Abstract
Context:
Goals-of-care discussions are associated with improved end-of-life care for patients and therefore may be used as a process measure in quality improvement, research, and reimbursement programs.
Objectives:
To examine three methods to assess occurrence of a goals-of-care discussion - patient report, clinician report, and documentation in the electronic health record (EHR) - at a clinic visit for seriously ill patients and determine whether each method is associated with patient-reported receipt of goal-concordant care.
Methods:
Secondary analysis of a multi-center cluster-randomized trial, with 494 patients and 124 clinicians caring for them. Self-reported surveys collected from patients and clinicians two weeks after a clinic visit assessed occurrence of a goals-of-care discussion. Documentation of a goals-of-care discussion was abstracted from the EHR. Patient-reported receipt of goal-concordant care was assessed by survey two weeks after the visit.
Results:
52% of patients reported occurrence of a goals-of-care discussion at the clinic visit; clinicians reported occurrence of a discussion at 66% of visits. EHR documentation occurred in 42% of visits (p<0.001 for each compared with other two). Patients who reported occurrence of a goals-of-care discussion at the visit were more likely to report receipt of goal-concordant care than patients who reported no discussion (β 0.441, 95% CI 0.190–0.692; p=0.001). Neither occurrence of a discussion by clinician report nor by EHR documentation was associated with goal-concordant care.
Conclusion:
Different approaches to assess goals-of-care discussions give differing results and yet each may have advantages. Patient report is most likely to correlate with patient-reported receipt of goal-concordant care.
Keywords: goals-of-care, palliative care, end-of-life
INTRODUCTION
Goals-of-care discussions are associated with improved end-of-life care for patients as well as lower risk of depression and anxiety in surviving family members [1–3]. As such, the occurrence of goals-of-care discussions may be an important metric for use in quality improvement initiatives, research, and reimbursement programs [4–8]. The Centers of Medicare and Medicaid Services (CMS) began reimbursement for advance care planning and goals-of-care discussions on January 1st 2016 [9].
The occurrence of a goals-of-care discussion may be determined in a number of ways: 1) through expert review of audiotaped discussions or direct observation; 2) from patient report; 3) from clinician report; or 4) from documentation in the electronic health record (EHR). Although direct observation or review of audiotaped discussions may provide the most precise evaluations, these are also the most complex, expensive, and time-consuming methods [10]. Prior studies that have demonstrated the value of goals-of-care discussions on improved patient and family outcomes have primarily relied on patient report [2–3] or direct observation by study investigators [1] to assess the occurrence of goals-of-care discussions. However, the use of clinician reports or EHR documentation is also possible, with ease of data collection from the EHR making this an attractive option.
Little is known about how these three approaches compare to each other, and prior studies suggest important differences between clinician and patient reports of communication about prognosis, treatment discussions, and care preferences [11–14]. In addition, patient reports and EHR documentation of goals-of-care discussions in hospitalized patients have been found to differ [15]. An understanding of differences in these methods and their association with patient-centered outcomes [8] may help guide the choice of measures in future intervention programs.
This study compares three measures of the occurrence of a goals-of-care discussion during a clinic visit for seriously ill patients who had participated in a randomized trial of the Jumpstart-Tips communication intervention [16–18]. We then investigate the association of each method of assessment with patient-reported receipt of goal-concordant care. We also examine factors associated with disagreement of clinician reports with patient reports and EHR documentation. We assess patient demographics (age, gender, race/ethnicity, education), patient illness characteristics (Charlson comorbidity score, self-perceived health status), clinician demographics (age, gender, primary vs. specialty care), and occurrence of prior discussions as predictors of patient-clinician disagreement. We hypothesize patient-clinician disagreement will be more likely in the absence of prior discussions, among male clinicians [19], among racial/ethnic minority patients [20], among patients with lower education level [21], and among sicker patients as indicated by Charlson score and self-perceived health status. We did not pre-specify a directional hypothesis for the remaining predictors. We hypothesize EHR-clinician disagreement will be more likely in situations where clinicians may feel less compelled to document discussions: when goals-of-care discussions occurred at prior visits, with patients who previously completed advance care planning documentation, with patients who have not been recently hospitalized, and when clinicians perceive patient goals align with default medical care (e.g. extending life). We also examine whether clinician age predicts clinician-EHR disagreement but did not pre-specify a direction.
METHODS
Study Design
We conducted a secondary analysis of a multi-center cluster-randomized trial of a patient-specific pre-conversation communication-priming intervention (Jumpstart Tips) [16]. The intervention targeted patients with serious illness and their clinicians and was designed to increase goals-of-care discussions compared to usual care in the outpatient setting. Clinicians were randomized to usual care or intervention, and patient assignment was based on the clinician’s assignment. Institutional review boards at all sites approved the study, and all participants provided written informed consent. Additional details of the randomized trial have been previously published [16–18].
Population and Setting
Eligible clinicians were physicians or nurse practitioners providing primary or specialty care to five or more eligible patients in their patient panel. Clinicians from two large health systems in the Pacific Northwest were initially contacted by mail or email and then recruited by phone and in-person between February 2014 and November 2015.
Eligible patients were 18 years of age or older, had two or more clinic visits with the eligible clinician within the prior 18 months, and had one or more qualifying conditions. Qualifying conditions were chosen to identify a group of patients with median survival of approximately 2 years and included: (1) metastatic cancer or inoperable lung cancer; (2) COPD with FEV1 <35% predicted or oxygen dependence, restrictive lung disease with TLC <50% predicted, or cystic fibrosis with FEV1 <30% predicted; (3) New York Heart Association Class III or IV heart failure, pulmonary arterial hypertension with six minute walk distance of <250 meters, LVAD, or ICD implant; (4) Child’s Class C cirrhosis or MELD score >17.5; (5) dialysis-dependent renal failure and diabetes; (6) age 75 years or older and at least one life-limiting chronic illness; (7) age 90 years or older; (8) hospitalization in the prior 18 months with a life-limiting chronic illness; and (9) Charlson comorbidity score of 6 or higher [22–26]. Life-limiting chronic illnesses were defined as any of the qualifying conditions listed above but that were not severe enough to be eligible outright. Using clinic schedules and EHRs, study coordinators identified consecutive eligible patients for each participating clinician. Patients were recruited initially by mail and then enrolled in person or by mail between March 2014 and May 2016.
Data Collection
Data were derived from patient and clinician questionnaires completed at enrollment and following an index clinic visit, and from the EHR record of the visit. Questionnaires were distributed to clinicians and patients within two weeks after the visit. For participants in the intervention arm, the index visit included the Jumpstart Tips intervention.
Determination of Discussion Variables
Patients assessed the occurrence of a goals-of-care discussion at the index visit with the question: “Did you discuss with this doctor, the kind of medical care you would want if you were too sick to speak for yourself”. Response options were “yes”, “no”, and “I don’t know”. We coded patient-reported occurrence of a discussion as a dichotomous variable: occurrence = “yes” and non-occurrence = “no” or “I don’t know”. This question was previously validated [27–30]. To help ensure the patient answered the survey questions in reference to the index clinic visit, the specific date of the clinic visit and clinician’s name were listed at the top of the survey.
Clinicians independently assessed the occurrence of a discussion by responding to the following question: “During this visit, did you talk with this patient about…”. Response options were “his/her goals of care”, “his/her preferences for end-of-life care”, and “neither of these was addressed”. If they endorsed “neither of these was addressed”, they were able to identify reasons for not having a discussion (i.e., “no time during the appointment”, “topics were addressed previously”, “not appropriate for this patient”, and “other”). We coded clinician-reported occurrence of a discussion as a dichotomous variable: occurrence = “goals of care” and/or “preferences for end-of-life care” and nonoccurrence = “neither”. This question was previously validated [27–30].
EHR documentation of a goals-of-care discussion was assessed through manual review of clinic notes from the visit. We defined EHR documentation of a discussion as a dichotomous variable with documentation classified as the presence of any of the following in the clinician’s note: prognosis discussion, advance care planning (ACP) discussion or Advance Directive (AD) completion, Physician Order for Life-Sustaining Treatment (POLST) form discussion or completion, discussion of palliative care, referral to palliative care, discussion of hospice, or referral to hospice. Abstractors were trained to identify evidence of a discussion in the clinic notes. To facilitate this, the protocol provided abstractors with specific examples of the types of phrases we considered as evidence of a discussion. For prognosis discussion, and example from the protocol was: “patient asked about chances of recovery and we had a long talk about his prognosis and what the future holds”. For ACP discussion or AD completion, an example from the protocol was: “patient and wife brought in Living Will today and we discussed patient preference if his illness should progress”. We conducted blinded co-reviews for 10% of records and found 95% agreement for all abstracted elements.
Outcomes
To investigate predictors of disagreement between assessment methods, we focused on the two most common and most clinically important types of disagreement: 1) clinicians reporting a discussion when the patient reported no discussion; and 2) clinicians reporting a discussion when no discussion was documented in the EHR. We computed two dichotomous outcomes: clinician-patient disagreement (1 = patient reported no discussion but clinician reported a discussion; 0 = both patient and clinician reported a discussion) and clinician-EHR disagreement (1 = no documentation in EHR but clinician reported a discussion; 0 = evidence of a discussion in both the clinician’s report and the EHR).
We also examined whether occurrence of a discussion – by each of the three methods – was associated with patient-reported receipt of goal-concordant care [8,10]. We assessed patient-reported receipt of goal-concordant care using two survey items from the SUPPORT study [31–32]. The first item asked patients about their goals: “If you had to make a choice at this time, would you prefer a plan of medical care that focuses on extending life as much as possible, even if it means having more pain and discomfort, or would you want a plan of medical care that focuses on relieving pain and discomfort as much as possible, even if that means not living as long?”. The second item asked patients, “Using those same categories, which of the following best describes the focus of the medical care you are currently receiving?” For both questions, patients could choose one of the two options or “I don’t know / not sure”. We defined patient-reported receipt of goal-concordant care as a dichotomous variable with concordance classified as matching patient goal with perception of current care plan. Patients who indicated “I don’t know / not sure” for either question were coded as not receiving goal-concordant care. Although many patients may value both life-extension and comfort, the “forced choice” structure helps identify a patient’s top priority [33–34].
Predictors
For analyses of predictors of disagreement between the measurement methods, we selected predictors a priori. Patient and/or clinician factors were explored as predictors of patient report of no discussion that was in contrast to the clinician’s report of having had a discussion; patient, clinician, and EHR factors were explored as reasons for absent documentation in the EHR when a clinician reported the occurrence of a discussion. Patient variables from enrollment questionnaires included: 1) age; 2) gender; 3) race/ethnicity; 4) education; 5) self-assessed health status; and 6) patient-reported occurrence of goals-of-care discussions prior to study enrollment. Variables obtained from the EHR included: 1) Charlson comorbidity score; 2) presence of a POLST, living will, or healthcare directive in the EHR prior to the index visit; and 3) hospitalization in 18 months prior to study enrollment. Clinician variables from screening and enrollment questionnaires included: 1) age; 2) gender; and 3) practice (specialty vs. primary care).
In analyses assessing the association of a goals-of-care discussion with receipt of goal-concordant care, each method of measurement – patient report, clinician report, EHR documentation – was used as a predictor variable.
Covariates:
We included covariate adjustment for any variable whose addition to a bivariate model changed the beta coefficient for the predictor of interest by 10% or more [35–37]. The clinician’s randomization group in the RCT was tested for confounding in all analytic models. For the models examining the associations between occurrence of a discussion and goal-concordant care, we also tested the following demographic variables for confounding: clinician age, gender, race, and practice; patient age, gender, race, marital/partner status, education, income, Charlson comorbidity score, self-perceived health status, advanced cancer diagnosis, depression, and hospitalization in 18 months prior to enrollment.
Statistical Methods
To assess differences in the proportion of participants with goals-of-care discussions, measured in the three different ways, we used unclustered paired difference-of-means tests. The remaining analyses were based on probit regression models, estimated with weighted least squares with mean and variance adjustment (WLSMV), and with patients clustered under clinicians. Initial models separately regressed each outcome on each predictor. In a final evaluation of predictors of clinician-patient disagreement, all predictors with p<0.20 in the initial bivariate models were included in a multi-predictor model. For all analyses we accepted a 2-sided p<0.05 as evidence of statistical significance. We used IBM SPSS Version 19 for descriptive statistics and difference-of-means tests and Mplus Version 8 for regression models.
RESULTS
A total of 124 clinicians had at least one patient with an index visit. Clinicians’ average age was 45.5 years and a slight majority were women (53.2%). Most clinicians were non-Hispanic and white (76.6%; Table 1).
Table 1.
Characteristics of Clinicians and Patients
| Characteristic | Valid n | n (%) | Median (IQR) |
|---|---|---|---|
| CLINICIANSa | |||
| Age | 124 | 45.5 (16) | |
| Male | 124 | 58 (46.8) | |
| Racial/ethnic minority | 124 | 29 (23.4) | |
| Primary care vs. specialty care | 124 | ||
| Primary care | 66 (53.2) | ||
| Family medicine | 29 (23.4) | ||
| Internal medicine | 34 (27.4) | ||
| Geriatrics | 3( 2.4) | ||
| Specialty care | 58 (46.8) | ||
| Oncology | 24 (19.4) | ||
| Pulmonology | 8 ( 6.5) | ||
| Cardiology | 16 (12.9) | ||
| Gastroenterology | 3 ( 2.4) | ||
| Nephrology | 7 ( 5.6) | ||
| Clinician-reported reasons for non-occurrence of discussion at index visitb | |||
| Lack of time during visit | 155 | 62 (40.0) | |
| Had already had this discussion; no need to revisit | 155 | 60 (38.7) | |
| Focus on something else this visit | 155 | 17 (11.0) | |
| Inappropriate for this patient | 155 | 33 (21.3) | |
| Reason patient was inappropriate | |||
| Patient not ready for this discussion | 33 | 7 (21.2) | |
| Another clinician has this responsibility | 33 | 9 (27.3) | |
| Not comfortable discussing end-of-life with this patient | 33 | 1 ( 3.0) | |
| Patient not sick enough | 33 | 18 (54.5) | |
| Other reason | 33 | 1 ( 3.0) | |
| Other reason | 155 | 16 (10.3) | |
| PATIENTS | |||
| Age | 494 | 75.6 (17.5) | |
| Male | 494 | 259(52.4) | |
| Racial/ethnic minority | 494 | 103 (20.9) | |
| Education | 493 | ||
| 8th grade or less | 12 (2.4) | ||
| Some high school | 29 (5.9) | ||
| High school diploma or equivalent | 68 (13.8) | ||
| Trade school or some college | 202 (41.0) | ||
| 4-year college degree | 83 (16.8) | ||
| Some graduate school | 23 (4.7) | ||
| Graduate or professional degree | 76 (15.4) | ||
| Charlson comorbidity score | 492 | 7 (3) | |
| Self-Assessed Health Status | 492 | ||
| Poor | 73 (14.8) | ||
| Fair | 147 (29.9) | ||
| Good | 177 (36.0) | ||
| Very good | 73 (14.8) | ||
| Excellent | 22 ( 4.5) | ||
| Goals-of-care discussion before study enrollment | 494 | 142 (28.7) | |
| Any advance directive in EHR | 492 | 292 (59.3) | |
| Not hospitalized in 18 months before study enrollment | 492 | 259 (52.6) | |
| Qualifying condition | 494 | ||
| Qualifying diagnoses | |||
| Advanced cancer | 90 (18.2) | ||
| Chronic lung disease | 47 ( 9.5) | ||
| Heart failure | 32 ( 6.5) | ||
| Liver failure | 3 ( 0.6) | ||
| Renal failure | 21 ( 4.3) | ||
| Other qualifying conditions | |||
| Age 75–89 w/chronic condition | 180 (36.4) | ||
| Age 90+ | 35 ( 7.1) | ||
| Hospitalization | 81 (16.4) | ||
| Charlson score 6+ | 410 (83.0) | ||
| Number of qualifying diagnoses | 494 | ||
| 0 | 304 (61.5) | ||
| 1 | 187 (37.9) | ||
| 2 | 3 ( 0.6) | ||
For all clinician characteristics except the reasons for non-occurrence of discussion at the index visit, the total possible sample size was 124 (the number of participating clinicians who had at least one patient with an index visit). For the reasons for non-discussion, the total possible sample size was 156 (a patient-level sample comprising patients whose clinicians indicated that they had neither a discussion of patient goals nor a discussion of patient preferences at the index visit).
Based on clinician responses on post-index clinic visit questionnaire
A total of 494 patients had an index visit. Their average age was 75.6 years and a slight majority were men (52.4%). Most patients were non-Hispanic and white (79.1%), and 45% reported poor-to-fair health status at the time of enrollment. The most common qualifying condition was advanced cancer (18.2%; Table 1).
Occurrence of a Goals-of-Care Discussion
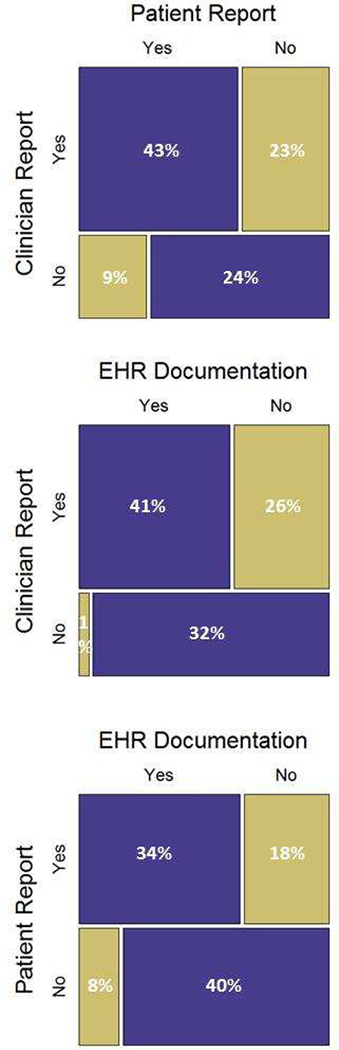
Complete questionnaire and EHR data were available for 356 index clinic visits. Fifty-two percent of patients reported the occurrence of a goals-of-care discussion (186 reported “yes”, 159 reported “no”, and 11 answered “I don’t know”); whereas clinicians reported the discussions at 66% of the visits, and the EHR documented these discussions at 42% of the visits (Figure). Each of these three assessments of whether a discussion occurred was significantly different than the other two (p<0.001). The most common reasons clinicians gave for not having a goals-of-care discussion are detailed in Table 1.
Figure.
Mosaic Plots of Occurrence of Goals-of-Care Discussions at Index Clinic Visit by Patient Report, Clinician Report, and EHR Documentationa
a Based on 356 cases with valid data for all three information sources. Purple boxes represent concordant assessments. Gold boxes represent discordant assessments.
Patients and clinicians disagreed as to whether a goals-of-care discussion occurred at 33% of visits. Twenty-seven percent of clinician reports and 26% of patient reports did not agree with EHR documentation of whether a discussion had occurred (Figure).
Predictors of Disagreement between Measurement Methods
Among the 236 visits at which the clinician reported occurrence of a goals-of-care discussion, 35.0% of patients did not agree that a discussion occurred at that visit. Three factors were significantly associated with this disagreement in a multi-predictor model (Table 2): 1) higher Charlson comorbidity score (β 0.117, 95% CI 0.040, 0.194; p=0.003); 2) patient report that no goals-of-care discussions had occurred with the clinician prior to study enrollment (β 0.444, 95% CI 0.041, 0.848; p=0.031); and 3) patients cared for by specialist, as opposed to primary care clinicians (β 0.358, 95% CI 0.006, 0.710; p=0.046).
Table 2.
Factors Associated with Clinician-Patient Disagreement and Clinician-EHR Disagreement, among Clinicians Who Reported Occurrence of a Goals-of-Care Discussion at Index Visita
| Disagreement Type | Predictor | Bivariate Models | Multi-Predictor Model | ||||
|---|---|---|---|---|---|---|---|
| nb | b (95% CI) | p | nb | b (95% CI) | p | ||
| Clinician-patient | Charlson comorbidity scorec | 236/ 99 | 0.132 ( 0.060, 0.204) | <0.001 | 236/99 | 0.117 ( 0.040, 0.194) | 0.003 |
| Male patientc | 237/100 | −0.048 (−0.447, 0.351) | 0.812 | ||||
| Patient agec | 237/100 | 0.002 (−0.011, 0.015) | 0.802 | ||||
| Patient in a racial/ethnic minority groupc | 237/100 | −0.365 (−0.880, 0.150) | 0.165 | −0.291 (−0.802, 0.220) | 0.265 | ||
| Patient reported no goals-of-care discussion with clinician prior to enrollmentc | 237/100 | 0.445 ( 0.075, 0.815) | 0.018 | 0.444 ( 0.041, 0.848) | 0.031 | ||
| Patient educationc | 237/100 | 0.051 (−0.070, 0.172) | 0.412 | ||||
| Patient self-assessed health statusc | 236/100 | −0.009 (−0.189, 0.171) | 0.924 | ||||
| Clinician agec | 237/100 | −0.005 (−0.023, 0.013) | 0.573 | ||||
| Specialty care clinicianc | 237/100 | 0.522 ( 0.166, 0.879) | 0.004 | 0.358 (0.006, 0.710) | 0.046 | ||
| Male clinicianc | 237/100 | −0.090 (−0.441, 0.260) | 0.614 | ||||
| Clinician-EHR | Patient-reported goals-of-care discussion with clinician prior to enrollmentc | 290/105 | −0.156 (−0.518, 0.207) | 0.400 | |||
| POLST form and/or living will/health care directive uploaded to EHR prior to index visitc | 290/105 | −0.132 (−0.516, 0.252) | 0.501 | ||||
| Clinician agec | 290/105 | 0.012 (−0.014, 0.039) | 0.368 | ||||
| No hospitalization in 18 months before study enrollmentc | 290/105 | −0.134 (−0.462, 0.195) | 0.425 | ||||
| Clinician identification of patient value as life-extension after index visitc | 284/104 | 0.113 (−0.244, 0.469) | 0.536 |
For the bivariate models, the two disagreement outcomes were regressed on each predictor separately, with randomization group added as a covariate if its addition changed the coefficient for the predictor by 10% or more. For clinician-patient disagreements, we ran a multi-predictor model, including all predictors with p<0.20 in the bivariate models, and with adjustment for randomization group. All models were based on complex probit regression (patients clustered under clinicians) and estimated with weighted least squares with mean and variance adjustment (WLSMV).
Patient n / clinician n
Adjusted for randomization group
Among the 236 visits at which the clinician reported occurrence of a goals-of-care discussion, 38.5% of the notes from those visits had no documentation of a discussion. None of the factors examined were associated with lack of EHR documentation (Table 2).
Association of Occurrence of a Goals-of-Care Discussion with Patient-Reported Goal-Concordant Care
Among the 371 patients who answered the questions assessing goal-concordant care, 180 (48.5%) reported receipt of goal-concordant care. Patients who reported occurrence of a goals-of-care discussion at the index visit were significantly more likely to report receipt of goal-concordant care than patients who reported no discussion at the index visit (β 0.441, 95% CI 0.190, 0.692; p=0.001; Table 3). However, neither clinician reports nor EHR documentation of goals-of-care discussions was significantly associated with patients’ reports of goal-concordant care.
Table 3.
Association of Occurrence of a Goals-of-Care Discussion with Patient-Reported Receipt of Goal-Concordant Carea
| Occurrence of Goals-of-Care Discussion as Determined by | nb | Proportion (95% CI) with Patient-Reportea Receipt of Goal-Concoraant Care | b (95% CI) | p | |
|---|---|---|---|---|---|
| No Discussion | Discussion Occurred | ||||
| Patient reportc | 368/118 | 0.40 (0.33, 0.47) | 0.57 (0.50, 0.64) | 0.441 (0.190, 0.692) | 0.001 |
| Clinician reportd | 335/114 | 0.52 (0.43, 0.62) | 0.48 (0.41, 0.54) | −0.234 (−0.506, 0.039) | 0.093 |
| EHR clinic notee | 345/116 | 0.50 (0.43, 0.57) | 0.49 (0.41, 0.58) | −0.087 (−0.404, 0.230) | 0.592 |
The outcome was regressed on each of the three predictors separately, using a probit regression model with patients clustered under clinicians and estimated with weighted least squares with mean and variance adjustment (WLSMV). Covariate adjustment was made for any of the following variables whose addition to the bivariate model changed the coefficient for the predictor by 10% or more: clinician age, gender, race, primary vs specialty care, randomization group; patient age, gender, race, marital/partner status, education level, income, Charlson comorbidity score, self-perceived health status, advanced cancer diagnosis, depression, and hospitalization in 18 months prior to study enrollment.
Number of patients / number of clinician clusters
Bivariate model
Covariate adjustment for randomization group
Covariate adjustment for patient age, gender, education, income, self-reported health status, and advanced cancer diagnosis; clinician race, primary vs specialty care, and randomization group
DISCUSSION
This study of older adults with serious illness is the first to compare these three methods of assessing the occurrence of goals-of-care discussions. We found discordance between patient- and clinician-report to be common, occurring in a third of visits. In addition, we found documentation of goals-of-care discussions in the EHR was often absent when either the clinician or patient reported the occurrence of a discussion. Although each of these methods may provide valuable information, we found that the occurrence of a goals-of-care discussion was associated with patient-reported receipt of goal-concordant care only when the patient reported such a discussion occurred, suggesting patient report may be most strongly associated with this patient-centered outcome. Although measurement using the EHR is a less burdensome method for assessing the occurrence of goals-of-care discussions, our study suggests that EHR documentation alone is not associated with patient reports of goal-concordant care and therefore may not predict other patient-centered outcomes.
Our finding of frequent discordance between patient and clinician reports of goals-of-care discussions suggests patient-clinician pairs may not have a shared understanding of what constitutes such discussion. Shared perspective is especially important to ensure patients receive care that is aligned with their goals and preferences near the end of life, a time when patients’ goals may diverge from those achieved by default medical care [31–32]. A shared understanding of goals-of-care, and how they are discussed in the context of an outpatient visit with the patient’s clinician, is likely a key initial step in the process of advance care planning; a lack of shared perspective may undermine the informed and shared decision-making required for successful end-of-life care [38–39]. Our findings are consistent with prior paired patient-clinician survey designs that examine quality of communication and have found considerable discordance between patients and clinicians in other aspects of end-of-life care including prognosis communication [13] and occurrence of discussions about advance directives [14]. Paired studies have also shown clinicians are commonly unaware of or misunderstand patients’ specific end-of-life care preferences including preference for CPR, symptom management, and location of death [12,14]. In the absence of shared understanding, prior studies have demonstrated adverse end-of-life outcomes, such as fewer DNR orders, delayed DNR orders, increased receipt of cardiopulmonary resuscitation (CPR), and higher hospital resource use among patients who preferred to forgo CPR [11–12].
Given the importance of shared perceptions, we sought to better understand differences between patient and clinician reports by focusing on predictors of patient disagreement when a clinician reported occurrence of a discussion. This scenario was common, occurring in over a third of the visits in which clinicians reported a discussion. First, we found that patients’ disagreement with clinicians’ reports of discussions were more likely when the patient reported no prior discussions with his/her clinician. It is possible that patients without prior discussions in which goals-of-care were introduced, defined, and considered may have had a more difficult time understanding and integrating what may be a novel and emotionally challenging subject. Some of the disagreement could also relate to inadequate communication skills among clinicians [4]. Second, we found more disagreement when the purported discussions occurred with specialists rather than primary care clinicians. Specialist and primary care physicians rate similarly the importance of goals-of-care discussions and report communication with older patients about goals-of-care at similar frequencies [40], suggesting the differences we observed may not be due to differential priority placed on these discussions. Our findings may be a product of differences in time spent and emphasis placed on goals-of-care discussions, or of the typically longer relationships between patients and primary care clinicians, creating a relationship which may add clarity to discussions. Third, we found patients with higher Charlson comorbidity scores were more likely to not report a discussion even though their clinicians did. Sicker patients may be less able to participate or less able to recall discussions with a particular clinician, especially if they receive care from a number of providers. Clinicians caring for patients with these features could consider being more explicit about goals-of-care discussions and “refreshers” over time to improve patient understanding and recall.
The EHR is an important tool for conveying a patient’s goals and care preferences to providers across the spectrum of medical care [41]. Yet we found no documentation of goals-of-care discussions for nearly forty percent of the visits for which clinicians reported occurrence of discussions on their after-visit questionnaires. We did not identify any clinician or patient characteristics associated with clinician-EHR disagreement. In this study, there was no standardized, centralized location in the EHR to document goals-of-care discussions and clinicians might be less likely to document discussions in clinic notes since they can be difficult to find in the EHR [40]. Future research is needed to identify effective ways to optimize documentation of goals-of-care discussions. Additional incentives and training may be needed to motivate clinicians to document goals-of-care discussions [41].
Our study has several important limitations. First, we did not audiotape the index visits and therefore cannot compare patient report, clinician report, and EHR documentation to audiotapes. However, we believe that knowing whether a discussion actually occurred may be less important than knowing whether participants think and agree that a discussion occurred [13]. Ultimately, effective goals-of-care discussions will likely require both patients and clinicians to agree about the occurrence and content of the discussion. Second, although multi-centered, our study took place in one region of the US with mostly white, non-Hispanic patients and may not generalize to other regions or populations. Third, our study is subject to recall bias, although the two-week time frame between index visit and survey distribution minimizes this concern. Fourth, we assessed the occurrence of goals-of-care discussions with different questions for patients, clinicians, and the EHR, which may contribute to some differences. However, it seems likely that different approaches are needed given differences in understanding or available information between patients, clinicians, and the EHR. Finally, it is important to highlight that the rate of patient-reported goals-of-care discussions in our study, in which approximately half of the patients and clinicians received a Jumpstart-Tips intervention designed to increase goals-of-care discussions, is higher than that found in many other studies [2,3,13]. However, the focus of the present study was less about how often goals-of-care discussions occur and more about comparing ways to measure whether they do.
In this study of older adults with serious illness, we found considerable disagreement between patient report, clinician report, and EHR documentation of whether a goals-of-care discussion occurred at a clinic visit. Of these methods used to measure the occurrence of a goals-of-care discussion, only patient report of the occurrence of a discussion was associated with patient-reported receipt of goal-concordant care. Although multiple approaches to assess goals-of-care discussions may be needed, our findings suggest that patient report may be the most likely to correlate with patient-reported outcomes.
ACKNOWLEDGEMENTS
Funding: This work was supported by the Patient Centered Outcomes Research Institute [IH-12–11-4596]; the National Institutes of Health [T32-HL007287–39]; and a grant from the Cambia Health Foundation.
Footnotes
DISCLOSURES & CONFLICTS OF INTEREST
There are no disclosures or conflicts of interest from any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomized controlled trial. BMJ 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300(14):1665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang B, Wright AA, Huskamp HH, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med 2009;169(5)480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academy Press; 2015. [PubMed] [Google Scholar]
- [5].National Quality Forum. National Voluntary Consensus Standards: Palliative Care and End-of-Life Care - A Consensus Report. 2012. April. Available at: http://www.qualityforum.org/Publications/2012/04/Palliative_Care_and_End-of-Life_Care%e2%80%94A_Consensus_Report.aspx. Accessed June 18, 2018.
- [6].Tulsky JA, Beach MC, Butow PN, et al. A research agenda for communication between health care professionals and patients living with serious illness. JAMA Intern Med 2017;177(9):1361–1366. [DOI] [PubMed] [Google Scholar]
- [7].Meier DE, Back AL, Berman A, Block SD, Corrigan JM, Morrison RS. A national strategy for palliative care. Health Aff (Millwood) 2017;36(7):1265–1273. [DOI] [PubMed] [Google Scholar]
- [8].Sudore RL, Heyland DK, Lum HD, et al. Outcomes that define successful advance care planning: a Delphi panel consensus. J Pain Symptom Manage 2018. February;55(2):245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Program Medicare. Revisions to payment policies under the physician fee schedule and other revisions to Part B for CY 2016 In: Services DoHaH, Services CfMM, eds. Federal Register: The daily journal of the United States government; 2015:70885–71386. [PubMed] [Google Scholar]
- [10].Sanders JJ, Curtis JR, Tulsky JA. Achieving goal-concordant care: a conceptual model and approach to measuring serious illness communication and its impact. J Palliat Med 2018;21(S2):S17–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Teno JM, Hakim RB, Knaus WA, et al. Preferences for cardiopulmonary resuscitation: physician-patient agreement and hospital resource use. J Gen Intern Med 1995;10:179–186. [DOI] [PubMed] [Google Scholar]
- [12].Wenger NS, Phillips RS, Teno JM, et al. Physician understanding of patient resuscitation preferences: insights and clinical implications. J Am Geriatr Soc 2000;48:S44–S51. [DOI] [PubMed] [Google Scholar]
- [13].Fried TR, Bradley EH, O’Leary J. Prognosis communication in serious illness: perceptions of older patients, caregivers, and clinicians. J Am Geriatr Soc 2003;51(10):1398–403. [DOI] [PubMed] [Google Scholar]
- [14].DesHarnais S, Carter RE, Hennessy W, Kurent JE, Carter C. Lack of concordance between physician and patient: reports on end-of-life care discussions. J Palliat Med 2007;10(3):728–40. [DOI] [PubMed] [Google Scholar]
- [15].Heyland DK, Barwich D, Pichora D, et al. Failure to engage hospitalized elderly patients and their families in advance care planning. ACCEPT (Advance Care Planning Evaluation in Elderly Patients) Study Team; Canadian Researchers at the End of Life Network (CARENET). JAMA Intern Med 2013;173(9):778–87. [DOI] [PubMed] [Google Scholar]
- [16].Curtis JR, Downey L, Back AL, et al. Effect of a patient and clinician communication-priming intervention on patient-reported goals-of-care discussions between patients with serious illness and clinicians: a randomized clinical trial. JAMA Intern Med 2018;178(7):930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fakhri S, Engelberg RA, Downey L, et al. Factors affecting patients preferences for and actual discussions about end-of-life care. J Pain Symptom Manage 2016;52(3):386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Coats H, Downey L, Sharma RK, Curtis JR, Engelberg RA. Quality of communication and trust in patients with serious illness: An exploratory study of the relationships of race/ethnicity, socioeconomic status, and religiosity. J Pain Symptom Manage 2018;56(4):530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roter DL, Hall JA, Aoki Y. Physician gender effects in medical communication: a meta-analytic review. JAMA 2002;288(6):756–64. [DOI] [PubMed] [Google Scholar]
- [20].Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health 2004;94(12):2084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Willems S, De Maesschalck S, Deveugele M, Derese A, De Maeseneer J. Socio-economic status of the patient and doctor-patient communication: does it make a difference? Patient Educ Couns 2005;56(2):139–46. [DOI] [PubMed] [Google Scholar]
- [22].McMurray JJ, Pfeffer MA. Heart failure. Lancet 2005;365(9474):1877–1889. [DOI] [PubMed] [Google Scholar]
- [23].Connors AF Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154(4, pt 1):959–967. [DOI] [PubMed] [Google Scholar]
- [24].Steinhauser KE, Clipp EC, Hays JC, et al. Identifying, recruiting, and retaining seriously-ill patients and their caregivers in longitudinal research. Palliat Med 2006;20(8):745–754. [DOI] [PubMed] [Google Scholar]
- [25].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62(1):10–29. [DOI] [PubMed] [Google Scholar]
- [26].Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK. Systematic review: the model for end-stage liver disease – should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther 2005;22(11–12):1079–1089. [DOI] [PubMed] [Google Scholar]
- [27].Curtis JR, Patrick DL, Caldwell E, Greenlee H, Collier AC. The quality of patient-clinician communication about end-of-life care: A study of patients with AIDS and their primary care clinicians. AIDS 1999;13:1123–31. [DOI] [PubMed] [Google Scholar]
- [28].Engelberg R, Downey L, Curtis JR. Psychometric characteristics of a quality of communication questionnaire assessing communication about end-of-life care. J Palliat Med 2006;9:1086–98. [DOI] [PubMed] [Google Scholar]
- [29].Curtis JR, Engelberg RA, Nielsen EL, Au DH, Patrick DL. Patient-physician communication about end-of-life care for patients with severe COPD. Eur Respir J 2004;24:200–5. [DOI] [PubMed] [Google Scholar]
- [30].Au DH, Udris EM, Engelberg RA, et al. A randomized trial to improve communication about end-of-life care among patients with COPD. Chest 2012;141:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA 1995;274(20):1591–8. [PubMed] [Google Scholar]
- [32].Teno JM, Fisher ES, Hamel MB, Coppola K, Dawson NV. Medical care inconsistent with patients’ treatment goals: association with 1-year Medicare resource use and survival. J Am Geriatr Soc. 2002;50(3):496–500. [DOI] [PubMed] [Google Scholar]
- [33].Finkelstein EA, Bilger M, Flynn TN, Malhotra C. Preferences for end-of-life care among community-dwelling older adults and patients with advanced cancer: a discrete choice experiment. Health Policy 2015;119(11):1482–1489. [DOI] [PubMed] [Google Scholar]
- [34].Flynn TN, Bilger M, Malhotra C, Finkelstein EA. Are efficient designs used in discrete choice experiments too difficult for some respondents? A case study eliciting preferences for end-of-life care. Pharmacoeconomics 2016;34(3):273–284. [DOI] [PubMed] [Google Scholar]
- [35].Modeling Greenland S. and variable selection in epidemiologic analysis. Am J Public Health 1989;79(3):340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mickey RM, Greendland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989;129(1):125–137. [DOI] [PubMed] [Google Scholar]
- [37].Kleinbaum DG, Kupper LL, Muller K. Applied Regression Analysis and Other Multivariable Methods. 2nd ed. Boston, MA: PWS-Kent Publishing Co; 1988. [Google Scholar]
- [38].Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med 2010;153(4):256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage 2017;53(5):821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].PerryUndem Research/Communication: Physicians’ Views Toward Advance Care Planning and End-of-life Care Conversations. 2016. Available at: www.cambiahealthfoundation.org/assets/files/Advance_Care_Report_F_Embargo.pdf. Accessed April 8, 2018.
- [41].Lamas D, Panariello N, Henrich N, et al. Advance care planning documentation in electronic health records: current challenges and recommendations for change. J Palliat Med 2018;21(4):522–528. [DOI] [PubMed] [Google Scholar]