Abstract
Despite the growing awareness regarding lutein’s putative roles in eyes and brain, its pharmacokinetics and tissue distribution in primates has been poorly understood. We hypothesized that 13C-lutein will be differentially distributed into tissues of an adult rhesus macaque (Macaca mulatta) three days following a single oral dose. After a year of pre-feeding a diet supplemented with unlabeled lutein (1 μmol/kg/d), a 19-year-old female was dosed with 1.92 mg of highly enriched 13C-lutein. Tissues of a non-dosed, lutein-fed monkey was used as a reference for natural abundance of 13C-lutein. On the third day post-dose, plasma and multiple tissues were collected. Lutein was quantified by HPLC-PDA and 13C-lutein tissue enrichment was determined by LC-Q-TOF-MS. In the tissues of a reference monkey, 12C-lutein with natural abundance of 13C-lutein was detectable. In the dosed monkey, highly enriched 13C-lutein was observed in all analyzed tissues except for the macular and peripheral retina, with the highest concentrations in the liver, followed by adrenal gland, and plasma. 13C-lutein accumulated differentially across six brain regions. In adipose depots, 13C-lutein was observed, with the highest concentrations in the axillary brown adipose tissues. In summary, we evaluated 13C-lutein tissue distribution in a non-human primate following a single dose of isotopically labeled lutein. These results show that tissue distribution 3 days following a dose of lutein varied substantially dependent on tissue type.
Keywords: lutein, biodistribution, rhesus macaque, isotopic tracer, mass spectrometry
1. Introduction
Lutein, a 40-carbon oxygenated carotenoid, cannot be endogenously synthesized in mammals. Thus, the amount of lutein in the body is determined by dietary sources including dark green leafy vegetables, fruits, and yellow and orange egg yolks. Lutein and its isomer zeaxanthin selectively accumulate in the macular region of the primate retina to form yellow coloration (termed macular pigment) and to protect the retina against harmful blue light and oxidative stress [1]. Epidemiological studies show that the consumption of lutein and zeaxanthin is strongly associated with a reduced risk of age-related macular degeneration, a leading cause of irreversible blindness in the United States [2, 3]. Recently, it has been suggested that lutein may also play a role in brain function. Lutein is the predominant carotenoid in neural tissues of both infants and older adults even though it’s not the major carotenoid in the US diet [4, 5]. When comparing dietary intake to brain function, several studies have reported associations between lutein and zeaxanthin supplementation and enhanced cognitive function in adults [6–8].
Despite growing interest in lutein, there has been limited research on its pharmacokinetics and tissue distribution in humans or nonhuman primates. Since it is difficult to completely deplete lutein in the body, especially in the retina [9], the use of isotopically labeled lutein is a good strategy to investigate its bioavailability and metabolism in vivo. Although a few studies have examined the time course of plasma responses to isotopically labeled lutein in human subjects, it is not possible in humans to obtain detailed information on tissue uptake [10, 11]. Previously, we established a carrot cell suspension culture system to produce enriched 13C-lutein and demonstrated proof-of-principle of tissue uptake through subsequent dosing of an adult female rhesus macaque [12]. Expanding on that work, this pilot study investigated how 13C-lutein was distributed into tissues of the adult rhesus macaque three days following a single oral dose. We hypothesized that 13C-lutein signals would be different across tissues.
2. Methods and Materials
2.1. Animals and Diet
Animal care and in vivo dosing were performed at the Oregon National Primate Research Center at Oregon Health and Science University. All procedures in this study were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A female, 19-year-old rhesus macaque was fed a standard laboratory diet (Monkey Diet Jumbo, LabDiet) (Table 1), supplemented daily with 570μg/kg diet/day of unlabeled lutein (FloraGloR beadlets, 5% lutein, DSM) mixed into various treats such as marshmallow, peanut butter and chocolate for the previous 12 months. The animal was anesthetized with ketamine (10 mg/kg) and dosed with 1.92 mg of 13C-lutein solubilized in a mixture of monoglyceride and diglyceride oil (Abbott Nutrition) via orogastric intubation. The bioproduction, purification, and analysis of the 13C-lutein dose and details of the dosing procedure were described previously [12]. In the lutein dose, the extent of labelling with 13C was as follows: all 40 carbons were labelled in 64.7 ± 0.9% of lutein molecules, 39 carbons in 27.0 ± 0.4%, and 38 carbons in 8.3 ± 0.9%. Three days after oral dosing and after an overnight fast, the monkey was humanely euthanized by a veterinary pathologist under deep pentobarbital anesthesia. Blood was collected in tubes containing ethylenediaminetetraacetic acid (EDTA) and processed to obtain plasma, and tissues were collected, snap-frozen in liquid nitrogen, and stored at −80° C until analysis. Tissues collected included samples of liver, heart, kidney, adrenal gland and quadriceps; adipose tissue from four sites (mesenteric, subcutaneous from abdomen and thigh, and brown axillary adipose tissues); samples of 6 brain regions (occipital, parietal and temporal cortex, medulla, pons, and subcortical white matter); a 4 mm punch of the macular retina, centered on the fovea; and peripheral retina. Tissues of a non-dosed monkey were used as a reference to determine the natural abundance of 13C-lutein. Throughout the text, 13C-lutein stands for the highly enriched 13C-lutein. Reference tissues were obtained from a male, 180-day-old rhesus macaque, who consumed unlabeled lutein via breast milk and supplemental foods for six months. Analytical measurements were conducted in accordance with and approved by University of Illinois at Urbana-Champaign institutional biosafety committee project.
Table 1.
Macronutrient composition of the diet1 (Monkey Diet Jumbo) fed to rhesus macaque monkeys
| Standard laboratory diet | |
|---|---|
| Protein, g/kg | 156 |
| Fat (ether extract), g/kg | 50 |
| Carbohydrate (nitrogen-free extract), g/kg | 600 |
| Fiber (crude), g/kg | 42 |
| Minerals2, g/kg | 52 |
| Vitamins3 | - |
| Gross energy, kcal/g | 4.08 |
Provided by Labdiet and the moisture content was 100g/kg.
Minerals: calcium, 9.0 g/kg; phosphorus, 9.3 g/kg; potassium, 7.5 g/kg; magnesium, 1.8 g/kg; sulfur, 2.4 g/kg; sodium, 2.5 g/kg; chloride, 3.7 g/kg; fluorine, 19 ppm; iron, 220 ppm; zinc, 110 ppm; manganese, 97 ppm; copper, 21 ppm; cobalt, 0.53 ppm, iodine, 1.3 ppm; chromium, 0.01 ppm, selenium, 0.37 ppm.
Vitamins: carotene, 1.7 ppm; vitamin K, 3.2 ppm; thiamin hydrochloride, 8.3 ppm; riboflavin, 8.6 ppm; niacin, 113 ppm; pantothenic acid, 60 ppm; choline chloride, 1200 ppm; folic acid, 7.9 ppm, pyridoxine, 14 ppm; biotin, 0.1 ppm; B12, 73 ppm; vitamin A, 20 IU/g, vitamin D3, 6.7 IU/g, vitamin E, 110 IU/kg, ascorbic acid, 0.5g/kg.
2.2. Purification and quantification of lutein from monkey tissues
Carotenoids were extracted in replicates with methods depending on the tissue type as previously described [13]. Each tissue extract was reconstituted in 40 μL of ethanol/methyl tert-butyl ether mixture (1:1, by vol) and injected onto an Alliance HPLC system (e2695 Separation Module) equipped with a 2998 photodiode array detector (PDA) (Waters). The extract was separated on a reverse-phase C30 column (4.6 × 150 mm, 3 μm; YMC) maintained at 18 °C. A phase gradient method used for carotenoid separation was based on the method of Yeum et al. [14]. Total lutein was identified via absorption spectrum, retention time, and standard comparison, and quantified by an external standard curve method. The lutein fraction was collected, aliquoted, and solvents were evaporated under argon.
2.3. Mass spectrometry
The dried lutein fraction was reconstituted in LC mobile phase (acetonitrile/methanol/10mM ammonium acetate=539:441:20, by vol; pH=4.5) prior to analysis. All samples were analyzed on a system consisting of a Thermo UltiMate 3000 UHPLC, a Bruker atmospheric pressure chemical ionization (APCI) II source, and a Bruker maXis 4G quadrupole time-of-flight mass spectrometer (Q-TOF-MS). The LC separation involved 30 min of isocratic elution at 0.3 mL/min flow rate at 20 °C on a Thermo Acclaim C30 column (3 μm, 2.1 × 150 mm), connected after a Thermo Acclaim C30 guard cartridge (5 μm, 2.1 × 10 mm). The operation parameters for the APCI source included: capillary 3500 V, corona 7000 nA, nebulizer 2 bar, dry gas 4 L/min, dry temperature 200 °C, vaporizer temperature 400 °C. Tandem MS analysis was performed for structure confirmation of target analytes, and high resolution and mass accuracy MS measurements were conducted for determination of 12C-lutein/ 13C-lutein ratios. Mass spectrometer calibration was performed with syringe infusion of the APCI/APPI calibrant solution (Fluka) before each sample run. Due to the low amount of 13C-lutein in adipose and brain tissues, the lutein fractions from 2–4 tissue extract replicates were combined and used for 13C-lutein detection.
2.4. Statistical analyses
All data were presented as mean from 1–4 replicates. Repeatability of measurements was shown as relative standard deviation (RSD, %) = standard deviation/mean × 100%.
3. Results
3.1. Accumulation of total lutein in rhesus macaque tissues
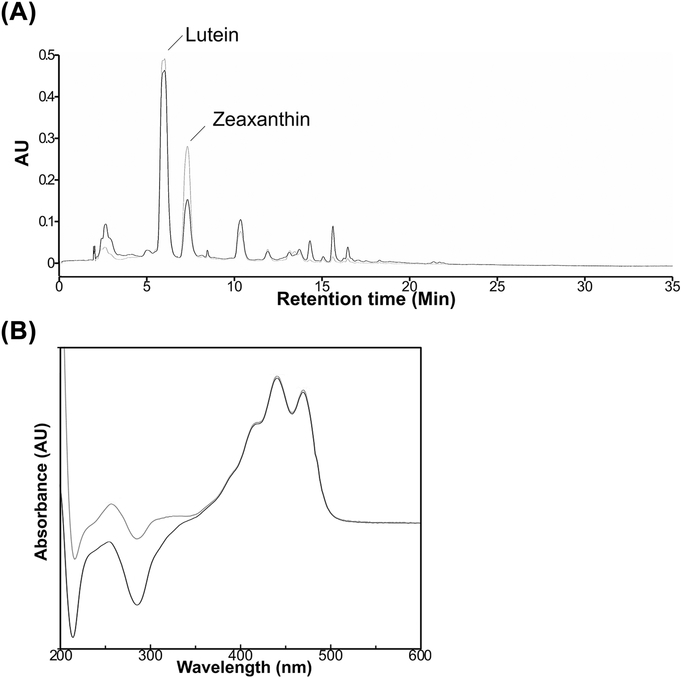
Total lutein (unlabeled + 13C-labeled) in monkey tissues was extracted and separated by HPLC-PDA. Fig. 1 depicts representative HPLC chromatograms of liver extracts of the monkey dosed with 13C-lutein and the reference monkey obtained at 445 nm as well as the ultraviolet/visible absorbance spectrum of lutein. The retention time and absorbance spectrum of lutein were identical between liver extracts of the dosed and the reference animals. Total lutein was detectable in all tissues examined and was differentially distributed depending on tissue type. Total lutein was most concentrated in the macular retina (5897 pmol/g), followed by adrenal gland and liver (3403 and 2677 pmol/g, respectively; Table 2).
Fig. 1.
HPLC chromatogram (A) and absorbance spectrum (B) of lutein peaks from liver extracts of an adult monkey dosed with 13C-lutein (Black) and a reference monkey (Grey).
Table 2.
Lutein isotopomer profiles in the multiple tissues of a monkey dosed with 13C-lutein1
| Organ | Total lutein [pmol/g] | Lutein Monoisotopic Peak Intensities Ratios |
13C-lutein [pmol/g] | ||||
|---|---|---|---|---|---|---|---|
| 13C40/12C40 | 13C3912C / 1240 | 13C38 12C2 /12C40 | 13C / 12C40 | ||||
| Plasma | 2762 | 7.07% | 3.23% | 0.96% | 11.3% | 27.92 | |
| Liver | 2677 | 6.56% | 3.12% | 0.92% | 10.6% | 257 | |
| Heart | 148 | 4.24% | 2.06% | 0.61% | 6.91% | 9.43 | |
| Kidney | 160 | 4.18% | 1.84% | 0.53% | 6.54% | 9.79 | |
| Adrenal gland | 3403 | 2.13% | 1.04% | 0.29% | 3.46% | 114 | |
| Quadriceps | 70.4 | 1.09% | 0.36% | nd3 | 1.45% | 0.74 | |
| Adipose | BAT | 250 | 0.58% | 0.15% | 0.04% | 0.76% | 1.94 |
| MAT | 191 | 0.17% | nd | nd | 0.17% | 0.32 | |
| ASAT | 222 | 0.26% | 0.06% | nd | 0.32% | 0.74 | |
| TSAT | 110 | 0.10% | nd | nd | 0.10% | 0.11 | |
| Brain | Temporal cortex | 88.2 | 1.77% | 0.64% | nd | 2.41% | 2.08 |
| Occipital cortex | 115 | 1.34% | 0.71% | 0.14% | 2.20% | 2.47 | |
| Parietal cortex | 71.3 | 1.29% | 0.56% | nd | 1.85% | 1.58 | |
| Medulla | 28.9 | 1.00% | nd | nd | 1.00% | 0.29 | |
| Pons | 33.6 | 0.88% | nd | nd | 0.88% | 0.29 | |
| SWM | 48.3 | 0.64% | nd | nd | 0.64% | 0.33 | |
| Eye | Macular retina | 5897 | nd | nd | nd | nd | nd |
| Peripheral retina | 9771 | nd | nd | nd | nd | nd | |
Data represent values from 1–4 replicates of tissue samples.
pmol/mL
nd = 13C-lutein not detected.
BAT, brown adipose tissue; MAT, mesenteric adipose tissue; ASAT, abdominal subcutaneous adipose tissue; TSAT, thigh subcutaneous adipose tissue; SWM, subcortical white matter.
3.2. 13C-lutein detection in rhesus macaque tissues
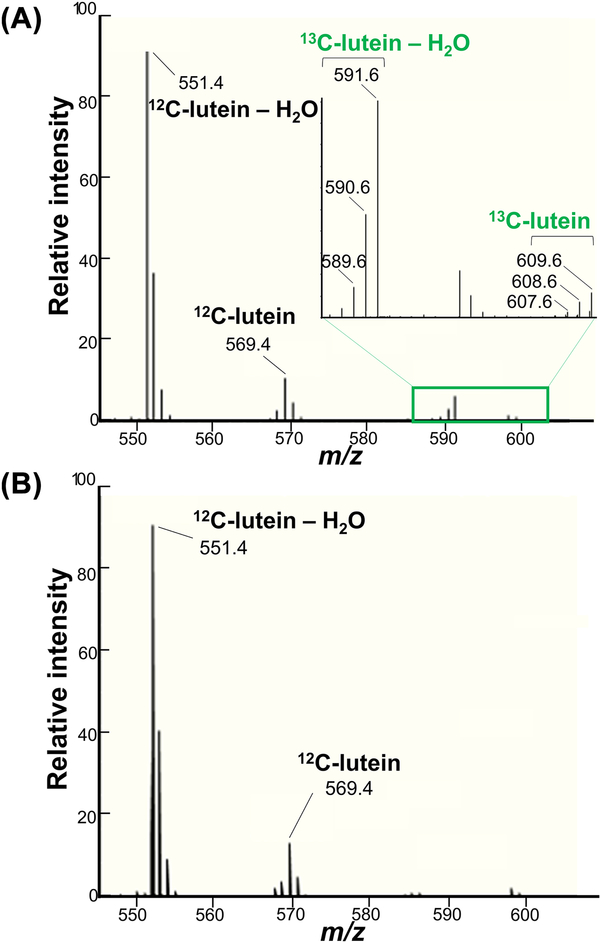
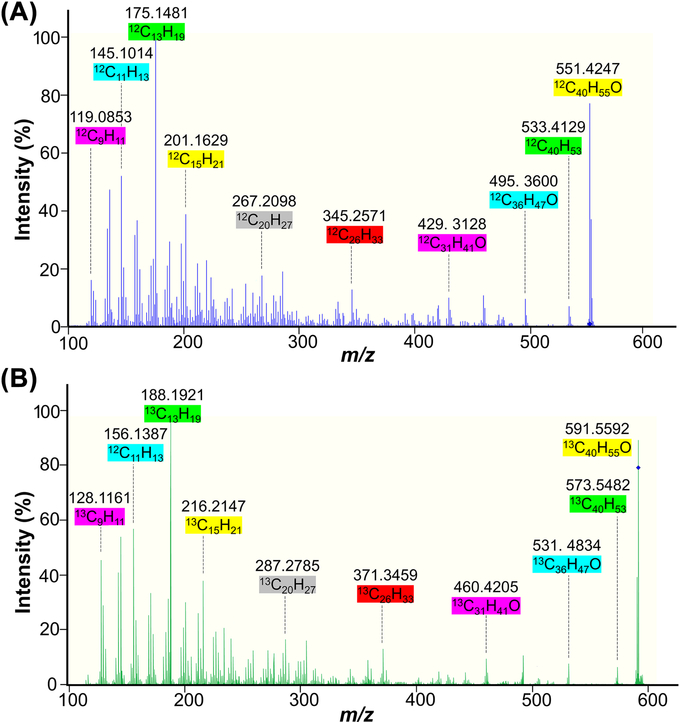
The total lutein concentrations and ratios of 13C-lutein and 12C-lutein in each tissue allow the determination of 13C-lutein concentrations. We compared our previous method [12], which utilized a triple quadrupole mass spectrometer operating in multiple reaction monitoring mode with a Q-TOF-MS instrument. The Q-TOF-MS was superior and enabled lutein measurement with high accuracy and monoisotopic signal detection (mass error < 5 ppm). The ratio of 12C-lutein and 13C-lutein was calculated from the intensities of corresponding [M–H2O+H]+ monoisotopic signals observed in the same mass spectrum (12C-lutein – H2O+H: m/z = 551.4253±0.0028 Da; 13C-lutein – H2O+H: m/z = 591.5593±0.0030 Da). Both 12C-lutein and 13C-lutein were observed in the dosed monkey liver, whereas 12C-lutein, but not 13C-lutein, was detectable in the reference monkey liver (Fig. 2). Measurements demonstrated good repeatability, with RSD ≤ 3.9% for triplicate evaluations of the liver (Supplemental Table S1). MS/MS analysis was also performed for structural confirmation of 12C-lutein and 13C-lutein detection. The representative MS/MS spectra are shown in Fig. 3. The matching of fragmentation patterns between unlabeled lutein standard (Fig. 3A) and the dosed monkey liver extract (Fig. 3B) confirmed that the m/z 591.5592 ion is a completely labeled 13C-lutein (13C40H56O) with the loss of one water molecule.
Fig. 2.
Mass spectra of the dosed monkey liver extract (A) and the reference monkey liver extract (B). The mass spectra were acquired during lutein elution from the column. Only the 545 to 610 region is presented.
Fig. 3.
Tandem mass spectra of the fragmentation of unlabeled standard of 12C-lutein (12C40H56O–H2O; m/z 551.4247) (A), and the 13C-lutein–H2O ion (m/z 591.5592) from the dosed monkey liver extract (B).
In the reference monkey, 12C-lutein, but not 13C-lutein, was detectable in all tissues examined. In contrast, 13C-lutein isotopomers were detected in multiple tissues of the 13C-dosed macaque, with the highest concentrations of 13C-lutein found in the liver (257 pmol/g), followed by adrenal gland (114 pmol/g), plasma (27.9 pmol/mL), kidney (5.57 pmol/g), and heart (5.36 pmol/g) (Table 2). As expected, lower signal intensity led to an increase in %RSD (Supplemental Tables S1-S3).
13C-lutein was differentially deposited across brain regions. Among six different brain regions examined (temporal cortex, occipital cortex, parietal cortex, medulla, pons, and subcortical white matter), the occipital cortex exhibited the highest 13C-lutein concentration (2.47 pmol/g), as well as the highest concentration of 12C-lutein. 13C-lutein also accumulated differentially among four different adipose depots, with the highest concentrations of 13C-lutein in axillary brown adipose tissue. 13C-lutein was undetectable in macular and peripheral retina samples, despite high concentrations of 12C-lutein. Except in samples with undetectable 13C-lutein isotopomers, the profile of 13C-lutein isotopomers found in tissues of the dosed monkey were similar to that in the dose, with the highest amount of uniformly labeled lutein, followed by lutein with 39 13C and 38 13C atoms, respectively. Information on 13C-lutein concentrations in tissues, organ weights, and estimated blood volume [15, 16] were used to estimate the % recovery of the 13C-lutein dose in selective tissues. The total recovery of the 13C-lutein dose from tissues (plasma, liver, heart, kidney, adrenal gland and brain) was 1.4%, with the majority being in the liver.
4. Discussion
Lutein preferentially accumulates in primate retina and brain, and this biological selectivity suggests critical or essential roles in these tissues. Long-term lutein supplementation leads to dose-dependent increases in serum lutein and macular pigment in human subjects [17]. In addition, we recently showed that the lutein deposition in multiple tissues of infant rhesus macaques, including retina and brain regions, varied in response to 6 months of breastfeeding or formula-feeding [18]. However, lutein pharmacokinetics are poorly understood. This pilot study is the first to demonstrate a determination of tissue bioaccumulation patterns of a single dose of 13C-lutein in an adult nonhuman primate.
We found that 1.4% of the highly enriched 13C-lutein dose was recovered in plasma and the measured tissues of the dosed rhesus monkey, while 13C-lutein was undetectable in any tissues of the reference monkey. Most of the consumed dose is assumed to be excreted via feces and urine, while additional dose accumulated in other unmeasured tissues. De Moura et al. reported that 45% of a 14C-lutein dose was excreted via feces and 10% via urine in an adult woman in the first 2 days following dosing [11]. That study also reported that 14C-lutein was absorbed and appeared in the bloodstream within one hour after consumption, reaching maximum blood levels at 14 hours after ingestion.
It is noteworthy that 13C-lutein was differentially distributed depending on tissue type, being particularly enriched in the liver and adrenal glands. Interestingly, after 12 months of unlabeled dietary lutein exposure, the adrenal glands accumulated higher concentrations of total lutein than the liver, whereas after tracer dosing, the ratio of 13C-lutein/12C-lutein (13C/12C) in the adrenal glands (3.5%) was relatively lower than in the liver (10.6%), heart (7.0%), and kidney (6.6%). It has been reported that the adrenal glands accumulate high concentrations of carotenoids in humans [19], possibly due to highly expressed LDL receptors and scavenger receptor class B type 1 (SR-B1), as well as a high rate of lipoprotein uptake [20–22]. Slow uptake, rapid metabolism, or enhanced export of lutein might explain the relatively low adrenal 13C/12C ratio. In the retina, no 13C-lutein was detected, even though 12C-lutein is highly concentrated, particularly in macular retina. This might be attributable to slow uptake and turnover of lutein in the retina, as suggested in previous studies in humans [23, 24]. Clearly, a limitation of this study is that only 1 dosed monkey was tested at one time point. Additional studies at other time points, including younger monkeys of both genders, are needed to clarify if these observations indicate differential kinetics of lutein tissue uptake, metabolism, and/or recycling into circulation.
This pilot study demonstrates that a single oral dose of 13C-lutein enabled the tissue distribution to be determined in a rhesus macaque. Our current work was able to detect 13C-lutein isotopomers in tissues of the dosed monkey by using LC-APCI-MS with high sensitivity, allowing determination of its absolute content. We provide evidence to support our hypothesis that 13C-lutein was differentially distributed across various tissues, including multiple brain regions; however, it was undetectable in the retina measured three days after a single dose. This suggests that distribution of lutein in the macaque is substantially dependent on tissue type. Follow-up studies are required to further investigate the pharmacokinetics of lutein.
Supplementary Material
Acknowledgment
We acknowledge the financial support provided by Abbott Nutrition through the Center for Nutrition, Learning and Memory, University of Illinois, Urbana-Champaign and NIH Grant P51OD011092. This work was funded by Abbott Nutrition and Matthew Kuchan is employed by Abbott Nutrition. No other authors have conflicts of interest to declare.
List of abbreviations:
- APCI
atmospheric pressure chemical ionization
- ASAT
abdominal subcutaneous adipose tissue
- BAT
brown adipose tissue
- MAT
mesenteric adipose tissue
- ND
not detected
- PDA
photodiode array detector
- Q-TOF-MS
quadrupole time-of-flight mass spectrometer
- RSD
relative standard deviation
- SWM
subcortical white matter
- TSAT
thigh subcutaneous adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- [1].Lutein Mares J. and zeaxanthin isomers in eye health and disease. Annu Rev Nutr 2016;36:571–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994;272:1413–20. [PubMed] [Google Scholar]
- [3].SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, Gensler G 3rd, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol 2007;125:1225–32. [DOI] [PubMed] [Google Scholar]
- [4].Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ. Lutein and preterm infants with decreased concentrations of brain carotenoids. J Pediatr Gastroenterol Nutr 2014;59:659–65. [DOI] [PubMed] [Google Scholar]
- [5].Johnson EJ, Vishwanathan R, Johnson MA, Hausman DB, Davey A, Scott TM, et al. Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J Aging Res 2013;2013:951786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bovier ER, Renzi LM, Hammond BR. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on neural processing speed and efficiency. PLoS One 2014;9:e108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bovier ER, Hammond BR. A randomized placebo-controlled study on the effects of lutein and zeaxanthin on visual processing speed in young healthy subjects. Arch Biochem Biophys 2015;572:54–7. [DOI] [PubMed] [Google Scholar]
- [8].Power R, Coen RF, Beatty S, Mulcahy R, Moran R, Stack J, et al. Supplemental retinal carotenoids enhance memory in healthy individuals with low levels of macular pigment in a randomized, double-blind, placebo-controlled clinical trial. J Alzheimers Dis 2018;61:947–61. [DOI] [PubMed] [Google Scholar]
- [9].Wang Y, Connor SL, Wang W, Johnson EJ, Connor WE. The selective retention of lutein, meso-zeaxanthin and zeaxanthin in the retina of chicks fed a xanthophyll-free diet. Exp Eye Res 2007;84:591–8. [DOI] [PubMed] [Google Scholar]
- [10].Yao L, Liang Y, Trahanovsky WS, Serfass RE, White WS. Use of a 13C tracer to quantify the plasma appearance of a physiological dose of lutein in humans. Lipids 2000;35:339–48. [DOI] [PubMed] [Google Scholar]
- [11].de Moura FF, Ho CC, Getachew G, Hickenbottom S, Clifford AJ. Kinetics of 14C distribution after tracer dose of 14C-lutein in an adult woman. Lipids 2005;40:1069–73. [DOI] [PubMed] [Google Scholar]
- [12].Smith JW, Rogers RB, Jeon S, Rubakhin SS, Wang L, Sweedler JV, et al. Carrot solution culture bioproduction of uniformly labeled 13C-lutein and in vivo dosing in nonhuman primates. Exp Biol Med 2017;242:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jeon S, Neuringer M, Johnson EE, Kuchan MJ, Pereira SL, Johnson EJ, et al. Effect of carotenoid supplemented formula on carotenoid bioaccumulation in tissues of infant rhesus macaques: a pilot study focused on lutein. Nutrients 2017;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, et al. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr 1996;64:594–602. [DOI] [PubMed] [Google Scholar]
- [15].Hobbs TR, Blue SW, Park BS, Greisel JJ, Conn PM, Pau FK. Measurement of blood volume in adult rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 2015;54:687–93. [PMC free article] [PubMed] [Google Scholar]
- [16].Summers L, Clingerman KJ, Yang X. Validation of a body condition scoring system in rhesus macaques (Macaca mulatta): assessment of body composition by using dualenergy X-ray absorptiometry. J Am Assoc Lab Anim Sci 2012;51:88–93. [PMC free article] [PubMed] [Google Scholar]
- [17].Bone RA, Landrum JT. Dose-dependent response of serum lutein and macular pigment optical density to supplementation with lutein esters. Arch Biochem Biophys 2010;504:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jeon S, Ranard KM, Neuringer M, Johnson EE, Renner L, Kuchan MJ, et al. Lutein Is differentially deposited across brain regions following formula or breast feeding of infant rhesus macaques. J Nutr 2018;148:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stahl W, Schwarz W, Sundquist AR, Sies H. cis-trans isomers of lycopene and beta-carotene in human serum and tissues. Arch Biochem Biophys 1992;294:173–7. [DOI] [PubMed] [Google Scholar]
- [20].Fong LG, Bonney E, Kosek JC, Cooper AD. Immunohistochemical localization of low density lipoprotein receptors in adrenal gland, liver, and intestine. J Clin Invest 1989;84:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996;271:518–20. [DOI] [PubMed] [Google Scholar]
- [22].Spady DK, Bilheimer DW, Dietschy JM. Rates of receptor-dependent and - independent low density lipoprotein uptake in the hamster. Proc Natl Acad Sci U S A 1983;80:3499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Johnson EJ, Hammond BR, Yeum K-J, Qin J, Wang XD, Castaneda C, et al. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr 2000;71:1555–62. [DOI] [PubMed] [Google Scholar]
- [24].Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr 2003;133:992–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.