variety of animal models of CNS pathologies and in postmortem brain tissue in human neurodegenerative disorders (Maeda et al., 2007; Rojas et al., 2007; Ji et al., 2008; Cosenza-Nashat et al., 2009; Maeda et al., 2011; Mattner et al., 2011; Lavisse et al., 2012; Arlicot et al., 2014; Wang et al., 2014; Dickens et al., 2014; Biesmans et al., 2015; Lavisse et al., 2015; Liu et al., 2015; Sérrière et al., 2015; Domene et al., 2016; Janssen et al., 2016; Israel et al., 2016; Nguyen et al., 2018). These series of studies showed that the dynamic nature of the TSPO glial cell response to brain injury represents a continuum of change based on the type of injury and whether the injury is acute or chronic. From this collection of papers, there are two studies that are notable for discussion. First, Ji et al., [2008] examined the cellular sources of the increased TSPO response in two mouse models of Alzheimer’s disease (APP23 and PS19 tau transgenic mice). Using double-label immunostaining, they found that in APP23 (mutant APP) mice, there was predominant localization of the TSPO signal to astrocytes in the vicinity of β-amyloid plaques with no detectable TSPO signal in Iba-1 positive microglia. Strikingly, and in contrast to the TSPO astrocyte response in APP23 mice, the reverse was found in the PS19 tau transgenic mice. That is, the build-up of phospho-tau in the PS19 mouse brain was associated with a TSPO signal in microglia and not in astrocytes. This study clearly indicated different glial sources of the TSPO response based on the animal model used. Although they did not do a timecourse profile in these two animal models of Alzheimer’s disease that would have potentially discovered additional glial cell TSPO colocalization at different time points as in the cuprizone studies presented above.
In the same publication, the authors also examined a variety of neurotoxicant-induced animal models to examine the cellular sources of the TSPO response using double-label immunostaining. They found that similar to the APP23 and PS19 mouse models, the cellular sources of the TSPO response was specific to the neurotoxicant used. In particular, when they examined the TSPO glial cell response in the striatum and corpus callosum of cuprizone-exposed animals to induce demyelination, they found that the vast majority of astrocytes in the striatum exhibited high levels of TSPO staining. They then performed in vivo microPET with [18F]FEDAA1106 and found increased uptake in the striatum of cuprizone-treated mice relative to nontreated mice. This finding was another demonstration that in vivo TSPO imaging is able to measure increased TSPO levels originating from astrocytes. In summary, this study provided evidence that TSPO increases in both microglia and astrocytes and may reflect deleterious and beneficial consequences of glial responses, respectively, in the injured brain.
The second notable study is related to the development of an animal model of selective astrocyte activation through lentiviral gene transfer of the cytokine ciliary neurotrophic factor (CNTF) into the striatum in the absence of neurodegeneration and microglia activation [Lavisse et al., 2012]. This approach resulted in extensive astrocyte activation visualized by GFAP staining with minimal or no increase in microglia activation markers. The authors showed that using two different TSPO radioligands i.e., [18F]DPA-713 and [11C]SSR180575 there was significant uptake of these two TSPO radioligands in the CNTF-injected striatum relative to the contralateral control striatum. Furthermore, the radioligand uptake was displaced by nonradioactive PK11195, demonstrating the pharmacologically selective binding of the radiotracer to TSPO. Examination of brain tissue indicated significantly increased TSPO mRNA and protein levels that colocalized with CNTF-activated astrocytes. This study showed a selective increase of TSPO levels in reactive astrocytes that yields a selective and increased radioligand TSPO signal using in vivo microPET imaging in the absence of microglia activation. In summary, these studies provided strong and confirmatory evidence that the sources of the PET radioligand uptake is also derived from astrocytes. It is notable that the dogma that the cellular source of the TSPO response in various CNS pathologies is exclusively from microglia is so erroneously entrenched in the literature that despite the fact that some studies have clearly shown and articulated that astrocytes significantly contribute to the TSPO signal, the astrocyte contribution to the TSPO signal was not reflected in the title of the publication [Dickens et al., 2014; Israel et al., 2016; Brackhan et al., 2016].
2. TSPO-PET literature indicating TSPO as an exclusive microglia biomarker:
Despite the fact that there are now many studies demonstrating an important contribution of astrocytes to the TSPO signal in brain injury and inflammation using both ex vivo and in vivo imaging methods, the TSPO-PET literature continues to have a significant number of studies taking the reductionist view that TSPO is a biomarker of neuroinflammation whose signal is originating from microglia (Table 1). This view is prevalent despite the fact that the TSPO response is defined by a complex cross-talk between microglia and astrocytes following brain injury [Streit et al., 2004; Masgrau et al., 2017; Schain and Kreisl, 2017], and neuroinflammation is not a selective microglia event, but it also involves other cells including astrocytes [Streit et al., 2004; Masgrau et al., 2017; Schain and Kreisl, 2017]. While it is scientifically expedient to believe that TSPO is a biomarker of brain injury and neuroinflammation whose signal is exclusively attributed to microglia and/or infiltrating peripheral macrophages, it is an inaccurate statement unless there is direct demonstration that a selective “microglia” TSPO signal occurs during the course of a specific brain disorder or pathology. Often, studies note that the TSPO response is due to microglia but they either do not examine TSPO expression in astrocytes or the immunostaining is only done at one time point or in one brain region and are highly likely missing the presence of an astrocyte-mediated increase in the TSPO signal at a different time point or brain region in the progression of the neuropathology or disease (Table 1). Consistent with this point, a study in which TSPO immunohistochemistry was performed with microglia/macrophage and astrocyte markers in the spinal cord of experimental autoimmune encephalomyelitis (EAE) rats shows an apparent selective increase of TSPO only in microglia and not in astrocytes [Abourbeh et al., 2012]. However, despite the fact that there was a longitudinal assessment of neurological score that ranged from 0–5 from approximately 8–15 days post-immunization, only animals that had the highest (peak) neurological score, i.e., 4–5 were used for TSPO and glial markers immunohistochemistry. Therefore, the authors missed the opportunity to examine the temporal profile of the TSPO glial cell response during the induction phase and resolution of the EAE disease [Abourbeh et al., 2012]. Based on a single time point in the continuum of the EAE, the authors conclude that the increased TSPO levels in EAE is from microglia/macrophages rather than astrocytes. While it is entirely possible that at the peak of the behavioral manifestation of the EAE the TSPO response originates from microglia, one cannot assume that the other phases of the disease have a similar TSPO glial cell response profile since signal colocalization and quantification were not performed. It is highly likely that the TSPO contribution from astrocytes was missed by the selection of a single time point in the disease continuum as it has been discussed with the cuprizone model above.
Table 1:
Representative animal and human studies in which the TSPO response is attributed only to microglia.
| Human Disease or Animal Model | Experimental Methods | Experimental Shortcoming | Reference |
|---|---|---|---|
| Autoimmune Encephalomyelitis (rats) | PET imaging; western blot and immunohistochemistry | Single time point; no signal colocalization analysis performed. | Abourbeh et al., 2012 |
| Excitotoxicity using quinolinic acid (rats) | Microglia in title; SPECT imaging; autoradiography and immunohistochemistry | No GFAP immunohistochemistry performed. | Arlicot et al., 2008 |
| Facial nerve axatomy (rats) | Emulsion microautoradiography; microglia immunohistochemistry | GFAP immunohistochemistry noted but not shown. | Banati et al., 1997 |
| Rasmussen’s encephalitis (human) | PET imaging; immunohistochemistry | Assumed astrocytes do not contribute to TSPO signal. GFAP immunostaining not performed. Emulsion autoradiography with GFAP staining not performed. | Banati et al., 1999 |
| Multiple Sclerosis (human, rats) | PET imaging; autoradiography and immunohistochemistry | GFAP immunohistochemistry noted but not shown. | Banati et al., 2000 |
| Status Epilepticus (rats) | PET imaging; autoradiography and immunohistochemistry | High degree of correlation of TSPO PET signal with both microglia and astrocytes. No double label of TSPO with glial markers. Only microglia correlation is noted in manuscript title. | Brackhan et al., 2016 |
| Alzheimer’s disease dementia (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Cagnin et al., 2001b |
| Multiple Sclerosis (human) | PET imaging; no assessment of microglia | No postmortem tissue immunohistochemistry performed. | Debruyne et al., 2003 |
| Lipopolysaccharide (LPS) (rats) | PET imaging; autoradiography and immunohistochemistry | Increased microglia and astrocyte staining; no double label with TSPO performed; only microglia is noted in manuscript title. | Dickens et al., 2014 |
| Multiple system atrophy (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Gerhard et al., 2003 |
| Corticobasal degeneration (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Gerhard et al., 2004 |
| Ischemic stroke (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Gerhard et al., 2005 |
| Idiopathic Parkinson’s disease (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Gerhard et al., 2006a |
| Progressive Supranuclear Palsy (humans) | PET imaging | No postmortem tissue immunohistochemistry performed. | Gerhard et al., 2006b |
| Parkinson’s Disease (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Ghadery et al., 2017 |
| Alzheimer’s Disease (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Groom et al., 1995 |
| Lipopolysaccharide (LPS) (non-human primates) | PET imaging; immunohistochemistry | Individual TSPO, microglia, and astrocyte imaging. No double label immunostaining performed. | Hannestad et al., 2012 |
| Corticobasal degeneration (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Henkel et al., 2004 |
| Microglia depletion and activation (nonhuman primates) | PET imaging | No postmortem tissue immunohistochemistry performed. | Hillmer et al., 2017a |
| Alcohol Dependence (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Hillmer et al., 2017b |
| Creutzfeldt-Jakob Disease (human) | PET imaging | No TSPO, microglia, or astrocyte postmortem tissue immunohistochemistry performed. | Iaccarino et al., 2017 |
| Fatal Familial Insomnia (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Iaccarino et al., 2018 |
| Head Injury (mice) | microPET imaging; autoradiography; immunohistochemistry | High degree of correlation of TSPO PET signal with both microglia and astrocytes. No double label of TSPO with glial markers. Only microglia correlation is noted in manuscript title. | Israel et al., 2016 |
| Lipopolysaccharide (LPS) (rabbit) | microPET imaging; immunohistochemistry | No astrocyte staining; no double label immunohistochemistry. | Kannan et al., 2007 |
| Amnestic mild cognitive impairment (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Knezevic et al., 2017 |
| Neuropathic Osteoarthritic Pain (rats) | Receptor binding; autoradiography; immunohistochemistry | No astrocyte staining performed; no TSPO double-label immunohistochemistry performed. | Miller et al., 2013 |
| Alzheimer’s Disease (5XFAD mice); postmortem human tissue from sporadic Alzheimer’s disease | microPET imaging; autoradiography; immunohistochemistry | One age of animals used. No signal colocalization and analysis performed. | Mirzaei et al., 2016 |
| Ischemic stroke (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Pappata et al., 2000 |
| Huntington’s Disease (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Politis et al., 2015 |
| Lipopolysaccharide (LPS) (human) | PET imaging | No postmortem tissue immunohistochemistry performed. Referenced nonhuman primate study of a single animal [Hannestad et al., 2012]. | Sandiego et al., 2015 |
| Global forebrain ischemia (rats) | Autoradiography; immunohistochemistry | One time point; no double label of TSPO with glial markers; visual inspection of TSPO binding with immunohistochemistry. | Stephenson et al., 1995 |
| Huntington’s Disease (human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Tai et al., 2007 |
| Alzheimer’s disease (human) | PET imaging | Presumed TSPO staining in arteries and veins in brain. No microglia or astrocyte immunohistochemistry. | Tomasi et al., 2008 |
| Alcohol (rats) | microPET imaging; histological staining of microglia | No staining of astrocytes. | Toyama et al., 2008 |
| Amyotrophic Lateral Sclerosis (ALS; human) | PET imaging | No postmortem tissue immunohistochemistry performed. | Turner et al., 2004 |
| AIDS (non-human primates) | PET imaging; autoradiography and immunohistochemistry | No double label or quantitative analysis signal colocalization. | Venneti et al., 2004 |
| Traumatic Brain Injury (TBI) (rats) | Autoradiography (in vitro and ex-vivo) and immunohistochemistry | No quantitative analysis of signal colocalization. | Venneti et al., 2007 |
| Cerebral Infarcts, Amyotrophic Lateral Sclerosis, Alzheimer Disease, Frontotemporal Dementia, and Multiple Sclerosis (humans) | Autoradiography and immunohistochemistry | No quantitative analysis signal colocalization. | Venneti et al., 2008 |
| Alzheimer’s disease postmortem tissue (human) APP/PS1 transgenic mice | MicroPET studies; autoradiography; immunohistochemistry | No quantitative analysis of signal colocalization. | Venneti et al., 2009 |
| Multiple Sclerosis (MS) (humans) and Experimental Autoimmune Encephalomyelitis (EAE) (mice) | PET imaging; autoradiography and immunohistochemistry | No astrocyte immunohistochemistry; no double label immunohistochemistry | Vowinckel et al., 1997 |
| Epilepsy (rats) | microPET imaging; | No postmortem tissue immunohistochemistry performed. | Yankam Njiwa et al., 2016 |
3. Endotoxin-induced neuroinflammation: TSPO in microglia and astrocytes?
Other studies have examined the TSPO glial cell response in endotoxin-induced neuroinflammation. Hannestad et al., [2012] examined the TSPO response in baboons injected with lipopolysaccharide (LPS; single injection) and TSPO brain levels imaged before and at 1 hr, 4hr, or 22 hr after i.v. injection of LPS using [11C]PBR28-PET. Whole brain analysis of PET scans revealed an increase in radioligand uptake in the whole brain that was increased only at the 4 hr time point and not at the 1 or 22 hr time points after LPS. However, when they used the animal’s own baseline PET scan, they found increased TSPO levels at 1 and 4 hrs suggestive of a transient increase since at the 22 hr time point PET analysis showed a marked decrease or no TSPO increase relative to the individual animal’s baseline PET [Hannestad et al., 2012]. Out of the 6 animals available, the authors euthanized 1 animal at 6 hr after LPS administration to perform immunohistochemistry for TSPO and CD68 for microglia/macrophages and GFAP staining for astrocytes. The immunostaining was only performed in the frontal lobe despite widespread increased brain TSPO uptake in the PET scan. From these limited immunostaining experiments the authors concluded that “cells that were immunoreactive (IR) for the antibody for TSPO are almost exclusively small, rod-like cells with short or no visible processes”. “These cells were very similar in morphology, size, and distribution to CD68 IR cells”. They further state: “Conversely, GFAP IR cells (astrocytes) were larger, much more abundant, and had more numerous and longer processes”. From this limited immunostaining in the absence of doublelabel studies they conclude that “Thus, TSPO immunoreactivity occurred almost exclusively in microglia, but not in astrocytes…”. This conclusion was representative of a single small area in the frontal lobe, from 1 animal, at a single time point after LPS (6 hr) in which double-label of TSPO with each individual glial marker was not performed. But yet, the conclusion based on visual inspection of presumed cellular morphology provides the evidence that the TSPO response from LPS administration is exclusively from microglia. Further, it is known that TSPO does not label the entire microglia or astrocyte but certain subcellular compartments; thus, the distinction cannot be made on presumed cellular size. Moreover, LPS not only activates microglia via TollLike Receptor 4, but it also activates the same receptors in astrocytes [Brahmachari et al., 2006; Gorina et al., 2011]. Finally, it is interesting to note that in the discussion of the study, the authors contradict their main conclusion that “TSPO immunoreactivity is occurring exclusively in microglia” by the following statement: “For the postmortem data, because double immunostaining was not performed it is possible that TSPO and CD68 expression occurred in different cell types, or that TSPO expression occurred in astrocytes in addition to microglia”.
This oversimplification of the cellular sources of the TSPO response was translated to a subsequent human study using LPS administration and [11C]PBR28-PET [Sandiego et al., 2015]. Eight healthy control volunteers had two [11C]PBR28-PET scans on the same day. They had a baseline scan and then a second scan in which LPS (10 ng/kg, i.v.) was administered 180 min before the second scan. They found a 30–60% increase in [11C]-PBR28 binding in multiple brain regions. Indeed, examination of the PET scan images after LPS indicated increased TSPO radioligand uptake throughout the entire brain. In the discussion, the authors conclude: “In the current study, the systemic LPS markedly increased the [11C]PBR28 binding in humans. [11C]PBR28 is a radioligand sensitive to TSPO levels expressed on activated microglia and is a putative biomarker of neuroinflammation. TSPO is also expressed in astrocytes; however, immunohistochemistry performed in nonhuman primate brain after LPS administration confirmed that TSPO expression occurred mainly in activated microglia [32]”. This conclusion referenced their previous baboon study [Hannestad et al., 2012] in which 1 single animal, at a single time point after LPS (6 hr), one brain area that was assessed by visual inspection of TSPO labeled cells with glial markers and in which double-label of TSPO with each individual glial marker and colocalization signal analysis was not performed. Further, as noted above, they acknowledge in the discussion of the baboon study, the uncertainty of the statement that the TSPO response is exclusively from microglia [Hannestad et al., 2012]. Despite these limitations and uncertainties in the baboon study, they indicate these two statements in the discussion of the human study: “In the current study, systemic LPS markedly increased [11C]PBR28 binding in humans. [11C]PBR28 is a radioligand sensitive to TSPO levels expressed on activated microglia and is a putative biomarker of neuroinflammation. TSPO is also expressed in astrocytes; however, immunohistochemistry performed in a nonhuman primate brain after LPS administration occurred mainly on microglia [32]”. They conclude: “thus, we have successfully extended the main findings of the nonhuman primate study to human subjects and established that LPS administration in humans is associated with a marked and significant increase in activated microglia”. While it is entirely possible that the early TSPO signal following LPS may be from microglia, the LPS response was examined at a single time point and may have missed the astrocytic TSPO response at other time points. In fact, a study from another group in which LPS was directly injected into the rat brain and in which a time-course study was performed using TSPO double label immunofluorescent imaging with microglia and astrocyte markers confirms that TSPO colocalized with microglia at the early time points with no TSPO astrocyte labeling but TSPO colocalized to astrocytes at a later time point when microglia do not express TSPO [Ory et al., 2015]. The study by Ory et al., [2015] support a significant body of evidence that the TSPO response in microglia and astrocytes have different temporal profiles. A limitation of the Ory et al., study was the lack of quantification of TSPO signal colocalization with the glial markers (see below).
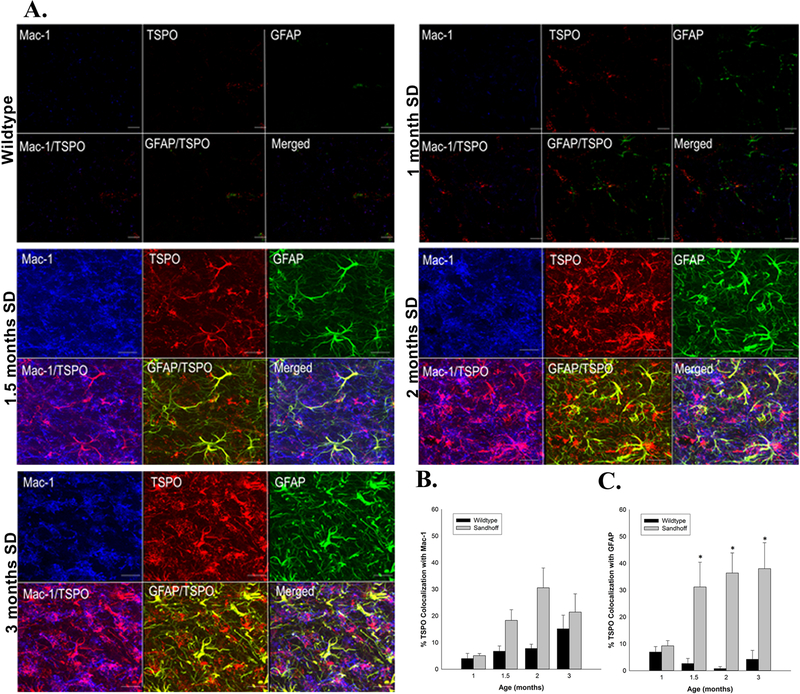
4. TSPO signal colocalization with microglia and astrocytic markers and computer-based quantitative image analysis–a way forward:
The last two decades of studies using TSPO as a biomarker of brain injury have witnessed a gradual improvement of methodological approaches used for assessing the cellular sources of the TSPO response. The current availability and use of state-of-the-art computer-based image analysis technology has facilitated the quantitative analysis of the colocalization of immunofluorescent signals from different proteins in the same cell with exquisite sensitivity and spatial resolution. An example of this new approach is depicted in a recent TSPO study using a mouse model of Sandhoff disease [Loth et al., 2016]. Sandhoff disease is an autosomal recessive disorder characterized by a deficiency of the lysosomal enzyme β-hexosaminidase leading to the accumulation of gangliosides and glycolipids in neurons, specifically GM2 and GA2 [Sango et al., 1995; Mahuran, 1999; Jeyakumar et al., 2002]. The accumulation and aggregation of gangliosides is the triggering event that leads to progressive and widespread neurodegeneration. The TSPO response was examined in this animal model as a function of age (neurodegeneration increases with age) using the second generation radioligand [3H]DPA-713 (autoradiography) and [125I]-iodo-DPA-713 (microSPECT) in parallel with behavioral and pathological expression of the disease. It was found that the increased TSPO response as measured by [3H]-DPA-713 autoradiography occurred much earlier in time in the thalamus (1.5 months) than the behavioral manifestation of disease (3 months). The thalamus is the brain region that exhibits the first neurodegenerative changes in this disease. Furthermore, increased [3H]DPA-713 specific binding to TSPO occurred simultaneously with the aggregation of GM2 gangliosides in neurons and before evidence of active neurodegeneration using Silver staining [Loth et al., 2016]. The increase in brain TSPO in the living anesthetized animal was measured using [125I]-iodo-DPA-713 microPET and brain uptake was blocked by the administration of non-radioactive PK11195. Double-label immunofluorescence imaging of TSPO (using an extensively validated TSPO antibody) with the microglia marker Mac-1 and the astrocyte marker GFAP was also performed in an age-dependent fashion (See Figure 1). Notably, this was the first study to provide a quantitative analysis of percent colocalization of the TSPO immunofluorescence signal with the Mac-1 or GFAP immunofluorescence signal using confocal imaging and computer-based image analysis. The results of this quantitative approach showed that there was a greater percent colocalization of the TSPO signal with GFAP (astrocyte) than with Mac-1 (microglia) and it changed with disease progression [Loth et al., 2016]. This state-of-the-art quantitative approach is an example of what is possible today using computer-based microscopy and image analysis software. During the writing of this review, a new study was published in a murine model of mesial temporal lobe epilepsy in which [18F]DPA-714 microPET/computed tomographic scanning, autoradiography, and double label immunofluorescence was performed using a longitudinal approach [Nguyen et al., 2018]. This study confirmed that the glial sources of the TSPO response was due to an early microglia component which decayed with time, and a predominant TSPO colocalization in astrocytes at the later time point providing further support that both microglia and astrocytes contribute to the TSPO signal. However, this study did not use computer-based microscopy and image analysis to determine the percent signal colocalization as performed by Loth et al., [2016], a method that is currently routinely used in many laboratories.
Figure 1:
Temporal colocalization of TSPO with glial markers in wildtype (WT) and Sandhoff disease (SD) mice in the thalamus. A) Representative triple labeled immunofluorescent confocal images in the thalamus of WT and SD mice at four different ages. Triple labeled immunofluorescent confocal imaging confirmed that TSPO colocalized with the microglial marker Mac-1 as indicated by the purple and magenta colors and astrocyte marker GFAP as indicated by the yellow color. B) In the thalamus, the percent colocalization of TSPO with Mac1 follows a similar pattern in both the WT and SD mice, with a more pronounced effect in the SD mice. The percent colocalization between TSPO and Mac-1 increases with age in both WT and SD mice and peaks at the 2 months of age. C) The percent colocalization between TSPO and GFAP also differs significantly between WT and SD mice, due to the low signal of both in the WT mice. In the SD mice, we observed a high degree of colocalization beginning at 1.5 months and remaining at 3 months of age. Each value represents the mean ± SEM. n = 3 – 4 animals and experiments.
“Reprinted from Neurobiology of Disease, 85, MK Loth, J Choi, JL McGlothan, MV Pletnikov, MG Pomper, TR Guilarte, TSPO in a murine model of Sandhoff disease: presymptomatic marker of neurodegeneration and disease pathophysiology, 174-186, 2016, with permission from Elsevier.”
Recommendation 1: Unlike in the early studies that examined the cellular sources of the TSPO response, today there are well-validated and specific antibodies that are commercially available for TSPO as well as glial markers. Furthermore, there are a number of computer-based microscopy and image analysis software that are readily available commercially and can provide unbiased quantitative TSPO immunofluorescence signal colocalization with microglia and astrocytes markers. Therefore, it is recommended and highly desirable that studies examining the cellular sources of the TSPO response should be done with the same level of experimental rigor and be standardized so that studies using different animal models of human disease with a longitudinal design can be compared. This approach can also assist with the interpretation of human PET studies.
In summary, a critical review of the literature indicates that the cellular sources of the TSPO response to brain injury is disease specific and has a temporal component. Temporal examination of the glial sources in any human disease of interest is critically important because glial cell dynamics are known to change as a function of age and disease progression [McNeela et al., 2017]. What is clear, however, is that it is inaccurate and a simplification of the complexity of glial responses to brain injury to continue to state that TSPO is a biomarker of microglia activation when astrocytes also play an important role in upregulating the TSPO signal in the neuroinflammatory response in many neurological and neurodegenerative disorders. Even within a single disease entity, the temporal profile of the TSPO signal originating from microglia/macrophages and/or astrocytes may vary depending upon the stage of the disease and unless a time-course in postmortem tissue is performed it is scientifically prudent to take great caution in making a generalized assumption, especially in PET studies. The most prudent approach is to note that TSPO is representative of a glial response rather than a specific glial type. The technological advances that exist today provide the appropriate means to rigorously examine this important question in brain tissue from any animal model or in human post-mortem brain tissue.
What has been extensively validated over the years is that the TSPO signal measured with [3H]R-PK11195 binding studies is driven by an increase in the maximal number of binding sites (Bmax) with no change in the affinity constant (Kd) of the protein-ligand interaction [Gehlert et al., 1997; Rao et al., 2000; Chen et al., 2004; Venneti et al., 2004; Chen and Guilarte, 2008]. What has yet to be discovered is the functional significance of the increased TSPO response to brain injury and neuroinflammation and whether the increased TSPO response has the same or different biological function(s) in microglia and astrocytes. A resolution to these important question(s) can bring about a new era in the development of novel TSPO radiotracers with a possible selectivity for microglia or astrocytes. If the latter is ever accomplished, it would be a major scientific breakthrough in the TSPO field.
C). Exploration of new findings in psychiatric disorders
1. TSPO in psychiatric disorders: an exception to the rule or alternative explanations?
The last decade has seen an exponential increase in the number of studies related to TSPO as a biomarker of brain injury and neuroinflammation. Technological advances in high resolution PET scanners and the development of second generation TSPO ligands with more favorable pharmacokinetics and lower non-specific binding than the prototypical TSPO ligand RPK11195, has facilitated this work [Dolle et al., 2009; Cumming et al., 2017; Dupont et al., 2017]. Further, TSPO as a biomarker of inflammation has been extended to study other organ systems [Hardwick et al., 2005; Fairweather et al., 2014]. Psychiatric disorders are no exception in the quest to use TSPO as a biomarker of neuroinflammation and the last 5 years have seen a number of reports with mixed results [see review by De Picker et al., 2017]. Paradoxically, some studies have provided the first description of decreased rather than increased brain TSPO levels in schizophrenia [Coliste et al., 2017; Benlloch et al., 2018] and in major depression [Li et al., 2018]. Overall, studies have found increased, decreased, or no change in brain TSPO levels in schizophrenia [De Picker et al., 2017] and in depression [Hannestad et al., 2013; Setiawan et al., 2015; Holmes et al., 2017; Li et al., 2018; Richards et al., 2018; Setiawan et al., 2018] compared to age-matched controls using PET imaging with radiolabeled R-PK11195 or with second generation TSPO PET ligands. There are many factors in study design such as TSPO radioligand used (R-PK11195 or second generation), PET imaging quantification with and without radioligand plasma input data, medicated vs naïve subjects, as well as corrections for the human TSPO polymorphism and vascular radioligand binding that have been discussed in the literature [Turkheimer et al., 2015; De Picker et al., 2017; Veronese et al., 2017] and could potentially account for some of the different findings of TSPO-PET in these psychiatric disorders. However, other factors that could potentially influence the interpretation of the PET results have yet to be investigated. This is critically important before the TSPO biomarker field takes a turn as it has recently occurred with studies on the function of TSPO [Morohaku et al., 2014; Tu et al., 2014; 2015]. Today, TSPO appears to be an orphan protein looking for a function because the longheld dogma of its principal role as the rate limiting step in the transfer of cholesterol from the outer to the inner mitochondria membrane for steroid synthesis has been questioned [Selvaraj and Stocco, 2015; Gut et al., 2015; Selvaraj and Tu, 2016]. While there are studies which seem to support and some that do not support the function of TSPO in steroid synthesis; this discourse has not yet occurred with the use of TSPO as a biomarker of brain injury and neuroinflammation. However, a reconceptualization of TSPO as a biomarker of neuroinflammation in psychiatric disorders has been proposed [Notter et al., 2017].
2. TSPO studies in schizophrenia prior to PET imaging-a historical review:
Before examining the TSPO-PET literature in schizophrenia, it is important to review the early literature in which TSPO levels were measured in cells or postmortem brain tissue from schizophrenia subjects. These studies may provide important information that may help the interpretation of current TSPO-PET findings. As far back as 1986, there were studies examining the levels of TSPO in platelets from schizophrenia subjects [Gavish et al 1986; Weizman et al., 1986]. Two different studies showed that the maximal number of [3H]PK11195 binding sites (Bmax) in platelets from untreated schizophrenia subjects did not differ from age-matched healthy controls. However, in one study, schizophrenia subjects with chronic neuroleptic treatment exhibited a 30% decrease in [3H]PK11195 Bmax with no change in Kd (affinity constant) compared to untreated schizophrenics and healthy controls [Gavish et al., 1986]. In the second study, medicated schizophrenia subjects with and without tardive dyskinesia had 31.3% and 21.1% decreased [3H]PK11195 Bmax relative to unmedicated patients and health controls [Weizman et al., 1986]. These studies showed no significant alterations in [3H]PK11195 binding parameters to TSPO in platelets from untreated schizophrenia subjects relative to healthy controls, but TSPO Bmax levels decreased significantly with schizophrenia medication or disease classification.
Later studies using platelets and other peripheral cells to measure TSPO levels supported the findings that TSPO binding parameters could be altered based on specific classifications of the disease. Additionally, co-morbidity factors such as alcohol and substance abuse also influenced TSPO binding parameters. For example, using granulocytes, Wodarz et al., [1998] found a 38% decrease in [3H]PK11195 Bmax in residual-type schizophrenics relative to controls. Similarly, Ritsner et al., [2003] found a 30% decrease in platelet [3H]PK11195 Bmax in aggressive schizophrenia relative to controls. Studies in peripheral cells from alcoholics, cocaine dependence, and after suicide attempts in schizophrenia subjects indicate that these factors can decrease [3H]PK11195 Bmax in peripheral cells [Suranyi-Cadotte et al., 1988; Javaid et al., 1994]. Collectively, all of these studies provided evidence of no change in peripheral cell [3H]PK11195 Bmax or Kd in unmedicated schizophrenia subjects relative to healthy controls but schizophrenia with specific classifications or co-morbidity factors decreased peripheral cell [3H]PK11195 Bmax with no change in Kd. These studies indicate that factors from disease classification to co-morbidity need to be carefully controlled to provide clarity to current findings from TSPO-PET studies in schizophrenia.
3. Relevant TSPO studies in brain tissue from schizophrenia subjects:
There is a paucity of studies examining TSPO levels in postmortem brain tissue from schizophrenia subjects. The only study found in PubMed was by Kurumaji et al., [1997] that examined postmortem brain tissue from schizophrenia subjects and controls. Analysis of 26 brain regions indicated decreased [3H]-PK11195 specific binding in superior parietal cortex, occipital cortex-visual area 1, and putamen that ranged from 23.1 to 41.5 %. Parenthetically, in this study they found decreases in both Bmax and Kd. That is, decreased number of binding sites with increased affinity.
Although the current review is focused on TSPO as a biomarker of neuroinflammation in schizophrenia, it was important to examine studies in which microglia and astrocyte morphology and viability have been examined in postmortem tissue from schizophrenia subjects. Notably, Wierzba-Bobrowicz et al., [2004] have described that in brain tissue from schizophrenia subjects there is evidence of degenerative changes in microglia including fragmentation of processes, apoptotic changes and, importantly, degenerated mitochondria. These findings are relevant because TSPO is highly localized in mitochondria and their degeneration can influence the available number of TSPO binding sites that can be measured in TSPO-PET studies. A PubMed search under “schizophrenia and microglia” resulted in 272 hits and “schizophrenia and astrocytes” resulted in 350 hits (January 27, 2018). Therefore, there are many studies, beyond the scope of this review, that may be relevant in understanding glial cell mitochondrial changes that may influence TSPO expression in schizophrenia.
4. What are potential but yet unexplored interpretations of TSPO PET findings in schizophrenia?
The current results of brain TSPO changes (increased, decreased, or no change) in schizophrenia relative to age-matched controls may depend on other potentially important factors that have yet to be considered but may need to be experimentally controlled and/or examined. Several points to this effect and relevant recommendations are provided below:
POINT 1: Disease modifying effects on peripheral TSPO levels or TSPO ligand binding proteins may alter the availability of TSPO radioligands for brain uptake:
It is well recognized that TSPO levels in peripheral organs including peripheral circulating blood cells are much higher than those expressed in normal or even in pathological brain tissue. Schizophrenia is thought to have a peripheral inflammatory component. Thus, it is entirely plausible that in peripheral tissues TSPO levels could be differentially altered from those in the brain at different stages of the disease so that peripheral TSPO is markedly altered and may modulate the amount of radiotracer delivered to the brain. A possible effect of increasing or decreasing peripheral TSPO levels could be reflected as an “apparent” decrease or increase in TSPO radioligand brain uptake measured by PET. In fact, such a modulatory effect of a treatment on [11C]PBR28 arterial plasma concentration and brain uptake has been reported with LPS and PLX3397 in nonhuman primates [Hillmer et al., 2017a]. PLX3397 is a selective colony-stimulating factor 1 receptor inhibitor used to deplete microglia in the brain.
Similarly, it is possible that in schizophrenia or in other disease conditions, there is induction of gene(s) that encode protein(s) in peripheral organs, blood, and possibly even in the brain neuropil that are able to bind R-PK11195 and/or second generation TSPO ligands; thus, potentially altering radiotracer delivery, uptake, or binding to TSPO in the brain. Such a protein exists and has already been described in the literature but has not been accounted for in TSPO-PET studies. The acute phase reactant 1-acid glycoprotein (AAG), an abundant protein in plasma, has been shown to bind PK11195 with high affinity [Lockhart et al., 2003]. While there is no current information on whether AAG also binds second generation TSPO ligands, it is highly likely that it does. Relevant to schizophrenia, plasma levels of AAG have been shown to be altered in schizophrenia subjects [Goodman et al., 1961] and AAG plasma concentrations are altered by drugs used for the treatment of schizophrenia [Levinson and Levine, 1995; Telford et al., 2012]. Furthermore, AAG protein levels are markedly increased in plasma, and potentially in the brain neuropil, by pathophysiological conditions including inflammation, depression, cancer, and autoimmune deficiency syndrome, altering the pharmacokinetic disposition of drugs that bind to AAG [Holladay et al., 1998; Garrido et al., 1999]. Notably, AAG genetic variants have been shown to alter the blood to brain transfer of AAG-binding drugs [Jolliet-Riant et al., 1998]. Finally, relevant to psychiatric disorders, studies have found a positive association between serum AAG with age in depressed female subjects [Young et al., 1999]. In summary, the abovementioned studies indicate that it is critically important to determine and understand the potential for disease-modified levels of AAG in plasma, and potentially in the brain, on TSPO ligand uptake and binding in the brain and its implications in the interpretation of TSPO-PET studies.
Recommendation 2: determine if AAG binds second generation TSPO radioligands with high affinity.
Recommendation 3: Examine plasma levels of AAG in schizophrenia subjects at the time of the TSPO-PET study, and under medication treatments, to determine if differences in AAG occur between schizophrenia subjects and age-matched healthy controls at different stages of the disease.
Recommendation 4: Determine in post-mortem brain tissue if AAG levels are altered in psychiatric disorders, neuroinflammatory disease, and other neuropathological conditions.
POINT 2: Microglia and astrocyte number in schizophrenia: analysis using rigorous unbiased stereological cell counting:
There is evidence of microglia number changes in schizophrenia [Frick et al., 2013; van Kesteren et al., 2017] as well as astrocytes [Williams et al., 2013; Kim et al., 2017]. Therefore, changes in microglia and/or astrocyte number in schizophrenia may influence TSPO-PET findings.
Recommendation 5: Determine microglia and astrocyte number in schizophrenia brain tissue and age-matched healthy controls using rigorous unbiased stereological cell counting methods.
POINT 3: Mitochondria number in microglia and astrocytes in schizophrenia: are they different? Microglia and astrocytes are the brain cells that express and increase TSPO levels in a variety of brain pathologies. Further, TSPO is a protein that is highly expressed in mitochondria. Therefore, one must consider the possibility that TSPO levels in schizophrenia subjects could be influenced by disease-modifying changes in glial cell mitochondria number; thus, altering the putative number of TSPO binding sites even under conditions where no glial cell activation or glia cell number change is observed. There is evidence of decreased mitochondria number in glial cells in the brain of schizophrenia subjects [Ben-Shachar, 2002; Somerville et al., 2012; Roberts, 2017] as well as changes in mitochondria genes that could modulate TSPO expression [Iwamoto et al., 2005].
Recommendation 6: Determine mitochondria integrity and number in microglia and astrocytes in postmortem brain tissue in schizophrenia using 3-dimensional scanning electron microscopy.
POINT 4: Modeling schizophrenia in preclinical animal models and their effect on brain TSPO levels - what do they tell us? Schizophrenia is a uniquely human disease and it is not possible to capture its complexity in an animal model. However, animal models of schizophrenia do provide a presumed behavioral and neuropathology phenotype and mimic certain aspects of the human disease. There is a paucity of studies using appropriate animal models of schizophrenia that also assess brain TSPO levels. A recent study by Notter et al. [2018] examined the relationship of the brain TSPO response in an inflammatory developmental animal model of schizophrenia; that is, the injection of polyriboinosinic-polyribocytidylic acid (poly:IC) in timepregnant mice. The offspring of these poly:IC exposed dams express deficits in prepulse inhibition of the acoustic startle response and social interaction similar to what has been described in schizophrenia subjects [Notter et al., 2017]. In the same study, the authors also performed TSPO-PET using [11C]DPA-713 in recent-onset schizophrenia subjects and matched healthy controls. They found a near (p=0.051), but non-significant decrease in TSPO levels by PET in the middle frontal gyrus of schizophrenic subject relative to controls.
In the animal portion of this study, the authors perform TSPO immunohistochemistry in frontal cortex and hippocampus of poly:IC exposed animals and controls. They found an apparent decrease in brain TSPO levels using immunofluorescent labeling that appears to be selective to the frontal cortex with no change in the hippocampus [Notter et al., 2018]. TSPO immunofluorescence signal was measured using relative optical intensity, a method that is not quantitative. In fact, a more quantitative method, receptor autoradiography, that they also used provided no evidence of a change in TSPO specific binding in the frontal cortex or hippocampus of poly:IC exposed animals compared to non-exposed. The authors recognized the discrepancy in results using TSPO immunohistochemistry and receptor autoradiography but they argue that the differences using these two methods are explained by the lower resolution of ex vivo radiotracer autoradiography compared to immunohistochemistry which can provide analysis at the cellular and subcellular level. They state that the immunohistochemistry technique is likely to be more suitable in detecting relatively small changes in TSPO expression, and thus confirmation of decreased TSPO levels in the frontal cortex of poly:IC exposed animals. While the authors make an argument for a significant and selective decrease in TSPO levels in the frontal cortex in the in utero exposed poly:IC animals based on the immunohistochemistry results, this argument needs further validation as they did not measure the TSPO signal specifically at the cellular and/or subcellular level. That is, they performed optical density readings at the gross anatomical level which not only includes a signal from TSPO labeled cells but also microvasculature and specific and non-specific primary and/or secondary antibody staining. It is likely that the receptor autoradiography data is providing the most accurate results. That is, no change in TSPO levels in frontal cortex or hippocampus of poly:IC animals compared to non-exposed controls as has been found in several of the TSPO-PET studies in schizophrenia subjects including results from the same investigators [Notter et al., 2018].
Recommendation 7: In animal studies it is important to use multiple methods to confirm a finding, including: 1) radioligand binding such as film and emulsion microautoradiography with the latter having resolution at the cellular level similar to confocal imaging immunofluorescence, 2) radioligand saturation isotherms with Scatchard analysis of brain tissue to determine Kd and Bmax values which is a more accurate representation of the radioligand interaction than a single radioligand concentration as it is typically used in autoradiography studies, and 3) protein analysis by quantitative Western blot.
POINT 5: Maintain independent variables the same in comparison groups: In the same study, a potentially important experimental design problem is that the animals used for TSPO immunohistochemistry received a behavioral experience, i.e., assessment of pre-pulse inhibition and social interaction [Notter et al, 2018-supplementary materials] while the animals used for TSPO autoradiography did not experience behavioral tests. Thus, it is possible that the behavioral experience, along with the handling of animals for behavior-a form of environmental enrichment- may differentially affect TSPO brain levels in poly:IC animals used for immunohistochemistry relative to those used for autoradiography. Although the authors waited a week prior to euthanizing the animals that experienced the behavioral protocol, the effect of the behavior on regional TSPO levels is not known and was not studied.
Recommendation 8: All animals in both treatment groups should have experienced an identical behavioral protocol in order to eliminate any potential modulatory effects from the behavior and manipulation which only one set of animals experienced. In future studies, it is possible to use one hemisphere for autoradiography studies and the other hemisphere for immunohistochemistry so that there is a direct comparison of the two different methods in the same animal. Alternatively, an entirely behaviorally naïve set of animals should have been used for both the immunohistochemistry and autoradiography studies.
D. Concluding remarks:
It is clear from the limited but increasing number of human TSPO studies in schizophrenia and other psychiatric disorders such as depression that the findings are equivocal in regards to TSPO as a biomarker of neuroinflammation. During the writing of this review, a report appeared of a meta-analysis in individual participant data of TSPO-PET studies in first-episode schizophrenia patients with psychosis and healthy controls [Plaven-Sigray et al., 2018]. The meta-analysis concludes that overall, brain TSPO levels are decreased in 75 schizophrenia participants relative to 77 healthy controls from five independent studies. This meta-analysis provides evidence of a decrease in TSPO levels in the schizophrenia brain relative to aged-matched healthy controls using PET imaging and provides a compelling reason to study the recommendations outlined in this review.
While there are differences amongst the TSPO-PET studies in schizophrenia in regards to TSPO radioligand used, PET imaging quantification with and without radioligand plasma input data and analytical methods used, medicated vs naïve subjects, as well as corrections for the human TSPO polymorphism and vascular radioligand binding; there are other important factors that have not been considered and are delineated in this review. From the perspective of using preclinical animal models, it is critically important that rigorous experimental design and interpretation of results be provided so that potential confounding factors are eliminated to reduce paradoxical findings and increase the reliability and reproducibility of the results. These considerations of rigor and authentication will go a long way to bring clarity so that the TSPO biomarker field is not turned up-side-down as has recently happened with studies on the function of TSPO. To this end, a remaining point of contention in the literature is the cellular sources of the TSPO response to brain injury and neuroinflammation which is often and still described by a significant number of publications in the literature as an exclusively microglial response. The current availability of well-validated and specific TSPO antibodies and computer-based software for microscopy and image analysis of confocal images now make it possible to provide accurate, reliable, and direct results on the cellular sources of the TSPO response with quantitative analysis of signal colocalization with glial markers in any human brain disease or preclinical model in which brain tissue samples are available. Using this type of approach, there is evidence that the TSPO response to different brain pathologies is derived from both microglia and astrocytes, although they may have different temporal profiles and degree of induction. The ready dismissal of the nature of the TSPO response to be less than a complex interaction of microglia and astrocytes, “a glial response”, in the context of diverse brain pathologies or neurodegenerative/mental disorders and their progression is no longer tenable and will not provide a near term solution, but it will delay the inevitable reality of how nature works. The progression of science will eventually provide the correct answer since “in science, truth always wins” (Nobel Prize winner Max Perutz; http://www.vega.org.uk/video/programme/1).
Acknowledgments
This review is dedicated to the many doctoral students, postdoctoral fellows, research staff, and collaborators that have contributed significantly to this work in my laboratory over the last 20 plus years. I am especially indebted to my senior research scientist Ms. Jennifer Dziedzic that has been involved in these studies over the last 20 years. The TSPO work from our laboratory has been continuously funded from the National Institute of Environmental Health Sciences grant number ES007062–20. I acknowledge that there are many more publications that could have been cited, but the ones cited in this review were selected to support a specific comment or scientific point of view.
Abbreviations
- Bmax
number of binding sites
- CBR
central benzodiazepine receptor
- CNS
central nervous system
- CNTF
cytokine ciliary neurotrophic factor
- EAE
experimental autoimmune encephalomyelitis
- GFAP
glial fibrillary acidic protein
- Kd
affinity constant
- LPS
lipopolysaccharide
- PBR
peripheral benzodiazepine receptor
- PET
positron emission tomography
- Poly
IC polyriboinosinic-polyribocytidylic acid
- SIV
simian immunodeficiency virus
- SIVE
simian immunodeficiency virus encephalopathy
- TBI
traumatic brain injury
- TMT
trimethyltin
- TSPO
translocator protein 18 kDa
Footnotes
Conflict of Interest Statement
The author declares that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abourbeh G, Theze B, Maroy R, Dubois A, Brulon V, Fontyn Y, Dolle F, Tavitian B, Boisgard R (2012) Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis rats by positron emission tomography using the mitochondrial 18 kDa translocator protein radioligand [18F]-DPA-714. J Neurosci 32, 5728–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholt RRH, Pedersen PL, De Suoza EB, Snyder SH (1986) The peripheral-type benzodiazepine receptor-localization to the mitochondrial outer membrane. J Biol Chem 261, 576–583. [PubMed] [Google Scholar]
- Arlicot N, Katsifis A, Garreau L, Mattner F, Vergote J, Duval S, Bodard S, Guilloteau D, Chalon S (2008) Evaluation of CLINDE as potent translocator protein (18 kDa) SPECT radiotracer reflecting the degree of neuroinflammation in a rat model of microglial activation. Eur J Nucl Med Mol Imaging 35, 2203–2211. [DOI] [PubMed] [Google Scholar]
- Arlicot N, Tronel C, Bodard S, Garreau L, de la Crompe B, Vandevelde I, Guilloteau D, Antier D, Chalon S (2014) Translocator protein (18 kDa) mapping with [125I]-CLINDE in the quinolinic acid rat model of excitotoxicity: a longitudinal comparison with microglial activation, astrogliosis, and neuronal death. Mol Imag 13, 1–11. [PubMed] [Google Scholar]
- Baestrup C, Squires RF (1977) Specific benzodiazepine receptors in rat brain characterized by high-affinity [3H]-diazepam binding. Proc Nat Acad Sci 74, 3805–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB, Myers R, Kreutzberg GW (1997) PK (‘peripheral benzodiazepine’) – binding sites in the CNS indicate early and discrete brain lesions: microautoradiography detection of [3H]-PK11195 binding to activated microglia. J Neurocytol 26,77–82. [DOI] [PubMed] [Google Scholar]
- Banati RB, Goerres GW, Myers R, Gunn RN, Turkheimer FE, Kreutzberg GW, Brooks DJ, Jones T, Duncan JS (1999) [11C]-R-PK11195 positron emission tomography imaging of activated microglia in Rasmussen’s encephalitis. Neurology 53, 2199–2203. [DOI] [PubMed] [Google Scholar]
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R (2000) The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as measure of disease activity. Brain 123, 2321–2337. [DOI] [PubMed] [Google Scholar]
- Benavides J, Quarteronet D, Imbault F, Malgouris C, Uzan A, Renault C, Dubroeucq MC, Gueremy C, LeFleur G, (1983) Labelling of “peripheral-type” benzodiazepine binding sites in the rat brain by using [3H]-PK11195, an isoquinoline carboxamide derivative: kinetic studies and autoradiographic localization. J Neurochem 41, 1744–1750. [DOI] [PubMed] [Google Scholar]
- Benavides J, Fage D, Carter C, Scatton B (1987) Peripheral type benzodiazepine binding sites are a sensitive indirect index of neuronal damage. Brain Res 421, 167–172. [DOI] [PubMed] [Google Scholar]
- Benavides J, Cornu P, Dennis T, Dubois A, Hauw JJ, MacKenzie ET, Sazdovitch V, Scatton B (1988) Imaging of human brain lesions with an omega 3 site radioligand. Ann Neurol 24, 708–712. [DOI] [PubMed] [Google Scholar]
- Benavides J, Dubois A, Gotti B, Buordiol F, Scatton B (1990) Cellular distribution of omega 3 (peripheral type benzodiazepine) binding sites in the normal and ischaemic rat brain: an autoradiographic study with the photoaffinity ligand [3H]-PK14105. Neurosci Lett 114, 32–38. [DOI] [PubMed] [Google Scholar]
- Benlloch JM, Gonzalez AJ, Pani R, Preziosi E, Jackson C, Murphy J, Barbera J, Correcher C, Aussenhofer S, Gareis D, Visvikis D, Bert J, Langstrom B, Farde L, Toth M, haggkvist J, Caixeta FV, Kullander K, Somial-Schweiger I, Schwaiger M (2018) The MINDVIEW project: First results. Eur Psych 50, 21–27. [DOI] [PubMed] [Google Scholar]
- Ben-Scachar D (2002) Mitochondria dysfunction in schizophrenia: a possible linkage to dopamine. J Neurochem 83, 1241–1251. [DOI] [PubMed] [Google Scholar]
- Biesmans S, Acton PD, Cotto C, Langlois X, Ver Donck L, Bouwknecht JA, Aelvoet S-A, Hellings N, Meert TF, Nuydens R (2015) Effect of stress and peripheral immune activation on astrocyte activation in transgenic bioluminescent Gfap-luc mice. GLIA 63, 11261137. [DOI] [PubMed] [Google Scholar]
- Brackhan M, Bascunana P, Postema JM, Ross TL, Bengel FM, Bankstahl M, Bankstahl JP (2016) Serial quantitative TSPO-targeted PET reveals peak microglia activation up to 2 weeks after an epileptogenic brain insult. J Nucl Med 57, 1302–1308. [DOI] [PubMed] [Google Scholar]
- Brahmachari S, Fung YK, Pahan K (2006) Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci 26, 4930–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin A, Myers R, Gunn RN, Lawrence AD, Stevens T, Kreutzberg GW, Jones T, Banati RB (2001) In vivo visualization of activated glia by [11C]-(R)-PK11195-PET following herpes encephalitis reveals projected neuronal damage beyond the primary focal lesion. Brain 124, 2014–2027. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE (2001) In vivo measurement of activated microglia in dementia. THE LANCET 358, 461–467. [DOI] [PubMed] [Google Scholar]
- Chen MK, Baidoo K, Verina T, Guilarte TR (2004) Peripheral benzodiazepine receptor imaging in CNS demyelination: functional implications of anatomical and cellular localization. Brain 127, 1379–1392. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR (2006) Imaging the peripheral benzodiazepine receptor response in central nervous system demyelination and remyelination. Toxicol Sci 91, 532–539. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR (2008) Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Therap 118, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coliste K, Plaven-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A, Amini N, Aeinehband S, Karolinska Schizophrenia Project (KaSP)., Erhardt S, Halidin C, Flyckt L, Farde L, Cervenka S (2017) Lower levels of the glial marker TSPO in drug-naïve first-episode psychosis patients as measured using PET and [11C]PBR28. Mol Psych 22, 850856. [DOI] [PubMed] [Google Scholar]
- Conway EL, Gundlach AL, Craven JA (1998) Temporal changes in glial fibrillary acidic protein messenger RNA and [3H]-PK11195 binding in relation to imidazoline-I2-receptor and α2-adrenoceptor binding to the hippocampus following transient global forebrain ischaemia in the rat. Neurosci 82, 805–817. [DOI] [PubMed] [Google Scholar]
- Cosenza-Nashhat M, Zhao M-L, Suh H-S, Morgan J, Natividad R, Morgello S, Lee SC (2009) Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropath Appl Neurobiol 35, 306–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV, Roosa HV, Gage KL, Stathis M, Rais R, Rojas C, McGlothan JL, Watkins CC, Sacktor N, Guilarte TR, Zhou Y, Sawa A, Slusher BS, Caffo B, Kassiou M, Endres CJ, Pomper MG (2014) Regional distribution of translocator protein using [11C]-DPA-713 PET in individuals infected with HIV. J Neurovirol 20, 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Munro CA, Ma S, Yue C, Chen MS, Airan R, Kim PK, Adams AV, Garcia C, Higgs C, Sair HI, Sawa A, Smith G, Lyketsos CG, Caffo B, Kassiou M, Guilarte TR, Pomper MG (2015) Neuroinflammation and brain atrophy in former NFL players: an in vivo multimodal imaging pilot study. Neurobiol Dis 74, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Minn I, Bienko N, Ambinder EB, Xu. X, Peters ME, Dougherty JW, Vranesic M, Koo SM, Ahn HH, Lee M, Cottrell C, Sair HI, Sawa A, Munro CA, Nowinski CJ, Dannals RF, Lyketsos CG, Kassiou M, Smith G, Caffo B, Mori S, Guilarte TR, Pomper MG (2017) Imaging of glial cell activation and white matter integrity in brains of active and recently retired National Football League players. JAMA Neurol 74, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming P, Burgher B, Patkar O, Breakspear M, Vasdev N, Thomas P, Liu G-J, Banati R (2017) Sifting through the surfeit of neuroinflammation tracers. J Cereb Blood Flow Metab 38, 204–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne JC, Versijpt J, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, Achten E, Slegers G, Dierckx RA, Korf J, De Reuck JL (2003) PET visualization of microglia in multiple sclerosis patients using [11C]-PK11195. Eur J Neurol 10, 257–264. [DOI] [PubMed] [Google Scholar]
- De Picker LJ, Morrens M, Chance SA, Boche D (2017) Microglia and brain plasticity in acute psychosis and schizophrenia illness course: a meta-review. Frontiers Psych 8, article 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens AM, Vainio S, Marjamaki P, Johansson J, Lehtiniemi P, Rokka J, Rinne J, Solin O, Haaparanta-Solin M, Jones PA, Trigg W, Anthony DC, Airas L (2014) Detection of microglial activation in an acute model of neuroinflammation using PET and radiotracers 11C-(R)-PK11195 and 18F-GE-180. J Nucl Med 55, 466–472. [DOI] [PubMed] [Google Scholar]
- Diorio D, Welner SA, Butterworth RF,, Meaney MJ, Suranyi-Cadotte BE (1991) Peripheral benzodiazepine binding sites in Alzheimer’s disease frontal cortex and temporal cortex. Neurobiol. Aging 12, 255–258. [DOI] [PubMed] [Google Scholar]
- Doble A, Malgouris C, Daniel M, Daniel N, Imbault F, Basbaum A, Uzan A, Gueremy C, LeFur G (1987) Labelling of peripheral-type benzodiazepine binding sites in human brain using [3H]-PK11195: anatomical and subcellular distribution. Brain Res Bull 18, 49–61. [DOI] [PubMed] [Google Scholar]
- Dolle F, Luus C, Reynolds A, Kassiuo M (2009) Radiolabelled molecules for imaging the translocator protein (18 kDa) using positron emission tomography. Curr Med Chem 16, 2899–2923. [DOI] [PubMed] [Google Scholar]
- Domene A, Cavanagh C, Page G, Bodard S, Klein C, Delarasse C, Chalon S, Krantic S (2016) Expression of phenotypic astrocyte marker is increased in a transgenic mouse model of Alzheimer’s disease versus age-matched controls: a presymptomatic stage study. Int J Alzheimers Dis 2016:5696241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Dierck RA, Klein HC (2008) PET imaging of the peripheral benzodiazepine receptor: monitoring disease progression and therapy response in neurodegenerative disorders. Curr Pharm Des 14, 3297–3315. [DOI] [PubMed] [Google Scholar]
- Dubois A, Benavides J, Peny B, Duverger D, Fage D, Gotti B, MacKenzie ET, Scatton B (1988) Imaging of primary and remote ischaemic and excitotoxic brain lesions. An autoradiographic study of peripheral type benzodiazepine binding sites in the rat and cat. Brain Res 445, 77–90. [DOI] [PubMed] [Google Scholar]
- Dupont AC, Largeau B, Santiago, Ribeiro MJ, Guilloteau D, Arlicot N (2017) Translocator protein-18 kDa (TSPO) positron emission tomography (PET) imaging and its clinical impact in neurodegenerative diseases. Int J Mol Sci 18, pii: E785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather D, Coronado MJ, Garton AE, Dziedzic JL, Bucek A, Cooper LT Jr., Brandt JE, Alikhan FS, Wang H, Endres CJ, Choi J, Pomper MG, Guilarte TR (2014) Sex differences in translocator protein 18 kDa (TSPO) in the heart: implications for imaging myocardial inflammation. J Cardiovasc Transl Res 7, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick LR, Williams K, Pittenger C (2013) Microglial dysregulation in psychiatric disease. Clin Dev Immunol 2013: 608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MJ, Valle M, Calvo R, Troconiz IF (1999) Altered plasma and brain disposition and pharmacodynamics of methadone in abstinent rats. J Pharm Exp Ther 288, 179–187. [PubMed] [Google Scholar]
- Gavish M, Weizman A, Karo L, Tyano S, Tanne Z (1986) Decreased peripheral benzodiazepine binding sites in platelets of neuroleptic-treated schizophrenics. Eur J Pharmacol 121, 275–279. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Yamamura HI, Wamsley JK (1985) Autoradiographic localization of “peripheral-type” benzodiazepine binding sites in the rat brain, heart, and kidney. Naunyn Schmiedebergs Arch Pharmacol 328, 454–460. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Stephenson DT, Schober DA, Rash K, Clemens JA (1997) Increased expression of peripheral benzodiazepine receptors in the facial nucleus following motor neuron axotomy. Neurochem Int 31, 705–713. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Banati RB, Goerres GB, Cagnin A, Myers R, Gunn RN, Turkheimer F, Good CD, Mathias CJ, Quinn N, Schwarz J, Brooks DJ (2003) [11C]-PK11195 PET imaging of microglial activation in multiple system atrophy. Neurology 61, 686–689. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Watts J, Trender-Gerhard I, Turkheimer F, Banati RB, Bhatia K, Brooks DJ (2004) In vivo imaging of microglial activation with [11C]-PK11195 PET in corticobasal degeneration. Mov Dis 19, 1221–1226. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Schwarz J, Myers R, Ise R, Banati RB (2005) Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. NeuroImage 24, 591–595. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ (2006a) In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis 21, 404–412. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Trender-Gerhard I, Turkheimer F, Quinn NP, Bhatia KP, Brooks DJ (2006b) In vivo imaging of microglia activation with [11C](R)-PK11195 PET in Progressive Supranuclear Palsy. Mov Dis 21, 89–93. [DOI] [PubMed] [Google Scholar]
- Ghadery C, Koshimori Y, Coakeley S, Harris M, Rusjan P, Kim J, Houle S, Strafella AP (2017) Microglia activation in Parkinson’s disease using [18F]-FEPPA. J Neuroinflammation 14, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, Luby ED, Froham CE, Gottlieb JS (1961) Orosomucoid in schizophrenia. Nature 192, 370–371. [DOI] [PubMed] [Google Scholar]
- Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkB signaling, MAPK, and Jak1/Stat1 pathways. GLIA, 59 242–255. [DOI] [PubMed] [Google Scholar]
- Groom GN, Junck L, Foster NL, Frey KA, Kuhl DE (1995) PET of peripheral benzodiazepine binding sites in the microgliosis of Alzheimer’s disease. J Nucl Med 36, 2207–2210. [PubMed] [Google Scholar]
- Guilarte TR, Kuhlmann AC, O’Callaghan JP, Micelli RC (1995) Enhanced expression of peripheral benzodiazepine receptors in the trimethyltin-exposed rat brain: a biomarker of neurotoxicity. Neurotoxicol 16, 441–450. [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS (2003) Methamphetamine-induced deficits of brain monoaminergic markers: distal axotomy or neuronal plasticity. Neurosci 122, 499–513. [DOI] [PubMed] [Google Scholar]
- Gut P, Zweckstetter M, Banati RB (2015) Lost in translocation: the functions of the 18-kD translocator protein. Trends Endocrinol Metabol 26, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay JW, Dewey MJ, Yoo SD (1998) Pharmacokinetics and antidepressant activity of fluoxetine in transgenic mice with elevated serum alpha1-acid glycoproteins levels. Drug Metabol Dispos 26, 20–24. [PubMed] [Google Scholar]
- Hammoud DA, Endres CJ, Chander AR, Guilarte TR, Wong DF, Sacktor NC, McArthur JC, Pomper MG (2005) Imaging glial cell activation with [11C]-R-PK1119 in patients with AIDS. J Neurovirol 11, 346–355. [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Gallezot J-D, Lim K, Nabulsi N, Esterlis I, Pittman B, Lee J-Y, O’Connor KC, Pelletier D, Carson RE (2013) The neuoinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression. Brain Beh Immun 33, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Schafbauer T, Lim K, Kloczynski T, Morris ED, Carson RE, Ding YS, Cosgrove KP (2012) Endotoxin-induced systemic inflammation activates microglia: [11C]-PBR28 positron emission tomography in nonhuman primates. NeuroImage 63, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick MJ, Chen M-K, Baidoo K, Pomper MG, Guilarte TR (2005) In vivo imaging of peripheral benzodiazepine receptors in mouse lungs: a biomarker of inflammation. Mol Imaging 4, 432–438. [DOI] [PubMed] [Google Scholar]
- Harhausen D, Sudmann V, Khojasteh U, Muller J, Zille M, Graham K, Thiele A, Dyrks T, Dirnagl U, Wunder A (2013) Specific imaging of inflammation with the 18 kDa translocator protein ligand DPA-714 in animal models of epilepsy and stroke. PLOS One 8, e69529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel K, Karitzky J, Schmid M, Mader I, Glatting G, Unger JW, Neumaier B, Ludolph AC, Reske SN, Landwehrmeyer GB (2004) Imaging activated microglia with PET and [11C]-PK11195 in corticobasal degeneration. Mov Dis 19, 817–821. [DOI] [PubMed] [Google Scholar]
- Hillmer AT, Holden D, Fowles K, Nabulsi N, West BL, Carson RE, Cosgrove KP (2017a) Microglia depletion and activation: a [11C]-PBR28 PET study in nonhuman primates. EJNMMI Res 7, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer AT, Sandiego CM, Hannestad J, Angarita GA, Kumar A, McGovern EM, Huang Y, O’Connor KC, Carson RE, O’Malley SS, Cosgrove KP (2017b) In vivo imaging of translocator protein, a marker of activated microglia, in alcohol dependence. Mol Psychiatry 22, 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, Gerhard A, Talbot PS (2017) Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol Psych 83, 61–69. [DOI] [PubMed] [Google Scholar]
- Iaccarino L, Moresco RM, Presotto L, Bugiani O, Iannaccone G, Tagliavini F, Perani D (2017) An in vivo [11C](R)-PK11195 PET and in vitro pathology study of microglia activation in Creutzfeldt-Jakob disease. Mol Neurobiol 55, 2856–2868. [DOI] [PubMed] [Google Scholar]
- Iaccarino L, Presotto L, Bettinardi V, Gianolli L, Roiter I, Capellari S, Parchi P, Cortelli P, Perani D (2018) An in vivo 11C-PK PET study of microglia activation in Fatal Familial Insomnia. Ann Clin Transl Neurol 5, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel I, Ohsiek A, Al-Momani E, Albert-Weissenberger C, Stetter C, Menci S, Buck AK, Kleinschnitz C, Samnick S, Siren A-L (2016). Combined [18F]-DPA-713 micropositron emission tomography and autoradiography imaging of microglia activation after closed head injury in mice. J Neuroinflammation 13, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Kato T (2005) Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder of schizophrenia, as revealed by large-size DNA microarray analysis. Human Mol Genet 14, 241–253. [DOI] [PubMed] [Google Scholar]
- Janssen B, Vugts DJ, Funke U, Molenaar GT, Kruijer PS, van Berckel BNM, Lammertsma AA, Windhorst AD (2017) Imaging of neuroinflammation in Alzheimer’s disease, multiple sclerosis, and stroke: recent developments in positron emission tomography. Biochim Biophys Acta 1862, 425–441. [DOI] [PubMed] [Google Scholar]
- Javaid JI, Notorangelo MP, Pandey SC, Reddy PL, Pandey GN, Davis JM (1994) Peripheral benzodiazepine receptors are decreased during cocaine withdrawal in humans. Biol Psych 36, 44–50. [DOI] [PubMed] [Google Scholar]
- Jeyakumar M, Butters TD, Dwek RA, Platt FM (2002) Glycosphingolipid lysosomal storage disease: therapy and pathogenesis. Neuropath Appl Neurobiol 28, 343–357. [DOI] [PubMed] [Google Scholar]
- Ji B, Maeda J, Sawada M, Ono M, Okauchi T, Inaji M, Zhang M-R, Suzuki K, Ando K, Staufenbiel M, Trojanowski JQ, Lee VMY, Higuchi M, Suhara T (2008) Imaging the peripheral benzodiazepine receptor expression as biomarkers of detrimental versus beneficial glial responses in mouse models of Alzheimer’s disease and other CNS pathologies. J Neurosci 28, 12255–12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliet-Riant R, Boukef MF, Duche JC, Simon N, Tillement JP (1998) The genetic variant A of human alpha1-acid glycoprotein limits the blood to brain transfer of drugs it binds. Pharmacol Lett 62, 219–226. [DOI] [PubMed] [Google Scholar]
- Junck L, Olson JMM, Ciliax BJ, Koeppe RA, Watkins GL, Jewett DM, McKeever PE, Wieland DM, Kilbourn MR, Starosta-Rubenstien S, Mancini WR, Kuhl DE, Greenberg HS, Young AB (1989) PET imaging of human gliomas with ligands for the peripheral benzodiazepine binding site. Ann Neurol 26, 752–758. [DOI] [PubMed] [Google Scholar]
- Kanaan NM, Kordower JH, Collier TJ (2010) Age-related changes in glial cells of dopamine midbrain subregions in rhesus monkeys. Neurobiol Aging 31, 937–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Saadani-Makki F, Muzik O, Chakraborty P, Mangner TJ, Janisse J, Romero R, Chugani DC (2007) Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with [11C]-R-PK11195 and small-animal PET. J Nucl Med 48, 946–954. [DOI] [PubMed] [Google Scholar]
- Kim R, Healey KL, Sepulveda-Orengo MT, Reissner KJ (2017) Astrogial correlates of neuropsychiatric disease: from astrocytopathy to astrogliosis. Prog Neuropsychopharmacol Biol Psych pii: S0278–5846(17)30485–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevic D, Verhoeff NPL, Hafizi S, Strafella AP, Graff-Guerrero A, Rajji T, Pollock BG, Houle S, Rusjan PM, Mizhari R (2017) Imaging microglia activation and amyloid burden in amnestic mild cognitive impairment. J Cereb Blood Flow Metab 1:271678X17741395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KE, Papadopoulos V (1990) Peripheral-type benzodiazepine receptors mediate translocationof cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J Biol Chem 265, 15015–15022. [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR (1997) The peripheral benzodiazepine receptor is a sensitive indicator of domoic acid neurotoxicity. Brain Res 751, 281–288. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR (1999) Regional and temporal expression of the peripheral benzodiazepine receptor in MPTP neurotoxicity. Toxicol Sci 48, 107–116. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR (2000) Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J Neurochem 74, 1694–1704. [DOI] [PubMed] [Google Scholar]
- Kurumaji A, Wakai T, Toru M (1997) Decreases in peripheral-type benzodiazepine receptors in postmortem brains of chronic schizophrenics. J Neural Transm 104, 1361–1370. [DOI] [PubMed] [Google Scholar]
- Lavisse S, Guillermier M, Herard A-S, Petit F, Delahaye M, Van Camp N, Haim LB, Lebon B, Remy P, Dolle F, Delzescaux T, Bonveto G, Hantraye P, Escartin C (2012) Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. Neurobiol Dis 32, 10809–10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavisse S, Inoue K, Jan C, Peyronneau MA, Petit F, Goutal S, Dauguet J, Guillermier M, Dolle F, Rhah-Vidal L, Van Camp N, Aron-Badin R, Remy P, Hantraye P (2015) [18F]DPA-714 PET imaging of translocator protein TSPO (18 kDa) in the normal and excitotoxically-lesioned nonhuman primate brain. Eur J Nucl Med Mol Imaging 42, 478–494. [DOI] [PubMed] [Google Scholar]
- Lemus MB, Bayliss JA, Lockie SH, Santos VV, Reichenbach A, Stark R, Andrews ZB (2015) A sterological analysis of NPY, POMC, Orexin, GFAP astrocyte, and Iba1 microglial cell number and volume in diet-induced obese male mice. Endocrinol 156, 17011713. [DOI] [PubMed] [Google Scholar]
- Levinson I, Levine S (1995) Negative correlation between Alpha-1-Acid-Glycoprotein plasma level and response to haloperidol in the acute treatment of schizophrenia. Biol Psych 38, 198200. [DOI] [PubMed] [Google Scholar]
- Li H, Sagar AP, Keri S (2018) Translocator protein (18 kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog Neuropsychpharm Biol Psych 83, 1–7. [DOI] [PubMed] [Google Scholar]
- Liu B, Le KX, Park MA, Wang S, Belanger AP, Dubey S, Frost JL, Holton P, Reiser V, Jones PA, Trigg W, Di Carli MF, Lemere CA (2015) In vivo detection of age- and disease-related increases in neuroinflammation by 18F-GE180 TSPO microPET imaging in wildtype and Alzheimer’s transgenic mice. J Neurosci 35, 15716–15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart A, Davis B, Matthews JC, Rahmoune H, Hong G, Gee A, Earnshaw D, Brown J (2003) The peripheral benzodiazepine receptor ligand PK11195 binds with high affinity to the acute phase reactant α1-acid glycoprotein: implications for the use of the ligand as a CNS inflammatory marker. Nucl Med Biol 30, 199–206. [DOI] [PubMed] [Google Scholar]
- Loth MK, Choi J, McGlothan JL, Pletnikov MV, Pomper MG, Guilarte TR (2016) TSPO in a murine model of Sandhoff disease: presymptomatic marker of neurodegeneration and disease pathophysiology. Neurobiol Dis 85, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda J, Higuchi M, Inaji M, Ji B, Haneda E, Okauchi T, Zhang M-R, Suzuki K, Suhara T (2007) Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Res 1157, 100–111. [DOI] [PubMed] [Google Scholar]
- Maeda J, Zhang M-R, Okauchi T, Ji B, Ono M, Hattori S, Kumata K, Iwata N, Saido TC, Trojanowski JQ, Lee VMY, Staufenbiel M, Tomiyama T, Mori H, Fukumura T, Suhara T, Higuchi M (2011) In vivo positron emission tomography imaging of glial response to amyloid-β and Tau pathologies in mouse models of Alzheimer’s disease and related disorders. J Neurosci 31, 4720–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahuran DF (1999) Biochemical consequences of mutations causing the GM2 gangliosidosis. Biochem Biophys Acta 1455, 105–138. [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Tarwater PJ, Adams RJ, Guilarte TR (2003) Elevated peripheral benzodiazepine receptor expression in simian immunodeficiency virus encephalitis. J Neurovirol 9, 94–100. [DOI] [PubMed] [Google Scholar]
- Maragos PJ, Patel J, Boulenger JP, Clark-Rosenberg R (1982) Characterization of peripheral-type benzodiazepine binding sites in brain using [3H]-Ro5–4864. Mol Pharmacol 22, 26–32. [PubMed] [Google Scholar]
- Masgrau R, Guaza C, Ransohoff RM, Galea E (2017) Should we stop saying “glial” and “neuroinflammation”. Trends Mol Med 23, 485–500. [DOI] [PubMed] [Google Scholar]
- Mattner F, Linares Bandin D, Staykova M, Berghofer P, Claude Gregoire M, Ballantyne P, Quinlivan M, Fordham S, Pham T, Willenborg DO, Katsifis A (2011) Evaluation of [123I]-CLINDE as a potent SPECT radiotracer to assess the degree of astroglia activation in cuprizone-induced neuroinflammation. Eur J Nucl Med Mol Imaging 38, 1516–1528. [DOI] [PubMed] [Google Scholar]
- McNeela AM, Bernick C, Hines RM, Hines DJ (2017) TSPO regulation in reactive gliotic diseases. J Neuro Res 96, 978–988. [DOI] [PubMed] [Google Scholar]
- Miller TR, Wetter JB, Jarvis MF, Bitner RS (2013) Spinal microglia activation in rat models of neuropathic osteoarthritic pain: an autoradiographic study using [3H]-PK11195. Eur J Pain 17, 692–703. [DOI] [PubMed] [Google Scholar]
- Mirzaei N, Pham Tang S, Ashworth S, Coello C, Plisson C, Passchier J, Selvaraj V, Tyacke RJ, Nutt DJ, Sastre M (2016) In vivo imaging of microglia activation by positron emission tomography with [11C]-PBR28 in the 5XFAD model of Alzheimer’s disease. GLIA, 64, 993–1006. [DOI] [PubMed] [Google Scholar]
- Miyazawa N, Diksic M, Yamamoto Y (1995) Chronological study of peripheral benzodiazepine binding sites in the rat stab wounds using [3H]-PK11195 as a marker of gliosis. Acta Neurochir (Wien) 137, 207–216. [DOI] [PubMed] [Google Scholar]
- Morohaku K, Pelton SH, Daugherty DJ, Butler WR, Deng W, Selvaraj V (2014) Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinol 155, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R, Manjil LG, Cullen BM, Price GW, Frackowiak RS, Cremer JE (1991) Macrophage and astrocyte populations in relation to [3H]-PK11195 binding in the cerebral cortex following a local ischaemic lesion. J Cereb Blood Flow Metab 11, 314–322. [DOI] [PubMed] [Google Scholar]
- Nguyen D-L, Wimberley C, Truillet C, Jego B, Caille F, Pottier G, Boisgard R, Buvat I, Bouilleret V (2018) Longitudinal positron emission tomography imaging of glial cell activation in a mouse model of mesial temporal lobe epilepsy: toward identification of optimal treatment windows. Epilepsia 59, 1234–1244. [DOI] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Sawa A, Meyer U (2017) Reconceptualization of translocator protein as a biomarker of neuroinflammation in psychiatry. Mol Psych 23, 36–47. [DOI] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M, Vernon AC, Benke D, Pomper MG, Sawa A, Meyer U (2018) Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psych 23, 323–334. [DOI] [PubMed] [Google Scholar]
- Ory D, Planas A, Dresselaers T, Gsell W, Postnov A, Celen S, Casteels C, Himmelreich U, Debyser Z, Van Laere K, Verbruggen A, Bormans G (2015) PET imaging of TSPO in a rat model of local neuroinflammation induced by intracerebral injection of lipopolysaccharide. Nucl Med Biol 42, 753–761. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M (2006) Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27, 402–409. [DOI] [PubMed] [Google Scholar]
- Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, Jones T, Kreutzberg GW, Banati RB (2000) Thalamic microglial activation in ischemic stroke detected in vivo by [11C]-R-PK11195. Neurology 55, 1052–1054. [DOI] [PubMed] [Google Scholar]
- Patel J, Marangos PJ (1982) Differential effects of GABA on peripheral and central type benzodiazepine binding sites in brain. Neurosci Lett 30, 157–160. [DOI] [PubMed] [Google Scholar]
- Plaven-Sigray P, Matheson GJ, Collste K, Ashok AH, Coughlin JM, Howes OD, Mizhari R, Pomper MG, Rusjan P, Veronese M, Wang Y, Cervenka S (2018) Positron Emission Tomography studies in the glial cell marker Translocator Protein in patients with psychosis: A meta-analysis using individual participant data. Biol Psych pii: S0006–3223, 3129831308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis M, Lahiri N, Niccolini F, Su P, Wu K, Giannetti P, Scahill RI, Turkheimer FE, Tabrizi SJ, Piccini P (2015) Increased central microglial activation associated with peripheral cytokine levels in premanisfest Huntington’s disease gene carriers. Neurobiol Dis 83, 115–121. [DOI] [PubMed] [Google Scholar]
- Praet J, Guglielmetti C, Berneman Z, Van der Linden A, Ponsaerts P (2014) Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev 47, 485–505. [DOI] [PubMed] [Google Scholar]
- Rao VLR, Dogan A, Bowen KK, Dempsey RJ (2000) Traumatic brain injury leads to increased expression of peripheral benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp Neurol 161, 102–114. [DOI] [PubMed] [Google Scholar]
- Richards EM, Zanotti-Fregonara P, Fujita M, Newman L, Farmer C, Ballard ED, Machado-Vieja R, Yuan P, Niciu MJ, Lyoo CH, Henter ID, Salvadore G, Drevets WC, Kolb H, Innis RB, Zarate CA Jr. (2018) EJNNMI Res 8, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsner M, Modai I, Gibel A, Leschneir S, Silver H, Tsinovoy G, Weizman A, Gavish M (2003) Decreased platelet peripheral-type benzodiazepine receptors in persistently violent schizophrenia patients. J Psych Res 37, 549–556. [DOI] [PubMed] [Google Scholar]
- Roberts RC (2017) Postmortem studies on mitochondria in schizophrenia. Schizophr Res 187, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas S, Martin A, Arranz MJ, Pareto D, Purroy J, Verdaguer E, Llop J, Gomez V, Gispert JD, Millan O, Chamorro A, Planas AM (2007) Imaging brain inflammation with [11C]PK11195 by PET and induction of the peripheral-benzodiazepine receptor after transient focal ischemia in rats. J Cereb Blood Flow Metab 27, 1975–1986. [DOI] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot J-D, Pittman B, Nabulsi N, Lim K, Lin S-F, Matuskey D, Lee J-Y, O’Conner KC, Huang Y, Carson RE, Hannestad J, Cosgrove KP (2015) Imaging robust microglia activation after lipopolysaccharide administration in humans with PET. Proc Nat Acad Sci 112, 12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sango K, Yamanaka S Hoffmann A, Okuda Y, Grinberg A, Westphal H, McDonald MP, Crawley JN, Sandhoff K, Suzuki K, Proia RL (1995) Mouse models of Tay-Sachs and Sandhoff diseases differ in neurologic and phenotype and ganglioside metabolism. Nat Genet 11, 170–176. [DOI] [PubMed] [Google Scholar]
- Schain M, Kreisl WC (2017) Neuroinflammation in neurodegenerative disorders-a review. Curr Neurol Neurosci Rep 17, 25. [DOI] [PubMed] [Google Scholar]
- Schoemaker H, Morelli M, Deshmukh P, Yamamura HI (1982) [3H]-Ro5–4864 benzodiazepine binding in the kainate lesioned striatum and Huntington’s disease basal ganglia. Brain Res 248, 396–401. [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Stocco DM (2015) The changing landscape in translocator protein (TSPO) function. Trends Endocrinol Metabol 26, 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V, Tu LN (2016) Current status and future perspectives: TSPO in steroid neuroendocrinology. J. Endocrinol 231, R1–R30. [DOI] [PubMed] [Google Scholar]
- Sérrière S, Tauber C, Vercouille J, Mothes C, Pruckner C, Guilloteau D, Kassiou M, Domene A, Garreau L, Page G, Chalon A (2015) Amyloid load and translocator protein 18 kDa in APPswePS1-dE9 mice: a longitudinal study. Neurobiol Aging 36, 1639–1652. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizhari R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, meyer JH (2015) Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psych 72, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Attwells S, Wilson AA, Mizhari R, Rusjan PM, Miler L, Xu C, Sharma S, Kish S, Houle S, Meyer JH (2018) Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psych 5, 339–347. [DOI] [PubMed] [Google Scholar]
- Somerville SM, Conley RR, Roberts RC (2012) Striatal mitochondria in subjects with chronic undifferentiated vs. chronic paranoid schizophrenia. Synapse 66, 29–41. [DOI] [PubMed] [Google Scholar]
- Starosta-Rubenstein S, Ciliax BJ, Penney JB, McKeever P, Young AB (1987) Imaging of a glioma using peripheral benzodiazepine receptor ligands. Proc Nat Acad Sci 84, 891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DT, Schober DA, Smaistig EB, Mincy RE, Gehlert DR, Clemens JA (1995) Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci 15, 5263–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WST (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 1, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suranyi-Cadotte B, Lafaille F, Dongier M, Dumas M, Quirion R (1988) Decreased density of peripheral benzodiazepine binding sites on platelets of currently drinking, but not abstinent alcoholics. Neuropharmacol 27, 443–445. [DOI] [PubMed] [Google Scholar]
- Tai YF, Pavese N, Gerhardt A, Tabrizi SJ, Barker RA, Brooks DJ, Piccini P (2007) Imaging microglial activation in Huntington’s disease. Brain Res Bull 72, 148–151. [DOI] [PubMed] [Google Scholar]
- Telford JE, Bones J, McManus C, Saldova C, Manning G, Doherty M, Leweke FM, Rothermundt M, Guest PC, Rahmoune H, Bahn S, Rudd PM (2012) Antipsychotic treatment of acute paranoid schizophrenia patients with olanzapine results in altered glycosyltion of serum glycoproteins. J Proteome Res 11, 3743–3752. [DOI] [PubMed] [Google Scholar]
- Tomasi G, Edison P, Bertoldo A, Roncaroli F, Singh P, Gerhard A, Cobelli C, Brooks DJ, Turkheimer FE (2008) Novel reference region model reveals increased microglial and reduced vascular binding of [11C]-R-PK11195 in patients with Alzheimer’s disease. J Nucl Med 49, 1249–1256. [DOI] [PubMed] [Google Scholar]
- Toyama H, Hatano K, Suzuki H, Ichise M, Momosaki S, Kudo G, Ito F, Kato T, Yamaguchi H, Katada K, Sawada M, Ito K (2008) In vivo imaging of microglial activation using a peripheral benzodiazepine receptor ligand: [11C]-PK11195 and animal PET following ethanol injury in rat striatum. Ann. Nucl Med 22, 417–424. [DOI] [PubMed] [Google Scholar]
- Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, Selvaraj V (2014) Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J Biol Chem 289, 27444–27454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LN, Zhao AH, Stocco DM, Selvaraj V (2015) PK11195 effect on steroidogenesis is not mediated through the translocator protein (TSPO). Endocrinol 156, 1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer FE, Rizzo G, Bloomfield PS, Howes O, Zanotti-Fregonara P, Bertoldo A, Veronese M (2015) The methodology of TSPO imaging with positron emission tomography. Biochem Soc Trans 43, 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, Leigh PN, Banati RB (2004) Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C]-(R)-PK11195 positron emission tomography study. Neurobiol Dis 15, 601609. [DOI] [PubMed] [Google Scholar]
- Van Kesteren CFMG, Gemmels H, Witte LD, Hoi EM, van Gool AR, Flakai PG, Kahn RS, Sommer IEC (2017) Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psych 7, e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti s., Lopresti BJ, Wang G, Bissel SJ, Mathis CA, Meltzer CC, Boada F, Capuano S III, Kress GJ, Davis DK, Ruszkiewicz SR, Reynolds IJ, Murphey-Corb M, Trichel AM, Wisniewski SR, Wiley CA (2004) PET imaging of brain macrophages using the peripheral benzodiazepine receptor in a macaque model of neuroAIDS. J Clin Invest 113, 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wiley CA (2006) The peripheral benzodiazepine receptor (Tanslocator Protein 18 kDa) in microglia: from pathology to imaging. Prog Neurobiol 80, 308–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Wagner AK, Wang G, Slagel SL, Chen X, Lopresti BJ, Mathis CA, Wiley CA (2007) The high affinity peripheral benzodiazepine receptor ligand DAA1106 binds specifically to microglia in a rat model of traumatic brain injury: implications for PET imaging. Exp Neurol 207, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Wang G, Nguyen J, Wiley CA (2008) The positron emission tomography ligand DAA1106 binds with high affinity to activated microglia in human neurological disorders. J Neuropath Exp Neurol 67, 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti SM, Lopresi BJ, Wang G, Hamilton RL, Mathis CA, Klunk WE, Apte UM, Wiley CA (2009) PK11195 labels activated microglia in Alzheimer’s disease and in vivo in a mouse model using PET. Neurobiol Aging 30, 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese M, Marques TR, Bloomfield PS, Rizzo G, Singh N, Jones D, Agushi E, Mosses D, Bertoldo A, Howes O, Roncaroli F, Turkheimer FE (2017) Kinetic modelling of [11C]PBR28 for translocator protein PET data: A validation study of vascular modelling in the brain using XBD173 and tissue analysis. J Cer Blood Flow Met 38, 1227–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowinckel E, Reutens D, Becher B, Verge G, Evans A, Owens T, Antel JP (1997) PK11195 binding to the peripheral benzodiazepine receptor as a marker of microglia activation in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci Res 50, 345353. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yue X, Kiesewetter DO, Niu G, Teng G, Chen X (2014) PET imaging of neuroinflammation in a rat traumatic brain injury model with radiolabeled TSPO ligand DPA714. Eur J Nucl Med Mol Imaging 41, 1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weizman R, Tanne Z, Karp L, Tyano., Gavish M (1986) Peripheral-type benzodiazepine-binding sites in platelets of schizophrenics with and without tardive dyskinesia. Life Sci 39, 549–555. [DOI] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Kosno-Knusewska E, Lechowicz W, Pasennik E, Schmidt-Sidor B (2004) Degeneration of microglial cells in frontal cortex and temporal lobes of chronic schizophrenics. Folia Neuropathol 42, 157–165. [PubMed] [Google Scholar]
- Williams RM, Hampton T, Pearce RKB, Hirsch SR, Ansorge O, Thom M, Maier M (2013) Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psych Clin Neurosci 263, 41–52. [DOI] [PubMed] [Google Scholar]
- Wodarz N, Rothenhofer C, Fischer R, Stober G, Kiehl B, Jungkunz G, Riederer P, Klein HE (1998) Peripheral-type benzodiazepine receptors in diagnostic subtypes of schizophrenia patients. Psych Res 81, 363–369. [DOI] [PubMed] [Google Scholar]
- Yankam Njiwa J, Costes N, Bouillot C, Bouvard S, Fieux S, Becker G, Levigoureux E, Kocevar G, Stamile C, Langlois JB, Bolbos R, Bonnet C, Bezin L, Zimmer L, Hammers A (2016) Quantitative longitudinal imaging of activated microglia as a marker of inflammation in the pilocarpine rat model of epilepsy using [11C]-(R)-PK11195 PET and MRI. J Cereb Blood Flow Metab 37, 1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EC, Patel A, Meyers BS, Kakuma T, Alexopoulos GS (1999) Alpha1-acid glycoprotein, age, and sex in mood disorders. Am J Geriatr Psych 7, 331–334. [PubMed] [Google Scholar]