Abstract
Purpose:
To compare long-term outcomes after bilateral lateral rectus recession (BLRc) or unilateral recess-resect (R&R) for primary treatment of childhood intermittent exotropia (IXT).
Design:
Multicenter randomized clinical trial
Participants:
197 children, age 3 to <11 years, with basic-type IXT, largest deviation by prism and alternate cover test at any distance of 15-40Δ, and near stereoacuity of at least 400 arc-seconds
Methods:
Random assignment to BLRc or R&R. Masked examinations were conducted every 6 months postoperatively for 3 years.
Main Outcome Measure:
The proportion of participants who met suboptimal surgical outcome by 3 years, defined as: 1) exotropia ≥10Δ at distance or near using simultaneous prism and cover test (SPCT), or 2) constant esotropia ≥6Δ at distance or near using SPCT, or 3) loss of ≥2 octaves stereoacuity from baseline, at ANY masked examination; or 4) reoperation without meeting any of these criteria.
Results:
The cumulative probability of suboptimal surgical outcome by 3 years was 46% (43/101) in the BLRc group versus 37% (33/96) in the R&R group (treatment-group difference of BLRc minus R&R = 9%; 95% CI = −6% to 23%). Reoperation by 3 years occurred in 9 (10%) participants in the BLRc group (8 of 9 met suboptimal surgical outcome criteria), and in 4 (5%) participants in the R&R group (3 of 4 met suboptimal surgical outcome criteria) (treatment-group difference of BLRc minus R&R = 5%; 95% CI = −2% to 13%). Among participants who completed the 3-year visit, 29% (25 of 86) in the BLRc group and 17% (13 of 77) in the R&R group underwent reoperation or met suboptimal surgical outcome criteria at the 3-year visit (treatment-group difference of BLRc minus R&R = 12% favoring R&R; 95% CI = −1% to 25%).
Conclusions:
We did not find a statistically significant difference in suboptimal surgical outcome by 3 years between children with IXT treated with BLRc compared with R&R. Based on these findings, we are unable to recommend one surgical approach over the other for childhood IXT.
Graphical abstract
Introduction
Intermittent exotropia (IXT) is the most common form of childhood-onset exotropia.1 If untreated, IXT may rarely lead to loss of binocular vision and can have negative psychosocial consequences.2, 3 When there is poor fusional control of an exodeviation, surgery is often the treatment of choice.4-6
The two most common surgical procedures are bilateral lateral rectus recession (BLRc) and unilateral lateral rectus recession combined with a medial rectus resection in the same eye (R&R). However, there is no agreement as to which procedure provides the greatest likelihood of short-term and long-term success.
We report the results of a large multi-center randomized trial comparing the effectiveness of BLRc with R&R for the surgical treatment of basic-type IXT in children 3 to <11 years old, with 3 years of post-operative follow-up.
Methods
The study was supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health, and was conducted according to the tenets of the Declaration of Helsinki by the Pediatric Eye Disease Investigator Group (PEDIG) at academic- and community-based clinical sites. The protocol and Health Insurance Portability and Accountability Act (HIPAA)–compliant informed consent forms were approved by institutional review boards, and a parent or guardian of each study participant gave written informed consent. An independent data and safety monitoring committee provided study oversight. The study is listed on www.clinicaltrials.gov (NCT01032603, accessed 6/01/18). The full study protocol is available on the PEDIG website (www.pedig.net, accessed 6/01/18).
Eligibility
We enrolled children aged 3 to <11 years with IXT, who had not undergone previous strabismus surgery, and had near stereoacuity of 400 arcsec or better on the Randot Preschool Stereotest (Stereo Optical Co., Chicago, IL). Eligibility and exclusion criteria are shown in Table 1, including five criteria specific to the magnitude of the IXT (manifest deviation) (see item #2).
Table 1:
Study Eligibility and Exclusion Criteria
| ELIGIBILITY CRITERIA |
|---|
| 1. Age 3 to < 11 years |
| 2. Intermittent exotropia (manifest deviation) meeting all of the following: |
| • Intermittent exotropia at distance OR constant exotropia at distance and either intermittent exotropia or exophoria at near |
| • Exodeviation at least 10Δ at distance AND near by PACT |
| • Exodeviation at least 15Δ at distance OR near by PACT |
| • Largest exodeviation at distance, near OR remote distance between 15 and 40Δ (inclusive) by PACT |
| • Basic type (distance and near exodeviation within 10Δ by PACT) |
| 3. Stereoacuity of 400 arcsec or better at near by Randot Preschool stereotest (better of 2 measures if initial test shows worse than 40 arcsec) |
| 4. Visual acuity in the worse eye 0.3 logMAR or better (20/40 by ATS HOTV for participants < 7 years old or 70 letters E-ETDRS testing for participants ≥ 7 years old) |
| 5. No hyperopia greater than +3.50 D spherical equivalent in either eye. |
| 6. Participants must be wearing spectacles or contact lenses for at least one week if refractive eR&Ror (based on cycloplegic refraction performed within 6 months prior to enrollment) meets any of the following: |
| • Myopia > −0.50 D spherical equivalent in either eye |
| • Anisometropia > 1.00 D spherical equivalent |
| • Astigmatism > 2.00 D in either eye if ≤ 5 years old and > 1.50D if > 5 years old |
| Refractive correction for participants meeting the above refractive eR&Ror criteria must meet the following guidelines: |
| • Anisometropia spherical equivalent must be within 0.25 D of full correction |
| • Astigmatism cylinder must be within 0.25 D of full correction and axis must be within 5 degrees of full correction. |
| • For hyperopia, the spherical component can be reduced at investigator discretion provided the reduction is symmetrical. Prescribing any refractive correction to yield lenses that are more myopic than −0.50 D spherical equivalent (SE) is considered deliberate overminus and is not allowed at enrollment. However, prescribing no correction or prescribing less than the full cycloplegic hyperopic correction (i.e., prescribing reduced plus) is not considered the same as overminusing for this protocol and is allowed because most participants without intermittent exotropia and hyperopic SE refractions in this range would not typically be prescribed a refractive correction. |
| • For myopia, the intent is to fully correct, but the spherical component can be undercorrected by investigator discretion provided the reduction is symmetrical and results in no more than −0.50 D SE residual (i.e., uncorrected) myopia. Prescribing a correction that yields more than 0.50 D more minus SE than the cycloplegic refraction SE is considered deliberate overminus and is not allowed at enrollment. |
| • Participants who have undergone treatment with prism or deliberate overminus refractive correction (as defined above) must have discontinued prism and/or any deliberate overminus for at least one week prior to enrollment. |
| Note that the refractive correction guidelines and the requirement to wear refractive correction for at least one week apply not only to participants who require refractive correction under the above criteria but also to any other participant who is wearing refractive correction. |
| 7. No atropine use within the last week |
| 8. Gestational age > 34 weeks |
| 9. Birth weight > 1500 grams |
| 10. Investigator plans to perform surgery, is willing to perform either surgical procedure, and is not planning to use adjustable sutures |
| 11. Parent understands protocol, has agreed to surgery, and is willing to accept randomization to one-eye surgery or two-eye surgery |
| 12. Parent has home phone (or access to phone) and is willing to be contacted by Jaeb Center staff |
| 13. Relocation outside of area of an active PEDIG site within next 3 years is not anticipated |
| EXCLUSION CRITERIA |
|---|
| 1. Coexisting vertical deviation, oblique muscle dysfunction, DVD, or A or V pattern, any of which the investigator plans to address with vertical transposition of horizontal rectus muscles, oblique surgery, or vertical rectus muscle surgery, i.e., only small vertical deviations, oblique muscle dysfunction, DVD, and A or V patterns not requiring surgery are allowed |
| 2. Limitation of ocular rotations due to restrictive or paretic strabismus |
| 3. Craniofacial malformations affecting the orbits |
| 4. Interocular visual acuity difference of more than 0.2 logMAR (2 lines on ATS HOTV for participants 3 to < 7 years old or 10 letters on E-ETDRS for participants ≥ 7 years old) and/or investigator plans to initiate amblyopia treatment at this time. |
| 5. High AC/A ratio (exclude > 6:1 by gradient method) |
| 6. Prior strabismus surgery or botulinum toxin injection |
| 7. Ocular disorders that would reduce visual acuity (except refractive error) |
| 8. Prior intraocular or refractive surgery |
| 9. Significant neurological impairment such as cerebral palsy. Participants with mild speech and/or learning disabilities are eligible. |
| 10. Investigator planning to change refractive correction at this time (if the participant is otherwise eligible, the investigator should consider prescribing refractive correction and bringing the participant back at a later time for enrollment). |
In addition to a primary cohort of basic-type IXT participants with angles ranging from 15 to 40Δ, the study had two secondary cohorts (participants with pseudo-divergence excess type IXT with 15 to 40Δ angles of deviation, and participants with either basic or pseudo-divergence excess type IXT with larger angles (45 to 50Δ) which will be reported in a separate manuscript. Pseudo-divergence-excess-type IXT was defined as a deviation at near which increased to within 10 prism diopters of the distance deviation when measured either with +3.00D lenses (unless high AC/A ratio ≥6:1 by −2.00 D gradient method in distance) or after 45 minutes of occlusion.
Enrollment/Randomization
At enrollment, near stereoacuity was measured using the Randot Preschool test at 1/3 m, control of the exodeviation was assessed at distance (6 m) and at near (1/3 m) using the Office Control Score,7 which ranges from 0 (phoria) to 5 (constant exotropia) (Table S2 – online only) and ocular alignment was assessed using the cover/uncover test, simultaneous prism and cover test (SPCT), and prism and alternate cover test (PACT) at distance (6 m) and at near (1/3 m). In addition, health-related quality of life (HRQOL) was assessed using the Intermittent Exotropia Questionnaire (IXTQ).8 This questionnaire consists of 3 components which assess IXT-related HRQOL as reported by children (separate versions for 5 to 7 year olds vs. those ≥8 years), the child’s HRQOL as perceived by the parents (proxy), and the parents’ own HRQOL on three subscales on psychosocial, functional, and surgical domains. All components use Likert-type response scales and Rasch-based scoring9, 10 transformed to a 0 (worst HRQOL) to 100 (best HRQOL) scale.
On the day of surgery or the day before, participants were randomly assigned (using a permuted block design stratified by site) with equal probability to BLRc or R&R, using the study website.
Surgical Procedures
Surgical dose was based on the largest preoperative exodeviation by PACT measurements at remote distance (at least 50 feet), distance (6 meters), or near (1/3 meter) (Table 3). Surgeons could adjust the surgical dose within 1.0 mm for each muscle at their discretion to account for participant variables, such as lateral incomitance and age. Adjustable techniques were not permitted, and hangback techniques were allowed only if episcleral bites also were taken at the intended scleral insertion site. For R&R procedures, choice of eye was at investigator discretion.
Table 3:
Surgical Dosea
| Bilateral Lateral Rectus Recession (BLRc) | |
|---|---|
| Magnitude of Largest Deviation by PACT |
Amount to Recess Each Lateral Rectus Muscleb |
| 16 Δ | 4.0 mm |
| 18 Δ | 5.0 mm |
| 20 Δ | 5.0 mm |
| 25 Δ | 6.0 mm |
| 30 Δ | 7.0 mm |
| 35 Δ | 7.5 mm |
| 40 Δ | 8.0 mm |
| 45 Δ | 8.5 mm |
| 50 Δ | 9.0 mm |
| Unilateral Lateral Rectus Recession with Medial Rectus Resection (R&R): | ||
|---|---|---|
| Magnitude of Largest Deviation by PACT |
Amount to Recess Lateral Rectus Muscleb |
Amount to Resect Medial Rectus Muscleb |
| 16 Δ | 4.0 mm | 3.0 mm |
| 18 Δ | 5.0 mm | 4.0 mm |
| 20 Δ | 5.0 mm | 4.0 mm |
| 25 Δ | 6.0 mm | 5.0 mm |
| 30 Δ | 7.0 mm | 5.5 mm |
| 35 Δ | 7.5 mm | 6.0 mm |
| 40 Δ | 8.0 mm | 6.5 mm |
| 45 Δ | 8.5 mm | 6.5 mm |
| 50 Δ | 9.0 mm | 7.0 mm |
Refers to largest angle by PACT at remote distance (at least 50 feet), distance (6 meters), or near (1/3 meter).
For recessions, the measurement of surgical dose was to be made from the insertion of the muscle after muscle disinsertion. For resections, the measurement of surgical dose was to be made from the insertion of the muscle prior to muscle disinsertion.
PACT = prism and alternate cover test; Δ = prism diopters
Data on surgical complications were collected at the 1-week and 8-week follow up visits.
Follow-up Visits
Postoperative visits occurred at 1 week (± 3 days) and 8 weeks (± 2 weeks), followed by masked exam visits at 6 months (± 1 month), and then every 6 months (± 2 month) through 3 years after randomization.
At each follow-up visit, a study-certified examiner (pediatric ophthalmologist, pediatric optometrist, or certified orthoptist) masked to the participant’s treatment group measured stereoacuity, exotropia control, and ocular alignment. If one or more protocol-specified criteria for suboptimal surgical outcome (Table 4) were met on the initial masked exam testing, testing specific to that criterion was repeated by the masked examiner after a 10-minute break, with suboptimal surgical outcome declared only when both the test and repeat test met the criteria.
Table 4:
Definition of Suboptimal Surgical Outcome by Three Years
| Suboptimal Surgical Outcome by Three Years (Primary Outcome) |
|---|
| A participant’s IXT was considered to be a suboptimal surgical outcome if at any visit occurring 6 months or later, ANY of the following criteria are present by masked examiner testing: |
| 1. Exotropia at distance OR near at any time during the exam (i.e., can be constant or intermittent; determined by a cover/uncover test) with a magnitude of ≥10Δ by SPCT, confirmed by a retest |
| 2. Constant esotropia at distance OR near (determined by at least 3 cover/uncover tests—one must be before any dissociation) with a magnitude of ≥6Δ by SPCT, confirmed by a retest |
| 3. Decrease in Randot Preschool near stereoacuity ≥2 octaves (≥0.6 log arcsec) from enrollment, or to nil, confirmed by a retest |
| Randot Preschool Stereotest (Stereo Optical, Chicago, Illinois) | |
|---|---|
| Baseline Stereoacuity, in arcsec |
Stereoacuity level needed at follow-up visit to meet suboptimal surgical outcome criteria, in arcsec |
| 40” | 200” or worse |
| 60” | 400” or worse |
| 100” | 400” or worse |
| 200” | 800” or worse |
| 400” | Nil |
| Participants who underwent reoperation (or treatment with botulinum toxin) without first meeting any of the above suboptimal surgical outcome criteria were also counted as suboptimal surgical outcomes in the primary analysis. |
IXT = intermittent exotropia; SPCT = simultaneous prism and cover test; Δ = prism diopter; arcsec = seconds of arc
At 6-month and 3-year visits, health-related quality of life (HRQOL) was reassessed using the IXTQ.8
Treatment during Post-operative Follow Up
If a constant esotropia ≥6 Δ by SPCT at distance and near was present at the 8-week postoperative visit, prism treatment was prescribed, selecting the minimum prism strength needed to neutralize the angle. At each subsequent visit, investigators were to attempt to reduce or discontinue prism at both distance and near.
Other than prism treatment at 8 weeks, any nonsurgical treatment of any recurrent or residual exodeviation, esodeviation, or diplopia was at investigator discretion throughout the study. Reoperation or treatment with botulinum toxin were permitted only between 6 months and 3 years and only after criteria for suboptimal surgical outcome were met (Table 4).
Statistical Methods
The sample size of 189 participants provided 90% power with a type 1 error rate of 5% to detect a difference if the treatment group failure rate was 50% in the BLRc group vs. 25% in the R&R group, assuming 10% loss to follow up.
The primary outcome was suboptimal surgical outcome by 3 years (originally termed “failure” and herein referred to as “suboptimal surgical outcome”), defined as: 1) ≥10Δ XT by SPCT at distance or near); 2) constant esotropia of ≥6Δ; or 3) loss of ≥2 octaves stereoacuity compared with baseline, at any masked follow-up examination between 6 months and 3 years (Table 4). We chose SPCT because we felt that from the standpoint of motor alignment, a manifest tropia of specific magnitude was more important than the total deviation after dissociation. We allowed a small angle esotropia because some patients with IXT are primary monofixators and may have a small angle tropia both pre-operatively and post-operatively, without diplopia. In addition, participants were also classified as having a suboptimal surgical outcome if they underwent reoperation without first meeting any of these criteria. For the primary analysis, the cumulative proportion of participants meeting criteria for suboptimal surgical outcome by 3 years was obtained using the Kaplan-Meier (K-M) method and compared between treatment groups using the Z test. A treatment-group difference and a corresponding 95% confidence interval were also calculated.
Each of the 3 individual components of the suboptimal surgical outcome criteria was specified post hoc as a secondary outcome. These cause-specific outcomes differ from the primary outcome in two ways: 1) the primary outcome refers to the first occurrence of any suboptimal surgical outcome criterion (or reoperation) being met, whereas the cause-specific outcomes refer to the first occurrence of a particular suboptimal surgical outcome criterion being met.
For each of the three cause-specific outcomes, participants who met any criteria other than the particular criterion being assessed remained “at risk” for the criterion of interest unless they underwent reoperation, in which case they were censored at the time of reoperation. The cumulative probability of each cause-specific outcome by 3 years and a 95% CI were obtained using the K-M method. It is acknowledged that the three cause-specific outcomes are not independent because reoperation is a competing risk for each (e.g., participants who met the exotropia outcome only and subsequently underwent reoperation were by definition censored without meeting the stereo loss or constant esotropia outcomes).
Additional secondary outcomes were suboptimal surgical outcome at the 3-year visit (pre-specified) and complete or near-complete resolution (specified post hoc) at the 3-year visit. Suboptimal surgical outcome at the 3-year visit was defined as meeting any of the three suboptimal surgical outcome criteria at the 3-year visit (regardless of whether the criterion had been met at an earlier visit), or undergoing reoperation at any time. Complete or near-complete resolution was defined as meeting all of the following at the 3 year visit: 1) exodeviation <10 Δ (tropia or phoria) by both SPCT and PACT at distance and near and ≥10 Δ reduction in PACT magnitude from the largest of the distance and near angles at enrollment, 2) esotropia <6 Δ at distance and near by SPCT, 3) no decrease in Randot Preschool stereoacuity of ≥2 octaves from the enrollment stereoacuity or to nil, 4) no reoperation or treatment with botulinum toxin, and 5) no non-surgical treatment for a recurrent or residual exodeviation. The proportion of participants with suboptimal surgical outcome at 3 years was compared between treatment groups using Barnard’s exact test, and an exact 95% CI on the treatment-group difference was calculated using Farrington-Manning scores.11
For all participants who completed the 3-year visit, (regardless of whether they had undergone reoperation and/or whether they had a suboptimal surgical outcome by 3 years), changes in exotropia control, PACT magnitude, and stereoacuity between baseline and 3 years timepoints were compared between treatment groups in linear regression models that adjusted for the corresponding baseline value.
The cumulative proportion of reoperation by 3 years was also compared between treatment groups using methods similar to the primary analysis.
For the each of the two age-specific versions of the child IXTQ, the proxy questionnaire, and for each of the three parent questionnaire subscales, mean Rasch-based HRQOL scores9, 10 at 3 years were compared between treatment groups using the Wilcoxon rank sum test.
All treatment-group differences were calculated as the BLRc group minus the R&R group. Analyses were conducted using SAS version 9.4 (SAS Institute Inc. Cary, NC).
Results
Baseline Characteristics
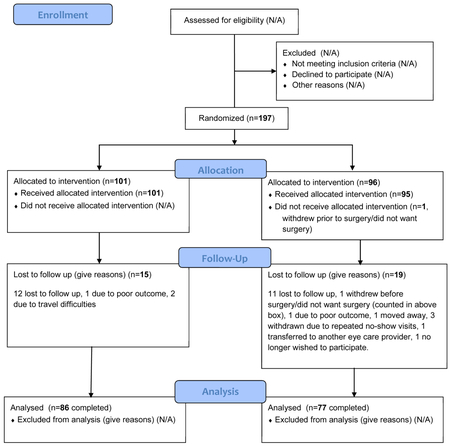
Between July 2010 and February 2014, 197 children were enrolled at 35 clinical sites with 101 assigned to the BLRc group and 96 assigned to the R&R group. Mean age was 6.2 (±2.0) years, 122 (62%) were female, and 113 (57%) were white. The mean preoperative angle (largest at distance, near, or remote distance) was 28Δ (±6Δ). Baseline demographic and clinical characteristics appeared similar for both treatment groups (Tables 5-7).
Table 5:
Baseline Demographic and Clinical Characteristics by Treatment Group (N=197)a
| BLRc | R&R | |||
|---|---|---|---|---|
| N | % | N | % | |
| All | 101 | 100 | 96 | 100 |
| GENDER | ||||
| Female | 64 | 63 | 58 | 60 |
| Male | 37 | 37 | 38 | 40 |
| RACE/ETHNICITY | ||||
| White | 58 | 57 | 55 | 57 |
| African American | 13 | 13 | 14 | 15 |
| Hispanic | 19 | 19 | 25 | 26 |
| Other | 11 | 11 | 2 | 2 |
| AGE AT RANDOMIZATION (YEARS) | ||||
| 3-<4 | 14 | 14 | 15 | 16 |
| 4-<5 | 19 | 19 | 24 | 25 |
| 5-<6 | 14 | 14 | 20 | 21 |
| 6-<7 | 15 | 15 | 10 | 10 |
| 7-<8 | 15 | 15 | 11 | 11 |
| 8-<9 | 10 | 10 | 4 | 4 |
| 9-<10 | 9 | 9 | 10 | 10 |
| 10-<11 | 5 | 5 | 2 | 2 |
| Mean (SD) | 6.4 (2.1) | 5.9 (1.9) | ||
| PRIOR NONSURGICAL TREATMENT | ||||
| No treatment | 49 | 49 | 49 | 51 |
| Patching alone | 36 | 36 | 29 | 30 |
| Overminus spectacles alone | 6 | 6 | 4 | 4 |
| Vision therapy alone | 2 | 2 | 3 | 3 |
| Combination or other | 8 | 8 | 11 | 11 |
| RANDOT PRESCHOOL STEREOACUITY (ARCSEC) | ||||
| 40 | 15 (15) | 16 (17) | ||
| 60 | 22 (22) | 20 (21) | ||
| 100 | 28 (28) | 31 (32) | ||
| 200 | 12 (12) | 13 (14) | ||
| 400 | 24 (24) | 16 (17) | ||
| Mean (SD) (log arcsec) | 2.1 (0.4) | 2.0 (0.3) | ||
| Range (log arcsec) | 1.6 to 2.6 | 1.6 to 2.6 | ||
| DISTANCE RANDOT STEREOACUITY (ARCSEC) | ||||
| 60 | 27 (27) | 25 (27) | ||
| 100 | 14 (14) | 19 (20) | ||
| 200 | 18 (18) | 16 (17) | ||
| 400 | 20 (20) | 13 (14) | ||
| Nil | 20 (20) | 20 (22) | ||
| Mean (SD) (log arcsec) | 2.3 (0.4) | 2.3 (0.4) | ||
| Range (log arcsec) | 1.8 to 2.9 | 1.8 to 2.9 | ||
BLRc= bilateral lateral rectus muscle recession; R&R = unilateral lateral rectus recession combined with a medial rectus resection in same eye
Note that percentages for each factor may not total 100% exactly due to rounding.
Participants were required to have 400 arcsec or better of stereoacuity at enrollment. arcsec = seconds of arc; SD = standard deviation
Table 7.
Baseline Exotropia Control by Treatment Group
| Distance | Near | |||||||
|---|---|---|---|---|---|---|---|---|
| BLRc (N=101) |
RR (N=96) |
BLRc (N=101) |
RR (N=96) |
|||||
| N | % | N | % | N | % | N | % | |
| Baseline Exotropia Control (in points) | ||||||||
| (0) No exotropia unless dissociated, recovers <1 secs (phoria) | 1 | <1 | 0 | 0 | 10 | 10 | 20 | 21 |
| (1) No exotropia unless dissociated, recovers 1-5 secs | 9 | 9 | 6 | 6 | 36 | 36 | 27 | 28 |
| (2) No exotropia unless dissociated, recovers >5 secs | 19 | 19 | 19 | 20 | 19 | 19 | 19 | 20 |
| (3) Exotropia <50% of 30-second observation | 23 | 23 | 19 | 20 | 24 | 24 | 18 | 19 |
| (4) Exotropia >50% of 30-second observation | 18 | 18 | 31 | 32 | 11 | 11 | 12 | 13 |
| (5) Constant exotropia | 31 | 31 | 21 | 22 | 1 | <1 | 0 | 0 |
| Mean (SD) | 3.4 (1.4) | 3.4 (1.2) | 1.9 (1.2) | 1.7 (1.3) | ||||
| Range | 0 to 5 | 1 to 5 | 0 to 5 | 0 to 4 | ||||
BLRc= bilateral lateral rectus muscle recession; R&R = unilateral lateral rectus recession combined with a medial rectus resection in same eye SD = standard deviation
Surgical Complications
There were no intraoperative complications reported in either group. Post-operative complications were reported at the 1-week or 8-week visits in 0 participants in the BLRc group and in 3 participants in the R&R group (one each of small epithelial cyst, dell, and conjunctival edema overlying MR resection site), none of which was serious, and all of which resolved without sequelae.
Visit Completion
In the BLRc and R&R groups respectively, 88% (89 of 101) and 84% (81 of 96) of participants contributed to the primary outcome analysis by either completing the 3-year visit or by meeting suboptimal surgical outcome criteria prior to the 3-year visit. There did not appear to be any meaningful baseline differences between those completing the 3-year visit, and those who did not (Table 8).
Table 8:
Clinical Characteristics According to Study Completion
| Participants Withdrawn from Study (N=34) |
Participants Completing 3-Year Visit (N=163) |
|||
|---|---|---|---|---|
| BLRc (N=15) |
R&R (N=19) |
BLRc (N=86) |
R&R (N=77) |
|
| Baseline age | 6.6 | 5.6 | 6.3 | 6.0 |
| Baseline stereo, log arcsec | 2.2 | 2.0 | 2.0 | 2.0 |
| Baseline control at distance, points | 2.7 | 3.4 | 3.5 | 3.5 |
| Baseline control at near, points | 1.8 | 1.9 | 2.0 | 1.7 |
| Baseline PACT at distance, Δ | 26 | 23 | 26 | 27 |
| Baseline PACT at near, Δ | 25 | 20 | 25 | 25 |
| Previous non-surgical treatment, N (%) | 4 (27) | 8 (42) | 48 (56) | 39 (51) |
| Pre-operative surgical exodeviation, Δ | 27 | 24 | 28 | 28 |
| Last follow up visit control at distance, points | 1.0 | 1.6 | 1.2 | 1.0 |
| Last follow up visit PACT at distance, Δ | −1 | 6a | 10 | 9 |
Distance PACT is missing at the last completed visit for one RR group participant who was withdrawn from the study.
BLRc= bilateral lateral rectus muscle recession; R&R = unilateral lateral rectus recession combined with a medial rectus resection in same eye
Δ = prism diopter; PACT = prism and alternate cover test; arcsec = seconds of arc
Postoperative Nonsurgical Treatment
Postoperative nonsurgical treatment was prescribed for XT, ET, and/or diplopia in 18 (18%) BLRc and 36 (38%) R&R group participants (Table S9- online only). Orthoptic exercises (alone or with other treatments) were prescribed for 6 (6%) and 8 (8%) of participants in the BLRc and R&R groups respectively (P = 0.53); patching (alone or with other treatments) was prescribed for 4 (4%) and 25 (26%) of participants in the BLRc and R&R groups, respectively (P <0.001). Over the 3 years, 11 (11%), 6 (6%), and 1 (<1%) participants in the BLRc group and 10 (10%), 23 (24%), and 3 (3%) participants in the R&R group were prescribed nonsurgical treatment for exodeviation, esodeviation, or for both exo-and eso-deviations, respectively.
Primary Outcome – Suboptimal Surgical Outcome by 3 Years
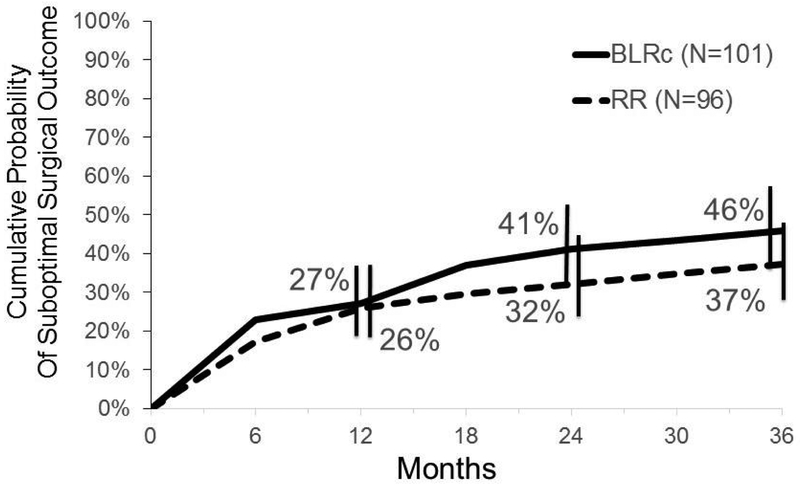
Suboptimal surgical outcome by 3 years (at a masked exam between 6 months and 3 years after surgery or reoperation at any time) occurred in 43 of 101 participants in the BLRc group and in 33 of 96 in the R&R group. The cumulative probability of suboptimal surgical outcome by 3 years was 46% in the BLRc group compared with 37% in the R&R group (treatment-group difference of BLRc minus R&R = 9%; 95% CI = −6% to 23%) (Figure 2) (Table 10). In both treatment groups, the first suboptimal surgical outcome criterion that was met most often was residual or recurrent exotropia (29 of 43 [67%] in BLRc group; 21 of 33 [64%] in R&R group).
Figure 2. Suboptimal Surgical Outcome By 3 Years (N=197).
Cumulative probability of suboptimal surgical outcome by 3 years from Kaplan-Meier analysis. Treatment-group difference of BLRc minus R&R = 9% (−6% to 23%). BLRc = bilateral lateral rectus muscle recession; R&R = unilateral lateral rectus recession combined with a medial rectus resection in same eye
Table 10:
Suboptimal Surgical Outcome by 3 Years (N=197)
| 6 Months |
12 Months |
18 Months |
24 Months |
30 Months |
36 Months |
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BLR | R&R | BLR | R&R | BLR | R&R | BLR | R&R | BLR | R&R | BLR | R&R | BLR | R&R | |
| N at risk | 96 | 93 | 71 | 75 | 66 | 61 | 58 | 57 | 53 | 52 | 48 | 50 | N/A | N/A |
| N with suboptimal surgical outcome | 23 | 16 | 3 | 8 | 9 | 3 | 4 | 2 | 2 | 2 | 2 | 2 | 43 | 33 |
| XT | 14 | 7 | 3 | 5 | 6 | 3 | 3 | 2 | 1 | 2 | 2 | 2 | 29 | 21 |
| Constant ET | 1 | 1 | 1 | 1 | 2 | |||||||||
| Stereo loss | 4 | 4 | 3 | 1 | 1 | 9 | 4 | |||||||
| Stereo loss and XT | 1 | 1 | 0 | |||||||||||
| Stereo loss and constant ET | 2 | 3 | 2 | 2 | 5 | |||||||||
| Reoperated without meeting suboptimal surgical outcome | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Cumulative probability of meeting suboptimal surgical outcome | 24% | 17% | 27% | 26% | 37% | 30% | 41% | 32% | 44% | 35% | 46% | 37% | N/A | N/A |
BLRc= bilateral lateral rectus muscle recession; R&R = unilateral lateral rectus recession combined with a medial rectus resection in same eye
N/A = not applicable
XT = exotropia ≥10Δ by SPCT at distance or near, confirmed by a retest
Constant ET = constant esotropia ≥6Δ by SPCT at distance or near, confirmed by a retest
Stereo loss = decrease in Preschool Randot near stereoacuity ≥2 octaves (≥0.6 log arcsec) from enrollment, or to nil, confirmed by a retest.
Reoperation by 3 Years
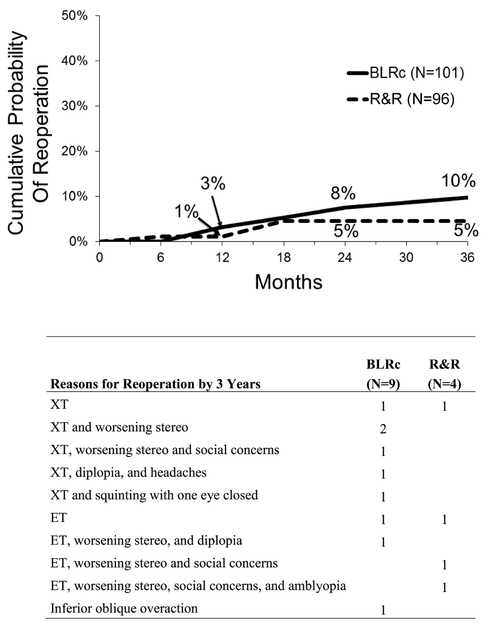
A reoperation was performed by 3 years in 9 (10%) participants in the BLRc group (8 of 9 met suboptimal surgical outcome criteria) and in 4 (5%) participants in the R&R group (3 of 4 met suboptimal surgical outcome criteria) (treatment-group difference of BLRc minus R&R = 5%; 95% CI = −2% to 13%); reasons for reoperation are listed in Figure 3.
Figure 3: Reoperation by 3 Years (N=197).
BLRc = bilateral lateral rectus muscle recession; R&R = unilateral lateral rectus recession combined with a medial rectus resection in same eye; XT = exotropia; ET = esotropia
Suboptimal Surgical Outcome at 3-year Visit
Among participants who completed the 3-year visit, 29% (25 of 86) in the BLRc group and 17% (13 of 77) in the R&R group met suboptimal surgical outcome criteria at the time of the 3-year visit (regardless of whether criteria were met at any previous visits) or had undergone reoperation (treatment-group difference of BLRc minus R&R = 12%; 95% CI = −1% to 25%).
Among the 38 BLRc group participants who met suboptimal surgical outcome criteria before 3 years and returned for the 3-year visit, 8 had undergone reoperation, 15 met the suboptimal surgical outcome criteria at 3 years, 5 met criteria for complete or near-complete resolution and 10 met neither (Table S11a – online only). Among the 26 R&R group participants who met suboptimal surgical outcome criteria before 3 years and returned for the 3-year visit, 4 had undergone reoperation, 7 met suboptimal surgical outcome criteria at 3 years, 7 met criteria for complete or near-complete resolution and 9 met neither (Table S11b – online only). Among the participants with suboptimal surgical outcomes identified before 3 years and who had not undergone reoperation, the proportion who no longer met suboptimal surgical outcome criteria at the 3-year visit was 50% (15 of 30) in the BLRc group compared with 70% (16 of 23) in the R&R group (P = 0.17).
Complete or Near-complete Resolution
Among participants who completed the 3-year visit, criteria for complete or near-complete resolution at the 3-year visit were met by 30% (26 of 86) in the BLRc group, and 45% (35 of 77) in the R&R group at the 3-year visit (treatment-group difference of BLRc minus R&R = −15%; 95% CI = −30% to −0.0003% favoring the R&R group). The proportions of patients who met complete or near-complete resolution at 3 years and never met suboptimal surgical outcome criteria were 24% (21 of 86) in the BLRc group and 36% (28 of 77) in the R&R group (treatment-group difference of BLRc minus R&R = −12%; 95% CI = −26% to 3%).
Constant Esotropia Suboptimal Surgical Outcome
The cumulative probability of constant esotropia ≥6Δ at distance and/or near at any exam from 6 months through 3 years was 3% in the BLRc group and 10% in the R&R group (treatment-group difference of BLRc minus R&R = −7%; 95% CI = −14% to 0.23%) (Table 12). In both treatment groups, 67% of participants who met the constant esotropia suboptimal surgical outcome were prescribed non-surgical treatment (2 of 3 in the BLRc group and 6 of 9 in the R&R group). Among participants who had constant esotropia who did not undergo reoperation and completed the 3-year visit, constant esotropia ≥6Δ at distance and/or near was no longer present in 1 of 2 in the BLRc group versus 7 of 7 in the R&R group.
Table 12:
IXT1 Suboptimal Surgical Outcomes by 3 Years (Primary Outcome) and Cause-Specific Outcomes By 3 Years (Secondary)
| Outcome | BLRc Group | R&R Group | Treatment-group differenceb (and 95% CI) |
P value | ||
|---|---|---|---|---|---|---|
| N with Outcome |
Kaplan- Meier Cumulative Probability |
N with Outcome |
Kaplan- Meier Cumulative Probability |
|||
| Suboptimal Surgical Outcome by 3 years | 43 | 46% | 33 | 37% | 9% (−6% to 23%) | 0.24 |
| XT by 3 yearsa | 31 | 34% | 22 | 26% | 8% (−6% to 21%) | 0.25 |
| Constant ET by 3 yearsa | 3 | 3% | 9 | 10% | −7% (−14% to 0.23%) | 0.06 |
| Stereo loss by 3 yearsa | 13 | 14% | 9 | 10% | 4% (−5% to 14%) | 0.36 |
BLRc= bilateral lateral rectus muscle recession; R&R = unilateral lateral rectus recession combined with a medial rectus resection in same eye
XT = exotropia ≥10Δ by SPCT at distance or near, confirmed by a retest
Constant ET = constant esotropia ≥6Δ by SPCT at distance or near, confirmed by a retest
Stereo loss = decrease in Preschool Randot near stereoacuity ≥2 octaves (≥0.6 log arcsec) from enrollment, or to nil, confirmed by a retest
Cause-specific criteria (XT, constant ET, stereo loss) refer to meeting the given criteria by 3 years, before any reoperation, and regardless of whether suboptimal surgical outcome was met by another criteria.
Treatment-group differences are calculated as BLRc minus R&R, so positive differences favor the R&R group.
Exotropia Suboptimal Surgical Outcome
The cumulative probability of having exotropia ≥10Δ at distance and/or near at any exam from 6 months through 3 years was 34% in the BLRc group and 26% in the R&R group (treatment-group difference of BLRc minus R&R = 8%; 95% CI = −6% to 21%) (Table 12). Of these cases, 23% (7 of 31) underwent reoperation in the BLRc group vs. 5% (1 of 22) in the R&R group. Among participants with residual exotropia who did not undergo reoperation and completed the 3-year visit, exotropia ≥10Δ at distance and/or near was no longer present in 9 of 20 (45%) in the BLRc group versus 8 of 15 (53%) in the R&R group.
Change in Exotropia Control, PACT Magnitude, and Stereoacuity Over 3 Years
In the BLRc and R&R groups, respectively, the proportion of participants with distance exotropia control of 2 or better at the 3-year visit were 29% (29 of 101) and 26% (25 of 96) at baseline and 81% (70 of 86) and 85% (64 of 77) at the 3-year visit. Mean improvement in exotropia control over 3 years was 2.3 vs. 2.5 points at distance (P=0.44) and 1.3 vs. 1.1 points at near (P=0.64) for the BLRc and R&R groups, respectively (Table 13). The mean reduction in PACT magnitude over 3 years was 16Δ vs. 18Δ at distance (P=0.21) and 15Δ vs. 16Δ at near (P=0.38) (Table 13) (Figure 4). In both treatment groups, mean improvement in stereoacuity at 3 years was 0.1 log arcsec at distance (P = 0.82) and 0.2 log arcsec at near (P = 0.93) (Table S14 – online only).
Table 13:
3-Year Exotropia Control and Ocular Alignment (N=163)
| DISTANCE | NEAR | |||
|---|---|---|---|---|
| BLRc (N=86) |
R&R (N=77) |
BLRc (N=86) |
R&R (N=77) |
|
| N (%) | N (%) | N (%) | N (%) | |
| Exotropia Controla | ||||
| At 3 years | ||||
| Not applicable (no exodeviation) | 19 (22) | 25 (32) | 20 (23) | 21 (27) |
| (0) No exotropia unless dissociated, recovers <1 secs | 25 (29) | 22 (29) | 36 (42) | 32 (42) |
| (1) No exotropia unless dissociated, recovers 1-5 | 14 (16) | 11(14) | 16 (19) | 13 (17) |
| (2) No exotropia unless dissociated, recovers >5 secs | 12 (14) | 6 (8) | 6 (7) | 4 (5) |
| (3) Exotropia <50% of 30-second observation | 5 (6) | 4 (5) | 1 (1) | 4 (5) |
| (4) Exotropia >50% of 30-second observation | 6 (7) | 4 (5) | 6 (7) | 3 (4) |
| (5) Constant exotropia | 5 (6) | 5 (6) | 1 (1) | 0 (0) |
| Mean (SD) | 1.2 (1.6) | 1.0 (1.6) | 0.7 (1.2) | 0.6 (1.1) |
| Range | 0 to 5 | 0 to 5 | 0 to 5 | 0 to 4 |
| Change between baseline and 3 yearsb | ||||
| Mean (SD) | 2.3 (1.7) | 2.5 (1.7) | 1.3 (1.6) | 1.1 (1.6) |
| Range | −3 to 5 | −2 to 5 | −3 to 4 | −3 to 4 |
| Prism and Alternate Cover Test (Δ)a | ||||
| At 3 years | ||||
| No deviation | 10 (12) | 21 (27) | 11 (13) | 15 (19) |
| 1-9Δ Exo | 23 (27) | 17 (22) | 21 (24) | 24 (31) |
| 10-14Δ Exo | 15 (17) | 12 (16) | 16 (19) | 9 (12) |
| 15-18Δ Exo | 10 (12) | 7 (9) | 11 (13) | 5 (6) |
| 20-25Δ Exo | 16 (19) | 12 (16) | 13 (15) | 14 (18) |
| 30-35Δ Exo | 3 (3) | 2 (3) | 3 (3) | 3 (4) |
| 40-45 Exo | 0 | 0 | 1 (1) | 0 |
| 10-14Δ Eso | 2 (2) | 0 | 1 (1) | 0 |
| 1-9Δ Eso | 7 (8) | 6 (8) | 9 (10) | 7 (9) |
| Mean (SD) | 10 (10) | 9 (10) | 10 (11) | 9 (10) |
| Range | −14 to 35 | −6 to 30 | −14 to 40 | −6 to 30 |
| Change between baseline and 3 yearsb | ||||
| Mean (SD) | 16 (11) | 18 (10) | 15 (12) | 16 (12) |
| Range | −10 to 44 | −5 to 36 | −14 to 40 | −12 to 41 |
BLRc= bilateral lateral rectus muscle recession; R&R = unilateral lateral rectus recession combined with a medial rectus resection in same eye; Δ = prism diopters; eso = esodeviation; exo = exodeviation, SD = standard deviation
For treatment group comparisons of mean 3-year control using linear regression models adjusting for baseline control, P values = 0.44 and 0.64 for distance and near control, respectively. For treatment group comparisons of mean 3-year PACT using linear regression models adjusting for baseline PACT, P values = 0.21 and 0.38 for distance and near PACT, respectively.
Change is defined as the baseline value minus the 3-year value, therefore positive change = improvement. If the 3-year PACT is an esodeviation, change in PACT from baseline is the reduction in the exodeviation plus the amount of the 3-year exodeviation.
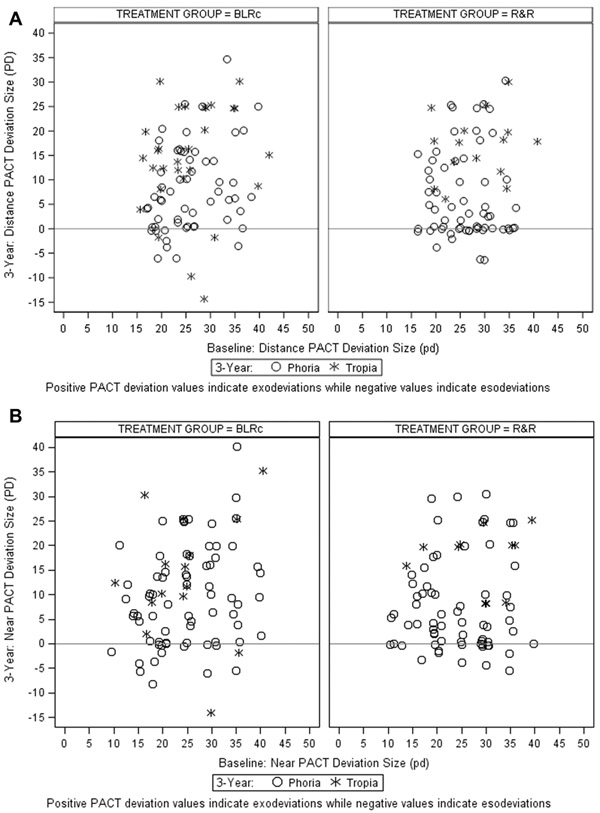
Figure 4a and 4b: Baseline vs. 3-Year PACT Magnitude (N=86 for BLRc and 77 for R&R) for Distance (4a) and Near (4b) Deviations.
A deviation is defined as a tropia (O) if a tropia was present by cover/uncover test at 3 years; otherwise it was defined as phoria (X). BLRc = bilateral lateral rectus muscle recession; R&R = unilateral lateral rectus recession combined with a medial rectus resection in same eye
Health-related Quality of Life
There were no significant differences between the BLRc and R&R treatment groups in the distribution of 3-year IXTQ scores as assessed by the child IXTQ (median = 91 vs. 86, P = 0.30 for participants 5 to 7 year olds at 3-year follow up; median = 82 vs. 82, P = 0.77 for participants 8 to 13 years olds at 3-year follow up); parent proxy IXTQ (median = 86 vs. 91, P = 0.51); and parent psychosocial (median = 97 vs. 100, P = 0.42), parent function (median = 83 vs. 86, P = 0.68), and parent surgery (median = 83 vs. 92, P = 0.64) subscales of the parent version of the IXTQ.
Discussion
In this randomized trial, we compared BLRc and R&R in 197 children with basic IXT aged 3 to less than 11 years. We found the cumulative probability of a suboptimal surgical outcome through 3 years of follow-up was 46% in the BLRc group and 37% in the R&R group, a difference of 9% in favor of the R&R group (with a 95% CI of −6% to 23%). Although the difference was not statistically significant, the confidence interval reasonably rules out a moderately-sized benefit in favor of BLRc but cannot rule out the possibility of a moderately-sized benefit in favor of R&R.
We also compared several secondary outcome measures. The first was the rate of suboptimal surgical outcome at the 3-year masked examination, ignoring the results from the earlier masked examinations. This analysis was performed to account for surgical overcorrections that improved spontaneously prior to the 3-year visit. The rates of suboptimal surgical outcome at 3 years did not differ statistically, nor did the reoperation rates differ between groups. However, it is noteworthy that 6 of 9 reoperations in the BLRc group were for recurrent exotropia whereas 3 of the 4 reoperations in the R&R group were for esotropia. Both procedures improved mean control score and reduced deviation magnitude similarly. Because a post-hoc outcome of "complete or near-complete resolution at 3 years” favored the R&R procedure, (45% in the R&R group versus 30% in the BLRc group), and because suboptimal surgical outcomes during the 3 years, at the end of 3 years, and rates of reoperation were in the direction of favoring R&R but were not statistically significant, there may be slightly more support for the R&R procedure than for BLRc. However, because these outcomes are correlated with each other, we might expect them to all favor the same procedure, regardless of whether the overall results of our particular study reflect the underlying truth in the population of interest.
Our study used a standard dose of surgery for each group. Differing amounts of surgery may have produced different results. Using a greater amount of recession than we used for bilateral lateral rectus muscle recessions (augmenting) has been advocated.12 While augmented recession may decrease failure rates due to residual exotropia, it might do so at the expense of increasing failure rates due to overcorrection.
Kushner reported the only other prospective randomized clinical trial comparing these two procedures, with a follow-up period ranging from 12 to 15 months.13 He found 82% of 17 patients who underwent R&R had a successful outcome, compared with only 52% of 19 patients who underwent BLRc (P<0.05).13 His definition of a satisfactory outcome was alignment ranging between 0 and 5Δ of esophoria and 0 and 10 Δ of exophoria. In addition, any intermittent or constant esotropia or exotropia was considered an unsatisfactory outcome, as was any reoperation, prism treatment after surgery, or adjustment of spectacle power to alter alignment. Although our primary outcome measure differs in many ways from that of Kushner, the results of the two studies are not entirely inconsistent because our study could not rule out a moderate benefit of R&R.
Only a small number of our participants had constant esotropia at or after the six-month follow-up examination, and nearly all of those who did not undergo reoperation resolved spontaneously (1 of 2 BLRc group; 7 of 7 in the R&R group). The resulting 11% (1 of 9) observed rate of initial overcorrections that were longstanding (i.e., at 3 years) is substantially lower than that reported by Buck et al14 who found that among initial surgical overcorrections, defined as any manifest esotropia at distance or near, 40% (10 of 25) remained at 2 years. However, direct comparison to our study is difficult because of differences in surgical doses and follow-up periods, and the lack of randomization in the Buck et al study.
The strengths of this study include the large sample size, the relatively long follow-up, a pre-defined masked outcome measure, and its multi-center design. Our study also has several limitations. Our secondary outcome analyses (suboptimal surgical outcome at 3 years, reoperation, and complete or near-complete resolution) were all subject to investigator bias, because reoperation was at investigator discretion once suboptimal surgical outcome criteria were met, and the previous type of surgical procedure may have impacted the decision to reoperate. An additional limitation of our secondary outcomes was that “complete or near-complete resolution” and the cause-specific suboptimal surgical outcomes were defined post hoc. Another limitation of the three cause-specific suboptimal surgical outcomes by 3 years is their non-independence because reoperation is a competing risk for each. The low overall rate of reoperation minimizes the impact of this lack of independence; however, because participants in the BLRc group were slightly more likely to be censored due to reoperation, we may have slightly underestimated the outcome rates in this group.
We did not find a statistically significant difference in the suboptimal surgical outcome rate by 3 years between children treated with BLRc compared with R&R. Our results reasonably rule out a moderately-sized benefit in favor of BLRc but cannot rule out the possibility of a moderately-sized benefit in favor of R&R. Nevertheless, based on our findings, we are unable to recommend one surgical approach over the other. Although the 3-year postoperative results reported here were obtained from over 80% of enrolled participants and represent the longest-term follow-up for a prospective randomized cohort of children with IXT who received surgery, 3 years of follow-up is a relatively short period of time for assessing treatment of IXT. Accordingly, an extension study has begun that will follow these children for an additional 5 years.
Supplementary Material
Figure 1. Flow of Participants through Study.
BLRc = bilateral lateral rectus muscle recession; R&R = unilateral lateral rectus recession combined with a medial rectus resection in same eye. *One participant in the R&R group was withdrawn from the study without undergoing surgery.
Table 6.
Baseline Ocular Alignment by Treatment Group
| Distance | Near | Remote Distance | Largest Angle by Remote Distance, Distance or Neara |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Exotropia Magnitude by PACT | BLRc (N=101) |
R&R (N=96) |
BLRc (N=101) |
R&R (N=96) |
BLRc (N=101) |
R&R (N=96) |
BLRc (N=101) |
R&R (N=96) |
||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| 10-14Δ | 0 | 0 | 0 | 0 | 10 | 10 | 12 | 13 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| 15-18Δ | 12 | 12 | 7 | 7 | 16 | 16 | 15 | 16 | 9 | 9 | 5 | 5 | 7 | 7 | 4 | 4 |
| 20-25Δ | 55 | 54 | 50 | 52 | 38 | 38 | 33 | 34 | 45 | 45 | 41 | 43 | 50 | 50 | 45 | 47 |
| 30-35Δ | 29 | 29 | 38 | 40 | 31 | 31 | 34 | 35 | 38 | 38 | 45 | 47 | 35 | 35 | 43 | 45 |
| 40Δ | 5 | 5 | 1 | 1 | 6 | 6 | 2 | 2 | 9 | 9 | 4 | 4 | 9 | 9 | 4 | 4 |
| Mean (SD) | 26 (6) | 26 (6) | 25 (8) | 24 (8) | 28 (6) | 27 (6) | 28 (6) | 27 (6) | ||||||||
| Range | 16 to 40 | 16 to 40 | 10 to 40 | 10 to 40 | 16 to 40 | 14 to 40 | 18 to 40 | 18 to 40 | ||||||||
BLRc= bilateral lateral rectus muscle recession; R&R = unilateral lateral rectus recession combined with a medial rectus resection in same eye
Surgical dose was based on the largest angle at remote distance, distance, or near.
SD = standard deviation; PACT = prism and alternate cover test; Δ = prism diopter
Acknowledgements
Research reported in this publication was supported by the National Eye Institute of the National Institutes of Health, under Award Numbers EY011751 and EY018810. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding/Support: Supported by National Eye Institute of National Institutes of Health, Department of Health and Human Services EY011751, EY023198, and EY018810. The funding organization had no role in the design or conduct of this research.
Writing Committee:
Sean P. Donahue, MD, PhD1; Danielle L. Chandler, MSPH2; Jonathan M. Holmes, BM, BCh 3 ; Brian W. Arthur, MD4 ; Evelyn A. Paysse, MD5 ; David K. Wallace, MD, MPH6 ; David B. Petersen, MD7 ; B. Michele Melia, ScM2 ; Raymond T. Kraker, MSPH2 Aaron M. Miller, MD8
1Vanderbilt University, Nashville, TN
2Jaeb Center for Health Research, Tampa, FL
3Mayo Clinic, Rochester, MN
4Children’s Eye Research Center, Kingston, ON, Canada
5Texas Children’s Hospital, Houston, TX
6Indiana University, Indianapolis, IN
7Rocky Mountain Eye Care Associates, Salt Lake City, UT
8Houston Eye Associates, Houston, TX
Clinical Sites
Sites are ordered by number of participants enrolled. Personnel are listed as (I) for Investigator, (C) for Coordinator or (E) for Examiner.
*Center received support utilized for this project from an unrestricted grant from Research to Prevent Blindness Inc., New York, New York.
† Center received grant support from the National Institutes of Health award number P30EY10572
The Woodlands, TX - Houston Eye Associates (38)
Aaron M. Miller (I); Jorie L. Jackson (C); Luis Arguijo (E); Kathleen M. Curtin (E); Angela C. Dillon (E); Suzanne S. LaRiviere (E); Maria N. Olvera (E); Cynthia R. Ramos (E); Leslie E. Robertson (E); Jay S. South (E)
Houston, TX - Texas Children’s Hospital - Department of Ophthalmology (31)
Evelyn A. Paysse (I); David K. Coats (I); Kimberly G. Yen (I); Gihan Romany (C); Amit R. Bhatt (E); Jane C. Edmond (E); Jane C. Edmond (E); Melynda J. Homann (E); Mohamed A. Hussein (E); Lingkun Kong (E); Paul G. Steinkuller (E)
Norfolk, VA - Virginia Pediatric Eye Center (18)
Earl R. Crouch, Jr. (I); Earl R. Crouch III (I); Gaylord G. Ventura (C)
Erie, PA - Pediatric Ophthalmology of Erie (15)
Nicholas A. Sala (I); Allyson Sala (C); Rhonda M. Hodde (E); Catherine Johnson (E); Jeanine M. Romeo (E); V. Lori Zeto (E)
Nashville, TN - Vanderbilt University Medical Center (15)*
Sean P. Donahue (I); Robert L. Estes (I); David G. Morrison (I); Scott T. Ruark (C); Ronald J. Biernacki (E); Megan K. Campbell (E); Lisa A. Fraine (E); Neva J. Fukuda (E); Kelsie J. Haskins (E); Jessica M. Kane (E)
Kingston, ON, Canada - Children’s Eye Research Center (12)
Brian W. Arthur (I); Christine Law (I); Lesley E. MacSween (C); Sarah D. Churchill (C)
Cranberry TWP, PA - Everett and Hurite Ophthalmic Association (11)
Darren L. Hoover (I); Pamela A. Huston (C); Kari E. Soros (C)
Salt Lake City, UT - Rocky Mountain Eye Care Associates (11)
David B. Petersen (I); Tori S. Pickens (C); J. R. McMurtrey (E); Beth A. Morrell (E)
Portland, OR - Casey Eye Institute (10)*,†
Daniel J. Karr (I); Allison I. Summers (I); Annie F. Kuo (I); Lorri B. Wilson (I); Paula K. Rauch (C); Pamela H. Berg (E); Yelena M. Bubnov (E); Grant A. Casey (E); Dusty S. Gronemyer (E); Laura M. Lenius (E); Srianna Narain (E); Rhea N. Nelson (E); Albert Romo (E); Ann U. Stout (E); Kevin M. Woodruff (E)
Chicago Ridge, IL - The Eye Specialists Center, LLC (9)
Benjamin H. Ticho (I); Deborah A. Clausius (C); Megan Allen (E); Sharon L. Giers (E); Lindsay A. Horan (E); Birva K. Shah (E)
Minneapolis, MN - University of Minnesota-Minnesota Lions Children’s Eye Clinic (9)
Raymond G. Areaux (I); Jill S. Anderson (I); Erick D. Bothun (I); Ann M. Holleschau (C); Kathy M. Hogue (E); Andrea M. Kramer (E); Kim S. Merrill (E); Anna I. Schweigert (E)
Mayfield Heights, OH - Rainbow Babies and Children’s Hospital Department of Ophthalmology (8)
Faruk H. Orge (I); Adriana P. Grigorian (I); LeslieRichards (C); Alicia M. Baird (E); Veronica M. Bontempo (E); Beth J. Colon (E); Florin Grigorian (E); Nina Mar (E); Sara E. Schoeck (E)
Lancaster, PA - Conestoga Eye (7)
David I. Silbert (I); Heather Modjesky (C); Linda K. Gehman (E); Garry L. Leckemby (E); Prucilla R. Shady (E)
Poland, OH - Eye Care Associates, Inc. (7)
S. Ayse Erzurum (I); Veronica Plessinger (C); Guy C. Barrett (E); Beth J. Colon (E); Zainab Dinani (E); Diana McOwen (E)
Baltimore, MD - Wilmer Institute (6)
Michael X. Repka (I); Hee-Jung S. Park (I); Xiaonong Liu (C); Alex Christoff (E); Carole R. Goodman (E)
Charleston, SC - Medical University of South Carolina, Storm Eye Institute (5)
Edward W. Cheeseman (I); Mae M. Peterseim (I); Carol U. Bradham (C); Richard A. Saunders (E); Ronald W. Teed (E)
Kansas City, MO – Children’s Mercy Hospitals and Clinics (5)
Justin D. Marsh (I); Amy L. Waters (I); Michelle M. Ariss (I); Rebecca J. Dent (C); Cindy J. Cline (E); Adriana P. Grigorian (E)
Little Rock, AR - Arkansas Children’s Hospital/ University of Arkansas Medical Sciences (5)
Robert S. Lowery (I); Paul H. Phillips (I); Shawn L. Cupit (C); Kelly D. To (C); Lynsey R. Tolleson (E)
Rochester, MN - Mayo Clinic (5)*
Jonathan M. Holmes (I); Brian G. Mohney (I); Suzanne M. Wernimont (C); Sarah R. Hatt (E); Julie A. Holmquist (E); Virginia X. Karlsson (E); Lindsay D. Klaehn (E); Rose M. Kroening (E); Laura Liebermann (E); Rebecca A. Nielsen (E); Debbie M. Priebe (E); Laura M. Taylor (E); Emily J. Treichel (E)
Rockville, MD - Stephen R. Glaser, MD, PC, Kids Eye Care of Maryland (5)
Stephen R. Glaser (I); Kasey L. Yost (C); Deandra B. Andrade (E); Odalis R. Flores (E); Laura L. Graham (E); Tara J. Guretzky (E); Nancy A. Morrison (E); Monica M. Pacheco (E); Aliza C. Shabanowitz (E)
Aberdeen, NC - Family Eye Care of the Carolinas (4)
Michael J. Bartiss (I); Tennille F. McGaw (C); Leah M. Kelly (E); Keith P. Poindexter (E)
Durham, NC - Duke University Eye Center (4)
Laura B. Enyedi (I); Robert J. House (C); Lois B. Duncan (E); Sandra Holgado (E); Sarah K. Jones (E); Namita Kashyap (E); Amanda P. Mestler (E); Tanya A. Plekan (E); Yos M. Priestley (E)
Baltimore, MD - Greater Baltimore Medical Center (3)
Mary Louise Z. Collins (I); Allison A. Jensen (I); Maureen A. Flanagan (C); Saman Bhatti (E); Kelsey A. Black (E); Cheryl L. McCarus (E); Srianna Narain (E)
Cleveland, OH - Cole Eye Institute (3)
Elias Traboulsi (I); Fatema F. Ghasia (I); Paul J. Rychwalski (I); Angela M. Borer (C); Susan W. Crowe (E)
Providence, RI - Pediatric Ophthalmology and Strabismus Associates (3)
David R. Tien (I); Tineke L. Chan (E); Samantha J. Garner (E); Shannon M. Lees (E); Myra B. McGuinness (E); Natalie T. Pepper (E); Gi H. Yoon-Huang (E)
Seattle, WA - Seattle Children’s Hospital (3)*
Erin P. Herlihy (I); Lyndsey A. Tews (C); Jennifer L. Brady (E); Amy Gladstone (E)
Spokane, WA - Northwest Pediatric Ophthalmology, P.S. (3)
George F. Whitehead (I); Christina N. Nye (I); Caroline J. Shea (I); SueAnn M. Stillman (C); Vicki P. Eglet (E)
Chicago, IL - Ann & Robert H. Lurie Children’s Hospital of Chicago (2)
Bahram Rahmani (I); Hantamalala Ralay Ranaivo (C); Bridget P. Farrell (E); Dina M. Johnson (E); Anthony J. Klauer (E); Kristyn M. Magwire (E); Erika A. Talip (E); Vivian Tzanetakos (E)
Madison, WI - University of Wisconsin, University Station (2)
Yasmin S. Bradfield (I); Angela M. Adler (C); Kristin A. Anderson (E); Gail V. Morton (E)
Atlanta, GA - The Emory Eye Center (1)*
Amy K. Hutchinson (I); Jason H. Peragallo (I); Scott R. Lambert (I); Judy L. Brower (C); Natario L. Couser (E); Marla J. Shainberg (E)
Indianapolis, IN - Riley Hospital for Children (1)
Kathryn M. Haider (I); Daniel E. Neely (I); Michele E. Whitaker (C); Dana L. Donaldson (E); Jay G. Galli (E); Jingyun Wang (E)
Lisle, IL - Progressive Eye Care (1)
Patricia L. Davis (I); Kathy A. Anderson (E); Carrie S. Bloomquist (E); Indre M. Rudaitis (E); Jackie M. Twite (E)
New York, NY - Mount Sinai School of Medicine (1)
Tamiesha A. Frempong (I); Edward L. Raab (E); Nalini Rambharose (E); Jennifer E. Williamson (E)
Philadelphia, PA - Children's Hospital of Philadelphia (1)
Brian J. Forbes (I); Gil Binenbaum (I); Meg M. Richter (C); Christopher B. Garvin (E); Boram Hong (E); Karen A. Karp (E); Malinda A. News (E)
Waterbury, CT - Eye Care Group, PC (1)
Tara H. Cronin (I); Andrew J. Levada (I); Susan H. Heaton (C); MaryJane J. Abrams (E); Cheryl Capobianco (E)
PEDIG Coordinating Center - Tampa, FL
Raymond T. Kraker, Roy W. Beck, Darrell S. Austin, Nicole M. Boyle, Courtney L. Conner, Danielle L. Chandler, Patricia Connelly, Trevano W. Dean, Quayleen Donahue, Brooke P. Fimbel, Graham M. Hardt, James E. Hoepner, Joseph D. Kaplon, Elizabeth L. Lazar, B. Michele Melia, Gillaine Alvarez, Diana E. Rojas, Jennifer A. Shah, Rui Wu.
Intermittent Exotropia Study Planning Committee
Jonathan M. Holmes (Planning Committee Chair), Sean P. Donahue (Protocol Chair, Intermittent Exotropia Study 1), Susan A. Cotter (Protocol Co-Chair, Intermittent Exotropia Study 2), Brian G. Mohney (Protocol Co-Chair, Intermittent Exotropia Study 2), Roy W. Beck, Eileen E. Birch, Danielle L. Chandler, Stephen P. Christiansen, Sarah R. Hatt, Raymond T. Kraker, David A. Leske, Michele Melia Mary O’Hara, Yi Pang, Michael X. Repka, Kenneth Romanchuck, Susanna M. Tamkins, David K. Wallace, David T. Wheeler
Strabismus Steering Committee
Eileen E. Birch, Danielle L. Chandler, Angela M. Chen (2016-present), Stephen P. Christiansen (2012-15), Susan A. Cotter, Eric R. Crouch (2015-present), Sean P. Donahue (2010-present), S. Ayse Erzurum (2016-present), Caroline C. Fang (2011-12), Sarah R. Hatt, Jonathan M. Holmes, Darren L. Hoover (2011-14), Lingkun Kong (2012-14), Raymond T. Kraker, Elizabeth L. Lazar, Ingryd Lorenzana (2014 - 2015), J. Ryan McMurtrey (2011-12), Michele Melia, Brian G. Mohney, Reena Patel (2016-2017), Karen Pollack (2014-2015), Michael X. Repka, Allyson Sala (2016-2017), Mitchell M. Scheiman (2011-14), Rosanne Superstein, Susanna M. Tamkins (2010-14), Tomohiko Yamada (2015-2016)
PEDIG Executive Committee
David K. Wallace (chair), William F. Astle (2013-15), Roy W. Beck, Eileen E. Birch, Angela M. Chen (2012-14, 2017-present), Melanie L. Christian (2012-14), Susan A. Cotter (2011-14, 2015-present), Earl R. Crouch Jr.(2012-14), Eric R. Crouch (2014-2015), Sean P. Donahue (2012-14), Laura B. Enyedi (2011-1, 2014-16), S. Ayse Erzurum (2016-17), Donald F. Everett, Sharon F. Freedman (2016-present), William V. Good, (2017-presesnt), Jonathan M. Holmes, Darren L. Hoover (2011-13), Jorie L. Jackson (2011-2012), Raymond T. Kraker, Scott R. Lambert (2013-15), Katherine A. Lee (2014-16), Vivian M. Manh (2016-present), Ruth E. Manny (2013-present), Aaron M. Miller (2011-2012), David B. Petersen (2011-12), Michael X. Repka, David L. Rogers (2011-13), Bonita R. Schweinler (2016-present), Jayne L. Silver (2014-16), Katherine K. Weise (2014-16), Lisa C. Verderber (2015-2017)
National Eye Institute - Bethesda, MD
Donald F. Everett
Data and Safety Monitoring Committee
Marie Diener-West (chair), John D. Baker, Barry Davis, Dale L. Phelps, Stephen W. Poff, Richard A. Saunders, Lawrence Tychsen
Footnotes
This article contains online-only material. The following should appear online-only: Tables S2, S9, S11a, S11b and S14.
Meeting Presentation: Content from this manuscript was presented at the April 2017 annual meeting of the American Association for Pediatric Ophthalmology and Strabismus in Nashville, TN.
An address for reprints will not be provided.
Conflict of Interest: No conflicting relationships exist for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Govindan M, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood exotropia: A population-based study. Ophthalmology 2005;112(1):104–8. [DOI] [PubMed] [Google Scholar]
- 2.Yamada T, Hatt SR, Leske DA, Holmes JM. Health-related quality of life in parents of children with intermittent exotropia. J AAPOS 2011;15:135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paysse EA, Steele EA, McCreery KM, et al. Age of the emergence of negative attitudes toward strabismus. J AAPOS 2001;5(6):361–6. [DOI] [PubMed] [Google Scholar]
- 4.von Noorden GK, Campos EC. Exodeviations In: Lampert R, Cox K, Burke D, eds. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. St. Louis CV Mosby, 2002. [Google Scholar]
- 5.Exotropia Wright K.. In: Wright KW, ed. Pediatric Ophthalmology and Strabismus. St Louis: Mosby Year Book, 1995. [Google Scholar]
- 6.Mitchell PR, Parks MM. Concomitant Exodeviations In: Tasman WS, ed. Duane's Clinical Ophthalmology. Philadelphia, PA: Lippincott Williams & Wilkins, 2000; v. 1. [Google Scholar]
- 7.Mohney BG, Holmes JM. An office-based scale for assessing control in intermittent exotropia. Strabismus 2006;14(3):147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatt SR, Leske DA, Yamada T, et al. Development and initial validation of quality-of-life questionnaires for intermittent exotropia. Ophthalmology 2010;117(1):163–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leske DA, Holmes JM, Melia M, on behalf of Pediatric Eye Disease Investigator Group. Evaluation of the Intermittent Exotropia Questionnaire using Rasch analysis. JAMA Ophthalmol 2015;133(4):461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leske DA, Hatt SR, Liebermann L, Holmes JM. Lookup Tables Versus Stacked Rasch Analysis in Comparing Pre- and Postintervention Adult Strabismus-20 Data. Transl Vis Sci Technol 2016;5(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med 1990;9(12):1447–54. [DOI] [PubMed] [Google Scholar]
- 12.Lee SY, Hyun Kim J, Thacker NM. Augmented bilateral lateral rectus recessions in basic intermittent exotropia. J AAPOS 2007;11(3):266–8. [DOI] [PubMed] [Google Scholar]
- 13.Kushner BJ. Selective surgery for intermittent exotropia based on distance/near differences. Arch Ophthalmol 1998;116(3):324–8. [DOI] [PubMed] [Google Scholar]
- 14.Buck D, Powell CJ, Sloper JJ, et al. Surgical intervention in childhood intermittent exotropia: current practice and clinical outcomes from an observational cohort study. Br J Ophthalmol 2012;96(10):1291–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.