Abstract
The purpose of this pilot study was to longitudinally quantify the T2 laminar integrity of knee cartilage in a subset of subjects with osteoarthritis from the Osteoarthritis Initiative at baseline, 1-year follow-up, and 2-year follow-up. Cartilage from 13 subjects was divided into six compartments and subdivided into deep and superficial layers. At each time point, mean T2 values in superficial and deep layers were compared. Longitudinal analysis included full-thickness mean T2, mean deep T2, mean superficial T2, mean T2 laminar difference, mean percentage T2 laminar difference, and two-dimensional measures of cartilage thickness. More compartments showed significantly higher superficial T2 than deep T2 values at baseline and 1-year follow-up compared to 2-year follow-up. No significant longitudinal changes of full-thickness mean T2 and superficial T2 values were observed. Significant longitudinal changes were observed in the deep T2 values, T2 laminar difference, and percentage T2 laminar difference. Cartilage thickness had no influence on T2 analysis. Results of this study suggest that laminar analysis may improve the sensitivity to detect longitudinal T2 changes and that disruption of the T2 laminar organization of knee cartilage may be present in knee osteoarthritis progressors. Further investigation is warranted to evaluate the potential of the presented methodology to better characterize evolution and pathophysiology of osteoarthritis.
Keywords: T2, cartilage, Osteoarthritis Initiative, OAI, MRI
MRI T2 relaxation time is a parameter sensitive to biochemical changes, particularly changes in water and collagen content and tissue anisotropy (1). In cross-sectional studies, differences in knee cartilage T2 have been observed between healthy controls and subjects with knee osteoarthritis (OA) (2–5) and between subjects with different levels of knee OA (4,6). Differentiation of normal hyaline cartilage from reparative tissue in terms of T2 has also been investigated (7), as well as T2 differences with respect to age (8), gender (9), and exercise (10).
The few longitudinal studies evaluating knee cartilage T2 changes that have been published have shown conflicting (or diverging) results (3,11–13). Studies have demonstrated that there is a laminar organization of T2 values in knee cartilage, with lower values close to the subchondral bone and higher values close to the articular surface (8,14–16). The potential of the T2 laminar analysis in evaluating cartilage degeneration has also been demonstrated (13,17,18).
The Osteoarthritis Initiative (OAI; http:/www.oai.ucsf.edu/) is a multicenter, 4-year, prospective observational study of men and women, designed to better understand how to prevent and treat knee OA. The study is designed to help find biochemical, genetic, and imaging-based biomarkers for development and progression of OA. The OAI will establish and maintain a natural history database that includes clinical evaluation data, radiographs, MR images, and biospecimen repository. MRI in the OAI also includes acquisitions for T2 quantification at each time point.
The hypotheses are that the laminar organization of knee T2 cartilage becomes disrupted with progression of OA and that by analyzing the T2 laminar pattern, early cartilage degeneration changes may be detected. To test these hypotheses and develop the methodology, the purpose of this pilot work was to longitudinally quantify the T2 laminar integrity of knee cartilage, using a subset of OA subjects from the OAI progression cohort at baseline, 1-year follow-up, and 2-year follow-up.
MATERIALS AND METHODS
Subjects
Thirteen subjects from the progression cohort of the OAI were included in this study, a subset of the 4796 participants involved in the OAI study. The inclusion criteria for this cohort required that both of the following conditions be present in at least one knee at baseline:
Radiographic signs of OA, defined as definite osteophytes based on posteroanterior fixed flexed radiographs (Osteoarthritis Research Society International (OARSI) atlas grade ≥1). Subjects with OARSI grade of 3 (severe narrowing) were excluded due to expected severe cartilage loss.
Presence of frequent knee symptoms, defined as pain,aching, or stiffness on most days of a month in the past year.
Subjects had a mean ± standard deviation age of 55.7 ± 10.6 years and body mass index mean ± standard deviation of 30.1 ± 3.7 kg/m2, as well as radiographic Kellgren-Lawrence scores (19) of 2 (n =7) and 3 (n = 6). Kellgren-Lawrence = 2 means definite osteophytes and definite narrowing of joint space, while Kellgren-Lawrence = 3 means moderate multiple osteophytes, definite narrowing of joint space, some sclerosis, and possible deformity of bone contour.
The study protocol, amendments, and informed consent documentation, including analysis plans, were reviewed and approved by the local institutional review boards. Data used in the preparation of this article were obtained from the OAI database, which is available for public access at http:/www.oai.ucsf.edu/. Baseline clinical dataset 0.2.2 was used in this study.
MRI
MRI studies of the right knee were used in this study since this side included sequences for cartilage morphology quantification and T2 mapping. Images were obtained at 3 T (Siemens Magnetom Trio; Siemens, Erlangen, Germany) using quadrature transmit-receive knee coils (USA Instruments, Aurora, OH). The sequence for cartilage morphology quantification (i.e., segmentation) consisted of a sagittal three-dimensional double echo in steady state (DESS) with water excitation (WE), pulse repetition time of 16.3 ms, echo time (TE) of 4.7 ms, bandwidth of 185 Hz/pixel, in-plane spatial resolution of 0.365mm 0.456 mm (0.365 mm 0.365 mm after reconstruction), and slice thickness of 0.7 mm. The sequence for T2 mapping consisted of a sagittal two-dimensional (2D) multiecho spin-echo with pulse repetition time of 2700 ms, seven TEs (10 ms, 20 ms, 30 ms, 40 ms, 50 ms, 60 ms, and 70 ms), bandwidth of 250Hz/pixel, in-plane spatial resolution of 0.313 mm 0.446 mm (0.313 mm 0.313 mm after reconstruction), slice thickness of 3.0 mm, and 0.5 mm gap. For further details of image acquisition parameters, please refer to the report of Peterfy et al. (20). Images used in this study are available for public access at http:/www.oai.ucsf.edu/. The specific image datasets used in this study were 0.B.1, 1.B.1, and 3.B.1.
Image Analysis
Cartilage was segmented semiautomatically from the DESS-WE images, as described in Carballido-Gamio et al. (21). Basically, Bezier splines were used to represent the bone-cartilage interface and the articular surface, and their control points were positioned on the cartilage boundaries using image enhancement and edge detection techniques. Cartilage was segmented into six distinct compartments: medial femoral condyle (MFC), lateral femoral condyle (LFC), trochlea (TRO), patella (PAT), medial tibia (MT), and lateral tibia (LT).
Studies have suggested that excluding the first echo from a multiecho Carr-Purcell-Meiboom-Grill sequence minimizes error from stimulated echoes in calculated T2 values for cartilage (14,22). For this reason, image quality for T2 calculations was measured at baseline in the second (TE2 = 20 ms) and last echo (TE7 = 70 ms) images in terms of signal-to-noise ratio (SNR). T2 maps at baseline, 1-year follow-up, and 2-year follow-up were then created on a pixel-by-pixel basis using in-house-developed software based on the Levenberg-Marquardt algorithm, and assuming monoexponential decay:
| [1] |
In Eq. 1, S is the signal intensity in a T2-weighted image with a certain TE, and S0 is the signal intensity when TE = 0 ms. T2 maps were calculated excluding the first echo (TE2 = 20 ms TE7 = 70 ms), and at baseline they were compared to those using all echoes (TE1 = 10 ms TE7 = 70 ms). Comparisons were made using two different metrics of goodness of fit, the root mean squared error (RMSE) and the adjusted coefficient of multiple determination (Adjusted R2), as given in Eqs. 2 and 3, respectively:
| [2] |
| [3] |
In Eqs. 2 and 3, Si represents the measured value at the ith TE, represents the predicted value at the ith TE, is the mean of the measured values, n is the number of echoes (seven for all echoes and six excluding the first echo), and m is the number of fitted coefficients estimated from the measured values (m = 2; S0 and T2).
As there was patient motion between the DESS-WE and T2 mapping acquisitions in most of the cases (34 out of 39), before cartilage segmentations were transferred from the DESS-WE to the T2 maps, three-dimensional automatic image registration was performed using mutual information (VTK CISG Registration Toolkit). The first echoes (TE1 = 10 ms) and the DESS-WE images were used to calculate the transformations that were applied to the T2 maps. Once images were aligned, the Bezier splines representing the bone cartilage interface and the articular surface were transferred from the DESS-WE images to the aligned T2 maps.
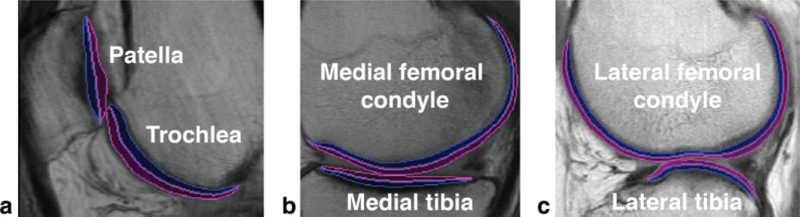
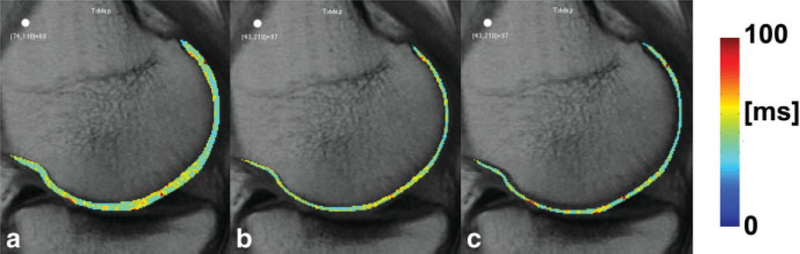
Full-thickness mean T2 values of each compartment were calculated at baseline, 1-year follow-up, and 2-year follow-up. T2 cartilage laminar analysis was also done for each compartment and time point, and it was performed automatically on a slice-by-slice basis using in-house-developed software implemented in MATLAB (The Mathworks Inc., Natick, MA). Knee cartilage was partitioned into two layers: deep and superficial. The deep layer corresponded to the bone-cartilage interface, and the superficial layer, to the articular surface. The Euclidean distances of all cartilage pixels to the splines representing the bone-cartilage interface and articular surface were calculated. Cartilage pixels were assigned to only one layer, which corresponded to the closest spline. Figure 1 shows representative examples of laminar regions of interest for each cartilage compartment used in this study. Cartilage was partitioned into two layers and not three (17) to minimize imminent partial-volume effects within the layers. Figure 2 shows a representative example of a MFC full-thickness T2 map and deep and superficial T2 layers. The difference between mean superficial and mean deep T2 values was also assessed with two different metrics, one expressed in milliseconds (Eq. 4) and another expressed in percentage (Eq. 5):
| [4] |
| [5] |
FIG. 1.
Representative laminar regions of interest for the (a) PAT and TRO, (b) MFC and MT, and (c) LFC and LT. Blue regions correspond to the deep layer, while magenta regions correspond to the superficial layer.
FIG. 2.
Example of T2 laminar analysis in the MFC. a: Full-thickness map. b: Deep layer. c: Superficial layer. Slightly elevated T2 values are observed in cartilage regions about 55° with respect to main magnetic field, possibly due to the T2 magic-angle effect
To investigate a possible relationship between laminar thickness changes and T2 changes, mean 2D cartilage thickness was computed using the splines of T2 maps in all compartments and time points. For each point in the spline representing the bone-cartilage interface, a normal vector was computed. The heads of these normal vectors ended at the articular surface, and the mean lengths of these vectors were taken as the 2D cartilage thickness values. It should be clarified that these measurements should not be confused with the actual cartilage thickness, which can be better computed in three dimensions using the DESS-WE images. The 2D measurements in this case better represent the thickness of the T2 regions of interest, and consequently the thickness of each layer (~one-half).
Statistical Analysis
Statistical analysis was done individually for each compartment using mean values.
The comparisons at baseline between T2 fittings using all echoes (TE1 = 10 ms -T7 = 70 ms) against those excluding the first echo (TE2 = 20 ms TE7 = 70 ms) were done with unpaired t tests for both RMSE and Adjusted R2 measures.
Paired t tests were used to compare the superficial and deep T2 values at each time point. Longitudinal changes (baseline, 1-year follow-up, 2-year follow-up) of full thickness mean T2 values, mean deep T2 values, mean superficial T2 values, T2 difference in milliseconds, T2 percentage difference, and mean 2D thickness values were analyzed using repeated-measures analysis of variance followed by Bonferroni corrections (23). Power analysis of longitudinal changes of T2 relaxation times was done using power techniques for repeated-measures analysis of variance (23). Statistical analysis was done using JMP (SAS Institute, Cary, NC) and MATLAB (The Mathworks Inc.). Results were considered significant if P values were less than 0.05.
RESULTS
T2 Fitting
Results for SNR measurements are summarized in Table 1 for each compartment. Mean SNR values were relatively low in the last echo (TE7 = 70 ms) but sufficient for T2 fitting. While the MT showed the lowest SNR, the TRO showed the highest, which could be due to the fact that this is a characteristic location for sampling at the magic angle (24). The ratio of mean cartilage signal over mean background signal in the last echo was on average 3.264.
Table 1.
Mean SNR of Knee Cartilage Compartments at Baseline
| Compartment | TE2 = 20 ms | TE7 = 70 ms |
|---|---|---|
| MFC | 16.476 | 6.745 |
| LFC | 19.981 | 7.967 |
| TRO | 20.000 | 8.704 |
| PAT | 18.325 | 7.292 |
| MT | 13.745 | 5.019 |
| LT | 18.450 | 6.699 |
In terms of the T2 fitting comparisons using all echoes against those excluding the first one (TE1 = 10 ms), RMSE and Adjusted R2 values were lower for the latter, as shown in Table 2. Since RMSE represents the error of fitted values with respect to measured signals and Adjusted R2 is a measure of correlation, lower RMSE values and higher Adjusted R2 values are desired. Interestingly, the MT showed the lowest RMSE; however, it also showed the lowest Adjusted R2. The highest RMSE was observed in the PAT, while the highest Adjusted R2 was observed in the LFC. Based on Table 2 and previous phantom studies that have shown that by excluding the first echo from multiecho spin-echo sequences, T2 accuracy improves (22), fittings excluding the first echo were used for subsequent analysis.
Table 2.
Comparison of Quality of T2 Fittings at Baseline
| Compartment | RMSE |
Adjusted R2 |
||
|---|---|---|---|---|
| TE1-TE7 | TE2-TE7 | TE1-TE7 | TE2-TE7 | |
| MFC | 38.823 | 32.391* | 0.816 | 0.752*** |
| LFC | 40.737 | 33.320** | 0.855 | 0.810*** |
| TRO | 43.871 | 35.946** | 0.815 | 0.766** |
| PAT | 43.942 | 36.149** | 0.799 | 0.750* |
| MT | 36.894 | 30.812* | 0.780 | 0.681** |
| LT | 40.859 | 33.265** | 0.836 | 0.777* |
Differences were significant at
P < 0.05
P < 0.01
P < 0.001.
T2 Analysis
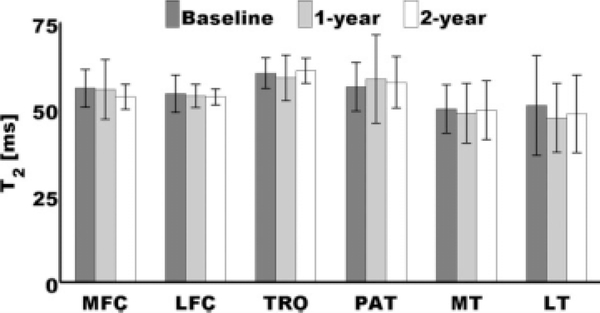
Repeated-measures analysis of variance indicated no significant longitudinal changes of full-thickness mean T2 values in all compartments, as can be appreciated in Fig. 3.
FIG. 3.
Bar plot showing full-thickness mean T2 longitudinal results. No significant changes were observed. Error bars represent standard deviations.
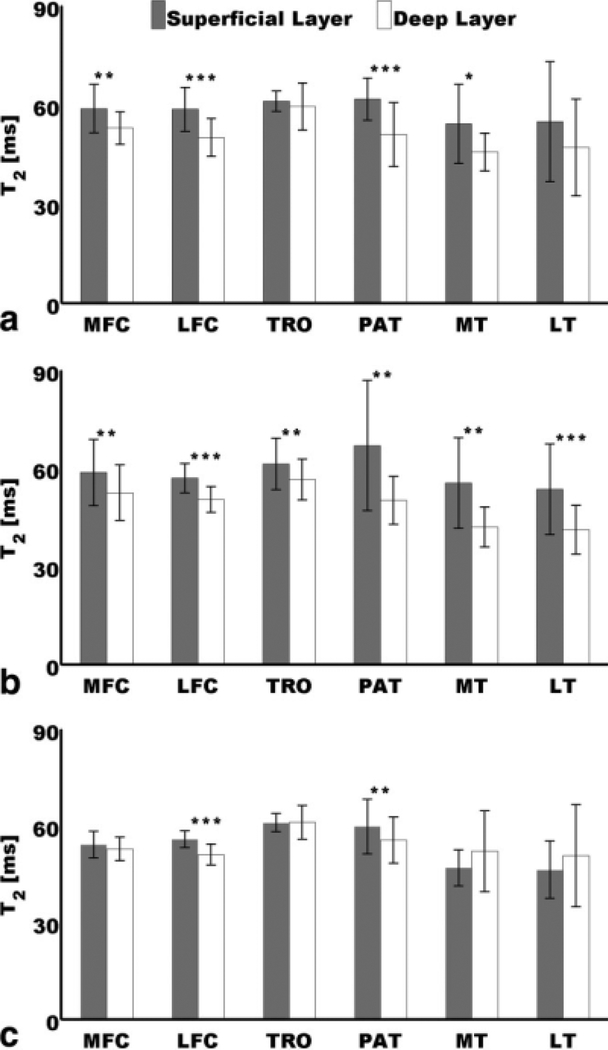
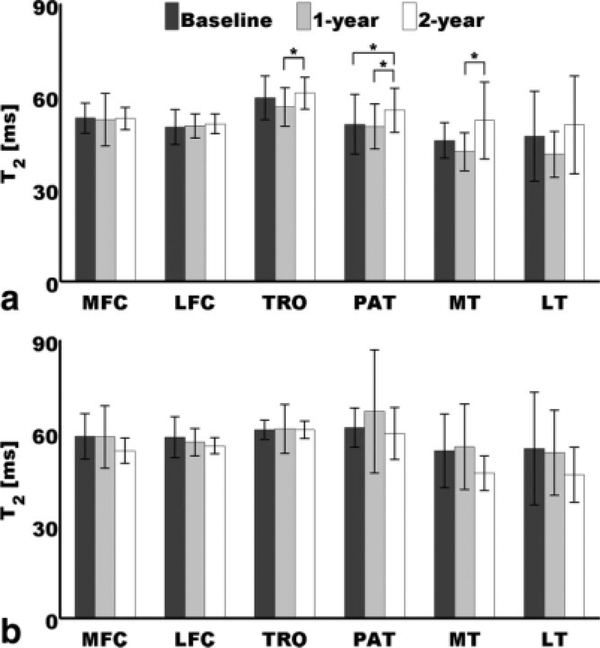
Figure 4 shows results of paired t tests done at each time point to compare mean deep and mean superficial T2 values. It can be appreciated that at baseline and 1-year follow-up, most of the mean superficial values were higher than the mean deep values, and most of these observations were significant. It can also be appreciated that at 2-year follow-up, mean superficial and mean deep T2 showed more similar values, and most of the significant observations at baseline and 1-year follow-up were not significant at this time point. While the LFC and the PAT showed significantly higher superficial values at the three time points, the MFC and the MT did it only at baseline and 1-year follow-up. The TRO and the LT showed this laminar pattern of higher mean superficial values then mean deep values at baseline and 1-year follow-up, but it was only significant at 1-year follow-up.
FIG. 4.
Comparison of mean superficial and mean deep T2 values at (a) baseline, (b) 1-year follow-up, and (c) 2-year follow-up. Mean superficial values were significantly higher than mean deep values at *P< 0.05, **P< 0.01, and ***P< 0.001. Error bars represent standard deviations.
Longitudinal analysis of the mean deep and mean superficial values are shown in Fig. 5a and b, respectively. No significant longitudinal changes were observed at the superficial layer in all compartments, and no significant longitudinal changes were observed between baseline and 1-year follow-up at the deep layer in all compartments. Only four significant changes were detected, and those were in the deep layer. The first was between 1year follow-up and 2-year follow-up in the TRO. The second and third were in the PAT, between 2-year follow-up and the other time points, baseline and 1-year follow-up. The fourth change was seen in the MT between 1-year follow-up and 2-year follow-up.
FIG. 5.
Longitudinal changes of (a) mean deep T2 values and (b) mean superficial T2 values. Longitudinal changes were significant at *P< 0.05. Error bars represent standard deviations.
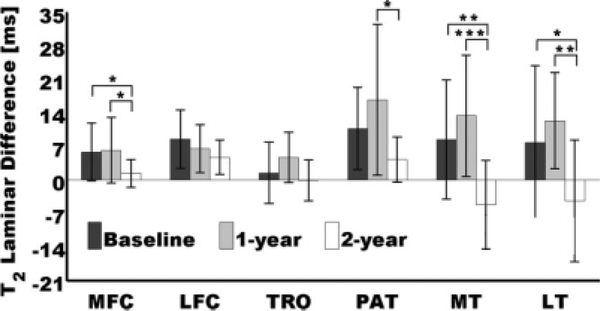
Significant longitudinal laminar changes were observed for the expression in Eq. 4, i.e., the difference of mean laminar values expressed in milliseconds. Consistent with the longitudinal changes observed in Fig. 5, no significant differences were observed between baseline and 1-year follow-up in all compartments. Although no significant changes were observed at the TRO, and between baseline and 2-year follow-up in the PAT, as could be expected from Fig. 5a, additional longitudinal changes were detected by the T2 laminar difference as shown in Fig. 6. The PAT showed significant T2 laminar difference changes between 1-year follow-up and 2-year follow-up, and the MFC, the MT, and the LT, showed significant longitudinal T2 laminar difference changes between 2-year follow-up and the other time points, baseline and 1-year follow-up.
FIG. 6.
Longitudinal changes of T2 laminar differences: mean superficial T2 – mean deep T2. Longitudinal T2 laminar difference changes were significant at *P < 0.05, **P < 0.01, and ***P < 0.001. Error bars represent standard deviations.
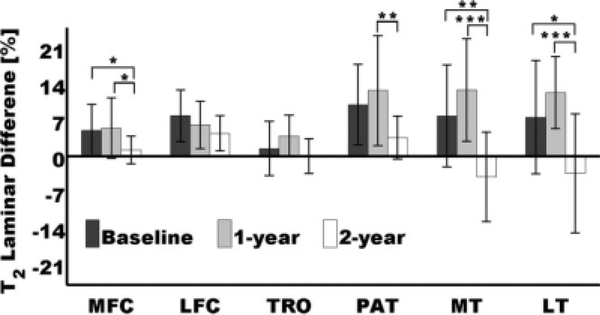
The last metric analyzed in this study was the T2 percentage difference (Eq. 5), and Fig. 7 is a longitudinal bar plot showing the corresponding results for all compartments. As can be observed, results in Fig. 7 and Fig. 6 are quite similar; P values in the PAT and LT being the only difference. While the longitudinal T2 difference change (expressed in milliseconds) between 1-year follow-up and 2-year follow-up in the PAT showed a significance of P < 0.05, the longitudinal T2 percentage difference (expressed in percent) showed a significance of P < 0.01. A similar result was observed in the LT, where the T2 difference change in milliseconds between 1-year follow-up and 2-year follow-up showed a significance of P < 0.01, and the T2 percentage difference showed a significance of P < 0.001.
FIG. 7.
Longitudinal changes of T2 laminar percentage differences (Eq. 5). Longitudinal T2 laminar percentage difference changes were significant at *P < 0.05, **P < 0.01, and ***P < 0.001. Error bars represent standard deviations.
Power analysis of longitudinal changes of T2 relaxation times for repeated-measures analysis of variance is summarized in Table 4. Table 4 also shows the minimum number of subjects that would be required to detect the smallest significant changes observed in this study with P < 0.05 but with a power of 0.90. Values corresponding to nonsignificant longitudinal changes were omitted in this table.
Table 4.
Power Analysis of Longitudinal T2 Relaxation Time Changes*
| Power values |
Number of subjects for minimum change and power = 0.90 | ||
|---|---|---|---|
| Baseline to 2 years | 1 Year to 2 years | ||
| TRO: Deep layer | NS | 0.550 | 26 |
| PAT: Deep layer | 0.672 | 0.778 | 26 |
| MT: Deep layer | NS | 0.672 | 23 |
| MFC: Difference in ms | 0.672 | 0.778 | 21 |
| PAT: Difference in ms | NS | 0.778 | 17 |
| MT: Difference in ms | 0.778 | 0.979 | 16 |
| LT: Difference in ms | 0.672 | 0.919 | 25 |
| MFC: Percentage difference | 0.672 | 0.778 | 21 |
| PAT: Percentage difference | NS | 0.861 | 16 |
| MT: Percentage difference | 0.861 | 0.990 | 15 |
| LT: Percentage difference | 0.778 | 0.957 | 20 |
All values at P < 0.05.
NS = power was not computed since the longitudinal change was not significant.
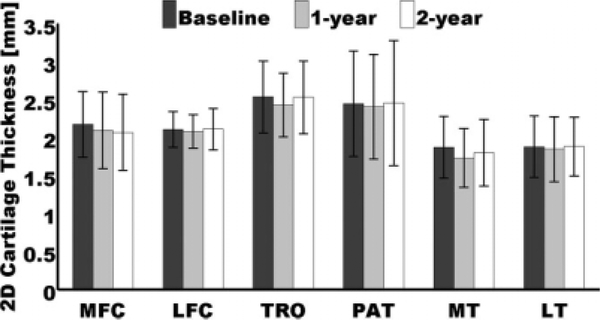
As mentioned in the previous section, 2D cartilage thickness values were also calculated to investigate the effect of the number of pixels traversing the regions of interest. Repeated-measures analysis of variance did not show any longitudinal change of this measurement in all compartments, discarding any possible effect on the T2 analyses. Figure 8 demonstrates these results, while Table 3 shows the mean thickness of a layer in each compartment expressed in pixels. Two scenarios are shown in Table 3, one conservative, where the cartilage layers are assumed to be parallel to the pixel sides, and one extreme, where the cartilage layers are assumed to be parallel to the diagonals of the pixels. For these two scenarios, the global mean thickness values were 3.420 pixels and 2.419 pixels, respectively, indicating that in general the superficial and deep layers had between two and three pixels of thickness.
FIG. 8.
Longitudinal changes of mean 2D knee cartilage thickness values in all compartments. No significant changes were observed. Error bars represent standard deviations.
Table 3.
Average Thickness of a Cartilage Layer Expressed in Pixels
| Compartments | Pixel side = 0.3125 mm | Pixel diagonal = 0.442 mm |
|---|---|---|
| MFC | 3.384 | 2.393 |
| LFC | 3.361 | 2.377 |
| TRO | 3.999 | 2.828 |
| PAT | 3.899 | 2.757 |
| MT | 2.886 | 2.040 |
| LT | 2.993 | 2.116 |
Values were obtained by dividing the mean 2D cartilage thickness by the pixel side or diagonal and then dividing by two.
DISCUSSION
In this pilot study, a comprehensive longitudinal analysis of T2 knee cartilage relaxation times in a subset of patients from the progression cohort of the OAI has been presented. Image quality of the multiecho spin-echo images was assessed quantitatively using SNR analysis, and goodness of fit of T2 maps was evaluated quantitatively using RMSE and Adjusted R2 measurements. T2 laminar analysis was done at each time point; longitudinal analysis of full-thickness mean T2, T2 laminar values, and T2 laminar differences with two different metrics was performed; and cartilage thickness effects on T2 measurements were discarded. Furthermore, image registration was applied between DESS and T2 maps to correct for patient motion, eliminating bone and fluid contamination. Results indicate a possible disruption of the T2 laminar organization of knee cartilage in knee OA progressors manifested as a reduction of the difference between mean superficial and mean deep T2 values.
OA studies of knee cartilage have consistently reported higher mean T2 values in subjects with OA than in healthy controls (2–6). However, longitudinal studies of full-thickness T2 values have yielded diverging results. While Blumenkrantz et al. (11), in a 2-year knee OA longitudinal study, reported significant increase in fullthickness mean T2 between baseline and 2-year followup in most compartments except the LT, Stahl et al. (3) could not demonstrate changes between baseline and 1year follow-up in patients with knee OA. Similarly, Blumenkrantz et al. (12), in a 9-month longitudinal study, observed a decrease in global knee T2 in patients with knee OA, but this change did not reach significance. Similar to most of the abovementioned follow-up studies, full-thickness mean T2 values in all compartments showed no longitudinal changes in this study.
Dardzinski et al. (17) obtained T2 knee cartilage maps of a 35-year-old subject with a history of arthroscopic surgery for free chondroid fragments. While cartilage of healthy subjects had shown a pattern of spatial dependency, i.e., a laminar organization, the T2 map and T2 profile of a damaged region in the PAT of this subject showed marked heterogeneity in the distribution of T2 values, with substantial increase in T2 of the radial and transitional zones, showing no laminar organization. The authors suggested that this observation was compatible with an increased mobility of water protons and that might also reflect the loss of normal anisotropy present in the radial zone of cartilage due to damage to the collagen-proteoglycan matrix.
Mosher et al. (10), in a feasibility study, investigated T2 laminar values of knee cartilage before and after running exercise. While no statistically significant change in T2 profiles of tibial cartilage was observed, there was a significant decrease in T2 of the superficial 40% of the weight-bearing femoral cartilage after exercise, suggesting that cartilage compression results in greater anisotropy of superficial collagen fibers.
In recent studies of knee cartilage repair tissue by Welsch et al. (13,18), the authors reported a gradual recovery of cartilage T2 zonal variation after surgery. Furthermore, the authors reported that differences between healthy cartilage and cartilage repair tissue were most obvious within the deep cartilage layer for both the PAT and the MFC (18).
The previously mentioned studies highlight the potential of knee cartilage T2 laminar analysis. Laminar results of this work are coherent with results of those studies since most of the compartments showed a laminar organization at baseline (MFC, LFC, PAT, and MT) and 1year follow-up (MFC, LFC, TRO, PAT, MT, and LT), but only two compartments (LFC and PAT) had this laminar pattern at 2-year follow-up. Furthermore, while no longitudinal changes were observed in the superficial layer, the deep layer manifested increase in T2 values from baseline to 2-year follow-up (PAT) and from 1-year to 2year follow-up (TRO, PAT, and MT).
Results of this and previous studies suggest a possible disruption of the knee T2 laminar organization in damaged articular cartilage. This hypothesis becomes stronger in this study by quantitative analysis using two additional metrics, the T2 laminar difference, and the T2 percentage laminar difference. Although both metrics provided similar results, demonstrating smaller differences between superficial and deep T2 values at 2-year follow-up, the T2 percentage laminar difference yielded higher significance in two compartments (PAT and LT). A possible explanation for this phenomenon is that the percentage difference takes into account individual T2 laminar increasing or decreasing. For example, if at baseline the superficial and deep layers had a mean value of 48 ms and 44 ms, respectively, but at 1-year follow-up they had a mean value of 50 ms and 46 ms, respectively, then the laminar difference at baseline and follow-up would be the same, 4 ms, while the percentage laminar difference at baseline would be 4.35% and at follow-up 4.17%, showing a decrease. The advantage of longitudinally looking at the differences of mean T2 laminar values rather than the individual mean T2 laminar values was also demonstrated by the power analysis, which also showed higher power values for the percentage difference metric than for the millisecond difference metric in most compartments.
The most interesting findings were perhaps those of the MFC and the medial and LT, since these compartments showed that at 2-year follow-up the difference between superficial and deep T2 values was smaller in comparison to baseline and 1-year follow-up. In fact, although high standard deviations were observed in the LT, probably due to partial-volume effects related to the convex shape of cartilage at this anatomic location, it is probably the first time that a longitudinal change of T2 values in this compartment is reported since, in the study by Blumenkrantz et al. (11), the LT was the only compartment showing no increase in T2 values at 2-year follow-up. The PAT also showed an interesting behavior. Smith et al. (14) demonstrated that in asymptomatic volunteers, the T2 difference between the superficial zone and the deep zone was larger in the PAT than in the femoral and tibial compartments. In this study, the PAT showed no longitudinal changes of full-thickness T2 values and consistently showed a laminar organization at the three time points, which may be related to the previously mentioned findings (14). However, although the thickness of patellar cartilage was the thickest after the TRO, with about three pixels across each layer, minimizing to some extent partial-volume effects, significant longitudinal changes of T2 -laminar values were detected at the PAT.
This study has four important limitations, in addition to the well-known T2 magic-angle effect (24). The main limitation is the small number of subjects. Studies with larger patient samples are currently in progress. The second limitation is that at the time this work was done, no phantom studies were available to the public validating the T2 sequences, which would have provided more material to choose between T2 fittings using all echoes and those discarding the first one. Since the authors dropped the first echo, short T2 cartilage components, which are important for normal cartilage homeostasis, might not be fully represented (22). The third limitation was the lack of images to test reproducibility of full-thickness T2 and laminar T2 values. The fourth limitation is the low SNR obtained in the last echo (7.071), resulting in Adjusted R2 values of less than 0.9. Studies evaluating T2 fittings that take into account the possible influence of noise are also in progress.
In this pilot study, we have established methodology for doing laminar analysis of longitudinal images. In summary, two important conclusions can be derived from this study: first, laminar analysis may improve the sensitivity to detect longitudinal T2 changes, and second, disruption of the T2 laminar organization of knee cartilage may be present in knee OA progressors, manifested as a decrease in the difference between the superficial and deep T2 values. Further investigation is warranted to evaluate the potential of the presented methodology for assessing the evolution and pathophysiology of OA.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: U01 AR-06-006.
REFERENCES
- 1.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Seminin Musculoskelet Radiol 2004;8: 355–368. [DOI] [PubMed] [Google Scholar]
- 2.Stahl R, Luke A, Li X, Carballido-Gamio J, Ma CB, Majumdar S, Link TM. T1rho, T(2) and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients: a 3.0-tesla MRI study. Eur Radiol 2009;19:132–143. [DOI] [PubMed] [Google Scholar]
- 3.Stahl R, Blumenkrantz G, Carballido-Gamio J, Zhao S, Munoz T,Hellio Le Graverand-Gastineau MP, Li X, Majumdar S, Link TM. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage 2007;15:1225–1234. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 2007;15:789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolbos RI, Zuo J, Banerjee S, Link TM, Ma CB, Li X, Majumdar S. Relationship between trabecular bone structure and articular cartilage morphology and relaxation times in early OA of the knee joint using parallel MRI at 3 T. Osteoarthritis Cartilage 2008;16: 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 2004;232:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsch GH, Mamisch TC, Domayer SE, Dorotka R, Kutscha-Lissberg F, Marlovits S, White LM, Trattnig S. Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures—initial experience. Radiology 2008;247:154–161. [DOI] [PubMed] [Google Scholar]
- 8.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2—preliminary findings at 3 T. Radiology 2000;214: 259–266. [DOI] [PubMed] [Google Scholar]
- 9.Mosher TJ, Collins CM, Smith HE, Moser LE, Sivarajah RT, Dardzinski BJ, Smith MB. Effect of gender on in vivo cartilage magnetic resonance imaging T2 mapping. J Magn Reson Imaging 2004;19:323–328. [DOI] [PubMed] [Google Scholar]
- 10.Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ,Smith MB. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology 2005;234:245–249. [DOI] [PubMed] [Google Scholar]
- 11.Blumenkrantz G, Lindsey CT, Dunn TC, Jin H, Ries MD, Link TM,Steinbach LS, Majumdar S. A pilot, two-year longitudinal study of the interrelationship between trabecular bone and articular cartilage in the osteoarthritic knee. Osteoarthritis Cartilage 2004;12:997–1005. [DOI] [PubMed] [Google Scholar]
- 12.Blumenkrantz G, Stahl R, Carballido-Gamio J, Zhao S, Lu Y, Munoz T, Hellio Le Graverand-Gastineau MP, Jain SK, Link TM, Majumdar S. The feasibility of characterizing the spatial distribution of cartilage T(2) using texture analysis. Osteoarthritis Cartilage 2008;16:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welsch GH, Mamisch TC, Marlovits S, Glaser C, Friedrich K, Hennig FF, Salomonowitz E, Trattnig S. Quantitative T2 mapping during follow-up after matrix-associated autologous chondrocyte transplantation (MACT): full-thickness and zonal evaluation to visualize the maturation of cartilage repair tissue. J Orthop Res 2009;27:957–963. [DOI] [PubMed] [Google Scholar]
- 14.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, Schmithorst VJ, Smith MB. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging 2001;14:50–55. [DOI] [PubMed] [Google Scholar]
- 15.Dardzinski BJ, Laor T, Schmithorst VJ, Klosterman L, Graham TB.Mapping T2 relaxation time in the pediatric knee: feasibility with a clinical 1.5-T MR imaging system. Radiology 2002;225:233–239. [DOI] [PubMed] [Google Scholar]
- 16.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rhorelaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging 2006;23:547–553. [DOI] [PubMed] [Google Scholar]
- 17.Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA, Smith MB. Spatialvariation of T2 in human articular cartilage. Radiology 1997;205: 546–550. [DOI] [PubMed] [Google Scholar]
- 18.Welsch GH, Mamisch TC, Quirbach S, Zak L, Marlovits S, Trattnig S.Evaluation and comparison of cartilage repair tissue of the patella and medial femoral condyle by using morphological MRI and biochemical zonal T2 mapping. Eur Radiol 2008. [DOI] [PubMed] [Google Scholar]
- 19.Kellgren J, Lawrence J. Radiologic assessment of osteoarthritis. AnnRheum Dis 1957;16:494–502. [Google Scholar]
- 20.Peterfy CG, Schneider E, Nevitt M. The Osteoarthritis Initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 2008;16: 1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carballido-Gamio J, Bauer JS, Stahl R, Lee KY, Krause S, Link TM,Majumdar S. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal 2008;12:120–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maier CF, Tan SG, Hariharan H, Potter HG. T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging 2003;17:358–364. [DOI] [PubMed] [Google Scholar]
- 23.Glantz SA. Primer of biostatistics. New York: McGraw-Hill, Inc.,Health Professions Division; 1992. 473 p. [Google Scholar]
- 24.Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB. MRimaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR Am J Roentgenol 2001;177:665–669. [DOI] [PubMed] [Google Scholar]