SUMMARY
Objective:
This study aimed to investigate the associations between patellofemoral cartilage T1ρ and T2 relaxation times and knee flexion moment (KFM) and KFM impulse during gait.
Method:
Knee magnetic resonance (MR) images were obtained from 99 subjects with and without patellofemoral joint (PFJ) osteoarthritis (OA), using fast spin-echo, T1ρ and T2 relaxation time sequences. Patellar and trochlear cartilage relaxation times were computed for the whole cartilage, and superficial and deep layers (laminar analysis). Subjects also underwent three-dimensional (3D) gait analysis. Peak KFM and KFM impulse were calculated during the stance phase. Linear regressions were used to examine whether cartilage relaxation times were associated with knee kinetics during walking while adjusting age, sex, body mass index (BMI) and walking speed.
Results:
Higher peak KFM and KFM impulse were significantly related to higher T1ρ and T2 relaxation times of the trochlear and patellar cartilage, with standardized regression coefficients ranging from 0.21 to 0.28. Laminar analysis showed that overall the superficial layer of patellofemoral cartilage showed stronger associations with knee kinetics. Subgroup analysis revealed that in subjects with PFJ OA, every standard deviation change in knee kinetics was related to greater increases in PFJ cartilage T1ρ and T2 (standardized coefficients: 0.29 to 0.41). Conversely, in subjects without OA, weaker relationships were observed between knee kinetics and PFJ cartilage T1ρ and T2.
Conclusions:
Our findings suggest that increased peak KFM and KFM impulse were related to worse cartilage health at the PFJ. This association is more prominent in superficial layer cartilage and cartilage with morphological lesions.
Keywords: Magnetic resonance imaging, Relaxation time, Gait, Patellofemoral joint
Introduction
Patellofemoral joint (PFJ) osteoarthritis (OA) is a prevalent knee condition1–3 that is highly associated with pain and dysfunction4,5. Mechanical loading is integral to the onset and progression of OA6,7, contributing to articular cartilage matrix deterioration and loss, the hallmark of OA6,7. It has been well-documented that increased external knee adduction moment and knee adduction moment impulse, which can result in higher mechanical load at the medial tibiofemoral joint (TFJ), are related to degeneration of the medial TFJ cartilage8–10. However, only few studies have investigated gait characteristics in individuals with PFJ OA11–15 and limited information has been reported regarding knee kinetics associated with PFJ cartilage health.
Sagittal plane knee kinetic variables are indicative of PFJ mechanical loading during gait. Knee flexion moment (KFM) is the external moment acting in the sagittal plane to flex the knee joint. During the stance phase of gait, the quadriceps femoris contracts to counteract this external moment16. As such, an increase in KFM is indicative of a higher quadriceps force. Because PFJ reaction force is the resultant force of quadriceps force and patellar tendon force, an increase in KFM can result in a higher PFJ reaction force and stress17,18. Moreover, KFM impulse is calculated as the integral of KFM with respect to time. Thus, it takes into account both duration and magnitude of loading, and may be more related to knee OA than peak joint moment19–22. Both increased KFM and KFM impulse can result in higher mechanical loading at the PFJ17,18,23 and have been shown to be related to morphological lesions of PFJ OA24,25. Yet, it remains unclear whether these biomechanical factors are associated with PFJ cartilage biochemical composition. Understanding these relationships is critical, as changes in cartilage composition can provide indications of early cartilage degeneration which can occur before morphological changes are visualized on radiographs and magnetic resonance (MR) images26–28.
Quantitative MR T1ρ and T2 relaxation times provide a noninvasive means to evaluate compositional changes related to cartilage degeneration. Specifically, an increase in cartilage T1ρ relaxation time is related to a loss of glycosaminoglycan29–31; and an increase in cartilage T2 relaxation time is associated with an increase in water content and disorganization of the collagen matrix30,32,33. Cartilage MR relaxation times can be quantified by the whole compartment or by separating the superficial and deep cartilage layers34. A previous study investigating knee OA following anterior cruciate ligament injury found that cartilage degeneration initiated from the superficial layer35. These imaging metrics have been widely used as imaging biomarkers of early cartilage degeneration26–28, but have not been utilized to identify biomechanical factors related to PFJ OA. In addition, degenerative cartilage is less stiff and may be more susceptible to mechanical load36.
The primary aim of this study was to evaluate the associations between sagittal plane knee kinetics during gait (i.e., peak KFM and KFM impulse) and PFJ cartilage T1ρ and T2 relaxation times. The secondary aim was to examine these associations for the superficial and deep layers PFJ cartilage (laminar analysis). The tertiary aim of this study was to evaluate these associations in individuals with and without PFJ OA (subgroup analysis). We hypothesized that higher peak KFM and KFM impulse would be related to higher PFJ cartilage T1ρ and T2 relaxation times and the superficial layer cartilage would show stronger correlation with knee kinetic variables than deep layer cartilage. Lastly, we hypothesized that the observed association would be stronger in individuals with PFJ OA than those without OA.
Methods
Subjects
Participants were recruited from the local community as a part of a longitudinal knee OA study. Fifty-six subjects with PFJ OA and 43 subjects without PFJ or TFJ OA (controls) were included in this study. All participants were above 35 years of age and did not have: (1) history of lower extremity or spine surgery, (2) total joint replacement of any lower extremity joint, (3) self-reported inflammatory arthritis, (4) any conditions that limited the ability to walk without assistant device, and (5) contraindications to MR imaging24. Because this study was a part of a primary study focused on TFJ OA, all participants underwent a weight-bearing, posteroanterior, fixed-flexion radiograph of the TFJs. The knee with higher Kellgren–Lawrence (KL) grade was chosen for MR imaging and biomechanical tests. When both knees presented the same KL grade, the test limb was determined randomly. The study was approved by the Committee of Human Research of the university. Prior to participation, all subjects signed a written informed consent. Moreover, all subjects completed the Knee injury and Osteoarthritis Outcome Score (KOOS) survey, which has a range from 0 to 10037. A higher KOOS score represents less pain and better function37.
MR acquisition
MR images of the knee were acquired using a 3-T MR 750w Scanner (General Electric, Milwaukee, WI) and an 8-channel phased-array knee coil (Invivo, Orlando, FL). All subjects were positioned in supine with their knee in neutral rotation and full extension. To reduce movement, the test foot was secured in place, the study knee was stabilized with padding, and a belt was secured across the subject’s waist. All subjects arrived at the imaging center and were unloaded (seated in a chair) for a 45-min period before imaging38,39. The following sequences were obtained for each participant: (1) high-resolution 3D intermediate-weighted fast spin-echo (FSE) sequence for clinical grading and cartilage segmentation, (2) 3D T1ρ relaxation time sequence and (3) 3D T2 relaxation time sequence (Table I).
Table I.
MRI sequences
| Sequence | Parameters |
|---|---|
| 3D intermediate-weighted fast spin-echo |
TR/TE = 1500/26.69 ms, field of view = 16 cm, matrix = 384 × 384, slice thickness =
0.5 mm, echo train length = 32, bandwidth = 37.5 kHz, number of excitations = 0.5, acquisition time = 10.5 min |
| 3D T1ρ relaxation time | TR/TE = 9/2.6 ms, time of recovery = 1500 ms, field of view = 14 cm, matrix = 256 ×
128, slice thickness = 4 mm, bandwidth = 62.5 kHz, TSL = 0/2/4/8/12/20/40/80 ms, frequency of spin-lock = 500 Hz, acquisition time = 11 min |
| 3D T2 relaxation time | Same as the T1ρ quantification except for magnetization preparation TE = 1.8/3.67.3/14.5/29.1/43.6/58.2, acquisition time = 11 min |
TR, Repetition time.
MR analysis
Quantitative cartilage T1ρ and T2 relaxation times
To facilitate image registration and cartilage segmentation, sagittal high-resolution FSE images were down-sampled to the same slice thickness as the T1ρ and T2 relaxation time map images. FSE and first echo of T2 images were then rigidly registered to the first echo of T1ρ images. Using this technique all images were aligned and differences in positioning were minimized. To account for potential motion artifacts during image acquisition of T1ρ and T2 sequences, echoes 2 through 8 were each registered to the first echo of T1ρ and T2 maps.
Whole compartment analysis
Patellar and trochlear cartilage were segmented semi-automatically (automated edge detection and manual correction) on multiple slices of the FSE images using an in-house program developed with MATLAB (MathWorks, Natick, MA) based on edge detection and Bézier splines40. Inferior border of the trochlear cartilage was defined by the anterior boundary of the medial and lateral menisci38. In this study, whole compartment refers to entire patellar or trochlear articular cartilage.
Laminar analysis
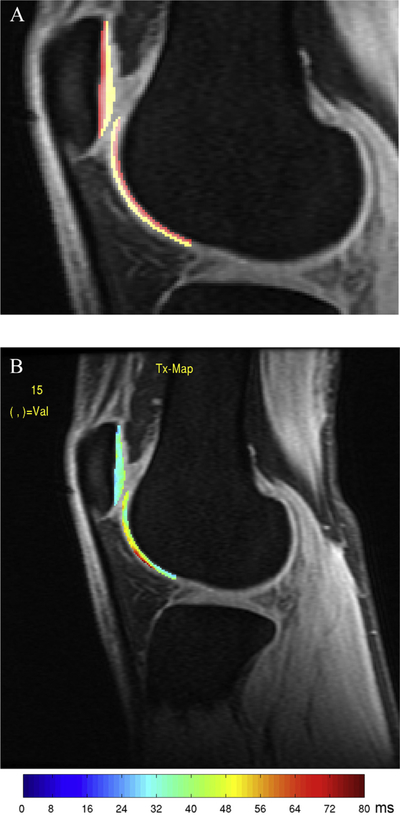
The segmented cartilage regions of interest were then partitioned automatically into two equal laminae: the deep layer (closer to the subchondral bone) and superficial layer (closer to articular surface) [Fig. 1(A)]34.
Fig. 1.
(A) Laminar analysis was performed by dividing the patellar and trochlear cartilage into two equal layers. Red regions correspond to deep layer cartilage. Yellow regions correspond to superficial cartilage. (B) T1ρ map of the PFJ for a control subject without OA.
T1ρ and T2 relaxation time quantification
T1ρ and T2 relaxation time maps were constructed by 3-parameter fitting for all eight of the T1ρ and T2 images, voxel by voxel, to the equations below using in-house developed program:
where S is the image signal at a given time point (time of spin-lock (TSL) for T1ρ maps or echo time (TE) for T2 maps), A is initial magnetization, and B is a constant.
The cartilage regions of interest were overlaid on the previously co-registered T1ρ and T2 maps. The cartilage splines were adjusted manually in order to avoid synovial fluid or surrounding anatomy. To eliminate artifacts due to partial volume effects with synovial fluid, voxels with relaxation time ≥130 ms for T1ρ or ≥100 ms for T2 maps were excluded from quantification [Fig. 1(B)]. Mean T1ρ and T2 values were calculated for defined cartilage regions.
Semiquantitative cartilage lesion assessment
Semiquantitative assessment of articular cartilage lesions was performed by a board-certified, fellowship-trained musculoskeletal radiologist using the modified Whole Organ Magnetic Resonance Imaging Score (WORMS)3,41. Cartilage lesions were graded using a scale from 0 to 6 as described previously24. Gradings were performed at the articular cartilage overlying six regions: patella, trochlea, medial and lateral femoral condyle, and medial and lateral tibia plateau. WORMS cartilage lesion score was used to define the presence of OA in this study. PFJ OA was defined as present when the WORMS score was 2 or higher for cartilage lesion of the patella or trochlea3. TFJ OA was defined when the WORMS score was 2 and higher for cartilage lesion of the tibia or femur3.
Gait analysis
3D lower extremity kinematics were recorded using a 10-camera motion capture system (Vicon, Oxford, UK) at a sampling rate of 250 Hz. Ground reaction force data were obtained using two embedded force platforms (Advanced Mechanical Technology, Watertown, MA) at a sampling rate of 1000 Hz. Marker and ground reaction force data were collected and synchronized using motion capture software (Nexus, Oxford, UK). Participants wore shorts and their personal sneakers during the evaluation.
Prior to the walking test, retro-reflective markers (14 mm spheres) were placed on the subjects bilateral lower limbs and pelvis as previously described24,25. Subjects were instructed to walk at a self-selected speed, which was described as “you have some place to be, but you are not late.” Five successful trials were obtained. A trial was considered successful when the foot of the tested limb fell within the borders of force platform from initial contact to toe-off. Walking speed of trial 2 to 5 was controlled to be within ±5% of the first successful trial.
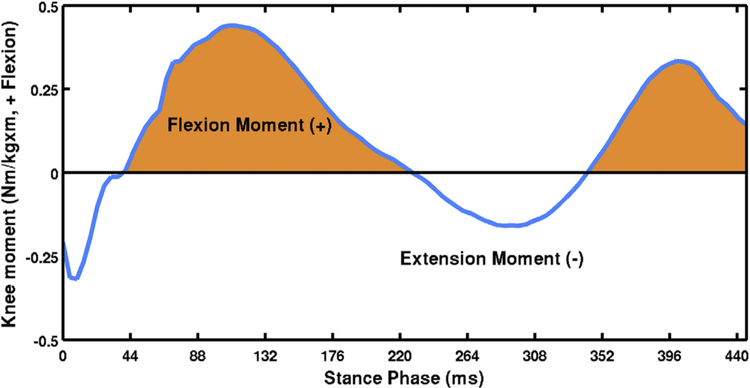
Kinematic and kinetic data were computed using Visual3D (C-Motion, Germantown, MD) and MATLAB software (The MathWorks, Natick, MA). Marker trajectory data were low-pass filtered using a fourth-order Butterworth filter with a cutoff frequency of 6 Hz. The hip, knee and ankle joints were assigned three degrees of freedom for rotations. Joint kinematics was calculated using a Cardan rotation sequence in the order of flexion/extension, abduction/adduction, and internal/external rotation. Net joint moments were reported as external moments and normalized to each participant’s body mass (kg) and height (m). The positive and negative values of the sagittal plane knee moment were used to indicate knee flexion and extension moments, respectively. KFM impulse was calculated as the integral of KFM (Nm/kg·m) with respect to time (ms) (Fig. 2)24. The stance phase of gait was defined during the time when the vertical ground reaction force was greater than 20 N. Peak KFM and KFM impulse during the stance phase were calculated. Average data from five successful trials were exported for statistical analyses.
Fig. 2.
Sagittal plane knee moment curve during the stance phase of walking of a sample trial. Joint moment is expressed as external moment. Positive and negative values indicate knee flexion and extension moments, respectively. KFM impulse was calculated as the area under curve when sagittal plane knee moment is positive.
Statistical analysis
Descriptive statistics were used to analyze the demographic characteristics, KOOS, knee kinetics and cartilage T1ρ and T2 relaxation times in control and PFJ OA subjects. Chi-squared and independent t-tests were used to compare group differences in demographics and cartilage T1ρ and T2 relaxation times. Linear regression models were built to examine whether PFJ cartilage relaxation times (i.e., patellar and trochlear cartilage T1ρ and T2 of the whole compartment, superficial layer and deep layer) were related to peak KFM and KFM impulse while adjusting for age, sex, body mass index (BMI) and walking speed. This was performed by entering covariates in the regression model and then adding the independent variable (peak KFM or KFM impulse). When there were subjects with TFJ OA, linear regression models were also controlled for the presence of TFJ OA. Regression models were performed for all subjects (primary and secondary analyses), and individuals with PFJ OA and controls (tertiary analysis). All statistical analyses were performed using SPSS Statistics version 23.0 (IBM Corporation, Armonk, NY) with a significance level set at 0.05.
Results
Subject characteristics
A total of 99 subjects completing the MR and gait analysis were included in this study (56 PFJ OA, 43 control). Demographics, KOOS, walking biomechanics, and cartilage relaxation time of the control and PFJ OA groups are presented in Table II. Significant group differences were found in sex (P = 0.003), age (P = 0.002) and KOOS–Sports (P = 0.035). Moreover, independent t-tests revealed that PFJ OA group exhibited significantly higher cartilage T1ρ of the patellar whole compartment (P = 0.015), deep layer (P = 0.010) and superficial layer (P = 0.042) compared to the controls. After adjusting for age, sex and BMI, only the patellar whole compartment and deep layer T1ρ remained significantly different between groups (P = 0.045 and 0.024, respectively). Overall, the T1ρ and T2 cartilage relaxation times were higher for the superficial layer than the deep layer (Table II).
Table II.
Descriptive statistics of subjects with PFJ OA and controls without TFJ or PFJ OA
| All | PFJ OA | Control | ||
|---|---|---|---|---|
| N = 99 | N = 56 | N = 43 | ||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Sex, Male/Female | 37/62 | 14/42 | 23/20* | |
| Age, years | 52.0 (49.9, 54.2) | 55.0 (52.2, 57.7) | 48.2 (45.1, 51.4)* | |
| BMI, kg/m2 | 24.6 (23.9, 25.2) | 24.6 (23.7, 25.6) | 24.5 (23.6, 25.5) | |
| OA type, Isolated PFJ OA/Mixed PFJ & TFJ OA | 34/22 | 34/22 | ||
| KOOS | ||||
| Pain | 86.4 (82.3, 89.5) | 84.2 (79.7, 88.7) | 89.3 (85.2, 93.4) | |
| Symptoms | 86.7 (83.7, 89.7) | 84.6 (80.2, 89.0) | 89.4 (85.4, 93.3) | |
| Activities of Daily Living | 91.8 (89.3, 94.3) | 89.9 (86.2, 93.6) | 94.4 (91.3, 97.4) | |
| Sports | 82.1 (77.9, 86.3) | 78.2 (71.8, 84.5) | 87.1 (82.3, 92.0)* | |
| Quality of Life | 76.0 (71.3, 80.7) | 73.3 (66.5, 80.1) | 79.5 (73.2, 85.7) | |
| Walking biomechanics | ||||
| Walking speed, m/s | 1.52 (1.48, 1.57) | 1.50 (1.43, 1.57) | 1.56(1.50, 1.61) | |
| Peak KFM, Nm/kg m | 0.36 (0.34, 0.39) | 0.36 (0.32, 0.40) | 0.37 (0.32, 0.41) | |
| KFM impulse, Nm ms/kg m | 53.6 (48.6, 58,6) | 52.7 (47.1, 60.4) | 53.5 (45.6, 61.3) | |
| Cartilage T1ρ relaxation times | ||||
| Trochlea | Whole compartment, ms | 32.9 (32.2, 33.7) | 33.1 (32.1, 34.2) | 32.6 (31.5, 33.8) |
| Superficial layer, ms | 36.0 (35.1, 36,8) | 36.4 (35.2, 37.6) | 35.4 (34.1, 36.7) | |
| Deep layer, ms | 29.8 (29.0, 30.5) | 29.8 (28.8, 30.9) | 29.6 (28.5, 30.8) | |
| Patella | Whole compartment, ms | 33.4 (32.6, 34.2) | 34.2 (33.3, 35.2) | 32.3 (31.1, 33.6)† |
| Superficial layer, ms | 37.1 (36.3, 38.0) | 37.9 (36.8, 38.2) | 36.1 (34.7, 37.6) | |
| Deep layer, ms | 29.9 (29.1, 30.7) | 30.8 (29.8, 31.9) | 28.7 (27.4, 29.9)† | |
| Cartilage T2 relaxation times | ||||
| Trochlea | Whole compartment, ms | 25.5 (25.0, 26.0) | 25.8 (25.0, 26.5) | 25.1 (24.6, 25.7) |
| Superficial layer, ms | 27.3 (26.7, 27.8) | 27.7 (26.9, 28.5) | 26.7 (26.1, 27.4) | |
| Deep layer, ms | 23.7 (23.1, 24.2) | 23.9 (23.0, 24.7) | 23.4(22.8, 24.1) | |
| Patella | Whole compartment, ms | 24.4 (23.8, 24.9) | 24.6 (23.9, 25.4) | 24.0 (23.2, 24.8) |
| Superficial layer, ms | 26.7 (26.2, 27.3) | 27.0 (26.2, 27.7) | 26.5 (25.6, 27.3) | |
| Deep layer, ms | 22.1 (21.5, 22.6) | 22.4 (21.6, 23.2) | 21.6 (20.8, 22.4) | |
CI, confidence interval.
Variables that reach statistical significant difference between PFJ OA and control groups (P < 0.05) are in bold.
Significant differences between PFJ OA and control groups (P < 0.05).
Significant differences between PFJ OA and control groups after adjusting for age, sex and BMI (P < 0.05).
Whole compartment analysis
Significant associations were found between knee kinetics and PFJ cartilage T1ρ and T2 relaxation times based on whole compartment analysis across all subjects (Table III). Higher peak KFM was significantly related to higher T1ρ and T2 of the trochlear and patellar cartilage, with standardized regression coefficients ranging from 0.21 to 0.25 after adjusting for age, sex, BMI, walking speed and presence of TFJ OA. Similarly, significant positive associations were observed between KFM impulse and T1ρ and T2 of the trochlear and patellar cartilage, with the standardized regression coefficients ranging from 0.22 to 0.28.
Table III.
Associations between knee kinetics and cartilage T1ρ and T2 relaxation times of the patella and trochlea for all subjects (n = 99)*
| Whole compartment analysis | Laminar analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trochlea | Patella | Trochlea | Patella | Trochlea | Patella | Trochlea | Patella | ||||||
| T1ρ | T1ρ | T2 | T2 | T1ρ | T1ρ | T2 | T2 | ||||||
| Whole | Whole | Whole | Whole | Superficial | Deep | Superficial | Deep | Superficial | Deep | Superficial | Deep | ||
| Peak KFM | β (95% CI) | 0.21 | 0.25 | 0.20 | 0.22 | 0.20 | 0.19 | 0.30 | 0.17 | 0.22 | 0.18 | 0.23 | 0.18 |
| (0.05, 0.41) | (0.04, 0.45) | (0.03, 0.40) | (0.05, 0.43) | (−0.003, 0.41) | (−0.02, 0.40) | (0.10, 0.51) | (−0.04, 0.40) | (0.18, 0.42) | (−0.03, 0.39) | (0.02, 0.44) | (−0.03, 0.39) | ||
| P value | 0.046 | 0.022 | 0.047 | 0.045 | 0.054 | 0.072 | 0.004 | 0.107 | 0.033 | 0.087 | 0.029 | 0.095 | |
| KFM | β (95% CI) | 0.28 | 0.25 | 0.26 | 0.22 | 0.25 | 0.27 | 0.33 | 0.18 | 0.25 | 0.22 | 0.25 | 0.18 |
| impulse | (0.08, 0.47) | (0.05, 0.45) | (0.07, 0.45) | (0.14, 0.42) | (0.05, 0.45) | (0.08, 0.47) | (0.14, 0.53) | (−0.03, 0.38) | (0.06, 0.44) | (0.02, 0.41) | (0.05, 0.45) | (−0.03, 0.39) | |
| P value | 0.005 | 0.014 | 0.008 | 0.036 | 0.014 | 0.007 | 0.001 | 0.090 | 0.011 | 0.029 | 0.015 | 0.087 | |
Linear regression adjusted for age, sex, body mass index, walking speed and presence of TFJ OA; β, standardized regression coefficients; CI, confidence interval. Associations that reach the statistical significance level (P < 0.05) are in bold.
Laminar analysis
Laminar analysis showed that overall, the superficial layer of PFJ cartilage showed stronger associations with knee kinetic variables (Table III). Both patellar and trochlear superficial cartilage T1ρ and T2 showed significant positive correlations with peak KFM and KFM impulse, with the relationship between the superficial trochlea T1ρ and peak KFM trending toward statistical significance (P = 0.054). The standardized regression coefficients ranged from 0.22 to 0.33. For the deep layers of patellar and trochlear cartilage, only trochlea T1ρ and T2 showed significant associations with KFM impulse. The standardized regression coefficients were 0.27 and 0.22, respectively.
Subgroup analysis
Results of linear regressions for the PFJ OA group are displayed in Table IV. Both peak KFM and KFM impulse showed significant positive correlations with trochlea T1ρ and T2 (whole compartment and superficial layer) and patella T1ρ (superficial layer). The standardized regression coefficients ranged from 0.29 to 0.40. KFM impulse was also significantly associated with T1ρ and T2 of deep layer trochlea (standardized regression coefficients = 0.34 and 0.38, respectively).
Table IV.
Associations between knee kinetics and cartilage T1ρ and T2 relaxation times of the patella and trochlea for subjects with PFJ OA (n = 56)*
| Whole compartment analysis | Laminar analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trochlea | Patella | Trochlea | Patella | Trochlea | Patella | Trochlea | Patella | ||||||
| T1ρ | T1ρ | T2 | T2 | T1ρ | T1ρ | T2 | T2 | ||||||
| Whole | Whole | Whole | Whole | Superficial | Deep | Superficial | Deep | Superficial | Deep | Superficial | Deep | ||
| Peak KFM | β (95% CI) | 0.30 | 0.27 | 0.29 | 0.18 | 0.30 | 0.25 | 0.33 | 0.21 | 0.29 | 0.23 | 0.22 | 0.14 (−0.25, 1.03) |
| (0.03, 0.57) | (−0.02, 0.55) | (0.01, 0.56) | (−0.10, 0.47) | (0.03, 0.57) | (−0.03, 0.53) | (0.06, 0.60) | (−0.08, 0.50) | (0.01, 0.57) | (−0.04, 0.51) | (−0.02, 0.50) | |||
| P value | 0.033 | 0.064 | 0.040 | 0.205 | 0.032 | 0.082 | 0.019 | 0.150 | 0.041 | 0.096 | 0.123 | 0.435 | |
| KFM impulse | β (95% CI) | 0.35 | 0.20 | 0.40 | 0.11 | 0.31 | 0.34 | 0.31 | 0.15 | 0.36 | 0.38 | 0.17 | 0.06 (−0.23, 0.35) |
| (0.09, 0.61) | (−0.08, 0.48) | (0.14, 0.66) | (−0.18, 0.40) | (0.05, 0.57) | (0.08, 0.60) | (0.04, 0.57) | (−0.14, 0.43) | (0.09, 0.62) | (0.12, 0.63) | (−0.11, 0.45) | |||
| P value | 0.009 | 0.154 | 0.003 | 0.440 | 0.021 | 0.013 | 0.024 | 0.304 | 0.011 | 0.005 | 0.222 | 0.683 | |
Linear regression adjusted for age, sex, body mass index, walking speed and presence of TFJ OA; β, standardized regression coefficients; CI, confidence interval. Associations that reach the statistical significance level (P < 0.05) are in bold.
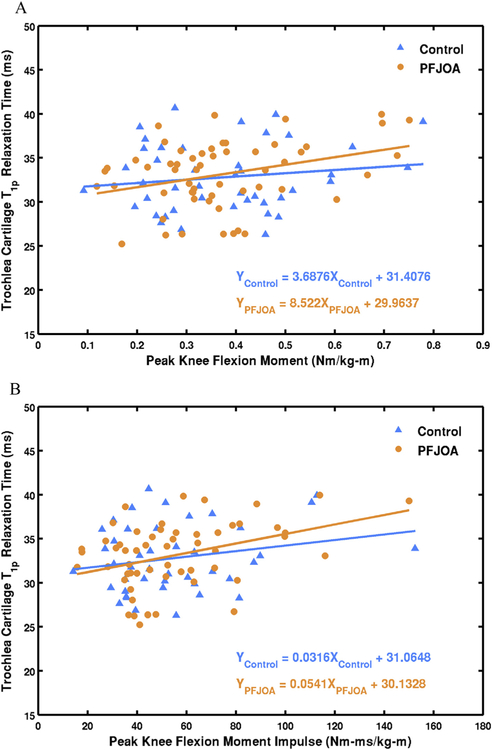
Table V displays results of linear regression for the control group. No significant associations were observed between knee kinetics and any of the whole compartment relaxation times. Laminar analysis showed significant correlations between KFM impulse and T1ρ and T2 of superficial layer patellar cartilage (standardized regression coefficients = 0.33 and 0.31, respectively). Fig. 3 shows scatter plots of cartilage relaxation time and knee kinetics in control and PFJ OA groups.
Table V.
Associations between knee kinetics and cartilage T1ρ and T2 relaxation times of the patella and trochlea for controls (n = 43)*
| Whole compartment analysis | Laminar analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trochlea | Patella | Trochlea | Patella | Trochlea | Patella | Trochlea | Patella | ||||||
| T1ρ | T1ρ | T2 | T2 | T1ρ | T1ρ | T2 | T2 | ||||||
| Whole | Whole | Whole | Whole | Superficial | Deep | Superficial | Deep | Superficial | Deep | Superficial | Deep | ||
| Peak KFM | β (95% CI) | 0.04 | 0.16 | 0.02 | 0.23 | −0.002 | 0.06 | 0.26 | 0.04 | 0.10 | −0.09 | 0.25 | 0.22 (−0.13, 0.57) |
| (−0.30, 0.39) | (−0.18, 0.51) | (−0.34, 0.37) | (−0.12, 0.58) | (−0.35, 0.34) | (−0.29, 0.40) | (−0.08, 0.60) | (−0.31, 0.39) | (−0.23, 0.43) | (−0.43, 0.26) | (−0.08, 0.58) | |||
| P value | 0.803 | 0.347 | 0.93 | 0.194 | 0.991 | 0.737 | 0.134 | 0.810 | 0.534 | 0.609 | 0.137 | 0.210 | |
| KFM impulse | β (95% CI) | 0.13 | 0.26 | −0.01 | 0.30 | 0.09 | 0.17 | 0.33 | 0.14 | 0.08 | −0.08 | 0.31 | 0.27 (−0.04, 0.60) |
| (−0.19, 0.45) | (−0.06, 0.58) | (−0.33, 0.32) | (−0.02, 0.61) | (−0.24, 0.41) | (−0.15, 0.49) | (0.03, 0.64) | (−0.19, 0.46) | (−0.23, 0.39) | (−0.41, 0.24) | (0.05, 0.61) | |||
| P value | 0.411 | 0.105 | 0.967 | 0.068 | 0.587 | 0.285 | 0.034 | 0.399 | 0.603 | 0.60 | 0.047 | 0.089 | |
Linear regression adjusted for age, sex, body mass index and walking speed; β, standardized regression coefficients; CI, confidence interval. Associations that reach the statistical significance level (P < 0.05) are in bold.
Fig. 3.
Scatter plots of trochlear cartilage T1ρ relaxation time (whole compartment) and peak KFM (A) and KFM impulse (B) with least-squares regression lines and equations for control and PFJ OA groups.
Discussion
This study aimed to investigate the associations between sagittal plane knee kinetics during walking and PFJ cartilage T1ρ and T2 relaxation times. At the whole compartment level, our findings showed that higher peak KFM and KFM impulse were significantly associated with higher T1ρ and T2 of patellar and trochlear cartilage. After adjusting for age, sex, BMI, walking speed and presence of TFJ OA, every one standard deviation increase in peak KFM and KFM impulse corresponded to 0.21 to 0.25 and 0.22 to 0.28 standard deviation increase in cartilage relaxation times, respectively (approximately 0.5–1.0 ms increase). In addition, laminar analysis revealed that both the superficial layer of patellar and trochlear cartilage showed significant positive correlations with knee kinetics in T1ρ and T2 relaxation times, whereas only the deep layer trochlear cartilage showed significant correlation with KFM impulse. Lastly, subgroup analysis showed significant positive associations between knee kinetics and trochlear and patellar T1ρ and T2 in individuals with PFJ OA, whereas only KFM impulse showed significant correlations with patellar superficial layer T1ρ and T2 in controls. Although statistically significant, it is important to keep in mind that peak KFM and KFM impulse only explain a small portion of variance in T1ρ and T2 values. Overall, our findings suggest that knee kinetics are related to PFJ cartilage composition and thus may play a role in the pathomechanics of PFJ OA.
The observed positive associations between KFM and PFJ cartilage T1ρ and T2 relaxation times are inconsistent with the previous study by Souza et al.42. In this previous study42, higher KFM during hopping was found to be related to lower averaged T1ρ and T2 of the entire knee cartilage. Young and healthy individuals were included in Souza et al. study (age, Mean ± SD, 22.7 ± 3.3 years), whereas older participants with more than half having PFJ OA were included in this current study (age, Mean ± SD, 52.0 ± 10.7 years). It has been reported that cartilage response to loading differed depending on disease state43 and age44. This may help explain the discrepancies in the observed relationships between the two studies. Caution should therefore be taken when generalizing findings of this current study to a younger population.
Findings of this study are consistent with our recent reports that individuals with PFJ cartilage lesions demonstrated higher peak KFM and KFM impulse compared to controls25, and that higher peak KFM and KFM impulse were predictive of progression of PFJ cartilage lesions and/or bone-marrow lesions at one year24. While these previous studies focused on morphological changes related to PFJ OA, this current study further revealed that these knee kinetic variables are also associated with OA-related cartilage composition in PFJ (i.e., loss of glycosaminoglycan, increase in water content and disorganization of collagen matrix). These compositional changes in cartilage may precede morphological changes and thus are imaging biomarkers of early stage cartilage degeneration26–28. Based on these findings, prevention and intervention protocols of PFJ OA may need to include the examination and reduction of peak KFM and KFM impulse.
With regard to laminar analysis, our findings suggest that increased peak KFM and KFM impulse had stronger associations with superficial cartilage relaxation times when compared to the deep layer cartilage. For example, an one-standard deviation increase in peak KFM and KFM impulse corresponded to around 0.30 to 0.33 standard deviation increase in superficial layer patella T1ρ, but only around 0.17 to 0.18 standard deviation increase in deep layer patella T1ρ. Using a finite element model, Halonen et al.45 reported higher stress and axial strain in the superficial layer of the knee cartilage than the deep layer during walking. As a result, the superficial layer PFJ cartilage may be more susceptible to mechanical load during walking and thus, show stronger association with knee kinetics than the deep layer cartilage. This finding is in line with a previous study by Li et al.,35 which showed that early cartilage degeneration after anterior cruciate ligament injury initiated from the superficial layer cartilage.
MR relaxation times of superficial PFJ cartilage have been shown to be important biomarkers of OA. Carballido-Gamio et al.34 reported that T1ρ and T2 of the superficial PFJ cartilage showed better accuracy in classifying radiographic knee OA compared to the deep layer PFJ cartilage. Moreover, Liebl et al.28 reported that higher T2 of the superficial patellar cartilage at baseline was predictive of incident radiographic TFJ OA at 4-year follow up. Drawing from the results of these previous studies and the current study, PFJ and TFJ OA may both be associated with increased peak KFM and KFM impulse.
This study also revealed stronger relationships between knee kinetics and PFJ cartilage relaxation times in individuals with PFJ OA compared to controls. In subjects with PFJ OA (defined by morphological lesions of PFJ cartilage), higher peak KFM and KFM impulse were associated with higher trochlea T1ρ and T2 (whole compartment, superficial and deep layers) and patella T1ρ (superficial layer). In control subjects, only the superficial patellar cartilage T1ρ and T2 showed significant correlations with KFM impulse. Together, this suggests that once the morphological lesions are present, the cartilage is more susceptible to mechanical overloading. This may be explained by the fact that degenerative cartilage shows lower stiffness, which lead to compromised resistance and greater deformation under mechanical loading36.
As demonstrated in Fig. 3 and Table III, control and PFJ OA subjects showed similar distributions in knee kinetics (without adjusting for covariates). The differences in correlations with cartilage relaxation time between the two groups might be driven by that PFJ OA subjects showed slightly higher T1ρ and T2 values. It is important to note that some subjects showed high T1ρ and T2 but low KFM and impulse. This suggests there might be subgroups in the PFJ OA subjects, which increased cartilage T1ρ and T2 were driven by other biological factors.
Interestingly, trochlear cartilage lesions showed a stronger relationship with knee kinetics than patellar cartilage in individuals with PFJ OA. This may be due to the fact that more severe cartilage lesions were observed in the patellar cartilage than the trochlear cartilage in PFJ OA subjects, with 25% of them having WORMS 4 cartilage lesions in patella. Due to the severe thinning and loss of the patellar cartilage, it may limit the effectiveness of cartilage relaxation time mapping on identifying changes in cartilage composition. Alternatively, this finding may suggest that trochlear cartilage showed greater change in composition under mechanical load than patellar cartilage and thus may be a more sensitive measure of PFJ pathology. Further studies are warranted to identify biomechanical factors related to patellar cartilage T1ρ and T2 in individuals with PFJ OA.
Significant difference in cartilage relaxation time was observed between PFJ OA and control groups. Individuals with PFJ OA demonstrated higher T1ρ of the patellar whole compartment and deep layer cartilage than controls after adjusting for age, sex and BMI. Interestingly, no significant differences were observed in patella T2 or trochlea T1ρ and T2 values between the two groups. Previous studies reported increased cartilage T1ρ and T2 in OA knees26–28. This discrepancy may be due to the fact that PFJ OA subjects in this study presented less advanced degenerative changes, with mean KOOS greater than 73 in all categories (Table II).
Age, sex and BMI were controlled in our statistical models. Post-hoc analysis showed that age, sex and BMI were significantly associated with knee kinetics and PFJ cartilage relaxation times. These findings are supported by previous studies. For instance, older age, female sex and higher BMI have been reported as risk factors of knee OA46 and may contribute to higher cartilage T1ρ and T2. In addition, age-, sex- and BMI-associated differences in gait patterns have been reported in the literature47e,49. More specifically, older age is related to slower walking speed47 and thus lower KFM and KFM impulse. Moreover, higher BMI was found to be related to higher KFM during walking49. Lastly, male and female adults showed different spatiotemporal characteristics in gait48, which can lead to differences in knee kinetics.
There were several limitations of this study. First, due to the cross-sectional design of the study, causality cannot be determined. Longitudinal studies are warranted to elucidate casual-effect relationships between knee kinetics and PFJ cartilage relaxation times. Second, contralateral TFJ OA was present in 20.2% of subjects and might influence the observed gait patterns. Post-hoc analysis was performed to compare knee kinetics between subjects with and without contralateral TFJ OA and found no significant difference. This finding is consistent with a recent study showing no difference in gait kinematics and kinetics between unilateral and bilateral TFJ OA patients50. Third, gait biomechanics associated with medial and lateral PFJ OA may be different but were not evaluated in this study. Further studies are needed to investigate biomechanical factors uniquely associated with medial or lateral PFJ OA. Fourth, significant but low associations were observed between knee kinetics and PFJ cartilage relaxation times. This suggests that other variables need to be considered when evaluating factors associated with PFJ cartilage relaxation times. Fifth, T1ρ and T2 relaxation time images were acquired using 4 mm slice thickness sequence, which might result in partial volume effect. However, decreasing slice thickness may result in lower signal-to-noise ratio as well as much longer image acquisition time, thereby introducing higher risk of motion artifacts. Lastly, laminar analysis was performed by dividing the cartilage into two equal layers. This method was chosen to avoid partial volume effects between layers, especially in knees with severe cartilage thinning. It is important to note that this method may not provide enough spatial resolution of cartilage composition changes from articular to bone surfaces. Additionally, using this method, half of the cartilage was arbitrarily determined as superficial layer cartilage, which might not correspond to histological findings.
In conclusion, this study revealed that increased T1ρ and T2 relaxation times of the patellar and trochlear cartilage are associated with higher peak KFM and KFM impulse after adjusting for age, sex, BMI, walking speed and presence of TFJ OA. Additionally, the observed associations were stronger for the superficial layer cartilage than the deep layer cartilage, suggesting that the superficial layer PFJ cartilage may be more susceptible to mechanical overload. Lastly, compared to controls, PFJ OA subjects showed stronger correlations between knee kinetics and PFJ T1ρ and T2. This indicates that the presence of cartilage morphological lesions may predispose PFJ cartilage to greater deterioration under mechanical loading than those without morphological lesions. Overall, our findings suggest that higher peak KFM and KFM impulse are related to worse PFJ cartilage composition in the presence of OA, and therefore may be important biomechanical factors of PFJ OA.
Acknowledgments
The project described was supported by Grant Numbers R01 AR062370, R01 AR046905, and P50 AR060752, from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, United States. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculo-skeletal and Skin Diseases or the National Institutes of Health.
Role of the funding source
The funding source had no role in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Conflict of interest
None of the authors have any financial and personal relationships with other people or organizations that could potentially and inappropriately influence (bias) this work and conclusions.
References
- 1.Duncan RC, Hay EM, Saklatvala J, Croft PR. Prevalence of radiographic osteoarthritiseit all depends on your point of view. Rheumatology (Oxford) 2006;45(6):757–60. [DOI] [PubMed] [Google Scholar]
- 2.McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis 1992;51(7):844–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefanik JJ, Niu J, Gross KD, Roemer FW, Guermazi A, Felson DT. Using magnetic resonance imaging to determine the compartmental prevalence of knee joint structural damage. Osteoarthritis Cartilage 2013;21(5):695–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornaat PR, Bloem JL, Ceulemans RYT, Riyazi N, Rosendaal FR, Nelissen RG, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology 2006;239(3):811–7. [DOI] [PubMed] [Google Scholar]
- 5.Duncan R, Peat G, Thomas E, Wood L, Hay E, Croft P. Does isolated patellofemoral osteoarthritis matter? Osteoarthritis Cartilage 2009;17(9):1151–5. [DOI] [PubMed] [Google Scholar]
- 6.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage 2013;21(1):10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andriacchi TP, Favre J. The nature of in vivo mechanical signals that influence cartilage health and progression to knee osteoarthritis. Curr Rheumatol Rep 2014;16(11):463. [DOI] [PubMed] [Google Scholar]
- 8.Chehab EF, Favre J, Erhart-Hledik JC, Andriacchi TP. Baseline knee adduction and flexion moments during walking are both associated with 5 year cartilage changes in patients with medial knee osteoarthritis. Osteoarthritis Cartilage 2014;22(11):1833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennell KL, Bowles KA, Wang Y, Cicuttini F, Davies-Tuck M, Hinman RS. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheum Dis 2011;70(10):1770–4. [DOI] [PubMed] [Google Scholar]
- 10.Foroughi N, Smith R, Vanwanseele B. The association of external knee adduction moment with biomechanical variables in osteoarthritis: a systematic review. Knee 2009;16(5): 303–9. [DOI] [PubMed] [Google Scholar]
- 11.Fok LA, Schache AG, Crossley KM, Lin Y-C, Pandy MG. Patellofemoral joint loading during stair ambulation in people with patellofemoral osteoarthritis. Arthritis Rheum 2013. July 26;65(8):2059–69. [DOI] [PubMed] [Google Scholar]
- 12.Farrokhi S, O’Connell M, Fitzgerald GK. Altered gait biomechanics and increased knee-specific impairments in patients with coexisting tibiofemoral and patellofemoral osteoarthritis. Gait Posture 2015;41(1):81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crossley KM, Dorn TW, Ozturk H, van den Noort J, Schache AG, Pandy MG. Altered hip muscle forces during gait in people with patellofemoral osteoarthritis. Osteoarthritis Cartilage 2012;20(11):1243–9. [DOI] [PubMed] [Google Scholar]
- 14.Culvenor AG, Schache AG, Vicenzino B, Pandy MG, Collins NJ, Cook JL, et al. Are knee biomechanics different in those with and without patellofemoral osteoarthritis after anterior cruciate ligament reconstruction? Arthritis Care Res 2014;66(10): 1566–70. [DOI] [PubMed] [Google Scholar]
- 15.Pohl MB, Patel C, Wiley JP, Ferber R. Gait biomechanics and hip muscular strength in patients with patellofemoral osteoarthritis. Gait Posture 2013;37(3):440–4. [DOI] [PubMed] [Google Scholar]
- 16.Besier TF, Fredericson M, Gold GE, Beaupré GS, Delp SL. Knee muscle forces during walking and running in patellofemoral pain patients and pain-free controls. J Biomech 2009;42(7): 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng H-L, Powers CM. Sagittal plane trunk posture influences patellofemoral joint stress during running. J Orthop Sports Phys Ther 2014;44(10):785–92. [DOI] [PubMed] [Google Scholar]
- 18.Besier TF, Gold GE, Beaupré GS, Delp SL. A modeling framework to estimate patellofemoral joint cartilage stress in vivo. Med Sci Sports Excerc 2005;37(11):1924–30. [DOI] [PubMed] [Google Scholar]
- 19.Bennell KL, Creaby MW, Wrigley TV, Bowles KA, Hinman RS, Cicuttini F, et al. Bone marrow lesions are related to dynamic knee loading in medial knee osteoarthritis. Ann Rheum Dis 2010;69(6):1151–4. [DOI] [PubMed] [Google Scholar]
- 20.Creaby MW, Wang Y, Bennell KL, Hinman RS, Metcalf BR, Bowles KA, et al. Dynamic knee loading is related to cartilage defects and tibial plateau bone area in medial knee osteoarthritis. Osteoarthritis Cartilage 2010;18(11):1380–5. [DOI] [PubMed] [Google Scholar]
- 21.Kean CO, Hinman RS, Bowles K-A, Cicuttini F, Davies-Tuck M, Bennell KL. Comparison of peak knee adduction moment and knee adduction moment impulse in distinguishing between severities of knee osteoarthritis. Clin Biomech 2012;27(5): 520–3. [DOI] [PubMed] [Google Scholar]
- 22.Maly MR, Robbins SM, Stratford PW, Birmingham TB, Callaghan JP. Cumulative knee adductor load distinguishes between healthy and osteoarthritic kneesea proof of principle study. Gait Posture 2013;37(3):397–401. [DOI] [PubMed] [Google Scholar]
- 23.Ho K-Y, Blanchette MG, Powers CM. The influence of heel height on patellofemoral joint kinetics during walking. Gait Posture 2012;36(2):271–5. [DOI] [PubMed] [Google Scholar]
- 24.Teng H-L, MacLeod TD, Link TM, Majumdar S, Souza RB. Higher knee flexion moment during the second half of the stance phase of gait is associated with the progression of osteoarthritis of the patellofemoral joint on magnetic resonance imaging. J Orthop Sports Phys Ther 2015;45(9):656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng H-L, MacLeod TD, Kumar D, Link TM, Majumdar S, Souza RB. Individuals with isolated patellofemoral joint osteoarthritis exhibit higher mechanical loading at the knee during the second half of the stance phase. Clin Biomech 2015;30(4): 383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 yearsedata from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2012;20(7):727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Benjamin Ma C, Link TM, Castillo D-D, Blumenkrantz G, Lozano J, et al. In vivo T1ρho and T2 mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 2007;15(7):789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebl H, Joseph G, Nevitt MC, Singh N, Heilmeier U, Subburaj K, et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis 2015;74(7):1353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keenan KE, Besier TF, Pauly JM, Han E, Rosenberg J, Smith RL, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1ρ and T2 MRI. Osteoarthritis Cartilage 2011;19(2):171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging 2011;29(3):324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med 2001;46(3):419–23. [DOI] [PubMed] [Google Scholar]
- 32.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 2004;8(4):355–68. [DOI] [PubMed] [Google Scholar]
- 33.Liess C, Lüsse S, Karger N, Heller MO, Glüer C-C. Detection of changes in cartilage water content using MRI T 2-mapping in vivo. Osteoarthritis Cartilage 2002;10(12):907–13. [DOI] [PubMed] [Google Scholar]
- 34.Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM. Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys 2009;36(9):4059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1rho and T2einitial experience with 1-year follow-up. Radiology 2011;258(2):505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansour JM. Biomechanics of cartilage In: CA O, Kinesiology: The Mechanics and Pathomechanics of Human Movement. Baltimore, MD: Lip- pincott Williams & Wilkins; 2003: 68–77. [Google Scholar]
- 37.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subburaj K, Kumar D, Souza RB, Alizai H, Li X, Link TM, et al. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med 2012;40(9):2134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Souza RB, Kumar D, Calixto N, Singh J, Schooler J, Subburaj K, et al. Response of knee cartilage T1rho and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthritis Cartilage 2014;22(10): 1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carballido-Gamio J, Bauer JS, Stahl R, Lee K-Y, Krause S, Link TM, et al. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal 2008;12(2):120–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;12(3):177–90. [DOI] [PubMed] [Google Scholar]
- 42.Souza RB, Fang C, Luke A, Wu S, Li X, Majumdar S. Relationship between knee kinetics during jumping tasks and knee articular cartilage MRI T1rho and T2 relaxation times. Clin Biomech 2012;27(4):403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Jt Surg Am 2009;91(Suppl 1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blazek K, Favre J, Asay J, Erhart-Hledik J, Andriacchi T. Age and obesity alter the relationship between femoral articular cartilage thickness and ambulatory loads in individuals without osteoarthritis. J Orthop Res 2013;32(3):394–402. [DOI] [PubMed] [Google Scholar]
- 45.Halonen KS, Mononen ME, Jurvelin JS, Töyräs J, Korhonen RK. Importance of depth-wise distribution of collagen and proteoglycans in articular cartilage–a 3D finite element study of stresses and strains in human knee joint. J Biomech 2013;46(6):1184–92. [DOI] [PubMed] [Google Scholar]
- 46.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 2010;18(1): 24–33. [DOI] [PubMed] [Google Scholar]
- 47.Ko SU, Hausdorff JM, Ferrucci L. Age-associated differences in the gait pattern changes of older adults during fast-speed and fatigue conditions: results from the Baltimore longitudinal study of ageing. Age Ageing 2010;39(6):688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho SH, Park JM, Kwon OY. Gender differences in three dimensional gait analysis data from 98 healthy Korean adults. Clin Biomech 2004;19(2):145–52. [DOI] [PubMed] [Google Scholar]
- 49.Harding GT, Hubley-Kozey CL, Dunbar MJ, Stanish WD, Wilson JLA. Body mass index affects knee joint mechanics during gait differently with and without moderate knee osteoarthritis. Osteoarthritis Cartilage 2012;20(11):1234–42. [DOI] [PubMed] [Google Scholar]
- 50.Messier SP, Beavers DP, Herman C, Hunter DJ, Devita P. Are unilateral and bilateral knee osteoarthritis patients unique subsets of knee osteoarthritis? A biomechanical perspective. Osteoarthritis Cartilage 2016;24(5):807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]