Abstract
A series of recent reports have questioned the ability of great apes to comprehend declarative communication and have suggested that this ability is biologically based and may have driven the evolution of human language. We tested three groups of differently reared chimpanzees and bonobos for their ability to understand declarative signals in an object-choice task. The scores of the two groups of apes that were reared in a sociolinguistically complex environment were significantly higher than the scores of the standard-reared group. The results further showed that bonobos did not outperform chimpanzees. Our results demonstrate that environmental factors, particularly access to a sociolinguistically rich environment, directly influence great apes’ ability to comprehend declarative signals and suggest that, contrary to recent claims, apes have the biological capacity to utilize purely informative communication.
Keywords: language evolution, apes, social rearing, declarative pointing
Human children are highly motivated to share information with other individuals (e.g., Tomasello, 2007). By 12 to 15 months of age, children begin spontaneously pointing imperatively to request objects, but also point declaratively—simply to draw the attention of adults to specific objects (Bates, Camaioni, & Volterra, 1975). Although recent studies in great apes (i.e., hominids: chimpanzees, bonobos, gorillas, and orangutans) have demonstrated evidence of imperative pointing (Leavens, Hopkins, & Bard, 2005), it has been suggested that comprehension and production of declarative gestures is absent in apes or is highly constrained to socially competitive contexts (Hare, 2007; but see Menzel, 2004). These findings have led some researchers to suggest that within the primate lineage, understanding declarative communication is unique to humans and is possibly driven by the same biological change that led to the development of human language (Tomasello, 2007).
The majority of studies on the comprehension of declarative signaling in nonhuman animals have employed the object-choice test, in which a human experimenter indicates the location of a hidden food item using a social cue (e.g., gaze direction, pointing, or vocalizing). Several studies utilizing this task have shown evidence of declarative comprehension in animals other than great apes, including lemurs, dolphins, seals, goats, corvids, and canines (e.g., Pack & Herman, 2007; Ruiz, Gómez, Roeder, & Byrne, 2009; see Miklósi & Soproni, 2006, for a recent review). However, results from studies of chimpanzees, gorillas, and orangutans have been much less clear.
Despite multiple reports that chimpanzees can readily follow the gaze of humans and conspecifics (e.g., Hostetter, Russell, Freeman, & Hopkins, 2007; Tomasello, Hare, & Agnetta, 1999), object-choice studies have provided contradictory evidence as to whether chimpanzees, gorillas, and orangutans can comprehend declarative signals (e.g., Barth, Reaux, & Povinelli, 2005; Call, Agnetta, & Tomasello, 2000). In the data published to date, the 32 chimpanzees tested on the object-choice task had a mean percentage correct of 61% (Lyn & Hopkins, 2009). Although this mean is significantly above chance level, of the 32 chimpanzees, only 9 performed significantly better than 50% correct. Thus, it appears that these few individual chimpanzees account for the significant results.
Different explanations have been proposed to account for species differences as well as individual differences in performance on the object-choice task. One hypothesis is that biological or genetic factors play a critical role. For example, Hare et al. (2005) studied two groups of foxes, one of which was selectively bred for reduced fearful and aggressive behaviors toward humans. The selectively bred foxes performed significantly better on the object-choice task than did the control foxes, a result suggesting that genetic factors play an important role in canids (Hare, 2007). With respect to chimpanzees (and potentially other great apes), some studies have found that performance on the object-choice task is influenced by whether the test paradigm is a competitive or a cooperative task (see Moll & Tomasello, 2007, for a review). For example, Hare and his colleagues (2007) found that chimpanzees that do not follow cooperative gestures can utilize a competitive gesture (grabbing for food) to infer the location of a food item; these authors hypothesized that the noncooperative nature of chimpanzee social behavior and organization is a biological difference from humans that makes it difficult for chimpanzees to perform above chance levels on the object-choice task.
According to another hypothesis (the sociolinguistic-construction hypothesis; adapted from Moll & Tomasello, 2007), after humans’ evolutionary split from noncooperative primate antecedents, humans developed linguistic and social interactions that fostered the emergence of both imperative and declarative communication. In this view, humans’ ability to comprehend and produce declarative communication occurred as the result of an interaction of their biologically based cooperative nature and their environment, an idea that is similar to the concept of social scaffolding proposed by Vygotsky (1978). However, Moll and Tomasello suggested that chimpanzees not only did not develop this environmental support for declarative communication, but also are unable to utilize such support because of their noncooperative nature.
This suggestion is suspect because environmental support in the form of human contact (enculturation) was implicated as an important factor in the abilities of primates in several object-choice studies (Call & Tomasello, 1994; Itakura, Agnetta, Hare, & Tomasello, 1999; Itakura & Tanaka, 1998). However, these studies were based on relatively few animals (2 enculturated chimpanzees and 2 enculturated orangutans), and their findings were contradictory, with enculturated animals sometimes performing better than standard-reared animals and sometimes not (e.g., Call et al., 2000; Call, Hare, & Tomasello, 1998). Indeed, later studies did not discuss the possibility that human contact affected results, even when testing the same individual animals (Tomasello, Call, & Gluckman, 1997).
The first aim of the study we report here was to examine the effects of exposure to different human communicative environments on the comprehension of declarative signals in two closely related great ape species of the genus Pan: chimpanzees (Pan troglodytes) and bonobos (Pan paniscus). According to the biological hypothesis, apes simply lack the capacity to comprehend declarative signals; if this hypothesis is correct, all apes should perform poorly on tests of declarative comprehension. However, according to the sociolinguistic-construction hypothesis, apes raised in human communicative environments should perform significantly better than other apes.
The second aim of this study was to directly compare the abilities of bonobos and chimpanzees to utilize declarative social cues in a noncompetitive context. To date, among great apes, 32 chimpanzees, 8 orangutans, and 7 gorillas have been tested on the object-choice task; no data are available for bonobos. Given the differing social structures of the two species of Pan, one might predict performance differences on the object-choice task. Specifically, it has been suggested that bonobos are a more tolerant and cooperative species than chimpanzees (de Waal, 1996; Hare, Melis, Woods, Hastings, & Wrangham, 2007). If this is true, and if tolerance and cooperation are important components of the evolution of declarative comprehension (Hare, 2007), then bonobos should perform better than chimpanzees on cooperative versions of the object-choice task. We tested this hypothesis by comparing similarly raised bonobos and chimpanzees on this task.
Method
Subjects
Three groups of apes were tested. One group consisted of 6 chimpanzees (2 males, 4 females) from the Yerkes National Primate Research Center of Emory University (YNPRC); their ages ranged from 11 to 38 years. A second group comprised 7 bonobos (4 males, 3 females) residing at the Great Ape Trust of Iowa (GATI); their ages ranged from 8 to 39 years. The third group consisted of 4 chimpanzees (2 males, 2 females) living at the Language Research Center of Georgia State University (LRC); they ranged in age from 22 to 38 years.
Both the GATI bonobos and the LRC chimpanzees spent much of their early years at the LRC as part of a larger project devoted to fostering two-way communication between human and nonhuman primates (Lyn, Greenfield, & Savage-Rumbaugh, in press; Savage-Rumbaugh, 1986; Savage-Rumbaugh et al., 1993). In 2005, the bonobos moved to the GATI facility; the 4 chimpanzees remained at the LRC facility. All 11 of the LRC and GATI apes are socially housed and are exposed to high levels of complex communicative interactions with human caregivers. The YNPRC subjects, with the exception of one wild-caught individual (Leslie), were born at the center and were reared following standard practices for captive apes. These animals are housed in groups of 2 to 6 chimpanzees and have regular contact with human caregivers. However, these interactions are typically limited to basic husbandry contexts, such as shifting animals from cage to cage and feeding, and to cognitive testing with no specific emphasis on understanding human communication.
Procedure
The American Psychological Association’s guidelines for the ethical treatment of animals were adhered to during all phases of this study.
In an object-choice task, subjects are required to choose between two containers in order to find a hidden food item. In this study, we presented two paper tubes, closed at one end, one of which was baited with a small piece of food (an M&M or a small piece of fruit). These tubes were placed equidistant from the center of a testing surface (a testing table or piece of cardboard). In most object-choice tasks, subjects are required to point to the desired container. However, in our task, the paper tubes allowed the apes to grasp and bring the tubes inside their enclosure (see Fig. 1). In this way, we eliminated the need for subjects to produce a gesture and ensured that individual differences in the production of a gesture were not confounded with ability to comprehend a declarative cue.
Fig. 1.
Illustration of the setup for the object-choice task. On each trial, two tubes were presented, and one of the tubes contained a piece of food. The subject was allowed to take one of the tubes only. In all trial types, the experimenter alternated her gaze between the correct tube and the ape. On a pointing trial (illustrated here), the experimenter pointed to the correct tube. On a vocalizing trial, the experimenter leaned toward the correct tube and vocalized. On a point-and-vocalize trial, the experimenter both pointed to the correct tube and vocalized. In this photograph, Julie (a Yerkes National Primate Research Center chimpanzee) is grasping her choice as the experimenter points to the correct item.
The apes were trained on the task so they would learn to choose only one tube and to select the one with the food reward. On the initial trials, the open ends of the tubes faced the subjects (open trials). Next, the apes were tested with the closed ends of the tubes facing them (closed trials) so that they had to watch to see which tube was baited in order to choose the correct one. Subjects moved from the training phase to the testing phase only after they chose correctly on at least 8 out of 10 consecutive trials in each training condition.
On all test trials, the placement of the food in the tube was hidden from the ape by a blind. Each test session included a pseudorandomized set of 24 trials, 8 trials in each of three declarative conditions: point, vocalize, and point and vocalize. All trials started with the experimenter saying the ape’s name to catch his or her attention. Immediately afterward, the experimenter indicated the correct tube. In the point condition, the experimenter used her whole arm with the index finger extended to point toward the correct tube (at a distance of 2–10 cm) while alternating her gaze between the subject and the correct tube. In the vocalize condition, the experimenter leaned toward the correct tube while vocalizing and alternating her gaze between the tube and the ape. For the YNPRC chimpanzees, the vocalizations consisted of replicating a chimpanzee food grunt. For the chimpanzees at the LRC, this food grunt was alternated with spoken English: ‘‘It’s in this one; the [name of food] is in here.’’ The bonobos at the GATI heard a simulated bonobo vocalization, a food peep, alternated with a similar English sentence. In the point-and-vocalize condition, the experimenter pointed at the correct tube while alternating her gaze and vocalizing.
The GATI bonobos and the LRC chimpanzees were presented with two test sessions in a row. Because of poor performance, the YNPRC chimpanzees were given an extra session of training trials between their test sessions; in these training trials, they could see the tube being baited, and the correct tube was also indicated by the experimenter. Additionally, the 24 test trials in the YNPRC chimpanzees’ second testing session were randomly interspersed with 24 training trials.
Results
Descriptive statistics
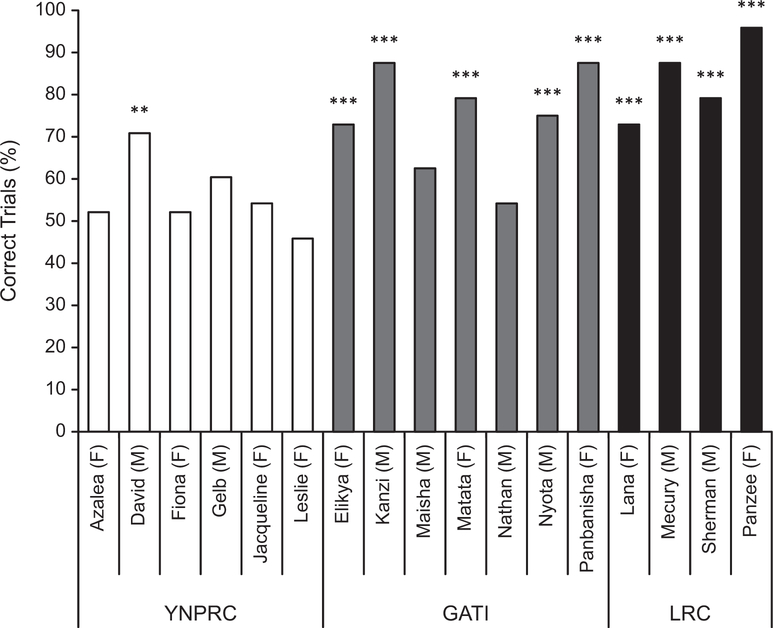
Individual performance on the task is summarized in Figure 2. Not unlike laboratory-living chimpanzees described in previous reports, only 1 of the 6 YNPRC subjects performed above chance levels, even though they were given extra training between the two test sessions. In contrast, 5 of the 7 GATI bonobos and all 4 LRC chimpanzees performed significantly above chance, with no explicit training. The YNPRC chimpanzees were correct on an average of 55.4% of the trials; however, the GATI bonobos were correct on 74.1% of the trials, and the LRC chimpanzees on 83.9% of the trials.
Fig. 2.
Percentage correct on the object-choice task across the three declarative conditions. Each subject is identified by name, sex (F = female, M = male), and group. We used binomial tests to evaluate whether each ape performed at better-than-chance levels, **p < .01, ***p < .001. YNPRC = Yerkes National Primate Research Center of Emory University; GATI = Great Ape Trust of Iowa; LRC = Language Research Center of Georgia State University.
Performance analyses
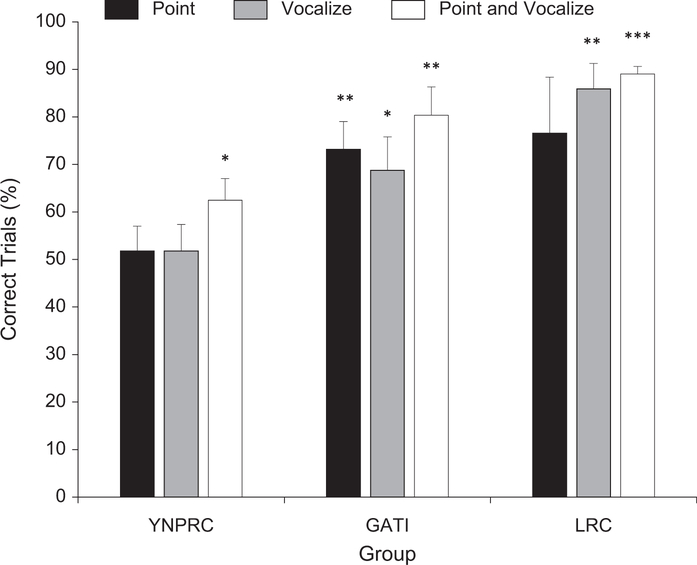
A repeated measures analysis of variance showed significant main effects for group, F(2, 14)=8.73, p = .003, η2 = .56, and test condition, F(2, 28) = 3.606, p = .04, η2 = .21, but no interaction between these two variables, F(4, 28) = 0.47, p >.05, η2 = .06 (see Fig. 3). With respect to the group effect, Tukey’s honestly significant difference tests showed that in every condition, the percentage of trials with a correct response was significantly lower for the YNPRC group compared with the GATI and LRC groups. Post hoc tests also showed that performance in the point-and-vocalize condition was significantly better than performance in either the point or the vocalize condition (see Fig. 3). This result supports earlier findings that vocalizations and other sounds enhance performance on a visual object-choice task and vice versa (a visual cue enhances performance on an auditory task; Call et al., 2000; Itakura et al., 1999). No other significant differences were found.
Fig. 3.
Mean percentage correct on the object-choice task for each group in each declarative condition. Performance was evaluated relative to chance (μ = 50%) using one-sample t tests, *p < .05, **p < .01, ***p < .001. Error bars represent standard errors. YNPRC = Yerkes National Primate Research Center of Emory University; GATI = Great Ape Trust of Iowa; LRC=Language Research Center of Georgia State University.
Discussion
The current findings indicate that apes raised in a complex social-communicative environment outperform other apes on the object-choice task. Therefore, although it seems that the ability to use purely informative social cues may be the result of biological changes in some species (such as canids), contrary to recent claims (Moll & Tomasello, 2007; Tomasello, 2007), at least two great apes share the biological capability for this behavior with humans, which can be observed when the apes are given the appropriate environment and rearing experiences. This finding supports the alternative, sociolinguistic-construction hypothesis in apes.
Additionally, the ability to comprehend declarative signals is not more developed in the allegedly more tolerant and cooperative bonobos than in chimpanzees when the two species are reared in similar environments. Therefore, either cooperation is not the determinant ability in declarative comprehension, contrary to recent hypothesizing, or cooperation is equally developed in bonobos and chimpanzees. The latter would perhaps not be surprising considering the multiple reports of chimpanzee cooperation in both wild (e.g., Gilby, 2006) and captive populations (Hirata & Fuwa, 2007; Savage-Rumbaugh, 1986; but see Silk et al., 2005).
Other studies have pointed to different mechanisms that may enhance performance on this task. For example, Miklósi and Soproni (2006) showed that proximal (close) pointing was easier for apes to follow than distal (distant) pointing. However, our procedure would be considered to involve proximal pointing, and the YNPRC chimpanzees still performed poorly. Also, although Povinelli, Reaux, Bierschwale, Allain, and Simon (1997) indicated that chimpanzees trained to look at a specific point indicated by the trainer’s gaze did not, as a group, generalize this response to other kinds of communicative gestures, Barth et al. (2005) showed that with some methodological changes (bringing the apes into the testing room after the gaze cue had been established rather than establishing the gaze cue after the choice was presented), these apes could follow gaze cues successfully.
Call, Agnetta, and Tomasello’s (2000) findings, similar to our findings, showed that the addition of vocalizations or sounds to a communicative cue increased performance, likely by bringing increased attention to the communicative signal. More recently, Ruiz et al. (2009) showed that lemurs that attend the most to a cue, as measured by their visual attention, are those most likely to follow that cue, a finding suggesting that perhaps the function of the social-communicative environment is to increase attentiveness to communicative cues. However, Povinelli et al. (1997) demonstrated that his chimpanzees attended to a communicative gesture (measured by the chimpanzee looking at the experimenter, then glancing at the correct box in an object-choice task), but that this did not predict successful choices. These results suggest that attention is not the only mechanism driving apes’ success on object-choice tasks. More research is required to begin to separate out possible mechanisms underlying the differences between individuals within and between species who are successful and unsuccessful on the object-choice task.
Vygotsky (1978) noted that the correct social environment could raise children of a given age to a level of abilities not normally seen at that age, as long as these abilities were with-in their ‘‘zone of proximal development.’’ Our results suggest that the comprehension of declaratives is within the apes’ ‘‘zone of proximal capability’’ and can be cultivated under socially and communicatively rich environments. Because the ability to acquire declarative comprehension is common to both apes and humans, researchers must look elsewhere for a candidate biological change that allowed for the evolution of human language and cognition.
Acknowledgments
We wish to thank Bill Fields, David Washburn, and Charles Menzel for their assistance in arranging for the data to be collected at the Great Ape Trust of Iowa and the Language Research Center of Georgia State University. We thank the research staff at all three locations for their assistance.
Funding
Funding for this study was provided by National Institutes of Health Grants HD-56232 and HD-38105.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Barth J, Reaux JE, & Povinelli DJ (2005). Chimpanzees’ (Pantroglodytes) use of gaze cues in object-choice tasks: Different methods yield different results. Animal Cognition, 8, 84–92. [DOI] [PubMed] [Google Scholar]
- Bates E, Camaioni L, & Volterra V (1975). The acquisition of performatives prior to speech. Merrill-Palmer Quarterly, 21, 205–224. [Google Scholar]
- Call J, Agnetta B, & Tomasello M (2000). Cues that chimpanzees do and do not use to find hidden objects. Animal Cognition, 3, 23–34. [Google Scholar]
- Call J, Hare B, & Tomasello M (1998). Chimpanzee gaze following in an object-choice task. Animal Cognition, 1, 89–99. [DOI] [PubMed] [Google Scholar]
- Call J, & Tomasello M (1994). Production and comprehension of referential pointing by orangutans (Pongo pygmaeus). Journal of Comparative Psychology, 108, 307–317. [DOI] [PubMed] [Google Scholar]
- de Waal FBM (1996). Good natured: The origins of right and wrong in humans and other animals Cambridge, MA: Harvard University Press. [Google Scholar]
- Gilby IC (2006). Meat sharing among the Gombe chimpanzees: Harassment and reciprocal exchange. Animal Behaviour, 71, 953–963. [Google Scholar]
- Hare B (2007). From nonhuman to human mind. What changed and why? Current Directions in Psychological Science, 16, 60–64. [Google Scholar]
- Hare B, Melis AP, Woods V, Hastings S, & Wrangham R (2007). Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Current Biology, 17, 619–623. [DOI] [PubMed] [Google Scholar]
- Hare B, Plyusnina I, Ignacio N, Schepina O, Stepika A, Wrangham R, & Trut L (2005). Social cognitive evolution in captive foxes is a correlated by-product of experimental domestication. Current Biology, 15, 226–230. [DOI] [PubMed] [Google Scholar]
- Hirata S, & Fuwa K (2007). Chimpanzees (Pantroglodytes) learn to act with other individuals in a cooperative task. Primates, 48, 13–21. [DOI] [PubMed] [Google Scholar]
- Hostetter AB, Russell JL, Freeman H, & Hopkins WD (2007). Now you see me, now you don’t: Evidence that chimpanzees understand the role of the eyes in attention. Animal Cognition, 10, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura S, Agnetta B, Hare B, & Tomasello M (1999). Chimpanzees use human and conspecific social cues to locate hidden food. Developmental Science, 2, 448–456. [Google Scholar]
- Itakura S, & Tanaka M (1998). Use of experimenter-given cues during object-choice tasks by chimpanzees (Pan troglodytes), an orangutan (Pongo pygmaeus), and human infants (Homo sapiens). Journal of Comparative Psychology, 112, 119–126. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, & Bard KA (2005). Understanding the point of chimpanzee pointing: Epigenesis and ecological validity. Current Directions in Psychological Science, 14, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyn H, Greenfield PM, & Savage-Rumbaugh ES (in press). Semiotic combinations in Pan: A cross-species comparison of communication in a chimpanzee and a bonobo. First Language
- Lyn H, & Hopkins WD (2009, September). Declarative comprehension and production in apes Paper presented at the annual meeting of the American Society of Primatologists, San Diego, CA. [Google Scholar]
- Menzel CR (2004). Unprompted recall and reporting of hidden objects by a chimpanzee (Pantroglodytes) after extended delays. Journal of Comparative Psychology, 113, 426–434. [DOI] [PubMed] [Google Scholar]
- Miklósi Á, & Soproni K (2006). A comparative analysis of animals’ understanding of the human pointing gesture. Animal Cognition, 9, 81–93. [DOI] [PubMed] [Google Scholar]
- Moll H, & Tomasello M (2007). Cooperation and human cognition: The Vygotskian intelligence hypothesis. Philosophical Transactions of the Royal Society B, 362, 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack AA, & Herman L (2007). The dolphin’s (Tursiops truncatus) understanding of human gazing and pointing: Knowing what and where. Journal of Comparative Psychology, 121, 34–45. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Reaux JE, Bierschwale DT, Allain AD, & Simon BB (1997). Exploitation of pointing as a referential gesture in children, but not adolescent chimpanzees. Cognitive Development, 12, 423–461. [Google Scholar]
- Ruiz A, Gómez JC, Roeder JJ, & Byrne RW (2009). Gaze following and gaze priming in lemurs. Animal Cognition, 12, 427–434. [DOI] [PubMed] [Google Scholar]
- Savage-Rumbaugh ES (1986). Ape language: From conditioned response to symbol New York: Columbia University Press. [Google Scholar]
- Savage-Rumbaugh ES, Murphy J, Sevcik RA, Brakke KE, Williams SL, & Rumbaugh DM (1993). Language comprehension in ape and child. Monographs of the Society for Research in Child Development, 58(3–4). [PubMed] [Google Scholar]
- Silk JB, Brosnan SF, Vonk J, Henrich J, Povinelli DJ, Richardson AS, et al. (2005). Chimpanzees are indifferent to the welfare of unrelated group members. Nature, 437, 1357–1359. [DOI] [PubMed] [Google Scholar]
- Tomasello M (2007). If they’re so good at grammar, then why don’t they talk? Hints from apes’ and humans’ use of gestures. Language Learning and Development, 3, 133–156. [Google Scholar]
- Tomasello M, Call J, & Gluckman A (1997). The comprehension of novel communicative signs by apes and human children. Child Development, 68, 1067–1081. [PubMed] [Google Scholar]
- Tomasello M, Hare B, & Agnetta B (1999). Chimpanzees, Pan troglodytes, follow gaze direction geometrically. Animal Behaviour, 58, 769–777. [DOI] [PubMed] [Google Scholar]
- Vygotsky LS (1978). Mind in society (Cole M, John-Steiner V, Scribner S, & Souberman E, Trans.). Cambridge, MA: Harvard University Press. [Google Scholar]