Abstract
Background:
Patients who achieve ≥10 METS during exercise SPECT myocardial perfusion imaging (MPI) have very low rates of significant ischemia and major adverse cardiac events (MACE). It is unknown how many older adults can achieve ≥10 METS, and if low risk extends to this subgroup.
Methods and Results:
We examined the workload achieved, prevalence and predictors of ischemia, and MACE (cardiac death, nonfatal MI, late revascularization) in a cohort of 382 patients ≥65 years of age who underwent exercise 99mTc SPECT MPI. The cohort was 64.4% male and 36.9% had known coronary artery disease (CAD). All achieved ≥85% of maximum age-predicted heart rate. A workload of ≥10 METS was achieved in 25.4%; 50.3% attained 7–9 METS, and 24.4% reached <7 METS. There was a stepwise decrease in prevalence of any ischemia and significant ischemia (≥10% of the left ventricle (LV)) as workload increased (p=0.037). Patients achieving ≥10 METS had a 3.1% prevalence of ≥10% LV ischemia (1.2% in those without ST depression). Cardiac death and MACE rates in the ≥10 METS subgroup were 0.6%/year and 2.6%/year over a median 7.0 years of follow-up.
Conclusions:
A substantial proportion of older adults who undergo exercise SPECT MPI can achieve ≥10 METS. This subgroup has low rates of significant LV ischemia and MACE. The favorable diagnostic and prognostic implications of achieving a high workload in an older adult population suggest it is feasible, with certain exceptions, to include this subgroup in workload-based strategies of provisional imaging.
Keywords: Coronary artery disease, Exercise stress testing, Myocardial perfusion imaging: SPECT, Outcomes research
Exercise capacity is inversely associated with all-cause mortality and cardiovascular mortality, both in the general population and in older adults.(1–8) Among patients ≥65 years of age, attaining a high workload has been associated with a reduction in all-cause mortality and future cardiac events.(1) We previously reported 0% high-risk ischemia and low mortality and non-fatal myocardial infarction (MI) rates in a consecutive series of patients of all ages reaching ≥85% of maximum age-predicted heart rate (MAPHR) who achieved ≥10 METS with no ischemic ST segment depression.(9, 10) We concluded that exercise ECG stress testing may be sufficient in this cohort and proposed a provisional imaging protocol in which patients achieving ≥85% MAPHR and ≥10 METS without abnormal stress ECG or hemodynamic changes would not be injected with an imaging agent. Other investigators subsequently assessed such a provisional imaging protocol but excluded patients age ≥65 years.(11, 12)
As our original cohort had an inadequate sample size, we assembled a larger population to test the hypothesis that patients ≥65 years of age, achieving both ≥85% MAPHR and ≥10 METS of workload, would also demonstrate a low prevalence of high-risk ischemia and a low cardiac event rate, allowing them to qualify for a provisional imaging protocol with exercise stress testing.
Methods
Study Cohort
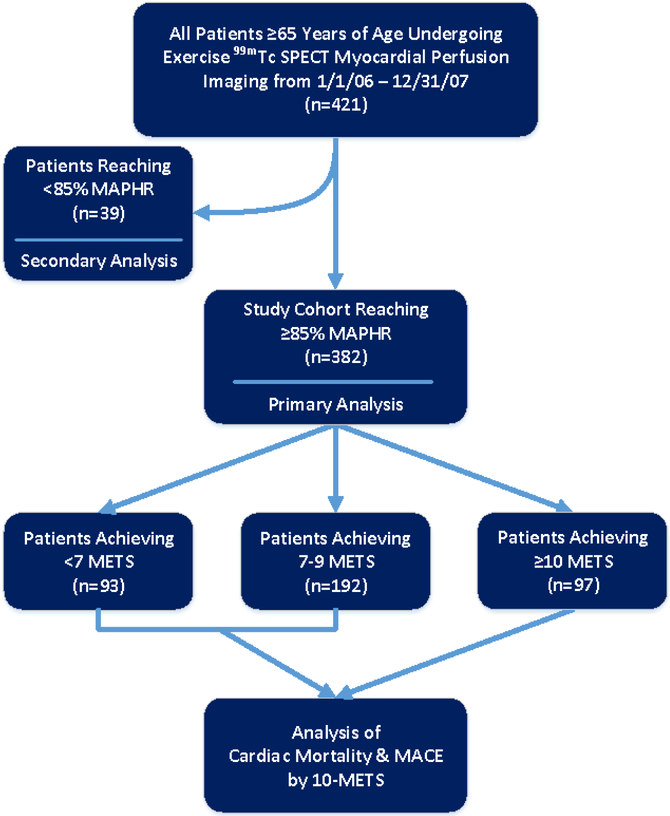
Prospectively collected data from the University of Virginia Nuclear Databank were analyzed in a cohort of consecutive patients who underwent exercise stress 99mTc SPECT MPI at the University of Virginia Health System between January 2006 and December 2007. The rate of exercise stress in this population was 39.4%. The study cohort for the primary analysis included 382 subjects who achieved ≥85% of their MAPHR. They were subdivided into 3 groups [<7 METs (n=93), 7 to 9 METs (n=192), and ≥10 METs (n=97)] as shown in Figure 1. The primary analysis was to determine the prevalence of a high exercise workload of ≥10 METs and the risk of high-risk LV ischemia (≥10%) at this workload in patients achieving a diagnostic stress test. Additional analysis on all-cause mortality and major adverse cardiac event (MACE) rates was performed in the subjects in the primary analysis cohort who had follow-up data available. A secondary analysis of the prevalence of any and significant ischemia was performed on 39 subjects who did not reach ≥85% of their MAPHR.
Figure 1.
Patient flow diagram showing derivation of the study cohort. The final study cohort for the primary analysis included 382 subjects. Additional analysis of cardiac mortality and major adverse cardiac events (MACE) was subsequently performed in those with follow-up available comparing those achieving < and ≥10 METS. A secondary analysis of the prevalence of positive ECG and ischemia was performed in the 39 subjects who achieved <85% MAPHR.
Clinical Information Collection and Management
Clinical information was collected from patients at the time of their exercise test and was entered into the University of Virginia Nuclear Databank. Exercise test parameters and SPECT results were also recorded.(13, 14) MACE, including nonfatal MI, revascularization, and cardiac death and all-cause mortality were identified thorough chart review and phone calls when needed. Protocol approval and waiver of informed consent were obtained from the University of Virginia Institutional Review Board.
Exercise Testing
All subjects underwent exercise treadmill stress with electrocardiographic monitoring using standard exercise protocols (93% Bruce or modified Bruce). Anti-ischemic medications were held at the discretion of the referring physician. Testing was symptom limited unless prematurely terminated as recommended in the exercise testing guidelines.(15) Exercise workload was defined as the total METs achieved.(16) Ischemic ST segment depression was defined as ≥1 mm horizontal or down-sloping depression of the ST-segment ≥80 ms after the J-point for 3 consecutive beats.
Radionuclide SPECT Imaging
99mTc sestamibi rest-stress, gated-SPECT MPI was performed with either a 1-day or 2-day protocol (for a body mass index ≥36 kg/m2) as described previously.(9) With the 1-day protocol, patients first received 10 mCi of 99mTc sestamibi at rest, and images were acquired after a 60-minute delay. Patients subsequently received 30 mCi of 99mTc sestamibi at peak stress, 1 minute prior to exercise cessation, with gated-SPECT imaging performed after a 30-minute delay. The 2-day protocol differed in that the patients received 30 mCi 99mTc sestamibi (45 mCi in patients with body mass index >45 kg/m2) before both rest and stress imaging.
Images were acquired with a dual-head GE Infinia camera (GE Medical Systems, Milwaukee, Wisconsin) with low-energy, high-resolution collimators. Each camera head rotated through 60 projections at 30 to 40 seconds/projection to acquire 180° of data with a standard 99mTc energy window. The data from the 2 heads were combined to give 360° of coverage. No scatter or attenuation correction was used.
Nuclear Imaging Interpretation
Experienced nuclear cardiology specialists performed visual and quantitative image analysis of the perfusion images according to a 17-segment model.(13) All borderline and abnormal studies were reclassified by the consensus of 2 additional readers blinded to all other clinical patient information, including data from the exercise study and the exercise ECG. Readers were aware that all patients had undergone exercise stress. Each segment was given a score from 0 to 4, with 0 representing normal perfusion and 1–4 representing mild, moderate, severe defects, and absent tracer uptake. Segmental scores were categorized by each reader who chose a score based on both quantitative perfusion data and a qualitative visual assessment. The semi-quantitative summed stress, rest, and difference values were calculated from these segmental scores. The 5 apical segments were weighted at 40% of the value of non-apical segments to so that each unit of myocardial volume was equal.(9) The percent myocardial ischemia, representing the extent and severity of LV inducible ischemia, was obtained by dividing the difference between the summed stress and summed rest scores by the maximum possible difference.(17, 18) Systolic and diastolic volumes and body surface area normalized volumes were also calculated.(13)
Outcomes
The primary outcome for this study was the prevalence of a high exercise workload of ≥10 METs and high-risk LV ischemia (≥10%) at this workload among patients 65 and older. The prevalence of varying degrees of LV ischemia was determined in the subgroups of patients achieving either <7, 7 to 9, or ≥10 METs. Secondary endpoints included predictors of ischemia and the prevalence of MACE (cardiac death, nonfatal MI, and late revascularization) amongst all patients and subdivided by exercise workload achieved, <10 versus ≥10 METs. Cardiac death was defined as any death with a demonstrable cardiac cause or without a clear non-cardiac cause. Nonfatal MI was designated if a patient presented with a history consistent with an acute coronary syndrome and had a troponin ≥2 times the upper limit of normal, with or without typical ischemic ECG changes. Late revascularization (or late invasive coronary angiography) was defined as >90 days post-MPI study unless the event was explicitly linked to the results of the MPI study.
Statistical Analysis
Continuous variables were described as medians with 25th and 75th percentiles and were compared by t tests with Satterthwaite approximations for unequal variances. Categorical variables were given as numbers of subjects with percentages and were compared with Pearson chi-square or Fisher’s exact testing. Event rates were calculated through person-years analysis. The total events in a subgroup over the entire study period were divided by the sum of the years of follow-up for all patients in that subgroup. This value was adjusted for one person-year of follow-up to give an annualized rate. The alpha level of significance was 0.05 for all analyses.
Univariable logistic regression analysis of possible predictors of LV ischemia was performed. Variables with p values <0.10 were entered into a multivariable logistic regression model predicting any LV ischemia. The C-statistic represents the discriminative power of the logistic equation (1.0 represents perfect prediction). Key assumptions for logistic regression validity were met. Kaplan-Meier survival analysis was performed to assess cardiac death and cardiac events over the 7-year median survival free of events. Cox proportional hazards analysis was performed to examine the relationship of ischemia and 10% LV ischemia to cardiac events. All statistics were performed with SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Study Population Characteristics
A substantial number of the 382 older adult subjects reaching target heart rate were able to achieve ≥10 METS of exercise capacity (97/382, 25.4%). The majority reached 7–9 METS (192/382, 50.3%), and 93/382 (24.4%) achieved a low workload of <7 METS. The median age of the entire cohort was 70.5 (25th, 75th: 67, 74). There were 161 subjects age 65–69 (42.2%), 131 age 70–74 (34.3%), and 90 subjects age 75 or greater (23.5%). The study population was 64.4% male and 36.9% had known CAD; 61.0% had chest pain, and 22.0% presented with dyspnea. The baseline characteristics related to workload achieved are provided in Table 1. The likelihood of achieving a high exercise workload was higher in younger adults and in men. Those with hypertension, diabetes mellitus, and elevated BMI ≥30 were less likely to achieve a high exercise workload. The presence of prior CAD and its sequelae did not appear to impact the likelihood of achieving a high workload.
Table 1.
Baseline Characteristics in Patients Achieving ≥85% of Their MAPHR Relative to Workload Attained.
| Characteristic | <7 METS*
Achieved (n (%)) |
7-9 METS Achieved (n (%)) |
≥10 METS Achieved (n (%)) |
P-value |
|---|---|---|---|---|
| Total number of patients | 93 (24.4) | 192 (50.3) | 97 (25.4) | - |
| Age (median, 25th, 75th percentiles) | 73.0 (68.0, 77.0) | 70.5 (67.0, 74.0) | 70.0 (67.0, 73.0) | <0.001 |
| Male | 55 (59.1) | 112 (58.3) | 79 (81.4) | <0.001 |
| Chest pain | 54 (58.1) | 117 (60.9) | 62 (63.9) | 0.71 |
| Hypertension | 71 (76.3) | 143 (74.5) | 59 (60.8) | 0.026 |
| Diabetes mellitus | 23 (24.7) | 34 (17.7) | 9 (9.3) | 0.019 |
| Hyperlipidemia | 61 (65.6) | 140 (72.9) | 65 (67.0) | 0.37 |
| Current tobacco use | 29 (31.2) | 45 (23.4) | 20 (20.6) | 0.21 |
| BMI ≥30 | 20 (21.5) | 34 (17.7) | 8 (8.3) | 0.034 |
| Known CAD* | 29 (31.2) | 71 (37.0) | 41 (42.3) | 0.29 |
| History of MI* | 15 (16.1) | 38 (19.8) | 16 (16.5) | 0.68 |
| Prior revascularization | 23 (24.7) | 64 (33.3) | 33 (34.0) | 0.28 |
| Abnormal resting ECG* | 21 (22.6) | 26 (13.5) | 11 (11.3) | 0.07 |
| Same-day beta-blocker use | 5 (5.4) | 15 (7.8) | 4 (4.1) | 0.44 |
CAD=coronary artery disease; ECG=electrocardiogram; METS=metabolic equivalents; MI=myocardial infarction.
Stress Findings
Exercise testing and stress electrocardiographic variables are presented by workload achieved in Table 2. There were no differences in percentage MAPHR achieved, maximum systolic and diastolic blood pressures, rate-pressure product, ischemic ST-depression, or chest pain during stress among the 3 workload groups. The median workload achieved decreased significantly for each age decile (8.5 METS for age 65–69, 8.0 METS for age 70–79, and 6.6 METS for age ≥80, p<0.001).
Table 2.
Exercise Test Variables in Patients Achieving ≥85% of Their MAPHR Relative to Workload Attained.
| Exercise Test Parameter | <7 METS* Achieved (n= 93) |
7-9 METS Achieved (n= 192) |
≥10 METS Achieved (n= 97) |
P-value |
|---|---|---|---|---|
| Exercise Duration (min) (median (25th, 75th percentiles)* | 5.5 (5.2, 6.2) | 8.0 (7.0, 8.5) | 11.0 (10.1, 12.0) | - |
| Percentage of MAPHR Achieved* | 95.4 (90.3, 102.0) | 95.3 (89.7, 100.7) | 97.9 (92.4, 103.3) | 0.15 |
| Maximum Systolic BP (mmHg) | 194 (178, 216) | 196 (169, 215) | 194 (180, 213) | 0.63 |
| Maximum Diastolic BP (mmHg) | 84 (76, 96) | 83 (73, 97) | 83 (73, 97) | 0.87 |
| Rate Pressure Product (×103) | 27.2 (24.4, 30.9) | 27.5 (24.1, 30.8) | 28.6 (25.0, 31.8) | 0.08 |
| ≥1mm ST-depression on ECG* (n (%)) | 19 (20.4) | 42 (21.9) | 16 (16.5) | 0.56 |
| Chest pain during stress (n (%)) | 16 (17.2) | 25 (13.0) | 10 (10.3) | 0.37 |
BP=blood pressure; ECG=electrocardiogram; HR=heart rate; MAPHR=maximum age-predicted heart rate; METS=metabolic equivalents; min=minutes.
SPECT Imaging Results and Predictors
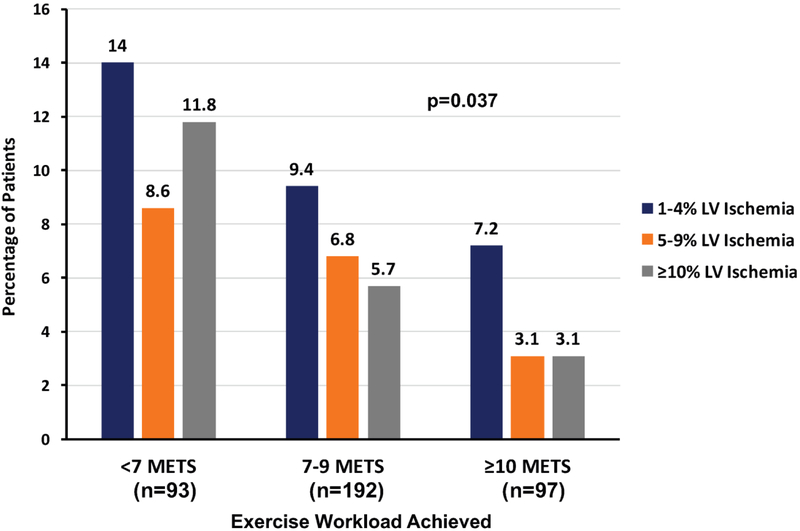
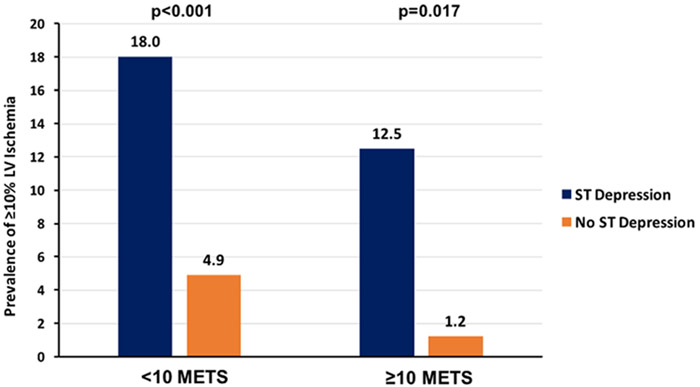
The prevalence of any reversible ischemia in the overall cohort of older adult patients achieving target heart rate was 22.8%, and 18.1% had a fixed defect with prior known infarction or wall-motion abnormality. The prevalence of significant LV ischemia (≥10%) in the entire cohort was 6.5%, and 0.8% had ≥20% LV ischemia. The median LVEF was 64% (25th, 75th percentiles: 58%, 70%). There were 34 subjects (8.9%) with a LVEF <50%. The prevalence of different levels of LV ischemia (mild or equivocal (1–4%), moderate (5–9%), and significant (≥10%)) are given by workload achieved in Figure 2. There is a significant stepwise decrease in all degrees of ischemia as workload increases (p=0.037). Significant LV ischemia (≥10%) was more prevalent in those with a lesser degree of workload achieved (11.8% for <7 METS versus 3.1% for ≥10 METS, p=0.042). Additional SPECT MPI results are analyzed by workload achieved in Table 3. Both the prevalence of a fixed perfusion defect and any reversible ischemia decreased by more than half as workload increased (p=0.005 and p=0.002, respectively). Similarly, the prevalence of significant LV ischemia (≥10%) decreased by almost three-quarters with increased workload (p=0.042). There were trends towards increased end-systolic and end-diastolic volume indices and decreased LVEF, but the differences were not significant. As shown in figure 3, subjects who had stress-induced ischemic ST depression ≥1mm had a higher prevalence of ≥10% LV ischemia both for those achieving <10 METS (18.0% versus 4.9%, p<0.001) and ≥10 METS (12.5% versus 1.2%, p=0.017).
Figure 2.
Chart of the percentage of patients who had 1–4%, 5–9%, and ≥10% LV ischemia divided by exercise workload achieved during stress testing. There was a stepwise decrease in all degrees of ischemia as exercise workload achieved increased (p=0.037).
Table 3.
Myocardial SPECT Imaging Results Versus Exercise Capacity in Patients Achieving ≥85% of Their MAPHR.
| Characteristic | <7 METS* Achieved (n= 93) |
7-9 METS Achieved (n= 192) |
≥10 METS Achieved (n= 97) |
P-value |
|---|---|---|---|---|
| Perfusion | ||||
| Fixed Perfusion Defects† | 27 (29.0) | 30 (15.6) | 12 (12.4) | 0.005δ |
| Any Reversible Ischemia | 32 (34.4) | 42 (21.9) | 13 (13.4) | 0.002 |
| Percentage LV Ischemia (mean ± SD) | 2.7±5.2 | 1.7±4.3 | 0.9±3.1 | |
| LV Ischemic burden | 0.037 | |||
| 0% Ischemic | 61 (65.6) | 150 (78.1) | 84 (86.6) | |
| 1-4% Ischemic | 13 (14.0) | 18 (9.4) | 7 (7.2) | |
| 5-9% Ischemic | 8 (8.6) | 13 (6.8) | 3 (3.1) | |
| ≥10% Ischemic | 11 (11.8) | 11 (5.7) | 3 (3.1) | |
| Volumes & Function | ||||
| ESVI* (median (25th, 75th percentiles) | 12 (9, 20) | 11 (8, 17) | 15 (11, 19) | 0.07 |
| ESVI ≥25 | 14 (15.2) | 19 (10.1) | 12 (12.5) | 0.45 |
| EDVI* | 45 (38, 55) | 43 (38, 51) | 46 (39, 56) | 0.053 |
| Ejection fraction | 65 (57, 69) | 65 (59, 71) | 63 (58, 68) | 0.07 |
EDVI=end-diastolic volume index; ESVI=end-systolic volume index; METS=metabolic equivalents.
All fixed perfusion defects had either known prior infarction in the affected territory or an associated wall-motion abnormality.
P-values are two-tailed with values <0.05 considered statistically-significant.
Figure 3.
Chart of the percentage of patients with significant (≥10%) left ventricular ischemia divided by workload achieved (<10 versus ≥10 METS) and presence or absence of diagnostic ST depression on stress electrocardiography. The prevalence of significant ischemia is very low in the group achieving ≥10 METS with no ST depression.
The predictors of any LV ischemia in univariable and multivariable analyses are given in Table 4. Results were similar when the dichotomous <10 or ≥10 METS variable was replaced with the trilevel METS variable (<7, 7–9 and ≥10). METS as a continuous variable was highly predictive of ischemia (HR 1.22, 95% CI 1.08–1.37, p=0.002) and for ≥10% LV ischemia (HR 1.32, 95% CI 1.06–1.66, p=0.014).
Table 4.
Uni- and Multivariable Logistic Regression Analysis Predicting Any Ischemia of the Left Ventricle (Global Wald X2 = 68.3, c=0.84).
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Predictors | X2 | Hazard Ratio (95% CI)* |
p-value | X2 | Hazard Ratio (95% CI) | p-value |
| LVEF (5% decrements)* | 53.4 | 1.76 (1.51-2.04) | <0.001† | 37.1 | 1.68 (1.42-1.99) | <0.001 |
| Male gender | 25.6 | 6.6 (3.17-13.56) | <0.001† | 12.6 | 4.54 (1.97-10.47) | <0.001 |
| <10 METS achieved | 6.3 | 2.27 (1.19-4.30) | 0.012† | 12.5 | 3.76 (1.80-7.84) | <0.001 |
| ST-Depression ≥1mm | 8.0 | 2.21 (1.28-3.81) | 0.005† | 6.9 | 2.42 (1.25-4.68) | 0.009 |
| Known CAD* | 21.7 | 3.22 (1.97-5.29) | <0.001† | 4.2 | 1.84 (1.02-3.31) | 0.041 |
| Prior MI* | 21.7 | 3.74 (2.15-6.53) | <0.001† | |||
| Prior revascularization | 20.5 | 3.14 (1.92-5.16) | <0.001† | |||
| Hypertension | 6.8 | 2.25 (1.22-4.12) | 0.009† | |||
| Age (5-year increments) | 2.9 | 1.24 (0.97-1.58) | 0.086 | |||
| Hyperlipidemia | 0.8 | 1.28 (0.75-2.19) | 0.37 | |||
| Chest pain | 0.6 | 0.83 (0.51-1.34) | 0.44 | |||
| Body mass index ≥30 | 0.5 | 0.78 (0.40-1.55) | 0.48 | |||
| Diabetes mellitus | 0.4 | 1.22 (0.66-2.25) | 0.53 | |||
| Tobacco use | 0.0 | 1.05 (0.60-1.82) | 0.87 | |||
CAD=coronary artery disease; CI=confidence interval; LVEF=left ventricular ejection fraction; METS=metabolic equivalents; MI=myocardial infarction.
P-values are two-tailed with values <0.05 considered statistically-significant.
Long-Term Events and Relationship of Ischemia
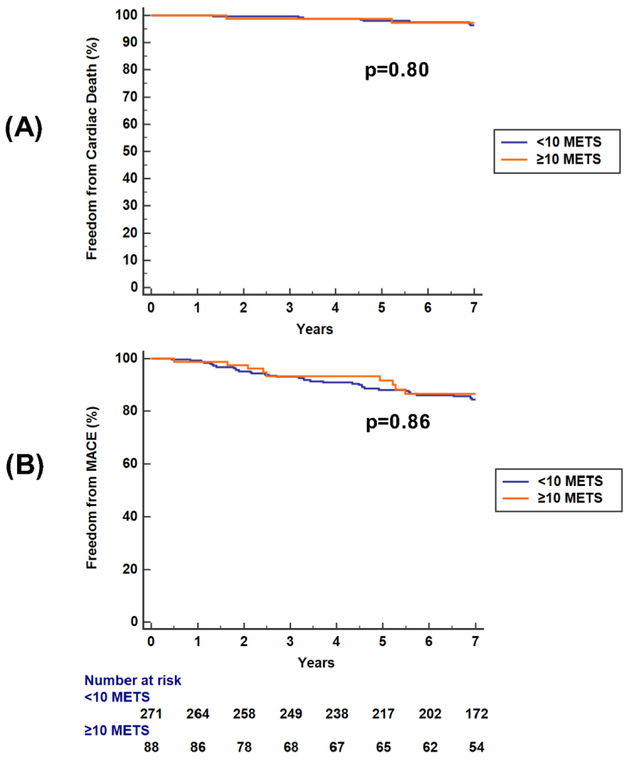
Follow-up for all-cause mortality and MACE were available in 372 (97.4%) and 359 (94.0%) of the entire cohort. The rate of 10-METS exercise capacity was not significantly different in those with and without follow-up (25.3% versus 22.2%, p= 0.83). The median follow-up was 7.7 years for all-cause mortality and 7.1 years for MACE. Early invasive coronary angiography was performed in 27 of the 359 patients (7.5%). The rate of early angiography was significantly lower in those reaching ≥10 METS: 3/88 (3.4%) versus 29/271 (10.7%), p=0.037. Of the three reaching ≥10 METS, 2 had known CAD and all had ≥5% LV ischemia. Subjects with early revascularization were excluded from subsequent outcomes analysis. Kaplan-Meier curves for cardiovascular death and MACE are provided in Figure 4 stratified by achievement of < or ≥10 METS. Yearly event rates are provided in Table 5 stratified by achievement of < or ≥10 METS. The annualized rate of cardiac death and nonfatal MI in patients achieving ≥10 METS were 0.6%/year and 0.8%/year, respectively. The rate of late revascularization was 1.4%/year. In those achieving ≥10 METS, neither ischemia (p=0.83) nor 10% LV ischemia (p=0.99) were significant predictors of MACE by Cox proportional hazards analysis. Eleven of the 13 subjects (84.6%) with MACE who had reached ≥10 METS had no ischemia during baseline imaging. Of the two with baseline ischemia, the median time to event was 3.9 years. There were no cardiac deaths or nonfatal MIs in the first 7 years of follow-up in those without known CAD who achieved ≥10 METS. Similarly, METS as a continuous variable was not a significant predictor of cardiac death (p=0.69) nor of MACE, though there was a trend (p=0.08).
Figure 4.
Graphs of Kaplan-Meier survival analysis for freedom from cardiac death (panel A) and freedom from cardiac events (panel B) over the 7-year median follow-up stratified by achievement of < or ≥10 METS in the 359 subjects with follow-up data on cardiac events available. The rate of both cardiac death and MACE was low in this older adult subgroup irrespective of exercise capacity.
Table 5.
Follow-up events by workload achieved
| Event | <10 METS* Achieved n (%/year) |
≥10 METS Achieved n (%/year) |
P- value† |
|---|---|---|---|
| Total patients | For Mortality: 278 For Cardiac Events: 271 |
For Mortality: 94 For Cardiac Events: 88 |
|
| All-cause mortality | 32 (1.7%/year) | 8 (1.3%/year) | 0.65 |
| Cardiovascular death | 12 (0.7%/year) | 3 (0.6%/year) | 0.68 |
| Nonfatal MI* | 14 (0.9%/year) | 4 (0.8%/year) | 0.82 |
| Late revascularization | 23 (1.4%/year) | 7 (1.4%/year) | 0.88 |
| Total MACE* | 41 (2.5%/year) | 13 (2.6%/year) | 0.94 |
MACE=major adverse cardiac events; METS=metabolic equivalents; MI=myocardial infarction.
P-values were determined using Chi-Square analysis.
Secondary Analysis in Subjects Failing to Reach Target Heart Rate
The 39 subjects who received an inadequate exercise stress of <85% MAPHR had a 12.8% rate of a positive stress ECG versus 20.2% for the study cohort with ≥85% MAPHR, p=0.27. There was a significant increased prevalence of ischemia, 46.2% versus 22.8% for those reaching target heart rate, p=0.001. However, there was no difference in the prevalence of significant ≥10% LV ischemia (5.1% for <85% MAPHR versus 6.5% for ≥85% MAPHR, p=0.73).
Discussion
Prior studies have shown that workload achieved on exercise stress testing is a good prognostic indicator of mortality and future cardiac events.(1, 2, 4, 10, 14, 19–22) We previously showed that patients achieving ≥85% of MAPHR, and ≥10 METS of workload on treadmill testing, had a low 0.4% prevalence of high-risk ≥10% LV ischemia on SPECT MPI.(9). Those achieving <7 METS had an 18-fold higher prevalence of high-risk ischemia, compared to those reaching ≥10 METS. A follow-up study of this cohort attaining ≥10 METS of exercise workload revealed a cardiac death rate of 0.1%/year and a nonfatal MI rate of 0.7%/year.(10) From these observations, we concluded that a provisional imaging protocol could be established in which low-risk patients reaching 85% MAPHR and ≥10 METS without ischemic ST depression would not be injected with an imaging agent at peak exercise unless other adverse exercise test endpoints were present (e.g. typical angina chest pain, exercise hypotension, serious arrhythmias).
Duvall et al. proposed such a provisional injection protocol for patients with chest pain in the emergency department, but excluded patients ≥65 years of age.(11) They performed a retrospective analysis of their existing stress test population data applying the hypothetical provisional protocol and found that only 5.9% of the patients qualifying for the provisional protocol had an abnormal MPI with a low 5-year all-cause mortality of 1.1%. This protocol could have avoided imaging in 29% of their patient population. This same group then undertook a prospective, non-randomized study in 965 low to low-intermediate risk patients age ≤65 years, with no known CAD and an interpretable ECG who were deemed able to exercise.(12) Patients who achieved target heart rate and ≥10 METS with no ST depression or exercise-induced anginal symptoms were not injected with a radioisotope (n=192). This provisional injection group had a similar all-cause mortality rate at an average follow-up of 42 months compared to those who had exercise imaging and achieved ≥10 METS (n=773). Only 1 cardiac death in the entire cohort was documented. Importantly, 22% of the original population of patients were not considered for the provisional protocol because of age >65 years.(12)
In our expanded study, we surmised that a provisional injection protocol for stress MPI might also be applied to a substantial number of patients ≥65 years of age. Approximately 25% of patients ≥65 years of age achieved ≥85% of their MAPHR and ≥10 METS on exercise testing. Those patients achieving ≥10 METs with no ischemic ST depression had a very low prevalence (1.2%) of high-risk ischemia on MPI and low rates of cardiac death (0.6%/year) and MACE (2.6%/year) over a median 7.0 years of follow-up. The MACE rate was similar in those with and without known CAD. Most patients achieving a high workload who had events did not have any ischemia on MPI. Of note, event rates were low for the entire population, with no difference in event rates between those achieving ≥10 METS vs <10 METS, possibly attributed to the lower risk of contemporary stress testing populations, particularly for patients able to exercise.
The favorable prognosis in this analysis for patients of advanced age achieving ≥10 METS is consistent with other groups (1, 6–8). Morise et al. showed that each 1-MET increment in peak treadmill workload was associated with an 18% reduction among those >65 years of age.(1) In a study of male veterans aged 65–92, every 1-MET increase in exercise capacity conferred a 12% lower risk of mortality.(8) Compared to the least fit veterans (≤4 METS), the adjusted hazard for death declined by 61% in those achieving >9 METS.
Certain limitations of employing a provisional imaging protocol in the clinical setting have been identified.(23) Referral physicians would have to agree to a provisional strategy. The costs of IV insertion and the imaging agent (even if not used) would need to be covered by insurance but would be offset by the cost savings related to not performing MPI. Pharmacologic stress imaging could be performed for patients requiring imaging for ST changes or angina symptoms during recovery. Potentially useful data on fixed perfusion defects and LV volumes and ejection fraction are not obtained. In addition, some high-risk patients undergoing stress ECG without imaging could benefit from CT coronary calcium imaging for further risk assessment as suggested previously.(24–27) The demonstration of a zero or very low coronary calcium score would provide further evidence of a low risk test result.
There are limitations to this study. First, it is a single-center study with a small cohort for assessing hard cardiac events. However, it is a valid patient population for determination of the percentage of older adult patients reaching target heart rate who can achieve ≥10 METS of exercise workload. A larger cohort of patients would be required to better ascertain event rates related to workload achieved.
Summary and Conclusions
In conclusion, this study shows that patients who are ≥65 years of age but achieve ≥85% of MAPHR and ≥10 METS on exercise stress testing have a very low prevalence of high-risk ischemia (≥10% LV ischemia) on MPI and low rate of MACE. Such patients, comprising approximately 25% of older adult patients who have a diagnostic exercise test, could avoid MPI for risk assessment. We suggest a larger study to evaluate the worth of a provisional imaging protocol for exercise testing, that includes patients ≥65 years of age, who are able to exercise to ≥10 METS without manifesting ST depression or exhibiting other adverse exercise test endpoints. The additional role of coronary calcium imaging in certain patients with a high pretest risk of CAD should also be evaluated in such a study design.
New Knowledge Gained
This analysis demonstrates that a substantial proportion of patients age ≥65 years undergoing stress testing can achieve ≥10 METS. This subgroup has a low prevalence of significant LV ischemia and early rate of subsequent MACE. These findings should facilitate the extension of the concept of a provisional imaging protocol to older adults, who make up a significant proportion of the population undergoing stress testing but have previously been excluded from such protocols.
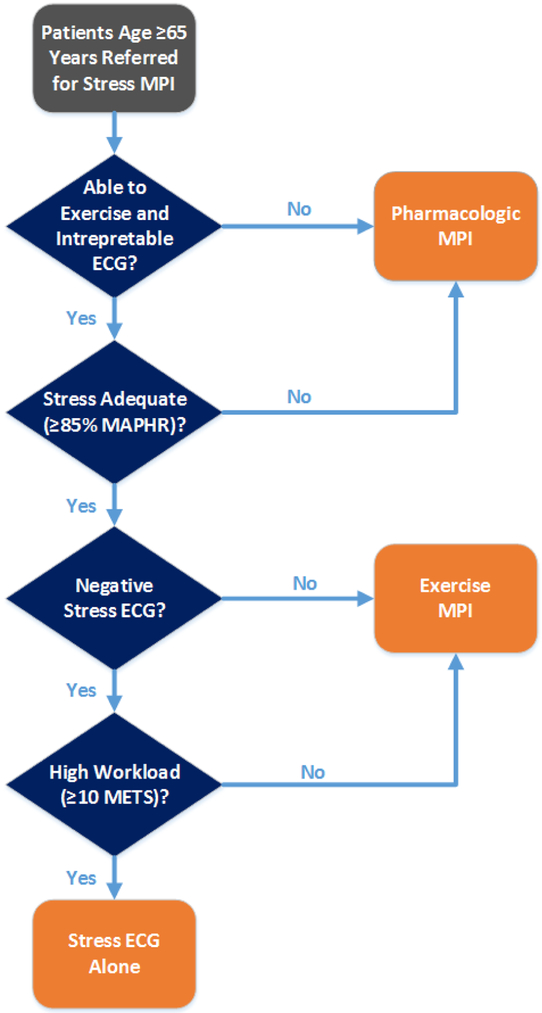
Figure 5.
Diagram of proposed provisional imaging algorithm. Older adult subjects ≥65 years of age who can exercise, have an interpretable ECG, reach target heart rate, have no concerning ECG changes, and reach a high exercise workload of ≥10 METS are appropriate for stress ECG alone due to a low risk of ischemia and subsequent MACE.
Acknowledgments
Funding: NIH K-Award 5K23HL119620–02
Relationships with Industry: Dr. Bourque receives research grant support from Astellas Pharma US Inc.
Abbreviations
- CAD
coronary artery disease
- CV
cardiovascular
- ECG
electrocardiography
- LV
left ventricular
- MAPHR
maximum age-predicted heart rate
- METS
metabolic equivalents
- MI
myocardial infarction
- MPI
myocardial perfusion imaging
- SPECT
single photon-emission computed tomography
Footnotes
Conflicts-of-Interest: None to report.
Conflict of Interest Disclosure Statement
Dr. Jamieson Bourque has received research grant support from Astellas Pharma US Inc. Drs. Smith, Myc, Watson, and Beller have no conflicts of interest to disclose.
Literature Cited
- (1).Morise AP, Jalisi F. Evaluation of pretest and exercise test scores to assess all-cause mortality in unselected patients presenting for exercise testing with symptoms of suspected coronary artery disease. J Am Coll Cardiol 2003;42:842–50. [DOI] [PubMed] [Google Scholar]
- (2).Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol 1997;30:641–8. [DOI] [PubMed] [Google Scholar]
- (3).Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 2009;301:2024–35. [DOI] [PubMed] [Google Scholar]
- (4).Goraya TY, Jacobsen SJ, Pellikka PA, Miller TD, Khan A, Weston SA et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Int Med 2000;132:862–70. [DOI] [PubMed] [Google Scholar]
- (5).Faselis C, Doumas M, Pittaras A, Narayan P, Myers J, Tsimploulis A et al. Exercise capacity and all-cause mortality in male veterans with hypertension aged >/=70 years. Hypertension 2014;64:30–5. [DOI] [PubMed] [Google Scholar]
- (6).Lee DS, Verocai F, Husain M, Al Khdair D, Wang X, Freeman M et al. Cardiovascular outcomes are predicted by exercise-stress myocardial perfusion imaging: Impact on death, myocardial infarction, and coronary revascularization procedures. Am Heart J 2011;161:900–7. [DOI] [PubMed] [Google Scholar]
- (7).Fine NM, Pellikka PA, Scott CG, Gharacholou SM, McCully RB. Characteristics and outcomes of patients who achieve high workload (>/=10 metabolic equivalents) during treadmill exercise echocardiography. Mayo Clinic Proceed 2013;88:1408–19. [DOI] [PubMed] [Google Scholar]
- (8).Kokkinos P, Myers J, Faselis C, Panagiotakos DB, Doumas M, Pittaras A et al. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation 2010;122:790–7. [DOI] [PubMed] [Google Scholar]
- (9).Bourque JM, Holland BH, Watson DD, Beller GA. Achieving an exercise workload of > or = 10 metabolic equivalents predicts a very low risk of inducible ischemia: does myocardial perfusion imaging have a role? J Am Coll Cardiol 2009;54:538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bourque JM, Charlton GT, Holland BH, Belyea CM, Watson DD, Beller GA. Prognosis in patients achieving >/=10 METS on exercise stress testing: was SPECT imaging useful? J Nucl Cardiol 2011;18:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Duvall WL, Levine EJ, Moonthungal S, Fardanesh M, Croft LB, Henzlova MJ. A hypothetical protocol for the provisional use of perfusion imaging with exercise stress testing. J Nucl Cardiol 2013;20:739–47. [DOI] [PubMed] [Google Scholar]
- (12).Duvall WL, Savino JA, Levine EJ, Hermann LK, Croft LB, Henzlova MJ. Prospective evaluation of a new protocol for the provisional use of perfusion imaging with exercise stress testing. Eur J Nucl Med Mol Imag 2015;42:305–16. [DOI] [PubMed] [Google Scholar]
- (13).Watson DD, Smith WH 2nd. The role of quantitation in clinical nuclear cardiology: the University of Virginia approach. J Nucl Cardiol 2007;14:466–82. [DOI] [PubMed] [Google Scholar]
- (14).Mark DB, Shaw L, Harrell FE Jr., Hlatky MA, Lee KL, Bengtson JR et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med 1991;325:849–53. [DOI] [PubMed] [Google Scholar]
- (15).Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 2002;40:1531–40. [DOI] [PubMed] [Google Scholar]
- (16).Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH et al. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation 2003;108:1554–9. [DOI] [PubMed] [Google Scholar]
- (17).Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107:2900–7. [DOI] [PubMed] [Google Scholar]
- (18).Hachamovitch R, Rozanski A, Hayes SW, Thomson LE, Germano G, Friedman JD et al. Predicting therapeutic benefit from myocardial revascularization procedures: are measurements of both resting left ventricular ejection fraction and stress-induced myocardial ischemia necessary? J Nucl Cardiol 2006;13:768–78. [DOI] [PubMed] [Google Scholar]
- (19).Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801. [DOI] [PubMed] [Google Scholar]
- (20).Peterson PN, Magid DJ, Ross C, Ho PM, Rumsfeld JS, Lauer MS et al. Association of exercise capacity on treadmill with future cardiac events in patients referred for exercise testing. Arch Int Med 2008;168:174–9. [DOI] [PubMed] [Google Scholar]
- (21).Morris CK, Morrow K, Froelicher VF, Hideg A, Hunter D, Kawaguchi T et al. Prediction of cardiovascular death by means of clinical and exercise test variables in patients selected for cardiac catheterization. Am Heart J 1993;125:1717–26. [DOI] [PubMed] [Google Scholar]
- (22).Bhat A, Desai A, Amsterdam EA. Usefulness of high functional capacity in patients with exercise-induced ST-depression to predict a negative result on exercise echocardiography and low prognostic risk. Am J Cardiol 2008;101:1541–3. [DOI] [PubMed] [Google Scholar]
- (23).Beller GA, Bateman TM. Provisional use of myocardial perfusion imaging in patients undergoing exercise stress testing: a worthy concept fraught with challenges. J Nucl Cardiol 2013;20:711–4. [DOI] [PubMed] [Google Scholar]
- (24).Bourque JM, Beller GA. Value of Exercise ECG for Risk Stratification in Suspected or Known CAD in the Era of Advanced Imaging Technologies. JACC Cardiovasc Imag 2015;8:1309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Rozanski A, Cohen R, Uretsky S. The coronary calcium treadmill test: a new approach to the initial workup of patients with suspected coronary artery disease. J Nucl Cardiol 2013;20:719–30. [DOI] [PubMed] [Google Scholar]
- (26).Chang SM, Nabi F, Xu J, Pratt CM, Mahmarian AC, Frias ME et al. Value of CACS compared with ETT and myocardial perfusion imaging for predicting long-term cardiac outcome in asymptomatic and symptomatic patients at low risk for coronary disease: clinical implications in a multimodality imaging world. JACC Cardiovasc Imag 2015;8:134–44. [DOI] [PubMed] [Google Scholar]
- (27).Shaw LJ. The exercise test is alive and well when coupled with coronary calcium scoring. JACC Cardiovasc Imag 2015;8:145–7. [DOI] [PubMed] [Google Scholar]