Abstract
Perinatal Mood and Anxiety Disorders (PMAD) are common and can cause significant morbidity and mortality for mother and child. A healthy perinatal period requires significant adaptations; however, systems can become imbalanced resulting in depressive and anxiety symptoms. The interface between the microbiome, the immune system, and the stress system may be a model for understanding mechanisms underlying PMAD. Emerging literature from general populations regarding immune, hormone, and HPA axis changes in relation to the microbiome combined with literature on immune, gonadotropin, and stress systems in the perinatal period provides a background. We systematically investigated literature in the developing field of the microbiome in relation to PMAD. Our inclusion criteria were 1) reporting measure of maternal mood, stress, or anxious or depressed behavior; 2) in the perinatal period, defined as pregnancy through one year postpartum; and 3) reporting measure of maternal microbiome including manipulations of the microbiome through prebiotics, probiotics, or interventions with microbial byproducts. The review identified research studying associations between stress and maternal microbiome; dietary impacts on microbial composition, mood, and stress; and the relationship between the microbiome and the immune system through immunoregulatory mechanisms. Important themes identified include: the importance of studying the maternal microbiome and measures of stress, anxiety, and depression and that multi-hit models will be needed as research strives to determine the effects of multiple mechanisms working in concert.
Keywords: Depression, Anxiety, Microbiome, Pregnancy, Stress, Immunity
1. Background
Perinatal Mood and Anxiety Disorders (PMAD), including Antenatal Depression (AND) and Postpartum Depression (PPD), are one of the most common postpartum complications affecting 10–20% of perinatal women (Andersson et al., 2006; Cooper et al., 1988; Dietz et al., 2007; Evans et al., 2001; Gaynes et al., 2005; Josefsson et al., 2001; O’Keane and Marsh, 2007). In severe cases, these disorders can be crippling (Abramowitz et al., 2010; Bernstein et al., 2008), and in developed nations, suicide is one of the largest contributors to maternal mortality (Palladino et al., 2011; Gentile, 2011; Oates, 2003; Lindahl et al., 2005). In addition to their negative impact on maternal health, PMAD can have long-term adverse effects for children, including delayed cognitive and socio-emotional development and poor mental health outcomes later in life (Kingston et al., 2012; Stein et al., 2014).
Currently studied risk factors have been unable to account for significant variance in PMAD cases, resulting in limited prevention, diagnosis, and treatment options (Ross et al., 2004; Kendler et al., 2002; Engel, 1977; Kimmel et al., 2015; Corwin and Pajer, 2008; Wisner et al., 2004). A biopsychosocial model of PMAD describes how biological (discussed later in this paper), psychological (i.e. coping skills and views of self), and social (i.e. social support or economic status) factors interact to produce depression and anxiety (Ross et al., 2004). Normal adaptive changes occurring during the perinatal period would otherwise be considered pathological in nonpregnant women (Skalkidou et al., 2012). The suspension of ovulation, development of placenta and fetus, and delivery require alterations of maternal stress systems (e.g., the Hypothalamic-Pituitary-Adrenal axis (HPA axis) and the parasympathetic/sympathetic systems), immune system, and metabolism. For most women, these alterations ensure good health through pregnancy; however, hormones and neurotransmitters altered by pregnancy have important implications for mental health (Skalkidou et al., 2012). In the case of PMAD, alterations of these systems have gone awry. Over the last decade, our understanding of the role the microbiome in human health, disease, and development has increased. Emerging evidence suggests that a woman’s microbiome may be a biological component influencing the metabolic states of pregnancy (Koren et al., 2012). Pregnancy-associated changes in microbial composition may have broader implications than metabolism. Current research suggests the microbiota also participate in the development and regulation of the immune and central nervous systems (CNS), thereby contributing to depressive or anxiety symptoms in the host (Sharon et al., 2016; Wang and Kasper, 2014; Collado et al., 2008; Koren et al., 2012; Smid et al., 2017; Nuriel-Ohayon et al., 2016; Naseribafrouei et al., 2014; Jiang et al., 2015; Maes et al., 2012). The microbiome may be a key biological component missing in current modeling of PMAD. To develop a working hypothesis regarding the microbiome’s contribution in PMAD, we conducted a systematic review of the literature examining the microbiome in relation to maternal mood, anxiety, or stress during the perinatal period. This emerging area of research intersects with existing literature regarding the microbiota-gut-brain axis, changes in the microbiome over pregnancy, and changes that occur across pregnancy in immune, gonadotropin, and stress systems.
1.1. The microbiome, depression and anxiety
The gut-brain axis involves bidirectional signaling between the CNS and the gut, which is regulated by the neuroendocrine and neuroimmune systems, the sympathetic and parasympathetic arms of the autonomic nervous system, and the enteric nervous system (ENS) (Dinan and Cryan, 2012). The ‘Old Friends’ hypothesis states that mammals co-evolved with an array of tolerable organisms, including bacteria, fungus, archaea, and viruses, that helped to develop human immunoregulatory circuits (Rook et al., 2013). In a seminal study, Sudo et al. were able to link intestinal microbiota to brain development, namely the hypothalamus, by comparing germ-free mice to conventionally raised mice (Sudo et al., 2004). Similarly, others have found that the microbiome is necessary for immune development and social behavior (Dinan and Cryan, 2017). These studies support the symbiotic relationship hypothesis that the host co-evolved with colonizing microorganisms to develop robust immune and neurological systems. Despite the evolutionary history behind this symbiotic relationship, intestinal microflora has only been added to the theoretical model of the gut-brain axis in the past few decades. In an important study by Turnbaugh et al., differences in microbial composition and abundance were observed between obese and lean individuals. These deviations from “normal” are commonly known as a microbial dysbiosis (Turnbaugh et al., 2006). Subsequent studies have followed, associating a dysbiotic microbiota to psychological disorders, including autism, stress, anxiety, major depressive disorder (MDD), and eating disorders (Dinan and Cryan, 2017; Kleiman et al., 2015). Current research focuses on the bidirectional communication between the microbiome and brain in order to understand the mechanisms behind these correlations (in part to elucidate cause or consequence of dysbiosis).
Communication between the gut microbiota and brain can involve the vagus nerve, gut-secreted neuropeptides, sensory nerves, and cytokines via direct interactions with the intestinal wall, gut permeability, or production of physiologically relevant microbial metabolites, such as tryptophan and Short-Chain Fatty Acids (SCFAs) (Dinan and Cryan, 2017; Holzer and Farzi, 2014). Vagal nerve stimulation has been associated with clinical antidepressant benefits and regulation of the HPA axis in patients with treatment refractory depression (Dinan and Cryan, 2012; Nemeroff et al., 2006; O’Keane et al., 2005). Studies have described a relationship between intestinal microbiota and depression via immune regulation. Experimental manipulation of the gut microbiota has been shown to influence depressive and anxious behaviors (Foster and McVey Neufeld, 2013). In one study, Bifidobacterium longum and Bifidobacterium breve were shown to reduce anxiety in an anxious mouse strain in a similar manner as a selective serotonin reuptake inhibitor (Savignac et al., 2014). Emerging evidence suggests the gut microbiota influences the serotonergic system and regulates 5-HT synthesis and secretion (Kelly et al., 2015; Desbonnet et al., 2015; O’Mahony et al., 2015; Clarke et al., 2013). Microbiota influence this system by altering tryptophan availability in the plasma. These mechanisms include 1) direct utilization of tryptophan by bacteria for cellular function or production of indole and, in some species, serotonin; 2) indirectly by inducing the up-regulation of hepatic In-doleamine-2,3-dioxygenase (IDO) and tryptophan-2,3-dioxygenase (TDO) through an inflammatory response, thereby shunting tryptophan into the kynurenic pathway and increasing production of kynurenic acid or quinolinic acid, known to be associated with psychological and neurological disorders (O’Mahony et al., 2015); and 3) stimulating serotonin synthesis in the enteroendocrine cells via SCFAs (O’Mahony et al., 2015; Clarke et al., 2013; Reigstad et al., 2015). Numerous animal studies demonstrate that manipulation of the gut microbiota increases corticosteroid reactivity and/or glucocorticoid levels (Foster and McVey Neufeld, 2013). Administration of Lactobacillus rhamnosus, considered an anti-inflammatory probiotic, reduced stress-induced corticosterone, as well as anxiety- and depression-related behavior in mice (Bravo et al., 2011). Two small studies have reported greater amounts of pro-inflammatory gut bacteria and reduced levels of antiinflammatory gut bacteria from individuals with MDD (Naseribafrouei et al., 2014; Jiang et al., 2015). Additionally, a cohort of chronically depressed patients had greater IgA and IgM antibodies against Enterobacteriaceae, which suggests increased translocation of bacteria in depressed patients (Maes et al., 2012). The perinatal period provides a unique opportunity to translate findings from current microbiome research to clinical research of PMAD for three reasons: 1) pregnancy can be accompanied by dramatic changes in the gut microbiota (Koren et al., 2012), 2) the perinatal period is a unique time of change in a number of systems implicated in the microbiota-gut-brain axis including the stress system and the immune system, and 3) the phenotypic presentation of PPD includes both anxious and depressive behaviors (Abramowitz et al., 2010; Bernstein et al., 2008).
1.2. Gonadotropins, stress, immune, and microbiome changes during the perinatal period
While biological mechanisms underlying PMAD remain unclear, research suggests that hormonal sensitivities, the stress system, and the immune system play a role. There is evidence that PPD is associated with sensitivity to fluctuating levels of the gonadal steroids estradiol and progesterone (Bloch et al., 2000). While similar levels of estradiol and estriol exist across euthymic and depressed women during the perinatal period, expression of estrogen-responsive genes during the third trimester is predictive of PPD and shows increased sensitivity to estrogen signaling, a mechanism for the development of PPD (Mehta et al., 2014). The gut microbiota is associated with systemic estrogen levels (Flores et al., 2012; Baker et al., 2017). For example, certain gut microbiota are associated with secretion of beta-glucoronidase which deconjugates estrogen into its most bioactive form (Baker et al., 2017). In the hypoestrogen postpartum state, deconjugation of estrogen may be a mechanism by which the microbiota participate in the balancing of fluctuating systems. Evidence regarding progesterone’s influence on the microbiota is limited. When given vaginally to prevent preterm birth, progesterone did not impact the vaginal microbiome (Kindinger et al., 2017).
Estrogen, progesterone, and the HPA axis are strongly linked, and HPA axis abnormalities in susceptible women may be associated with PMAD (Meltzer-Brody, 2011). In a healthy pregnancy, maternal levels of Corticotrophin-Releasing Hormone (CRH), Adrenocorticotropic Hormone (ACTH), and cortisol increase during pregnancy and peak in the third trimester. During normal parturition, increases in CRH, ACTH, and cortisol are observed, which drop to prepartum levels within the first 1–4 days and slowly return to prepregnancy levels after several weeks (Mastorakos and Ilias, 2003). In non-depressed women, there is a blunting of the response of ACTH to CRH for a few weeks while women with PPD may have continued blunting of ACTH to CRH for 6 to 12 weeks (Meltzer-Brody, 2011; Magiakou et al., 1996). Maternal CRH levels greatly increase during first trimester due to CRH production in the placenta, decidua, and fetal membranes (Corwin and Pajer, 2008; Mastorakos and Ilias, 2003). Placental production of CRH leads to further fetal cortisol which in turn causes increased placental CRH secretion. However, while fetal cortisol increases placental CRH secretion, it surpresses maternal CRH secretion, resulting in maternal CRH suppression that has been shown to last at least 6 weeks after delivery while returning to normal by 12 weeks (Corwin and Pajer, 2008; Mastorakos and Ilias, 2003). Maternal ACTH levels rise during pregnancy similarly to cortisol level thought they remain within normal limits (Mastorakos and Ilias, 2003). Women who have postpartum depressive symptoms exhibit a more blunted ACTH response to CRH than euthymic women in postpartum (Mastorakos and Ilias, 2003). Results from Bloch et al. have also shown that women with a history of PPD have HPA axis changes associated with estradiol and progesterone increases mimicking pregnancy levels (Bloch et al., 2005). While the relationship with the microbiome is yet to be studied in a perinatal population, there is evidence that bacteria play a critical role in HPA axis development and responsiveness (Sudo et al., 2004; O’Mahony et al., 2015). Dynamic system changes in the perinatal period may serve as a critical time period for exhibiting these alterations or reconstructing systems.
The HPA axis interfaces with the immune system, which has also been implicated in depression. As discussed by Dantzer et al., the brain monitors peripheral inflammation through several innate immune system pathways including 1) cytokine activation of nerves (i.e. the vagal nerve), 2) increased volume of pro-inflammatory cytokines by stimulation of Toll-like receptors on macrophage-like cells and these cytokines entering the brain through diffusion, 3) systemic circulating of pro-inflammatory cytokines that gain access through cytokine transporters at the blood-brain barrier, and 4) IL-1 receptors located on perivascular macrophages and endothelial cells of brain venules, which produce prostaglandin E2, which signals the brain (Dantzer et al., 2008). While pregnancy was previously thought to be a period of immune suppression, inflammation fluctuates throughout pregnancy, relying on the maternal immune system to successfully adapt to each developmental stage (Mor et al., 2017). Across pregnancy, the maternal innate and adaptive immune systems are involved in local and systemic immunomodulation (Schminkey and Groer, 2014). Local inflammation occurs during implantation and development of the placenta during the first trimester and early second trimester, followed by reduced inflammation in the second trimester. Inflammation again increases in the third trimester in preparation for birth, and inflammatory homeostasis is regained postpartum (Mor and Cardenas, 2010). These changes in inflammation along with the interrelated hormonal changes may affect blood flow to mucous membranes and gastric-intestinal (GI) motility, and account for increased nausea in the first trimester with stabilization during the second trimester (Lawson et al., 1985; Lee and Saha, 2011). Macrophages are an important modulator of these systems by switching from a more pro-inflammatory state (with elevated levels of TNF-α, IL-6, and IL-1β) in the first and third trimesters to a more anti-inflammatory state (with increasing levels of IL-10, IL-4, and TGF-β) in the second trimester and postpartum period (Osborne and Monk, 2013). The inflammatory response of macrophages to produce proinflammatory cytokines is likely related to changing estrogen and progesterone levels, as well as to changes in serotonin levels, leukemic inhibitory factor, and prostaglandins (Kraus et al., 2012; Edvinsson et al., 2017; Luppi et al., 2002; Mjösberg et al., 2010; Sykes et al., 2012). Edvinsson et al. found that three inflammatory markers were downregulated in patients with AND compared to women without depression and that these women also had a dysregulated HPA axis response (Edvinsson et al., 2017). During implantation and delivery, elevated levels of macrophages creating inflammation is necessary, but at other stages, excessive inflammation may result in imbalance and contribute to comorbidity (Osborne and Monk, 2013; Kraus et al., 2012). A review by Osborne and Monk identifies the complexity of research to date. Some studies show associations between PMAD with cytokines levels such as IL-6, TNF-α, and IL-1β, while others have not shown associations that are as clear, reflecting the complexity and careful regulation of the immune system in pregnancy (Osborne and Monk, 2013; Shelton et al., 2015; Bränn et al., 2017).
Throughout the perinatal period, the immune system interacts with microorganisms. The fetal/placental unit is actively involved in these immune changes over the perinatal period and is involved in complex responses to microorganisms (Mor and Cardenas, 2010). While there is evidence of a placental microbiome, it is controversial whether the uterus and placenta have microbiota in healthy states (Mor et al., 2017; Perez-Muñoz et al., 2017; Stout et al., 2013; Cao and Mysorekar, 2014). There is evidence that placental microbiota may induce some of the immune changes occurring throughout pregnancy, including tolerance of microorganisms at the maternal-fetal interface (Mor et al., 2017; Racicot et al., 2016). Regardless of whether there are microbes associated with the placenta and uterus, maternal microbes such as those found in the vagina and gut may contribute to immunological changes across pregnancy. The vaginal microbiota of pregnant women has a distinctly different compostion compared to non-pregnant women (Aagaard et al., 2012; Romero et al., 2014). Evidence suggests pregnant women have a more stable microbiota over time than that of nonpregnant women and that bacterial communities are less diverse and dominated by Lactobacillus during normal pregnancy (Aagaard et al., 2012; Romero et al., 2014; Walther-António et al., 2014). It is proposed that this increased stability promotes resilience against infections, which are risk factors for preterm birth and other obstetric complications (Romero et al., 2014). A study by Koren et al. shows that by the third trimester gut microorganisms that induce a pro-inflammatory response increase, and conversely, organisms that support an anti-inflammatory response decrease (Koren et al., 2012). In support of these findings, other studies have shown that the gut microbiome can modulate the immune system, including how it develops and operates (Lima-Ojeda et al., 2017; Cénit et al., 2014; Jandhyala, 2015).
Compositional changes in the gut microbial community across pregnancy have been reported, but to date, research is limited. Alterations include increases in abundance of members of the Phylum Actinobacteria and Proteobacteria and decreases in abundance of certain butyrate (a SCFA)-producing bacteria including Faecalibacterium, Eubacterium, and Roseburia (Koren et al., 2012). Multiple studies have found a decrease in alpha-diversity (the combination of microbial richness, number of different species, evenness, and abundance of present species within a sample) over the course of pregnancy, and one study noted an increase in beta-diversity (the differences in microbial composition or species between subjects) (Koren et al., 2012; Collado et al., 2008; Smid et al., 2017). This implies that through pregnancy a subject has a reduction in observed microbial species, but the altered microbial communities between different women becomes more divergent. Studies have also shown that microbial composition may be relatively stable between the third trimester and postpartum (DiGiulio et al., 2015; Koren et al., 2012; Jost et al., 2014). Variation in study sampling and design may account for descrepencies in findings, and their ultimate significance is not yet known.
Understanding the influences mediating microbial composition, as well as the consequences of those alterations on the host, is an enormous challenge. In the context of this review, the interface between microorganisms and the immune system offers an initial point of exploration; however, it is important to remember that numerous factors influence microbial composition. In one example, Mor et al. propose a “double-hit hypothesis” with regards to the microbiota associated with preterm labor and suggest that a viral infection changes how the placenta responds to bacterial products, which in turn, alters the response to the “normal “microbiota (Mor et al., 2017). In fact, a multi-hit hypothesis may be more accurate, where various biological factors and external influences drive individual microbial composition and response to microbota. Examples of important factors include maternal diet, fluctuating cortisol levels, and antibiotic use (Nuriel-Ohayon et al., 2016; Shelton et al., 2015). Higher use of antibiotics in women with AND suggests that AND is associated with infection during pregnancy (Field et al., 2006). From these investigations, it is difficult to draw conclusions regarding the direction of the relationship between anti-biotic-induced microbial dysbiosis and depressive symptoms, but antibiotic exposure has been linked to anxiety and depression in the general population as well (Lurie et al., 2015).
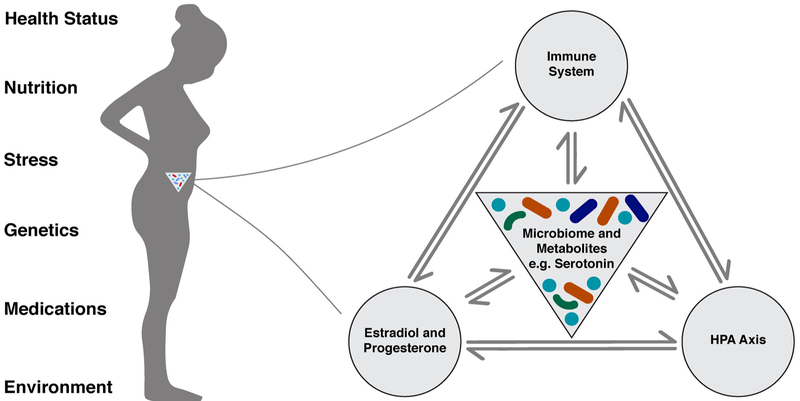
Ultimately, the relationship between the maternal microbiome and biological systems is bidirectional, whereby the microbial community must adapt to the changes occurring throughout pregnancy which, in turn, impacts pregnancy progression and a woman’s adaptation to pregnancy (Fig. 1). To understand these associations more completely, a systematic review was undertaken to identify research findings observing the relationship of the maternal microbiome to stress, depression, and anxiety.
Fig. 1.
Factors that influence microbial composition and the host systems that communicate with the microbiota during pregnancy.
2. Methods
We conducted a systematic review of peer-reviewed literature to identify sources investigating the relationship between maternal microbiome and maternal mood/behavior in the perinatal period. We searched for studies and reviews in MEDLINE® (via PubMed), Embase, and Scopus from date of database inception through January 8, 2018 using terms for microbiome, perinatal period, and mood disorders. We used either medical subject headings (MeSH) or Embase subject headings (Emtree) where available and keywords when applicable. Searches were supplemented with hand searching of references citing or cited by included articles.
After duplicate references were removed, at team of reviewers independently examined titles and abstracts for inclusion (HR, KW, MK). Next, the full texts of the remaining studies were obtained and each was independently examined by two reviewers for inclusion. At each stage of the review, discrepancies between reviewers were resolved through discussion. Studies were selected based on a set of predetermined inclusion and exclusion criteria. Inclusion criteria were: 1) reporting a measure of maternal mood, stress, or anxious or depressed behavior; 2) maternal population in the perinatal period defined as pregnancy through one year postpartum; and 3) reporting a measure of maternal microbiome, including manipulations of the microbiome through prebiotics or probiotics or interventions with microbial byproducts. Both animal studies and human subject studies were included. Studies were excluded if they were not available in English. Reviews were excluded. Abstracts meeting criteria, while not explicitly peer reviewed, were included.
3. Results
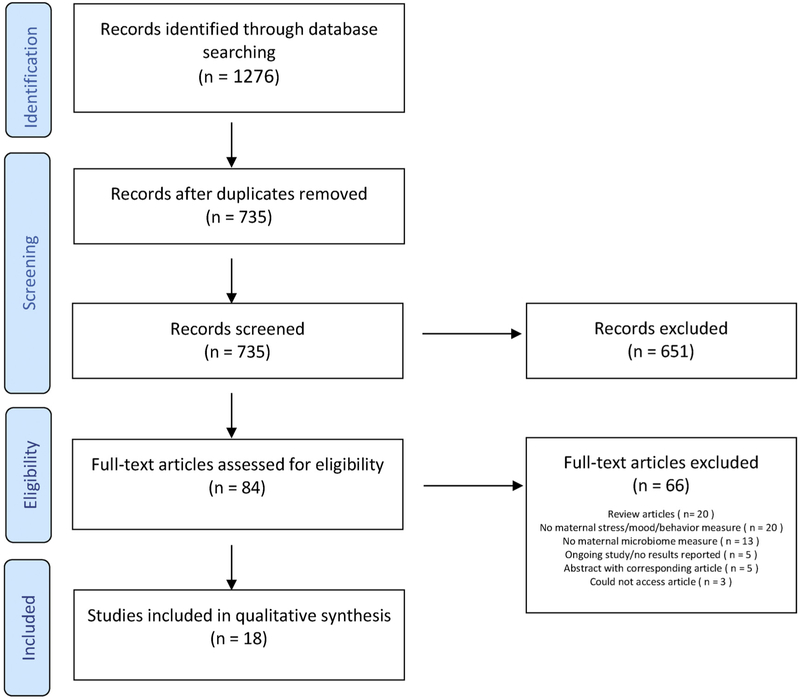
A total of 1276 sources were identified, and after removing duplicates, 735 were evaluated (Supplement 1). The number of articles removed through the screening and exclusion process is displayed in Fig. 2. Six hundered fifty-one articles were deemed irrelevant from title and abstract screening, and an additional 66 did not meet inclusion criteria after full text review. Seventeen peer-reviewed publications were identified for inclusion in this review. One additional abstract was also reviewed given this is an emerging area of study. Ten studies were conducted with lab mice, with the remaining eight conducted in human populations.
Fig. 2.
Records excluded during screening process.
3.1. Study findings
3.1.1. Associations between stress and maternal microbiome
Of the studies reviewed, five evaluated the effect of stress on the vaginal microbiome, and three evaluated the effect of stress on the gut microbiome. Stress has been associated with alterations in the vaginal microbiome during the perinatal period. Early research into stress and bacterial vaginosis (BV) showed that those with moderate-high or high chronic stress had significantly higher odds of positive BV status (OR = 2.2 and 2.3 respectively) (Culhane et al., 2001). In research investigating racial health disparities, Paul et al. found that while stressful life events were associated with BV infection in both white and African American women, neither perceived stress nor social support was related to BV status, suggesting that objective experience of a stressful event may be more important in impacting microbial composition (Paul et al., 2008). In contrast, Uscher-Pines et al. found a higher prevalence of BV among African American women (46% in African American women, 14% in non-African American women), but when controlling for race, sexual partners, marital status, and other behavioral risk factors, stress was not associated with BV status or any specific bacteria (Uscher-Pines et al., 2009). Recent research on stress and the vaginal microbiome has sought to characterize composition and function in this dynamic. Results from Jasarevic et al. show that vaginal Lactobacillus abundance was decreased in dams exposed to stress relative to control dams (p = 0.0398), and the authors suggest this is likely associated with an expansion of other microbiota (Jašarević et al., 2015). In a follow-up study, stress-exposed females showed increases of the Phylum Proteobacteria in early pregnancy, driven predominantly by members of the Helicobacter genus (Jašarević et al., 2017).
Stressors also impact intestinal microbial composition during pregnancy. Gur et al. found dams exposed to prenatal stress had significantly different fecal microbial community composition than samples from non-stressed control dams (Gur et al., 2017). In addition, microbial composition differed in the placentas from stressed versus non-stressed dams, but the difference did not remain statistically significant after controlling for multiple comparisons. Gur et al. suggest this may be due to low bacterial load within the placenta and difficulty measuring given the relative abundance of host DNA in the placental samples (Gur et al., 2017). Researchers stressed dams between embryonic day (E) 10-E16. On E17, dams were tested in an elevated plus maze to examine anxiety-like behavior. The association between stress exposure and higher anxiety-like behavior approached significance (t (14) = 2.010, p = 0.0641) (Gur et al., 2017). Similarly to their vaginal microbiome findings, Jasarevic et al. found that the gut microbial communities differed between the control and stressed dams (Jašarević et al., 2017). In control females, variation between individuals (Beta-diversity) was significantly greater in early pregnancy compared to late pregnancy, while for stressed dams less inter-individual variation was observed in both early and late stages of pregnancy. Taxa differences were found in stress-exposed females such as increase of Rikenellaceae, Odoribacter, and Mucispirillum and decrease of Bacteroides seen in early pregnancy through parturition. Stress increased the Desulfovibrionaceae family within the Proteobacteria phylum found late in pregnancy. Stress-exposed females had some significant associations of certain bacteria not found in control dams (Jašarević et al., 2017). This work indicates maternal stress during the first week of pregnancy induced a lasting gut and vaginal dysbiosis. In an abstract from human subjects research, Togher et al. reported reduced microbial diversity in gut and vaginal samples associated with higher levels of depression in the second trimester. However, this association disappeared in the third trimenster (Togher et al., 2017). This is preliminary work but may indicate the importance of the timing of factors in pregnancy. These studies provide a small but unified body of evidence exhibiting the effect of stress on the maternal microbiome, but are insufficient for drawing conclusions regarding the impact of microbial composition on experiences of stress, behavior or mood.
3.1.2. Dietary impacts on microbial composition, mood, and stress
Due to the impact of diet on host microbial composition (David et al., 2014), factors such as prebiotic and probiotic consumption have the potential to effect mood with host microbiota on the causal pathway. Of the studies reviewed, six evaluated the relationship between dietary factors, microbial composition and mood or stress. There is evidence that prebiotic consumption may impact the effect of stressors on maternal outcomes. In open field mouse experiments, dams exposed to Acrylamide (ACR, a known neurotoxin) displayed increased anxiety-like behavior compared to control dams, while supplementation with prebiotics (fructo- and xylo-oligosaccharide, non-digestible carbohydrates that are easily fermented by intestinal organisms) restored exploratory behavior in ACR-exposed dams, indicative of reduced anxiety (Krishna et al., 2015). ACR-exposed dams also supplemented with prebiotics showed a reduction in markers of oxidative stress and a recovery of antioxidant enzymes in the maternal brain to control levels. Glutathione S transferase levels in the maternal brain increased in both ACR-exposed dams and ACR-exposed dams supplemented with probiotics (Krishna et al., 2015). Enumerations of Bifidobacteria and Lactobacilli (commensal intestinal organisms that selectively ferment xylo-oligosaccharide and fructo-oligosaccharide, respectively) on selective media were significantly higher in ACR-exposed dams supplemented with prebiotics compared to ACR only-exposed dams and control dams. Postive correlations between Bifidobacteria and Lactobacilli abundance and maternal brain dopamine and GABA levels were also significant. This study only enumerated Bifidobacteria and Lactobacilli on selective media and did not explore other possible contributing species (Krishna et al., 2015). A second study by this same group found similar findings with the oligosaccharide inulin, another non-digestible carbohydrate, and exposure to ACR (Krishna and Muralidhara, 2015). These studies suggest that fermentation of non-digestible carbohydrates by intestinal microorganisms mitigate the neurotoxic affects in the brain, and suggest a beneficial link between diet, microbes and the brain.
In a human randomized control trial of 423 women, Slykerman et al. investigated the effects of perinatal supplementation of the probiotic Lactobacillus rhamnosus HN001 had on postpartum depression and anxiety (Slykerman et al., 2017). Women were randomized to receive HN001 at a dose of 6 × 109 colony-forming units (CFU) or placebo to be taken daily from enrollment (14–16 weeks) through six months postpartum. Mothers in the probiotic treatment group reported significantly lower depression scores (effect size, −1.2 points, Edinburgh Postnatal Depression Scale; EPDS) and anxiety scores (effect size −1.1, State Trait Anxiety Inventory 6; STAI-6) than those in the placebo group. When using a clinically significant cutoff for depression and anxiety levels and correcting for infant colic and time since delivery due to retrospective measurement of symptoms, probiotic supplementation remained significantly associated with reduced anxiety, but not depression (Slykerman et al., 2017). Cowan et al. found that maternal separation, with or without probiotic treatment, did not alter dams’ anxiety-like behavior or locomotor activity or responsiveness to their pups (Cowan et al., 2016). The systematic search also identified a study by Freeman et al. that found 2.6% of psychiatrically-ill women reported consumption of a probiotic supplement (Freeman et al., 2016). However this paper did not compare results to any other pregnant population, and it is difficult to know if probiotic consumption differs in the general perinatal population.
Bruce-Keller et al. manipulated the microbiome of dams through depletion and repopulation with fecal transplant from males fed with a high fat diet (HFD) (Bruce-Keller et al., 2017). Dams experienced a temporary 5% weight loss, but overall there was no difference in weight outcomes between dams repopulated with the HFD-fed male fecal samples (HFD dams) and dams repopulated with the control diet-fed male fecal samples (CD dams). For all three time points (before mating, during pregnancy, during lactation), statistically significant differences in microbial community composition were detected between CD and HFD dams (beta-diversity); however, no significant differences in diversity of communities within each subject (alpha-diversity) were observed at any time. Pre-pregnancy group-based differences were statistically significant, and both pregnancy and lactation introduced shifts in community composition of both CD- and HFD-manipulated dams while maintaining group differences. The taxonomical distribution within groups at Phylum levels for the maternal fecal samples revealed that before pregnancy, HFD-reconstituted dams show a statistically significantly elevated level of Bacteroidetes compared to CD dams, which had higher Verrucomicrobia. Bacteroidetes occupied a higher proportion of community composition postnatally in both groups. No other phylum-level changes could be observed (Bruce-Keller et al., 2017). Bruce-Keller et al. utilized time spent with pups as a measure for maternal behavior, and despite microbial composition differences, no significant differences in time spent with pups between CD and HFD dams were noted (Bruce-Keller et al., 2017). Notably, analyses of types of interactions between mother and pups, such as time spent grooming or feeding the pups or measures of maternal depressive or anxious behaviors, were not included. Evidence from these studies exhibit the complexity of dietary, prebiotic, and probiotic interventions, which encompass a variety of factors that may have dissimilar impacts on microbial composition, and subsequently, on maternal mood and behavior. Targeted prebiotics may promote growth among specific genera that appear to decrease inflammation and improve anxiety, while other dietary components, such as fat consumption, impact different genera. New evidence suggests probiotics have the potential to improve maternal mood and anxiety symptoms, but it is limited. It is unclear from existing research which dietary components are most important to consider in relation to mood, anxiety, or stress during the perinatal period.
3.1.3. Immunoregulation, PMAD, and the microbiome
The microbiota-gut-brain axis is highly regulated and influenced by immunological status and immune responses to microbial dysbiosis and translocation of microbial products. Of the studies reviewed, six articles assess the relationship between host immunoregulation, stress, and the microbiome during the perinatal period: one studying prenatal stress in relation to microbial communities in a transgenerational mouse model; two indentifying inflammatory responses to specific bacteria and have studied in relation to perinatal depression and anxiety in a humans; and three studying lipolysaccharide (LPS), a bacterial endotoxin, as a marker of dysbiosis and an effector of immunoregulation in the perinatal period.
To relate stess induced microbial dybiosis to inflammation in utero, Gur et al. analyzed cytokine expression. They found that IL-1β was significantly elevated in the placentas associated with female offspring and fetal brains from stressed dams when compared to non-stressed dams (Gur et al., 2017). Furthermore, Brain Derived Neurotrophic Factors (BDNF) was decreased in the placenta and in the brains of adult female offspring, which is attributed to changes in IL-1β levels. Immune alterations associated with maternal bacterial dysbiosis are also transmitted to female offspring, indicating that microbiota-gut-brain signaling related to stress may be transgenerational.
In humans, Roomruangwong et al. found lowered IgM-mediated autoimmune responses to oxidative specific epitopes (OSEs), molecules expressed on outer and inner cell membranes, during pregnancy, indicating a suppression of immune regulation toward cellular debris, and resulting in the enhancement of oxidative stress. This IgM-mediated response was significantly associated with gram-negative bacteria and suggests there is a reduction in bacterial translocation leading to the reduced IgM-mediated response found in all pregnancies, including mothers with prenatal depression. Alcohol use was associated with greater IgM-mediated responses and therefore possibly greater bacterial translocation. IgM-mediated responses were inversely associated with the tryptophan catabolite (TRYCAT) pathway (also known as the kynurenine pathway). Elevated TRYCAT pathway activity was strongly predictive of women with a lifetime history of depression and premenstrual syndrome (PMS), possibly indicating a chronic activation of the TRYCAT pathway in this group. Though an association was not detected with perinatal depression, this study points to complex immune regulation buffering the response to bacteria that warrants further investigation (Roomruangwong et al., 2017a). Subsequently, Roomruangwong et al. found that while perinatal anxiety was not associated with IgM responses, postnatal anxiety was significantly associated with IgA responses to Pseudomonas aeroguinosa, a common environmental bacteria and opportunistic pathogen (Roomruangwong et al., 2017b). Anxiety may have unique associations independent from depression, especially as related to immunity.
Criteria for review also included microbial byproducts that may be associated with stress, anxiety or depression during the perinatal period. Microbial byproducts may provide insight into mechanisms by which the microbiota participates in the gut-brain axis. Solati et al. found that acute exposure of pregnant dams during gestation to LPS is associated with increased anxiety as indicated by decreased time and number of entries in the open arms of the elevated plus maze (EPM) (Solati et al., 2015). Increased serum corticosterone levels and concentrations of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, 1.5 h post-injection were also observed in LPS exposed dams (Solati et al., 2015). Male offspring of dams injected with LPS displayed opposite results from their mothers using these same behavioural tests and cytokine assays (Solati et al., 2015).
Confirming the transgenerational findings by Gur et al., Walker et al. also found that elevated stress and anxiety induced in neonates via an inflammatory response to LPS persists into adulthood where treated rats exhibited more anxiety, reduced maternal behaviours with their own offspring, and spent more time in non-maternal behaviors. Second generation females then displayed greater anxiety and altered corticosterone reactivity. Second generation males had increased anxiety levels, with no changes in corticosterone reactivity. Cross-fostering with untreated mothers reversed these effects (Walker et al., 2012).
In research investigating stress, LPS, and fetal loss, Friebe et al. found that LPS injections or acute sound stress in mice led to increase in fetal loss via similar mechansims (Friebe et al., 2011). Blocking the Toll-like Receptor-4 (TLR-4) via TLR-4 antibody or pretreating before the stressor with bacteriocidal permeability increasing protein (BPI) that binds to LPS kept fetal loss from occurring with the stressor present, but only BPI prevented fetal loss in LPS-injected mice. Blocking TLR-4 also prevented stress-triggered immune responses, increased T regulatory cells, and reduced migration of dendritic cells to the lymph nodes, but TLR-4 blockage only partially decreased the HPA axis response and did not impact progesterone release (Friebe et al., 2011). Stress often induces intestinal permeability and thereby increases translocation of LPS into the blood and contributes to stress-induced fetal loss through the initiation of the inflammatory response via TLR-4 and proliferation of dendritic cells. This research provides additional evidence connecting psychological stress to systems that respond to bacterial LPS during pregnancy and supporting a multi-hit hypothesis, where stress alone may not initiate fetal loss without a “bacterial danger signal.”
From these studies, we see that stressed animals have microbial dysbiosis associated with greater pro-inflammatory responses and that certain bacteria are associated with greater inflammation and anxiety. Because dysbiosis leads to increased permeability and increased translocation of bacterial byproducts, LPS may be a mechanism by which microbes alter mood as well as an indicator of dysbiosis. Certain factors, such as alcohol, may increase translatocation. Immunological responses to microbes during the perinatal period are an important factor in etiology of the maternal psychological status and warrant further investigation.
4. Discussion
Multiple findings from this review demonstrate a relationship between stress, the microbiome, and health and behavior outcomes during the perinatal period. Our first finding is that maternal stress results in changes in the vaginal and gut microbiota and that specific bacterical abundance and community structure are associated with inflammation and psychological status (Culhane et al., 2001; Paul et al., 2008; Uscher-Pines et al., 2009; Jašarević et al., 2015, 2017; Gur et al., 2017; Togher et al., 2017). Interestingly, bacteria identified in this review in association with stress exposure have been identified in other studies relating to psychological outcomes. For example, differences in relative abundance of Rikenellaceae as found by Gur et al. were also associated with fear as a component of child temperament among 18–36 month-olds (Christian et al., 2015). Similarly, Jasarevic found an increase in Desulfovibrionaceae families within the Proteobacteria phylum, and while Rikenellaceae increased, Bacteroidetes decreased overall (Jašarević et al., 2015, 2017). Proteobacteria is thought to be more pro-inflammatory and has been found in greater amounts in a sample of healthy women by Koren et al. (2012). Conversely decreased abundance of certain bacteria are associated with inflammation. Highlighted in this review, Gur et al. found decreases in Bifidobacteria in stressed female offspring while Krishna et al. showed prebiotic supplementation increased Bifidobacteria and decreased inflammation (Gur et al., 2017; Krishna et al., 2015).
Another primary finding of this review is that a majority of studies providing direct intervention in maternal microbiome (i.e. high-fat diet, prebotics, or probiotics) or mimicking microbial effects (i.e. LPS injection) detect significant changes in anxious or depressive behaviors or symptoms (Krishna et al., 2015; Krishna and Muralidhara, 2015; Slykerman et al., 2017; Solati et al., 2015; Walker et al., 2012). Notably, all of these studies evaluating maternal behavior or symptoms antenatally detected an effect, while only half of the studies detected an effect in postpartum evaluation (Krishna et al., 2015; Krishna and Muralidhara, 2015; Slykerman et al., 2017; Cowan et al., 2016; Bruce-Keller et al., 2017; Solati et al., 2015; Walker et al., 2012). Evidence for intervening through the microbiota-brain axis is promising, though there are limitations to these studies. The majority of these studies are animal models (Krishna et al., 2015; Krishna and Muralidhara, 2015; Cowan et al., 2016; Bruce-Keller et al., 2017; Solati et al., 2015; Walker et al., 2012). The only human study regarding probiotics and PMAD retrospectively assessed mood (Slykerman et al., 2017). Inconsistencies in probiotic study findings may be due to different bacteria included, different impacts on commensal bacteria, the makeup of commensal bacteria prior to probiotic intervention, or host factors (Slykerman et al., 2017; Cowan et al., 2016). Additionally, while Roomruangwong et al. did not find perinatal anxiety associated with IgM responses, they did find postnatal anxiety was significantly associated with IgA responses to Pseudomonas aeroguinosa. Roomruangwong et al. also found history of depression and history of sensitivity to hormonal change were also important factors, therefore an account of historical systems along with current symptoms needs to be considered (Roomruangwong et al., 2017a, 2017b). For instance, Walker et al. showed a multigenerational transmission of altered maternal behavior that was initiated with a neonatal inflammatory stress response in the first generation and differing maternal care (Walker et al., 2012). Therefore, maternal behavior and mother-baby interactions should be considered in further research. Animal studies tended to examine anxious behaviors in relation to bacterial exposure and inflammation, while human PMAD studies focused on depressive symptoms. Future human and animal research would benefit from consideration of both anxiety and depressive symptoms. In light of findings that suggest a change in microbial composition in conjunction with other physiological changes over the course of the perinatal period, longitudinal studies provide a unique investigative opportunity (Koren et al., 2012; Collado et al., 2008; Smid et al., 2017; Bruce-Keller et al., 2017).
A number of studies found in our search addressed transgenerational transmission and only focused on offspring measures, not maternal measures, and were therefore excluded. More specifically, many studies stress the mothers but do not assess its impact on the mother before assessing the impact on the offspring. Other studies, while describing offspring microbiota, did not determine maternal microbial composition, which makes it difficult to understand whether the microbiota is associated with symptoms identified in the mother, associated with offspring measures, or if the microbial composition is transferred. One excluded article by Zijlmans et al. found that infants of mothers with high stress in the third trimester had greater abundance at that phylum-level of Proteobacterial groups, considered more inflammatory, and lower relative abundances of lactic acid bacteria and Bifidobacteria species, considered anti-inflammatory; however, this study did not evaluate maternal microbiome profiles (Zijlmans et al., 2015). The emerging literature indicates that the microbiota-brain axis is an important mechanism of transmission of stress from mother to baby. Paul et al. who found stressful events, and not perceived stress, were associated with differences in vaginal microbiota. Stress, particularly for human studies, is a broad term, and these findings suggest further study should include multiple stress measures and dimensions (Paul et al., 2008). Furthermore, the immune system is a key component of this communication and developmental factors such as BDNF are important endpoints of communication. The microbiota, immune system, HPA axis, hormones (i.e. estradiol, progesterone, allopregnanalone, serotonin) and the CNS all communicate simultaneously during their development; therefore, it must be remembered that a mother’s system is greatly influenced by her mother, and both will be transmitted in the development of the next generation (Koren et al., 2012; O’Mahony et al., 2015; Meltzer-Brody, 2011; Lima-Ojeda et al., 2017; Jolley et al., 2007; Gold et al., 2002).
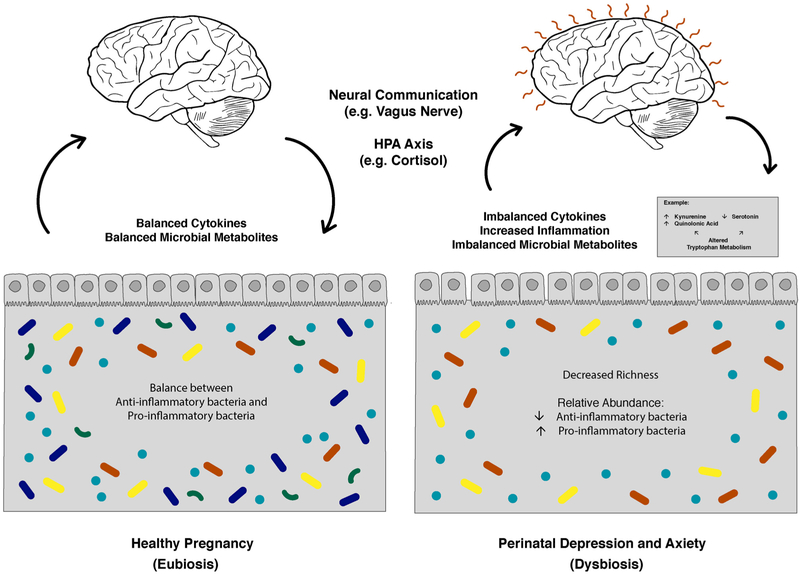
This review highlights possible mechanisms of stress communication utilizing the microbiota-brain axis and its relationships to the development of anxiety and depressive symptoms during the perinatal period (Fig. 3). The critical role of the HPA axis is one important pathway hypothesized and supported by evidence from this review. LPS provides a model of how certain bacterial byproducts lead to inflammation and activation of the HPA axis. Similar to findings in the general population, those who experience stress during pregnancy may have an increased risk of PMAD due to changes in their microbiome shifting toward proinflammatory composition and increased translocation of bacterial products, such as LPS (Maes et al., 2012; Solati et al., 2015; Walker et al., 2012). However, Roomruangwong et al. found that, while immune responses to gut bacteria may influence onset of prenatal immune response and postnatal anxiety, pregnancy itself may be protective against translocation (Roomruangwong et al., 2017a, 2017b). Roomruangwong et al. emphasize that the TRYCAT pathway is highly regulated over the course of the perinatal period, which makes it difficult to study (Roomruangwong et al., 2017a, 2017b, 2018). Due to the decreases in gonadotropins after delivery, translocation may be more likely during the postpartum period. Sex-based differences noted in results suggest that estrogen and progesterone may be important components of this relationship (Solati et al., 2015; Walker et al., 2012). Other research shows activation of the TLR-4 pathway occurs with stress and LPS exposure. Results from these studies, therefore, support the multi-hit model (Friebe et al., 2011). The microbiome provides opportunities to understand how the immune system develops, acts, and adapts over the perinatal period. Future research in this area is needed to study how gonadotropins, the HPA axis, and the immune system work together.
Fig. 3.
The microbiota-gut-brain axis in pregnancy without elevated symptoms of depression and anxiety and in perinatal depression and anxiety highlighting some hypothesized changes in the gut microbiota, immune system, and metabolism. Tryptophan metabolism is an example of a metabolic pathway impacted by microbial dybiosis that possibly impacts the brain.
Current research in this area is natally focused, but we need research that strives to better understand maternal outcomes and mechanisms. Future work should combine maternal psychological data with biological measures, including the microbiome and immune and stress system indicators, and factors that may mediate or moderate these effects such as social or environmental variables. Sample sizes must be large enough to evaluate multiple factors and to account for multiple comparisons. Designs should utilize nuanced phenotyping with the goal of understanding symptomatic components and trajectories through the perinatal period in order to appropriately define outcomes. While individual species and families may be associated with outcomes, future research should continue to evaluate the impact of microbial community interactions and composition in relation to host facotrs. Research that translates animal model findings to human populations and the human studies that produce mechanistic hypotheses which can be tested in animal models for proof of principle are important future directions.
4.1. Conclusions
Emerging literature suggests that the microbiota-brain axis in relation to PMAD is an important area of study that has the potential to provide us with better identification and treatment for women, ultimately improving outcomes for mother and child. An individual’s microbial signature may serve as both a complex biomarker that can identify differing personalized mixes of etiologies (e.g., of HPA axis changes and of immune system changes) and also serve as an important symbiont and communicator between the host and the environment, responding to transitions as well as driving transitions (i.e., via metabolites, vagus nerve, immune system modulator). Dietary components including prebiotic and probiotic consumption may be mediating factors and future points of intervention. Important themes identified include: the importance of studying the maternal microbiome and measures of stress, anxiety, and depression; and that multi-hit models will be needed as research strives to determine the effects of multiple mechanisms working in concert.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institutes of Mental Health 1 K23 MH110660-01, the Brain and Behavior Foundation NARSAD Young Investigator Award, the P&S Fund, and The Foundation of Hope Seed Grant.
Footnotes
Conflicts of interest
None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.psyneuen.2018.05.020.
References
- Aagaard K, Riehle K, Ma J, et al. , 2012. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy In: Ratner AJ (Ed.), PLoS ONE, pp. e36466 10.1371/journal.pone.0036466. 7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramowitz JS, Meltzer-Brody S, Leserman J, et al. , 2010. Obsessional thoughts and compulsive behaviors in a sample of women with postpartum mood symptoms. Arch. Womens Ment. Health 13 (6), 523–530. 10.1007/s00737-010-0172-4. [DOI] [PubMed] [Google Scholar]
- Andersson L, Sundström-Poromaa I, Wulff M, Åström M, Bixo M, 2006. Depression and anxiety during pregnancy and six months postpartum: a follow-up study. Acta Obstet. Gynecol. Scand 85 (8), 937–944. 10.1080/00016340600697652. [DOI] [PubMed] [Google Scholar]
- Baker JM, Al-Nakkash L, Herbst-Kralovetz MM, 2017. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas 103, 45–53. 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- Bernstein IH, Rush AJ, Yonkers K, et al. , 2008. Symptom features of postpartum depression: are they distinct? Depress. Anxiety 25 (1), 20–26. 10.1002/da.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR, 2000. Effects of gonadal steroids in women with a history of postpartum depression. Am. J. Psychiatry 157 (6), 924–930. 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bloch M, Rubinow DR, Schmidt PJ, Lotsikas A, Chrousos GP, Cizza G, 2005. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J. Clin. Endocrinol. Metab 90 (2), 695–699. 10.1210/jc.2004-1388. [DOI] [PubMed] [Google Scholar]
- Bränn E, Papadopoulos F, Fransson E, et al. , 2017. Inflammatory markers in late pregnancy in association with postpartum depression—A nested case-control study. Psychoneuroendocrinology 79, 146–159. 10.1016/j.psyneuen.2017.02.029. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, et al. , 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci 108 (38), 16050–16055. 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Fernandez-Kim S-O, Townsend RL, et al. , 2017. Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice In: Rosenfeld CS (Ed.), PLOS ONE, pp. e0175577 10.1371/journal.pone.0175577. 12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Mysorekar IU, 2014. Intracellular bacteria in placental basal plate localize to extravillous trophoblasts. Placenta 35 (2), 139–142. 10.1016/j.placenta.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Cénit MC, Matzaraki V, Tigchelaar EF, Zhernakova A, 2014. Rapidly expanding knowledge on the role of the gut microbiome in health and disease. Biochim. Biophys. Acta BBA - Mol. Basis Dis 1842 (10), 1981–1992. 10.1016/j.bbadis.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Christian LM, Galley JD, Hade EM, Schoppe-Sullivan S, Kamp Dush C, Bailey MT, 2015. Gut microbiome composition is associated with temperament during early childhood. Brain Behav. Immun 45, 118–127. 10.1016/i.bbi.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, et al. , 2013. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 18 (6), 666–673. 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Collado MC, Isolauri E, Laitinen K, Salminen S, 2008. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr 88 (4), 894–899. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Campbell EA, Day A, Kennerley H, Bond A, 1988. Non-psychotic psychiatric disorder after childbirth. A prospective study of prevalence, incidence, course and nature. Br. J. Psychiatry J. Ment. Sci 152, 799–806. [DOI] [PubMed] [Google Scholar]
- Corwin EJ, Pajer K, 2008. The psychoneuroimmunology of postpartum depression. J. Womens Health 17 (9), 1529–1534. 10.1089/jwh.2007.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CSM, Callaghan BL, Richardson R, 2016. The effects of a probiotic formulation (Lactobacillus rhamnosus and L. Helveticus) on developmental trajectories of emotional learning in stressed infant rats. Transl. Psychiatry 6 (5). 10.1038/tp.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhane JF, Rauh V, McCollum KF, Hogan VK, Agnew K, Wadhwa PD, 2001. Maternal stress is associated with bacterial vaginosis in human pregnancy. Matern. Child. Health J 5 (2), 127–134. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9 (1), 46–56. 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, et al. , 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505 (7484), 559–563. 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, et al. , 2015. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav. Immun 48, 165–173. 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC, 2007. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am. J. Psychiatry 164 (10), 1515–1520. 10.1176/appi.ajp.2007.06111893. [DOI] [PubMed] [Google Scholar]
- DiGiulio DB, Callahan BJ, McMurdie PJ, et al. , 2015. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci 112 (35), 11060–11065. 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF, 2012. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37 (9), 1369–1378. 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF, 2017. Microbes, immunity, and behavior: psychoneuroimmunology meets the microbiome. Neuropsychopharmacology 42 (1), 178–192. 10.1038/npp.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson Å, Bränn E, Hellgren C, et al. , 2017. Lower inflammatory markers in women with antenatal depression brings the M1/M2 balance into focus from a new direction. Psychoneuroendocrinology 80, 15–25. 10.1016/j.psyneuen.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Engel G, 1977. The need for a new medical model: a challenge for biomedicine. Science 196 (4286), 129–136. 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- Evans J, Heron J, Francomb H, Oke S, Golding J, 2001. Cohort study of depressed mood during pregnancy and after childbirth. BMJ 323 (7307), 257–260. 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Hernandez-Reif M, Diego M, 2006. Risk factors and stress variables that differentiate depressed from nondepressed pregnant women. Infant. Behav. Dev 29 (2), 169–174. 10.1016/j.infbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Flores R, Shi J, Fuhrman B, et al. , 2012. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J. Transl. Med 10 (1), 253 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld K-A, 2013. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36 (5), 305–312. 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Sosinsky AZ, Moustafa D, Viguera AC, Cohen LS, 2016. Supplement use by women during pregnancy: data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Arch. Womens Ment. Health 19 (3), 437–441. 10.1007/s00737-015-0586-0. [DOI] [PubMed] [Google Scholar]
- Friebe A, Douglas AJ, Solano E, et al. , 2011. Neutralization of LPS or blockage of TLR4 signaling prevents stress-triggered fetal loss in murine pregnancy. J. Mol. Med 89 (7), 689–699. 10.1007/s00109-011-0743-5. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, et al. , 2005. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes. Agency for Healthcare Research and Quality (US), Rockville, MD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile S, 2011. Suicidal mothers. J. Inj. Violence Res 3 (2), 91–97. 10.5249/jivr.v3i2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Gabry KE, Yasuda MR, Chrousos GP, 2002. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol. Metab. Clin. North. Am 31 (1), 37–62. 10.1016/S0889-8529(01)00022-6. [DOI] [PubMed] [Google Scholar]
- Gur TL, Shay L, Palkar AV, et al. , 2017. Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav. Immun 64, 50–58. 10.1016/j.bbi.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Holzer P, Farzi A, 2014. Neuropeptides and the Microbiota-Gut-Brain Axis In: In: Lyte M, Cryan JF (Eds.), Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease, vol. 817 Springer; New York, New York, NY, pp. 195–219. 10.1007/978-l-4939-0897-4_9. [DOI] [Google Scholar]
- Jandhyala SM, 2015. Role of the normal gut microbiota. World J. Gastroenterol 21 (29), 8787 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Howerton CL, Howard CD, Bale TL, 2015. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 156 (9), 3265–3276. 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Howard CD, Misic AM, Beiting DP, Bale TL, 2017. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep 7, 44182 10.1038/srep44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, et al. , 2015. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun 48, 186–194. 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Jolley SN, Elmore S, Barnard KE, Carr DB, 2007. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol. Res. Nurs 8 (3), 210–222. 10.1177/1099800406294598. [DOI] [PubMed] [Google Scholar]
- Josefsson A, Berg G, Nordin C, Sydsjö G, 2001. Prevalence of depressive symptoms in late pregnancy and postpartum. Acta Obstet. Gynecol. Scand 80 (3), 251–255. 10.1034/j.1600-0412.2001.080003251.x. [DOI] [PubMed] [Google Scholar]
- Jost T, Lacroix C, Braegger C, Chassard C, 2014. Stability of the maternal gut microbiota during late pregnancy and early lactation. Curr. Microbiol 68 (4), 419–427. 10.1007/s00284-013-0491-6. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP, 2015. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci 9 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA, 2002. Toward a comprehensive developmental model for major depression in women. Am. J. Psychiatry 159 (7), 1133–1145. 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- Kimmel M, Hess E, Roy PS, et al. , 2015. Family history, not lack of medication use, is associated with the development of postpartum depression in a high-risk sample. Arch. Womens Ment. Health 18 (1), 113–121. 10.1007/s00737-014-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindinger LM, Bennett PR, Lee YS, et al. , 2017. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 5 (1). 10.1186/s40168-016-0223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston D, Tough S, Whitfield H, 2012. Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child. Psychiatry Hum. Dev 43 (5), 683–714. 10.1007/s10578-012-0291-4. [DOI] [PubMed] [Google Scholar]
- Kleiman SC, Watson HJ, Bulik-Sullivan EC, et al. , 2015. The intestinal Microbiota in acute anorexia nervosa and during renourishment: relationship to depression, anxiety, and eating disorder psychopathology. Psychosom. Med 77 (9), 969–981. 10.1097/PSY.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O, Goodrich JK, Cullender TC, et al. , 2012. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150 (3), 470–480. 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus TA, Engel SM, Sperling RS, et al. , 2012. Characterizing the pregnancy immune phenotype: results of the Viral Immunity and Pregnancy (VIP) study. J. Clin. Immunol 32 (2), 300–311. 10.1007/s10875-011-9627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G, Muralidhara, 2015. Inulin supplementation during gestation mitigates acrylamide-induced maternal and fetal brain oxidative dysfunctions and neurotoxicity in rats. Neurotoxicol. Teratol 49, 49–58. 10.1016/j.ntt.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Krishna G, Divyashri G, Prapulla SG, Muralidhara, 2015. A combination supplement of Fructo- and Xylo-oligosaccharides significantly abrogates oxidative impairments and neurotoxicity in Maternal/Fetal milieu following gestational exposure to acrylamide in rat. Neurochem. Res 40 (9), 1904–1918. 10.1007/s11064-015-1687-x. [DOI] [PubMed] [Google Scholar]
- Lawson M, Kern F, Everson GT, 1985. Gastrointestinal transit time in human pregnancy: prolongation in the second and third trimesters followed by postpartum normalization. Gastroenterology 89 (5), 996–999. 10.1016/0016-5085(85)90199-4. [DOI] [PubMed] [Google Scholar]
- Lee NM, Saha S, 2011. Nausea and vomiting of pregnancy. Gastroenterol. Clin. North Am 40 (2), 309–334. 10.1016/j.gtc.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Ojeda JM, Rupprecht R, Baghai TC, 2017. “I am I and my bacterial circum-stances”: linking gut microbiome, neurodevelopment, and depression. Front. Psychiatry 8 10.3389/fpsyt.2017.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl V, Pearson JL, Colpe L, 2005. Prevalence of suicidality during pregnancy and the postpartum. Arch. Women’s Ment. Health 8 (2), 77–87. 10.1007/s00737-005-0080-1. [DOI] [PubMed] [Google Scholar]
- Luppi P, Haluszczak C, Trucco M, Deloia JA, 2002. Normal pregnancy is associated with peripheral leukocyte activation. Am. J. Reprod. Immunol 47 (2), 72–81. 10.1034/j.1600-0897.2002.1o041.x. [DOI] [PubMed] [Google Scholar]
- Lurie I, Yang Y-X, Haynes K, Mamtani R, Boursi B, 2015. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J. Clin. Psychiatry (November), 1522–1528. 10.4088/JCP.15m09961. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis J-C, Berk M, 2012. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J. Affect Disord 141 (1), 55–62. 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Magiakou M-A, Mastorakos G, Rabin D, et al. , 1996. The maternal hypothalamic-pituitary-adrenal axis in the third trimester of human pregnancy. Clin. Endocrinol (Oxf.) 44 (4), 419–428. http://dx.doi.Org/10.1046/j.1365-2265.1996.683505.x. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I, 2003. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann. N. Y. Acad. Sci 997 (1), 136–149. 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- Mehta D, Newport DJ, Frishman G, et al. , 2014. Early predictive biomarkers for postpartum depression point to a role for estrogen receptor signaling. Psychol. Med 44 (11), 2309–2322. 10.1017/S0033291713003231. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, 2011. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin. Neurosci 13 (1), 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjösberg J, Berg G, Jenmalm MC, Ernerudh J, 2010. FOXP3+ regulatory T cells and T Helper 1, T Helper 2, and T Helper 17 cells in human early pregnancy decidua1. Biol. Reprod 82 (4), 698–705. 10.1095/biolreprod.109.081208. [DOI] [PubMed] [Google Scholar]
- Mor G, Cardenas I, 2010. The immune system in pregnancy: a unique complexity: immune system in pregnancy. Am. J. Reprod. Immunol 63 (6), 425–433. 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Aldo P, Alvero AB, 2017. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol 17 (8), 469–482. 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, et al. , 2014. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil 26 (8), 1155–1162. 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Mayberg HS, Krahl SE, et al. , 2006. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 31 (7), 1345–1355. 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- Nuriel-Ohayon M, Neuman H, Koren O, 2016. Microbial changes during pregnancy, birth, and infancy. Front. Microbiol 7 10.3389/fmicb.2016.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keane V, Marsh MS, 2007. Depression during pregnancy. BMJ 334 (7601), 1003–1005. 10.1136/bmj.39189.662581.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keane V, Dinan TG, Scott L, Corcoran C, 2005. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol. Psychiatry 58 (12), 963–968. 10.1016/j.biopsych.2005.04.049. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF, 2015. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res 277, 32–48. 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Oates M, 2003. Perinatal psychiatric disorders: a leading cause of maternal morbidity and mortality. Br. Med. Bull 67 (1), 219–229. 10.1093/bmb/ldgOll. [DOI] [PubMed] [Google Scholar]
- Osborne LM, Monk C, 2013. Perinatal depression—The fourth inflammatory morbidity of pregnancy? Psychoneuroendocrinology 38 (10), 1929–1952. 10.1016/j.psyneuen.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino CL, Singh V, Campbell J, Flynn H, Gold KJ, 2011. Homicide and suicide during the perinatal period: findings from the national violent death reporting system. Obstet. Gynecol 118 (5), 1056–1063. 10.1097/AOG.0bOl3e31823294da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K, Boutain D, Manhart L, Hitti J, 2008. Racial disparity in bacterial vaginosis: the role of socioeconomic status, psychosocial stress, and neighborhood characteristics, and possible implications for preterm birth. Soc. Sci. Med 67 (5), 824–833. 10.1016/j.socscimed.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Perez-Muñoz ME, Arrieta M-C, Ramer-Tait AE, Walter J, 2017. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5 (1). 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racicot K, Kwon JY, Aldo P, et al. , 2016. Type I interferon regulates the placental inflammatory response to bacteria and is targeted by virus: mechanism of polymicrobial infection-induced preterm birth. Am. J. Reprod. Immunol 75 (4), 451–460. http://dx.doi.org/10.1111/aji.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF, et al. , 2015. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29 (4), 1395–1403. 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Hassan SS, Gajer P, et al. , 2014. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2 (1), 4 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GAW, Lowry CA, Raison CL, 2013. Microbial “Old Friends”, immunoregulation and stress resilience. Evol. Med. Public Health 2013 (1), 46–64. 10.1093/emph/eot004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomruangwong C, Kanchanatawan B, Sirivichayakul S, et al. , 2017a. IgA/IgM responses to gram-negative bacteria are not associated with perinatal depression, but with physio-somatic symptoms and activation of the tryptophan catabolite pathway at the end of term and postnatal anxiety. CNS Neurol. Disord. Drug Targets 16 (4), 472–483. 10.2174/1871527316666170407145533. [DOI] [PubMed] [Google Scholar]
- Roomruangwong C, Kanchanatawan B, Sirivichayakul S, et al. , 2017b. IgM-mediated autoimmune responses to oxidative specific epitopes, but not nitrosylated adducts, are significantly decreased in pregnancy: association with bacterial translocation, perinatal and lifetime major depression and the tryptophan catabolite (TRYCAT) pathway. Metab. Brain Dis 32 (5), 1571–1583. 10.1007/s11011-017-0040-2. [DOI] [PubMed] [Google Scholar]
- Roomruangwong C, Anderson G, Berk M, Stoyanov D, Carvalho AF, Maes M, 2018. A neuro-immune, neuro-oxidative and neuro-nitrosative model of prenatal and postpartum depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 81, 262–274. 10.1016/j.pnpbp.2017.09.015. ((Roomruangwong C.; Maes M., michaelmaes@hotmail.com) Department of Psychiatry, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand). [DOI] [PubMed] [Google Scholar]
- Ross LE, Sellers EM, Gilbert Evans SE, Romach MK, 2004. Mood changes during pregnancy and the postpartum period: development of a biopsychosocial model. Acta Psychiatr. Scand 109 (6), 457–466. 10.1111/j.1600-0047.2004.00296.x. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Kiely B, Dinan TG, Cryan JF, 2014. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil 26 (11), 1615–1627. 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- Schminkey DL, Groer M, 2014. Imitating a stress response: a new hypothesis about the innate immune system’s role in pregnancy. Med. Hypotheses 82 (6), 721–729. 10.1016/j.mehy.2014.03.013 [DOI] [PubMed] [Google Scholar]
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK, 2016. The Central nervous system and the gut microbiome. Cell 167 (4), 915–932. 10.1016/j-cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton MM, Schminkey DL, Groer MW, 2015. Relationships among prenatal depression, plasma cortisol, and inflammatory cytokines. Biol. Res. Nurs 17 (3), 295–302. 10.1177/1099800414543821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalkidou A, Hellgren C, Comasco E, Sylvén S, Poromaa IS, 2012. Biological aspects of postpartum depression. Womens Health 8 (6), 659–672. 10.2217/WHE.12.55. [DOI] [PubMed] [Google Scholar]
- Slykerman RF, Hood F, Wickens K, et al. , 2017. Effect of lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine(September). 10.1016/j.ebiom.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid M, Ricks N, Panzer A, et al. , 2017. Maternal gut microbiome biodiversity in pregnancy. Am. J. Perinatol(July). 10.1055/s-0037-1604412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solati J, Kleehaupt E, Kratz O, Moll GH, Golub Y, 2015. Inverse effects of lipopolysaccharides on anxiety in pregnant mice and their offspring. Physiol. Behav 139, 369–374. 10.1016/j.physbeh.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, et al. , 2014. Effects of perinatal mental disorders on the fetus and child. Lancet 384 (9956), 1800–1819. 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- Stout MJ, Conlon B, Landeau M, et al. , 2013. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am. J. Obstet.Gynecol 208 (3), 226.el–226.e7. 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, et al. , 2004. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol 558 (1), 263–275. 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes L, MacIntyre DA, Yap XJ, Teoh TG, Bennett PR, 2012. The Th1:Th2 dichotomy of pregnancy and preterm labour. Mediat. Inflamm 2012, 1–12. 10.1155/2012/967629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togher K, Khashan A, Kenny L, et al. , 2017. Depressive symptoms during pregnancy disrupt gut microbiome dynamics during critical prenatal and postnatal time windows. Neurogastroenterol. Motil 29, 34–35. 10.1111/nmo.13180. ((Togher K.; Khashan A.; Kenny L.; Stanton C.; Carafa I.; Murphy K.; O’Keeffe G.; Ryan A.; Cryan J.F.; Dinan T.; Clarke G.) University College Cork, Dept. of Anatomy and Neuroscience, Ireland). [DOI] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI, 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444 (7122), 1027–1131. 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Uscher-Pines L, Hanlon AL, Nelson DB, 2009. Racial differences in bacterial vaginosis among pregnant women: the relationship between demographic and behavioral predictors and individual BV-related microorganism levels. Matern. Child. Health J 13 (4), 512–519. 10.1007/s10995-008-0372-y. [DOI] [PubMed] [Google Scholar]
- Walker AK, Hawkins G, Sominsky L, Hodgson DM, 2012. Transgenerational transmission of anxiety induced by neonatal exposure to lipopolysaccharide: implications for male and female germ lines. Psychoneuroendocrinology 37 (8), 1320–1335. 10.1016/j.psyneuen.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Walther-António MRS, Jeraldo P, Berg, Miller ME, et al. , 2014. Pregnancy’s stronghold on the vaginal microbiome In: Gilbert JA (Ed.), PLoS ONE, pp. e98514 10.1371/journal.pone.0098514. 9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kasper LH, 2014. The role of microbiome in central nervous system disorders. Brain Behav. Immun 38, 1–12. 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Peindl KS, Hanusa BH, Piontek CM, Findling RL, 2004. Prevention of postpartum depression: a pilot randomized clinical trial. Am. J. Psychiatry 161 (7), 1290–1292. 10.1176/appi.ajp.161.7.1290. [DOI] [PubMed] [Google Scholar]
- Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C, 2015. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 53, 233–245. 10.1016/j.psyneuen.2015.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.