Abstract
Purpose:
The delivery of radiation therapy to cure gastrointestinal (GI) cancers is often limited by normal tissue toxicity of the GI tract. Studies using genetically engineered mice have demonstrated an essential role of the cyclin-dependent kinase (CDK) inhibitor p21 in protecting against the GI acute radiation syndrome (GI-ARS). Here, we examined the impact of the FDA-approved, selective CDK4/6 inhibitor palbociclib (PD-0332991) on the development of the GI-ARS induced by single dose vs. fractionated radiation in mice.
Methods and materials:
For the single dose radiation study, C57BL/6J mice were treated with palbociclib or vehicle 28 and 4 hours before subtotal-body irradiation (SBI). For the fractionated radiation study, C57BL/6J mice were exposed to fractionated SBI for 5 consecutive days. These mice were treated with palbociclib or vehicle either 28 and 4 hours before the 1st dose of irradiation or 4 hours before the 1st, 3rd, and 5th dose of irradiation.
Results:
Our data indicate that treatment with palbociclib before, but not after, a single fraction of SBI significantly ameliorated the GI-ARS, improved the integrity of the GI barrier and increased the number of surviving crypts in the small intestine. In addition, palbociclib did not protect tumor cell lines from radiation in vitro. In contrast to the results from the single dose exposure, treatment with palbociclib before 5 daily fractions of SBI did not prevent the GI-ARS. Moreover, we unexpectedly observed that the GI-ARS was exacerbated in mice treated with palbociclib before and during 5 daily fractions of SBI.
Conclusions:
Our results demonstrate that treatment with palbociclib before a single dose of SBI protects mice from the GI-ARS. In contrast, treatment with palbociclib before and during 5 daily fractions of SBI exacerbates the GI-ARS in mice. These results emphasize the importance of conducting preclinical studies of radioprotectors with single dose and fractionated radiation therapy.
INTRODUCTION
Delivery of adequate doses of radiation therapy to treat tumors in the abdomen is often limited by normal tissue toxicity of the GI tract (1). The GI-ARS occurs after high dose abdominal radiation exposure, which induces extensive damage to crypt stem cells of the small intestines. Severe damage to intestinal stem cells impairs regeneration of the intestinal epithelium, which can result in atrophy of the villi, loss of mucosal barrier, and sepsis (2). Currently, there are no drugs approved by the US FDA to specifically treat the GI-ARS beyond standard supportive care. Therefore, therapeutic strategies that preserve the crypt cells and promote regeneration of the GI epithelium after radiation are needed to manage GI-ARS, and these approaches may also allow escalation of radiation dose to treat intra-abdominal cancers.
Numerous studies have demonstrated that one essential protein that regulates the radiosensitivity of GI epithelial cells is the tumor suppressor p53 (3–5). For example, genetically engineered mice that lack two alleles of p53 in the GI epithelium are sensitized to the GI-ARS, whereas “Super p53” mice, which harbor an extra copy of p53, are more resistant to the GI-ARS (3). p53 functions as a transcription factor to regulate a number of genes including Cdkn1a, which encodes for the cyclin-dependent kinase (CDK) inhibitor p21 that controls the G1/S cell cycle checkpoint (6, 7). Remarkably, deletion of p21 sensitizes both p53 wild-type (3–5) and “Super p53” mice (8) to the GI-ARS, which indicates the crucial role of the p53-p21 axis in protecting the GI epithelium from acute radiation injury. Collectively, these findings suggest that blocking cyclin-dependent kinases that control the G1/S cell cycle checkpoint may be a promising approach to ameliorate the GI-ARS.
The G1/S cell cycle transition is normally controlled by integrating intracellular signal transduction pathways that regulate the activity of Cyclin D-CDK4/6 complexes (9). Palbociclib (PD-0332991), an FDA-approved drug for the treatment of advanced breast cancer, is a small molecule inhibitor that selectively inhibits CDK4/6 (10, 11). Here, we show that treatment with palbociclib before a single dose of radiation significantly protected mice from the GI-ARS; however, the GI-ARS was exacerbated when palbociclib was given before and during a course of fractionated abdominal irradiation.
METHODS AND MATERIALS
Statistical considerations.
For all experiments, measurements are presented as mean ± SEM. Each data point represents one mouse. Student’s t-test (two-tailed) was performed to compare the means of two datasets. Two-way ANOVA followed by Bonferroni post-hoc test was performed to examine the interaction between palbociclib and radiation treatment. For the GI-ARS studies, Kaplan–Meier estimate was performed followed by the log-rank test. We considered the evidence against a null hypothesis to be significant if the unadjusted P-value for the corresponding test was less than 0.05. GraphPad Prism 7 (GraphPad Software, Inc.) was used for carrying out these statistical analyses.
Mouse strains and palbociclib treatment.
All animal procedures for this study were approved by the Institutional Animal Care and Use Committee (IACUC) at our institution. Seven-week-old male C57BL/6J mice (Stock. No. 000664) were purchased from The Jackson Laboratory and acclimated for at least one week before being used for experiments. Palbociclib (PD-0332991) HCl (Cat. No. S1116, Selleckchem.com) was dissolved in sterile water at 10 mg/ml and administered to mice at a dose of 120 mg/kg body weight via oral gavage.
Radiation treatment.
All mice for the radiation study were irradiated between 2 to 5 p.m. To prevent the development of hematopoietic acute radiation syndrome, mice were irradiated using SBI (3). Unanesthetized mice were held in jigs and placed under lead shielding to protect the head and front limbs (Figure S1). SBI was performed 50 cm from the radiation source with a dose rate of 204 cGy/ min with 320 kVp X-rays, using 12.5mA and a filter consisting of 2.5mm Al and 0.1mm Cu (X-RAD 320 Biological Irradiator, Precision X-ray). The dose rate was measured with an ion chamber by members of the Radiation Safety Division at our institution.
GI epithelium permeability assay.
FITC-dextran (4 kD Sigma #46944) was dissolved in sterile water at 40 mg/ml. Mice were gavaged with 0.6 mg/g body weight of the FITC-dextran solution 4 hours before sacrifice to collect serum (12). The levels of FITC-dextran in serum were obtained after a standard curve was constructed using mouse serum from age-matched male C57BL/6 mice spiked with increasing amounts of FITC dextran. Samples were measured in duplicate in a 96-well plate using a Synergy 2 multi-mode reader (BioTek) and the results were averaged.
Quantification of surviving crypts
The number of surviving crypts in the small intestine 96 hours after SBI was quantified according to methods described previously (2). To label proliferating cells, mice received intraperitoneal injections of 200 μl BrdU in PBS at 10 mg/ml (Sigma-Aldrich) 4 hours before euthanization. A 12-cm piece of jejunum was fixed in 10% formalin overnight and then transferred to 70% ethanol. The intestines were cut into 2-cm pieces and bundled before embedding in paraffin to obtain the ideal orientation of the crypts (2). Immunohistochemistry staining was performed as described previously (13) using the rat anti-BrdU antibody (abcam, #ab6326, 1: 500 dilution). A surviving crypt was defined as one that had 10 or more tightly packed BrdU positive cells (excluding Paneth cells). For each mouse, at least 10 circumferences that were orientated correctly and did not contain Peyer’s patches were scored. The number of surviving crypts per circumference was corrected for crypt diameter and quantified by two observers (C-L.L. and P.O.) blinded to the treatment and the results were averaged.
Methods and Materials for Immunohistochemistry, Cell line studies and Immunoblotting are described in Supplementary Materials (available online at www.redjournal.org).
RESULTS
Palbociclib treatment decreases cell proliferation of intestinal crypt cells
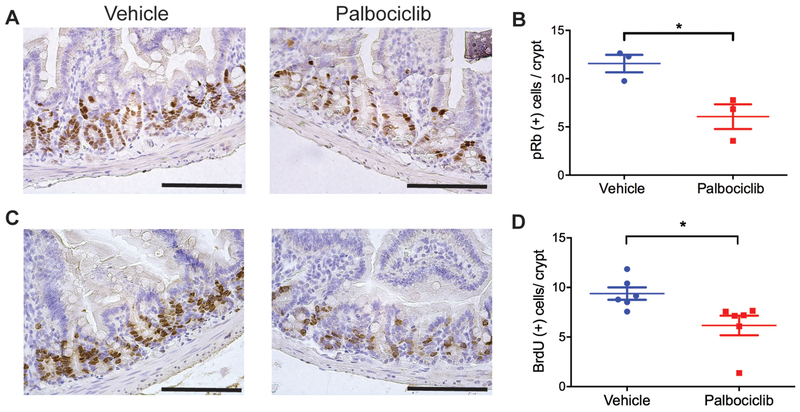
Palbociclib is a selective CDK4/6 inhibitor that blocks the phosphorylation of the retinoblastoma (Rb) protein and, as a result, delays cell cycle progression from G1 to S phase (14). To examine the effects of palbociclib on the phosphorylation of Rb and the proliferation of GI epithelial cells in the absence of irradiation, C57BL/6J mice were administered with 2 doses of palbociclib 24 hours apart before being euthanized for histology. Two hours before sacrifice, these mice were injected with BrdU to label cells that were proliferating. Immunohistochemistry staining showed that palbociclib significantly decreased the number of phosphorylated Rb (pRb) positive cells (Figure 1, A and B) as well as the number of BrdU positive cells per crypt (Figure 1, C and D). These data indicate that palbociclib is effective to decrease Rb phosphorylation and proliferation of crypt epithelial cells.
Figure 1. Treatment with palbociclib inhibits Rb phosphorylation and decreases proliferation of crypt cells in the absence of irradiation.
C57BL/6J mice that received two doses of palbociclib or vehicle 24 hours apart were euthanized for histology 8 hours after the last treatment. Two hours before sacrifice, mice were injected with BrdU to label proliferating cells. (A) Representative tissue sections of the small intestine stained with anti-phosphorylated Rb (pRb) antibody. Scale bars, 100 μm. (B) Quantification of pRb positive cells per crypt. Each dot represents the averaged result from one mouse. *P<0.05 by Student’s t test. (C) Representative tissue sections of the small intestine stained with an anti-BrdU antibody. (D) Quantification of BrdU positive cells per crypt. Each dot represents the averaged result from one mouse. *P<0.05 by Student’s t test. Scale bars, 100 μm.
Palbociclib treatment before, but not after, a single dose of radiation protects mice against radiation-induced GI toxicity
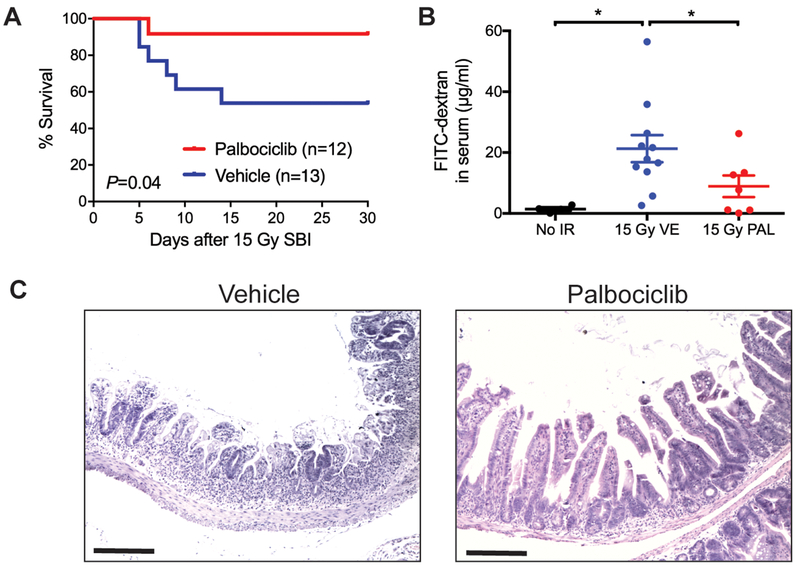
To evaluate the impact of palbociclib on the GI-ARS, C57BL/6J mice were treated with either palbociclib or vehicle 28 and 4 hours before exposure to a single dose of SBI that causes 40% of mice developed the GI-ARS within 10 days (LD40/10). As shown in two independent experiments, pre-treatment with palbociclib before irradiation significantly protected mice against the GI-ARS (Figure 2A and Figure S2). Next, we assessed the integrity of the GI epithelium by giving mice FITC-dextran via oral gavage and quantifying the amount of FITC-dextran in the serum (12). Although 15 Gy SBI significantly increased the leakiness of the GI epithelium 6 days after irradiation, palbociclib treatment improved the integrity of the GI epithelium of irradiated mice (Figure 2B). Histological analysis also showed that palbociclib treated mice had relatively intact crypt-villi structures 6 days after 15 Gy SBI compared to irradiated mice that received vehicle (Figure 2C).
Figure 2. Treatment with palbociclib before a single dose of sub-total-body irradiation prevents the development of the gastrointestinal acute radiation syndrome.
(A) C57BL/6J mice were treated with palbociclib (n=12) or vehicle (n=13) 28 and 4 hours before 15 Gy SBI and were followed for the development of the GI-ARS for at least 30 days after irradiation. P value was calculated by log-rank test. (B) Quantification of FITC-dextran in serum from unirradiated mice (No IR) or irradiated mice that received either vehicle (VE) or palbociclib (PAL) before 15 Gy SBI. Six days after irradiation, mice were given FITC-dextran via oral gavage 4 hours before sacrifice. Serum was collected from each mouse and the concentration of FITC-dextran in serum was quantified. Each dot represents the averaged result from one mouse. *P < 0.05 by Student’s t test. (C) Representative tissue sections of the small intestine 6 days after 15 Gy SBI. Mice were treated with either palbociclib or vehicle before irradiation. Scale bars, 100 μm.
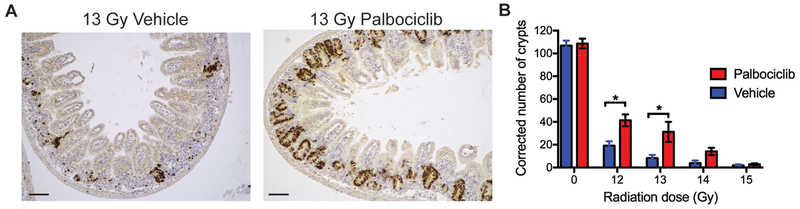
To examine the impact of palbociclib on the survival of clonogenic cells in the intestinal crypts, mice were treated with palbociclib or vehicle 28 and 4 hours before various doses of SBI (0, 12, 13, 14 and 15 Gy) and the number of surviving crypts in the small intestine were assessed 96 hours after irradiation (2). SBI markedly decreased the number of surviving crypts in a dose-dependent manner in vehicle-treated mice, but treatment with palbociclib significantly increased the number of surviving crypts at 96 hours after 12 and 13 Gy SBI (Figure 3, A and B).
Figure 3. Treatment with palbociclib before a single dose of sub-total-body irradiation increases the number of surviving crypts in the small intestine.
Mice were treated with palbociclib or vehicle 28 and 4 hours before 0, 12, 13, 14 and 15 Gy SBI. Proximal small intestines were harvested for histology 96 hours after irradiation. (A) Representative tissue sections of the small intestine 96 hours after 13 Gy SBI stained with an anti-BrdU antibody. Scale bars, 100 μm. (B) Quantification of surviving crypts 96 hours after various doses of SBI. To quantify the number of surviving crypts, tissue sections were stained for BrdU, which labels proliferating crypt cells. Data are presented as means ± SEM (n =3 mice group). *P < 0.05 by two-way ANOVA with Bonferroni’s post hoc test.
To evaluate whether palbociclib can function after irradiation to ameliorate radiation-induced GI toxicity, we assessed the number of surviving crypts in mice that were treated with palbociclib or vehicle 4 and 24 hours after 13 Gy SBI. However, palbociclib treatment after 13 Gy SBI did not increase the number of surviving crypts 96 hours after irradiation (Figure S3). Together, these results demonstrate that treatment with palbociclib before, but not after, a single dose of SBI protects mice against radiation-induced GI toxicity.
Palbociclib does not protect cancer cells from radiation in vitro
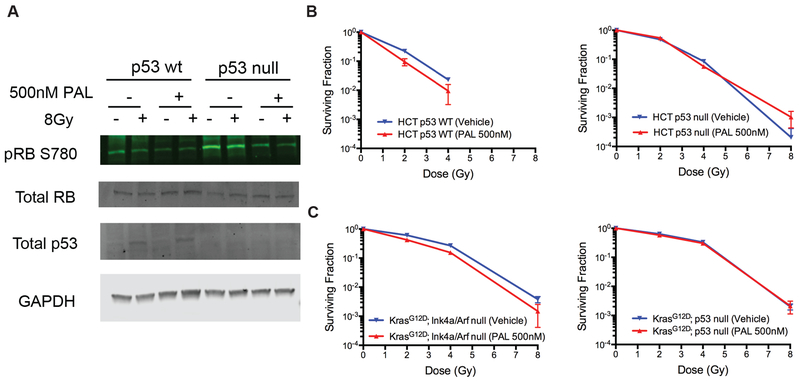
To study the impact of palbociclib on tumor cell response to radiation, we assessed the effect of palbociclib on the radiosensitivity of HCT116 human colorectal carcinoma cells that are wild-type for p53 (HCT p53 WT) and their isogenic counterparts that have p53 deleted (HCT p53 null) (15). Western blots showed that treatment with palbociclib markedly decreased phosphorylation of Rb in both HCT p53 WT and HCT p53 null cells regardless of radiation exposure, but it did not affect p53 accumulation after irradiation in HCT p53 WT cells (Figure 4A). However, palbociclib treatment did not alter the radiosensitivity of both p53 WT and p53 null HCT tumor cells in vitro (Figure 4B). We also conducted independent studies using mouse soft-tissue sarcomas cells generated by either deleting Ink4a/Arf (p53 WT) or p53 (p53 deficient) (16) and showed that palbociclib treatment did not impact the sarcoma cell response to radiation in vitro (Figure 4C). Together, these results indicate that although palbociclib decreases phosphorylation of Rb in both p53 WT and p53 null cancer cells, it does not affect the response of cancer cells to radiation in vitro.
Figure 4. Blocking CDK4/6 does not alter radiosensitivity of human and mouse cancer cells in vitro.
(A) Immunoblotting to examine the expression of phosphorylated RB (pRB S780), total Rb and total p53 protein in human HCT116 p53 WT cells and p53 null cells. Cells were treated with palbociclib (PAL) or vehicle for 24 hours before irradiation and were harvested 4 hours after irradiation to analyze protein expression. (B) Clonogenic survival assay of HCT116 p53 WT and p53 null cells after irradiation. Cells were treated with palbociclib (PAL) or vehicle for 24 hours before irradiation and were kept in palbociclib (PAL) for additional 24 hours after irradiation. (C) Clonogenic survival assay was repeated using KrasG12D; p53 null and KrasG12D; Ink4a/Arf null mouse sarcoma cells. No statistically significant difference in clonogenic survival was observed between vehicle and PAL treated cells. (n=3 independent experiments)
Palbociclib treatment before and during fractioned irradiation exacerbates radiation-induced GI toxicity
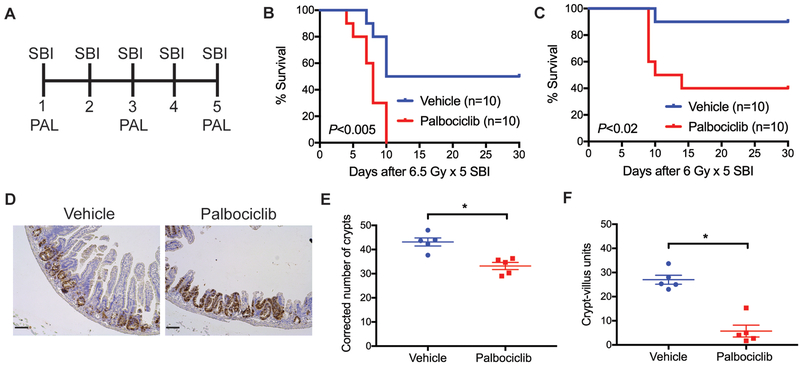
Although a single high dose of abdominal irradiation is often used to study the GI-ARS in mice (3, 17), in patients, abdominal irradiation is typically delivered in several fractions. Thus, we evaluated the impact of palbociclib treatment on the development of GI toxicity using 5 daily fractions of 6.5 Gy SBI, which is relevant to a fractionated schedule used in clinical trials of pancreatic cancer patients treated with SBRT (18). However, we found that pre-treatment with palbociclib 28 and 4 hours before the first fraction of 6.5 Gy × 5 SBI did not protect mice from the GI-ARS (Figure S4). Next, we examined whether treatment with palbociclib or vehicle 4 hours before the 1st, 3rd, and 5th fraction of SBI would prevent the GI-ARS (Figure 5A). Unexpectedly, treatment with palbociclib before and during fractionated SBI significantly exacerbated the development of the GI-ARS. Similar results were observed in two independent experiments in which mice were exposed to either 6.5 Gy × 5 or 6 Gy × 5 SBI (Figure 5, B and C). Histological examination showed that the mice that received palbociclib before and during 6 Gy × 5 SBI had significantly fewer surviving crypts and intact crypt-villus units of the small intestine (Figure 5, D to F). Collectively, these findings suggest that while treatment with palbociclib before a single fraction of SBI ameliorates the GI-ARS, the GI-ARS is exacerbated in mice treated with palbociclib before and during 5 daily fractions of SBI.
Figure 5. Palbociclib treatment before and during fractionated SBI exacerbates the GI-ARS.
(A) C57BL/6J mice were exposed to fractioned SBI for 5 consecutive days. Mice received one treatment of palbociclib or vehicle 4 hours before the 1st, 3rd, and 5th dose of irradiation. (B and C) Kaplan-Meier survival analysis of mice treated with palbociclib (n=10) or vehicle (n=10) before and during 6.5 Gy × 5 or 6 Gy × 5 SBI. P value was calculated by log-rank test. (D) Representative tissue sections of the small intestine 96 hours after the last dose of 6 Gy × 5 SBI stained with an anti-BrdU antibody. Scale bars, 100 μm. (E and F) Quantification of surviving crypts and intact crypt-villus units 96 hours after 6 Gy × 5 SBI. To quantify the number of surviving crypts, tissue sections were stained for BrdU, which labels proliferating crypt cells. Each dot represents the averaged result from one mouse. *P<0.05 by Student’s t test. Scale bars, 100 μm.
DISCUSSION
Preclinical studies using palbociclib (PD-0332991) have shown promising results in protecting hematopoietic stem/progenitor cells against acute toxicity induced by ionizing radiation or chemotherapy (19, 20). Here, we demonstrate that palbociclib treatment before a single dose of SBI significantly prevents the GI-ARS, improves the integrity of the GI mucosal barrier and increases the number of surviving crypts 96 hours after irradiation. (Figure 2 and 3). An independent study published by Wei et al. also demonstrated that blocking CDK4/6 using palbociclib and another small molecule inhibitor prevented the development of GI-ARS induced by a single dose of 15 Gy total-abdominal irradiation in mice (21). Their data showed that palbociclib treatment before 15 Gy total-body irradiation (TBI) reduced proliferation and apoptosis of crypt epithelial cells and increased the number of surviving crypts 96 hours after irradiation. In their study, the number of BrdU+ surviving crypts after 15 Gy TBI in the control mice was approximately 15, which was increased to around 30 crypts when palbociclib was administered prior to irradiation (Figure 1H in Wei et al., The Journal of Clinical Investigation 2016). In our study, about 10 surviving crypts per circumference were scored after a dose of 13 Gy sub-total-body irradiation (SBI), and palbociclib treatment before SBI also increased the number of BrdU+ surviving crypts to approximately 30 (Figure 3). Therefore, these results indicate that palbociclib treatment before a single dose of TBI or SBI causes a similar effect on improving the survival and regeneration of intestinal crypt epithelial cells. Collectively, the findings from Wei et al. and our study provide compelling evidence to support the use of palbociclib as a radioprotector to ameliorate the GI-ARS when given before a single dose of radiation exposure.
While palbociclib shows remarkable effects on preventing the GI-ARS induced by a single dose of radiation, we unexpectedly observed that palbociclib before and during treatment with clinically relevant 5 daily fractions of SBI exacerbated the GI-ARS in mice. These findings suggest that while transient inhibition of cell proliferation by blocking CDK4/6 before a single dose of radiation improves the survival of clonogenic cells of the small intestine (Figure 3), prolonged inhibition of CDK4/6 during 5 days of fractionated radiation impairs the regeneration of intestinal crypts (Figure 5). Of note, one potential limitation of extrapolating the results of our study to the clinic is that our sub-total-body irradiation method exposed the whole abdomen to radiation, which may differ in important ways to much smaller SBRT fields used to treat pancreatic cancer. Therefore, additional investigations are warranted to evaluate the impact of palbociclib on radiation-induced GI injury using focal radiation therapy that targets a spatially confined portion of the small intestine (22).
One general concern of using radioprotectors during radiation therapy is whether such compounds also increase radioresistance of tumor cells. Intriguingly, studies using preclinical models of brain tumors have shown that palbociclib does not protect tumor cells from radiation. In contrast, palbociclib treatment sensitizes both tumor cell lines and autochthonous mouse tumors, especially those that are p53 wild-type, to a single dose of radiation therapy (23–25). Our results indicate that palbociclib treatment does not alter the radiosensitivity of human or mouse cancer cells that are either WT or deficient for p53 (Figure 4). Collectively, these results suggest that palbociclib may be a promising drug to increase the therapeutic window of radiation therapy delivered in a single high dose.
In summary, our data demonstrate that the selective CDK4/6 inhibitor palbociclib (PD-0332991) protects against radiation-induced acute GI toxicity after a single dose of radiation. However, treatment of palbociclib before and during 5 daily fractions of radiation exacerbates GI acute radiation injury. This finding may have important implications for cancer therapy because there are currently at least 38 clinical trials using palbociclib to treat various types of tumors. Our findings also underscore the importance of completing appropriate pre-clinical studies that mimic the radiation schedule used to treat patients before translating promising combinations of radiation therapy and new drugs into the clinic. Although treatment of mice with the FDA-approved drug palbociclib before a single dose of radiation protects the GI tract from radiation toxicity, extrapolating these findings to multiple doses of palbociclib during multi-fraction SBRT in the clinic may exacerbate GI toxicity. Therefore, when designing clinical trials to combine palbociclib or other drugs with radiation therapy, it is important to first evaluate the combination using a clinically relevant fractionation schedule of radiation therapy to avoid unanticipated toxicity.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Bert Vogelstein for providing HCT116 human colorectal carcinoma cells. We thank Lixia Luo and Stephanie Hasapis for assisting with animal experiments. This work was supported by National Institute of Health grants 1R35 CA197616 (DGK).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicting financial interests.
REFERENCES
- 1.Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J. Gastroenterol 2013;19:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth C, Tudor G, Tudor J, et al. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012;103:383–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirsch DG, Santiago PM, di Tomaso E, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komarova EA, Kondratov RV, Wang K, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene. 2004;23:3265–3271. [DOI] [PubMed] [Google Scholar]
- 5.Leibowitz BJ, Qiu W, Liu H, et al. Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Mol. Cancer Res 2011;9:616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulić V, Kaufmann WK, Wilson SJ, et al. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. [DOI] [PubMed] [Google Scholar]
- 7.el-Deiry WS, Harper JW, O’Connor PM, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 8.Sullivan JM, Jeffords LB, Lee CL, et al. p21 protects “Super p53” mice from the radiation-induced gastrointestinal syndrome. Radiat. Res 2012;177:307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertoli C, Skotheim JM, de Bruin RAM. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol 2013;14:518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med 2016;375:1925–1936. [DOI] [PubMed] [Google Scholar]
- 11.Turner NC, Ro J, Andre F, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med 2015;373:209–219. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi CM, Miao YR, Diep AN, et al. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci. Transl. Med 2014;6:236ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang X, Falls DL, Li X, et al. Antigen-retrieval procedure for bromodeoxyuridine immunolabeling with concurrent labeling of nuclear DNA and antigens damaged by HCl pretreatment. J. Neurosci 2007;27:5837–5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin. Cancer Res 2014;20:3379–3383. [DOI] [PubMed] [Google Scholar]
- 15.Bunz F, Dutriaux A, Lengauer C, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. [DOI] [PubMed] [Google Scholar]
- 16.Kirsch DG, Dinulescu DM, Miller JB, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat. Med 2007;13:992–997. [DOI] [PubMed] [Google Scholar]
- 17.Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat. Res 2010;173:557–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrelli F, Comito T, Ghidini A, et al. Stereotactic Body Radiation Therapy for Locally Advanced Pancreatic Cancer: A Systematic Review and Pooled Analysis of 19 Trials. Int. J. Radiat. Oncol. Biol. Phys 2017;97:313–322. [DOI] [PubMed] [Google Scholar]
- 19.Johnson SM, Torrice CD, Bell JF, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J. Clin. Invest 2010;120:2528–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts PJ, Bisi JE, Strum JC, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J. Natl. Cancer Inst 2012;104:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei L, Leibowitz BJ, Wang X, et al. Inhibition of CDK4/6 protects against radiation-induced intestinal injury in mice. J. Clin. Invest 2016;126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verginadis II, Kanade R, Bell B, et al. A Novel Mouse Model to Study Image-Guided, Radiation-Induced Intestinal Injury and Preclinical Screening of Radioprotectors. Cancer Res 2017;77:908–917. [DOI] [PubMed] [Google Scholar]
- 23.Whiteway SL, Harris PS, Venkataraman S, et al. Inhibition of cyclin-dependent kinase 6 suppresses cell proliferation and enhances radiation sensitivity in medulloblastoma cells. J. Neurooncol 2013;111:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barton KL, Misuraca K, Cordero F, et al. PD-0332991, a CDK4/6 inhibitor, significantly prolongs survival in a genetically engineered mouse model of brainstem glioma. PLoS One. 2013;8:e77639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaud K, Solomon DA, Oermann E, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70:3228–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.