Abstract
Purpose:
To identify the effect of the benzimidazalone derivative, NS1619, on modulating pulmonary vascular tone in lungs from rats exposed to normoxia (21% FiO2) or chronic hypoxia (10% FiO2) for 3 weeks.
Methods:
Isolated perfused lungs were preconstricted (U46619) and dose-dependent vasodilation to NS1619 was assessed. To elucidate the mechanisms responsible, NS1619 vasodilatory responses were assessed following inhibition of large-conductance Ca2+-activated (BKCa; iberiotoxin and paxilline), L-type Ca2+ (nifedipine), K+ (tetraethylammonium), Cl− (niflumic acid) and cation/TRP (lanthanum) channels, as well as nitric oxide synthase (L-NAME).
Results:
Compared to normoxia, NS1619-induced vasodilation was significantly greater following hypoxia, however, NO-dependent vasodilation and BKCa-mediated vasodilation, in response to NS1619, was similar in the normoxic and hypoxic lungs. In contrast, direct activation of L-type Ca2+ and non-BKCa K+ channel was involved in the NS1619-induced vasodilation only in hypoxic lungs.
Conclusions:
NS1619 causes pulmonary vasodilation by affecting multiple complementary pathways including stimulation of NO production, activation of BKCa channels, other TEA-sensitive K+ channels and L-type Ca2+ channels, and could be considered as a therapeutic agent in hypoxic PH.
Keywords: isolated perfused lung, NS1619, hypoxia, pulmonary hypertension, pulmonary vasodilation
INTRODUCTION
The development of pulmonary hypertension (PH), particularly in response to prolonged hypoxia, is of critical importance in patients suffering from chronic lung diseases and is associated with significant morbidity and mortality [1]. The underlying pathophysiology of PH associated with chronic hypoxemia includes endothelial dysfunction, an enhanced vascular tone and vascular remodeling [2–4]. While the structural remodeling predominantly occurs in the large pulmonary arteries, the increase in pulmonary vascular resistance is largely attributed to pathological changes occurring within the pre-capillary arterioles. However, with the exception of supplemental oxygen therapy, there are currently no approved treatment strategies that effectively target the pulmonary resistance vasculature in PH associated lung disease.
In hypoxia-induced PH, changes in the activity and expression of the L-type Ca2+ channels [5], as well as voltage-gated (Kv) [6] and large conductance Ca2+-activated potassium channels (BKCa) [7,8], have been observed and contribute, in part, to an elevated vascular tone and hypersensitivity to vasoconstrictor stimuli [9]. Previously we[10], and others [11,12], have shown that the benzimidazalone derivative, NS1619, activates endothelial and vascular smooth muscle BKCa channels, and causes both endothelial dependent and independent vasodilation. Additionally, NS1619 has been reported to promote vasorelaxation through inhibitory actions on voltage-dependent Ca2+ channels [11,13]. Hence, NS1619 has the potential to favorably alter the activity of pulmonary vascular ion-channels that have been shown to be important in hypoxic lung vasculature. Therefore, the purpose of the current study was to determine the effect of NS1619 on pulmonary circulation in normoxic and chronically hypoxic animals using the isolated perfused lung technique, and to elucidate the underlying mechanisms responsible for the differences observed within hypoxic lung vasculature.
METHODS
Animals
All procedures were approved by the Animal Care and Use Committee at the Providence Veterans Affairs Medical Center and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Adult male Sprague–Dawley rats (n = 85, ~ 250 g; Charles River Laboratories, Wilmington, MA) were used in this study. Rats were housed at 23◦C, maintained on a 12:12-hr light-dark cycle in either normoxic (n = 38; 21% FiO2) or normobaric hypoxic (n = 49; 10% FiO2, Biospherix A chamber + Pro-Ox controller, Biospherix Ltd., Lacona, NY) environment for three weeks, and provided rat chow and water ad libitum.
Assessment of RV Hypertrophy
At the end of three weeks, the rats (n = 7/group) were anesthetized with isofluorane (2%/O2 balance) and a transthoracic echocardiogram (Visualsonics Vevo 2100, FUJIFILM Visualsonics Inc., Toronto, Canada) was performed. The pulse-wave Doppler recording, at the right ventricular outflow tract, was used to measure pulmonary acceleration time to confirm development of pulmonary hypertension in the rats exposed to hypoxia, as previously reported [14].
Isolated ventilated perfused lungs
At the end of three weeks, the rats were anesthetized (sodium pentobarbital, 60mg/kg ip), injected with ip heparin, and the trachea was cannulated and connected to a volume-cycled ventilator. Catheters were placed into the pulmonary artery (via the right ventricle) and left atrium (via the left ventricle) and secured. The heart and lungs were then trimmed free from the thorax and diaphragm, removed en bloc, and suspended. The pulmonary vasculature was continuously perfused at 7 ml/min with Earle’s balanced salt solution, maintained at 37 °C, and aerated with 95% O2–5% CO2 [15] and 21% O2/5% CO2/74% N2 [16] for the normoxic and hypoxic lung preparations, respectively. Select experiments were repeated using 21% O2/5% CO2/74% N2 aerated perfusate in normoxic lung preparations. A solution pH of 7.30–7.45 was maintained throughout the procedure.
Once suspended, the lungs were preconstricted with a thromboxane mimetic, U46619, until a pressor response (PAPU46619-PAPBaseline) of ~10 mmHg was achieved and maintained. Peak PAPNS1619 was assessed following incremental doses of the putative BKCa channel activator, NS1619 (3/30/150 μM), added to the perfusate. In select experiments, to isolate the mechanism of the NS1619-induced responses in determining PAPNS1619, lung preparations were perfused with iberiotoxin (IbTx; 200 nM; BKCa channel inhibitor [17]) paxilline (3 μM; BKCa channel inhibitor[18]), tetraethylammonium (TEA; 10 mM; non-selective K+ channel blocker[10]), L-NAME (100 μM; nitric oxide synthase inhibitor [10]), lanthanum (La3+; 100 μM; non-specific cation/TRP channel blocker [10]), niflumic acid (NFA; 30 μM; Cl− channel inhibitor[19]), or nifedipine (1 μM; L-type Ca2+ channel blocker[19]) prior to the addition of NS1619.
The baseline PAP (PAPBaseline) at was calculated as a mean pressure over forty seconds prior to the addition of U46619. Data is reported as percent preconstriction, or percent vasorelaxation, and is determined by the following equations;
All chemicals were obtained from Sigma Inc. (St. Louis, MO) with the exception of Ibtx, which was purchased from Anaspec (Fremont, CA).
Statistical Analysis
Dose-response curves were compared using a Student’s t test, or repeated measures two-way ANOVA with Tukey’s post hoc analyses for pairwise comparisons, as appropriate. Values are expressed as means ± SE. Statistical significance was defined as P < 0.05.
RESULTS
Animal characteristics
As expected, hypoxic rats weighed significantly less than their normoxic counterparts at the time of sacrifice (Normoxic: 401.3 ± 7.6g, Hypoxic: 354.1 ± 8.3g; P < 0.05). Heart rate (Normoxic: 376 ± 15 bpm vs. Hypoxic: 363 ± 11 bpm; P = NS) and LV ejection fraction (Normoxic: 87.6 ± 1.7% vs. Hypoxic: 86.5 ± 2.8%; P = NS) were not significantly different between rats exposed to normoxia or hypoxia. However, RV thickness by echocardiogram (Normoxic: 0.61 ± 0.06 mm vs. Hypoxic: 1.5 ± 0.06 mm; P < 0.05; n = 7), , and RV/LV weight ratio (Normoxic: 0.53 ± 0.02 vs. Hypoxic: 0.87 ± 0.1; P < 0/05; n = 4) was significantly higher, and pulmonary acceleration time (Normoxic: 39.8 ± 1.5 msec vs. Hypoxic: 25.9 ± 2.3 msec; P < 0.05; n = 7) significantly lower, in hypoxic animals, validating the efficacy of the PH protocol.
NS1619-induced vasodilatory response in normoxic and hypoxic lungs
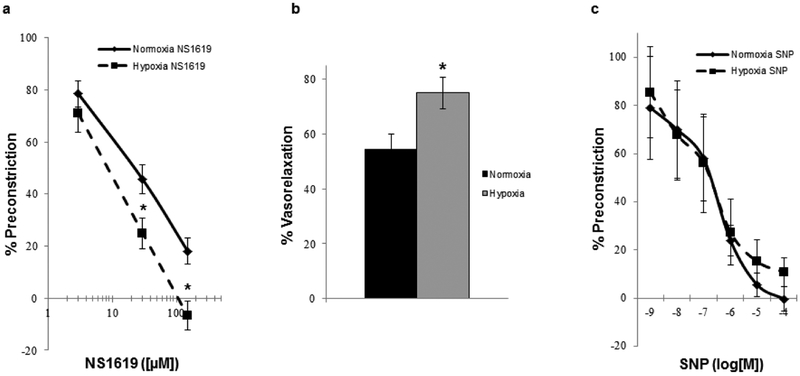
The selective BKCa channel opener NS1619 elicited a dose-dependent vasodilation in the U46619-preconstricted lungs from normoxic and hypoxic rats, however, NS1619 mediated vasodilation was significantly greater (~20% more) in lungs from hypoxic animals compared to normoxic (Fig. 1a & b). Additionally, a subset (n=4) of lungs perfused with 21% O2/5% CO2 were investigated to assess the potential influence of variable perfusate O2 concentrations as a confounding factor, however the NS1619 mediated vasodilation remained significantly less compared to that observed in hypoxic lungs (63.8 ± 3.4% of maximal vasoconstriction with 30μM NS1619). Vasodilatory responses to the exogenous nitric oxide donor, sodium nitroprusside (SNP; 10−9-10−4 M), elicited dose-dependent vasodilation within both groups, but there was no significant difference in SNP-induced dilation between groups (Fig. 1c), confirming vasodilatory capacity was not affected by hypoxic conditions.
Fig. 1.
Concentration-response relation (a) of isolated perfused lungs from normoxic and hypoxic rats (n = 6/group) to the BKCa channel activator, NS1619 (vehicle). Graphical representation (b) of the resultant percent vasorelaxation in response to NS1619 ([30μM]) in normoxic and hypoxic lungs. Concentration-response relation (c) of isolated perfused lungs from normoxic and hypoxic rats (n = 4/group) to the exogenous NO-donor, sodium nitroprusside (SNP). Values are means ± SE. *P < 0.05 vs. normoxia
Differential effect of ion channel blockers on NS1619 mediated vasodilation in hypoxic lungs
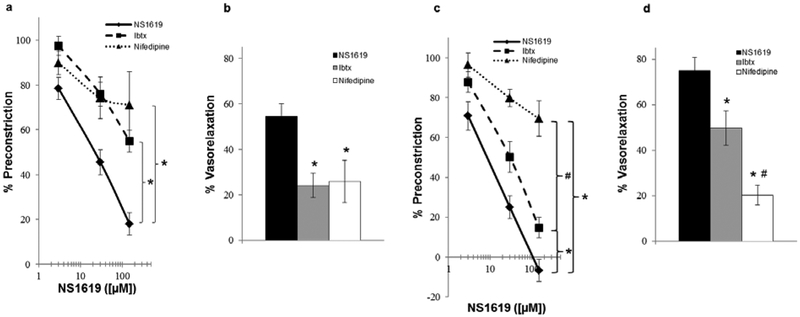
The mechanism underlying the vasodilatory effect of BKCa channel activation is membrane hyperpolarization, and the concomitant inhibition of L-type Ca2+ channels, in vascular smooth muscle cells [20]. Hence, we tested the effect of BK channel blocker, Ibtx, and L-type Ca2+ channel blocker, nifedipine, on NS1619-induced vasodilation. Pretreatment with Ibtx and nifedipine significantly attenuated NS1619-induced vasodilation in lung preparations from normoxic rats to a similar extent (Fig. 2a & b). However, although Ibtx significantly attenuated the vasodilation in lungs from hypoxic animals (Fig. 2c & d), the inhibition was significantly less compared to the effect of nifedipine (Fig. 2c & d), suggesting that NS1619 causes vasodilation in hypoxic lungs, in part, by a non-BKCa mechanism. Also, pretreatment of the lungs with paxilline, a structurally unrelated BK blocker, resulted in attenuation of vasodilation similar to that by Ibtx (Normoxia: 64.7 ± 4.1% vs. 76.0 ± 5.3%, Hypoxia: 42.2 ±6.2% vs. 50.2 ± 7.6%, respectively).
Fig. 2.
Respective concentration-response relation and resultant percent vasorelaxation ([NS1619]= 30μM) of isolated perfused lungs from normoxic (a & b) and hypoxic (c & d) rats (n = 3–6/group) to the BKCa channel activator, NS1619, in the absence (vehicle) and presence of iberiotoxin (Ibtx; 200 nM; membrane BKCa channel inhibitor) and nifedipine (1 μM; L-type Ca2+ channel blocker). Values are means ± SE. *P < 0.05 vs. vehicle. #P < 0.05 vs. Ibtx
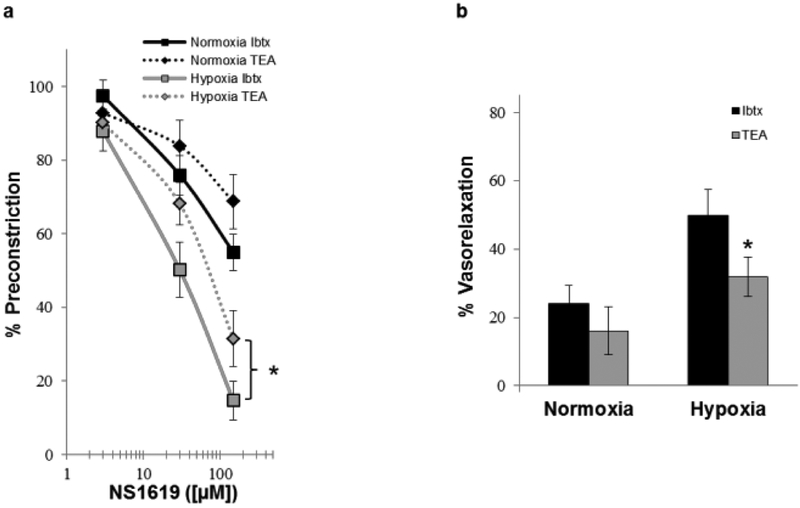
In order to assess contribution of alternate potassium channels, we next compared the effect of Ibtx with a non-specific potassium channel blocker, TEA, on NS1619-induced vasodilation. Both TEA and Ibtx caused similar attenuation of the NS1619-induced vasodilation in normoxic lung (Fig. 3a & b). In contrast, TEA caused significantly more inhibition of NS1619-induced vasodilation in hypoxic lungs compared to Ibtx (Fig. 3a & b), suggesting a role of non-BKCa K+ channels in mediating the NS1619-induced vasodilation.
Fig. 3.
Concentration-response relation (a) and graphical representation of the resultant percent vasorelaxation (b; [NS1619]= 30μM) of isolated perfused lungs from normoxic and hypoxic rats (n = 3–7) to the BKCa channel activator, NS1619, in presence of iberiotoxin (Ibtx; 200 nM; selective BKCa channel inhibitor) and tetraethylammonium (TEA; 10 mM; non-specific K+ channel blocker). Values are means ± SE. *P < 0.05 vs. Ibtx within same condition
To determine if Cl− channel activation may underlie the effect of NS1619 in addition to K+ channels, we pretreated the lungs with Cl− channel blocker, NFA, in addition to TEA and observed no additional attenuation of the vasodilation with TEA alone (Normoxia: 68.9 ± 5.8% vs. 83.9 ± 7.0, Hypoxia: 60.3 ± 5.3% vs. 68.2 ± 5.8%, respectively).
NO-dependent effects of NS1619
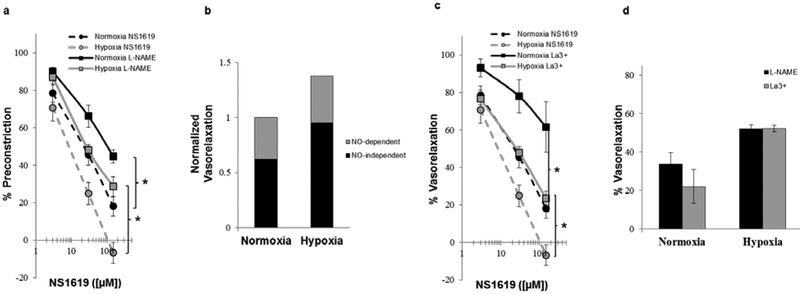
Since activation of BKCa channels is associated with nitric oxide (NO) production in endothelial cells (related to an increase in intracellular Ca2+[10], we next assessed the NO-dependent vasodilation in normoxic and hypoxic lungs. In both normoxic and hypoxic lungs, inhibition of NO production (L-NAME; Fig. 4a) significantly reduced NS1619-induced vasodilation, compared to vehicle controls. However, the magnitude of reduction in both normoxic and hypoxic lungs was similar (Fig. 4b). We have previously shown that NS1619 activates BKCa channels in endothelial cells and causes NO release dependent on La3+ sensitive Ca2+ entry pathways [21]. Pretreatment of lungs with La3+ resulted in attenuation of NS1619-induced vasodilation (Fig. 4c) similar to that of L-NAME (Fig. 4d).
Fig. 4.
Concentration-response relation of isolated perfused lungs from normoxic and hypoxic rats (n = 4–6/group) to NS1619 in the absence (vehicle) and presence of (a) L-NAME (100 μM; nitric oxide synthase inhibitor) and (c) lanthanum (La3+; 100 μM; non-specific cation/TRP channel blocker). Qualitative representation of the relative contribution of NO-dependent and NO-independent mechanisms to vasorelaxation (b). Comparison of the resultant percent vasorelaxation, in response to NS1619 ([30μM]), of normoxic and hypoxic lungs in the presence of L-NAME and La3+ (d). Values are means ± SE. *P < 0.05 vs. vehicle within same condition
DISCUSSION
While vascular remodeling is a critical component of PH associated with hypoxia [3,4] there is evidence to support that tonic vasoconstriction plays a significant role in the high pulmonary vascular resistance characteristic of PH [22]. Although vascular ion channels play an important role in vasoregulation of the pulmonary circulation [9], there is significant heterogeneity in the distribution of ion channels between the macro- and microvasculature throughout the pulmonary circulation [23]. Furthermore, the expression and activity of several of these vascular ion-channels is altered in chronically hypoxic lungs [9,5,8]. Hence, using an isolated perfused lung preparation we sought to determine the effect of the benzimidazalone derivative, NS1619, on the vasoreactivity of the preconstricted pulmonary circulation and to identify the relative contribution of specific vascular ion channels in mediating the response in both normoxic and chronically hypoxic lungs. Our results demonstrate that the NS1619 causes significantly higher vasodilatory response in lungs from hypoxic animals. Furthermore, we show that the NS1619-induced vasodilation exhibits both NO-dependent and NO-independent components, and is primarily mediated via effects on vascular K+ and L-type Ca2+ channels. Current therapies in pulmonary hypertension target vascular L-type calcium channels, endothelin pathway or nitric oxide pathway. Our observations suggest that another approach may be to study small molecules such as NS1619 that favorably affect pulmonary vascular function by targeting multiple pathophysiologically relevant pathways. Further studies in preclinical models are needed to confirm this approach and compare it to other available therapies.
BKCa channels present in the pulmonary vascular endothelial (PVEC) and smooth muscle cells (PVSMC) open in response to depolarization and elevated intracellular calcium ([Ca2+]i) levels, resulting in membrane hyperpolarization [20]. In endothelial cells, the hyperpolarization may result in a further increase in [Ca2+]i resulting in release of vasodilatory mediators such as NO [24], whilst within vascular smooth muscle cells, the hyperpolarization can promote vasodilation by decreasing the activity of voltage-dependent L-type Ca2+ channels [25]. In chronic hypoxia, BKCa channel activation has been shown to underlie the vasodilatory and anti-mitogenic effects of carbon monoxide inhalation [26] and dehydroepiandrosterone (DHEA) administration [27], respectively. Correspondingly, we found that activation of BKCa channels resulted in vasodilation of the pulmonary vasculature from both normoxic and hypoxic animals (~ 29% in normoxia and 25% in hypoxic lungs). Although it has been demonstrated that chronic hypoxia promotes an increased expression of BKCa channels in rat PVSMC [8], in the current study the activation of the channels with NS1619 did not show an increased BKCa mediated vasodilation in the hypoxic lungs. This apparent discrepancy may be related to the observation that the BKCa channels already have a higher basal activity in settings of hypoxia, which is considered to be a compensatory mechanism associated with increased β1-subunit expression [8] and increased intracellular calcium levels in PVSMC [28].
Nitric oxide plays an important role in pulmonary vascular reactivity and is considered a therapeutic target in pulmonary hypertension [29]. Previously, we have demonstrated a NS1619-induced hyperpolarization of PVEC and increased NO production in vivo [10], and that the hyperpolarization is attenuated by blocking non-specific cation channels in vitro [21]. Consistent with the mechanism we previously observed in cell culture [21], pretreatment with L-NAME (NOS inhibitor) and La3+ (TRP inhibitor) attenuated the NS1619-induced vasodilation, suggesting that the NS1619-induced vasodilation is partly endothelial dependent and related to an increase in extracellular Ca2+ entry within the endothelium. However, in the current study, there was no apparent difference in the endothelial dependent vasodilation between the normoxic and hypoxic lungs. Prior studies have demonstrated that eNOS expression and activity is augmented in hypoxic lungs and results in enhanced endothelial dependent vasodilation in response to certain vasodilators, such as histamine[30]. In contrast, exogenous NO donors show an impaired vasodilatory response in hypoxic lungs likely due to endothelium derived ROS and ET-1 counteracting the actions of exogenous NO in hypoxic lungs [31]. These studies suggest that in order to improve endothelium dependent vasodilation, a simultaneous increase in NO release and inhibition of endothelial ROS and ET-1 may be required. In the current study, we did not observe an enhanced NO dependent vasodilation in hypoxic lungs in response to NS1619. It is possible that although NS1619 increases NO release in hypoxic vasculature, it may not directly affect ROS and/or ET-1 release similar to other endothelial dependent vasodilators, such as histamine.
Thus, we found that the extent of NO-dependent vasodilation and BKCa mediated vasodilation, in response to NS1619, was similar in the normoxic and hypoxic lungs. However, pretreatment with L-type Ca2+ channel blocker, nifedipine, had a larger inhibitory effect in hypoxic lungs than in normoxic lungs. This would be expected as a result of NS1619 inhibiting either L-type Ca2+ channels directly or by activating other K+ channels in VSMC resulting in hyperpolarization and indirect inhibition of L-type Ca2+ channels. We find that pretreatment with TEA, a BKCa and non-specific K+ channel blocker, had similar effect as nifedipine in hypoxic lungs and caused significantly more inhibition than Ibtx alone. Hence, it would appear the higher NS1619-induced vasodilation in hypoxia could be a result of activation of other TEA-sensitive PVSMC K+ channels involved in the hypoxic pulmonary vasoconstrictor response, such as Kv 2.1 [32,33].
The results from our study have to be interpreted in context of certain limitations. Since, we did not simultaneously block both L-type Ca2+ channels and K+ channels, it is possible that some effect of NS1619 is mediated by direct inhibition of L-type Ca2+ channels that have been shown to be upregulated in hypoxic lungs as well [5]. Also, we chose to use similar pressor response instead of similar U46619 doses as previously used by others [30] in our preconstriction protocol. Hence, we cannot completely rule out that use of varying doses of U46619 could have had an effect on subsequent dose-response curves. Although we performed select experiments with 21% O2 perfusate conditions to demonstrate that NS1619 response remained significantly higher in chronically hypoxic lungs as with 95% O2 perfusate conditions, we can not rule out the effect of 21% O2 perfusate in chronically hypoxic lungs on the dose responsiveness of NS1619 in presence of other inhibitors.
In conclusion, we demonstrate that NS1619 causes robust vasodilation in the pulmonary circulation that is further accentuated in lungs from hypoxic animals. Also, NS1619 mediated the vasodilation by affecting multiple complementary pathways including stimulation of NO production, activation of BKCa channels, other TEA-sensitive K+ channels and L-type Ca2+ channels. Hence, compounds like NS1619 represent a novel therapeutic strategy of targeting multiple channels in hypoxic pulmonary hypertension and could be considered for further preclinical evaluation.
Acknowledgement of Support:
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development: Biomedical Laboratory Research and Development Service (MERIT Review Award to GC, IBX000711A). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Footnotes
Conflict of Interest: None
REFERENCES
- 1.Ghofrani HA, Voswinckel R, Reichenberger F, Weissmann N, Schermuly RT, Seeger W, Grimminger F (2006) Hypoxia- and non-hypoxia-related pulmonary hypertension - established and new therapies. Cardiovasc Res 72 (1):30–40. doi: 10.1016/j.cardiores.2006.07.025 [DOI] [PubMed] [Google Scholar]
- 2.Budhiraja R, Tuder RM, Hassoun PM (2004) Endothelial dysfunction in pulmonary hypertension. Circulation 109 (2):159–165. doi: 10.1161/01.CIR.0000102381.57477.50 [DOI] [PubMed] [Google Scholar]
- 3.Stenmark KR, Fagan KA, Frid MG (2006) Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99 (7):675–691. doi: 10.1161/01.RES.0000243584.45145.3f [DOI] [PubMed] [Google Scholar]
- 4.Pak O, Aldashev A, Welsh D, Peacock A (2007) The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J 30 (2):364–372. doi: 10.1183/09031936.00128706 [DOI] [PubMed] [Google Scholar]
- 5.Wan J, Yamamura A, Zimnicka AM, Voiriot G, Smith KA, Tang H, Ayon RJ, Choudhury MS, Ko EA, Wang J, Wang C, Makino A, Yuan JX (2013) Chronic hypoxia selectively enhances L- and T-type voltage-dependent Ca2+ channel activity in pulmonary artery by upregulating Cav1.2 and Cav3.2. Am J Physiol Lung Cell Mol Physiol 305 (2):L154–164. doi: 10.1152/ajplung.00313.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet S, Archer SL (2007) Potassium channel diversity in the pulmonary arteries and pulmonary veins: implications for regulation of the pulmonary vasculature in health and during pulmonary hypertension. Pharmacol Ther 115 (1):56–69. doi: 10.1016/j.pharmthera.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 7.Peinado VI, París R, Ramírez J, Roca J, Rodriguez-Roisin R, Barberà JA (2008) Expression of BK(Ca) channels in human pulmonary arteries: relationship with remodeling and hypoxic pulmonary vasoconstriction. Vascul Pharmacol 49 (4–6):178–184. doi: 10.1016/j.vph.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 8.Resnik E, Herron J, Fu R, Ivy DD, Cornfield DN (2006) Oxygen tension modulates the expression of pulmonary vascular BKCa channel alpha- and beta-subunits. Am J Physiol Lung Cell Mol Physiol 290 (4):L761–L768. doi: 10.1152/ajplung.00283.2005 [DOI] [PubMed] [Google Scholar]
- 9.Weir EK, Cabrera JA, Mahapatra S, Peterson DA, Hong Z (2010) The role of ion channels in hypoxic pulmonary vasoconstriction. Advances in experimental medicine and biology 661:3–14. doi: 10.1007/978-1-60761-500-2_1 [DOI] [PubMed] [Google Scholar]
- 10.Vang A, Mazer J, Casserly B, Choudhary G (2010) Activation of endothelial BK(Ca) channels causes pulmonary vasodilation. ascul Pharmacol. 2010 Sep-Oct;53(3–4):122–9. doi: 10.1016/j.vph.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Holland M, Langton PD, Standen NB, Boyle JP (1996) Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol 117 (1):119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhlmann CR, Trumper JR, Abdallah Y, Wiebke Ludders D, Schaefer CA, Most AK, Backenkohler U, Neumann T, Walther S, Piper HM, Tillmanns H, Erdogan A (2004) The K+-channel opener NS1619 increases endothelial NO-synthesis involving p42/p44 MAP-kinase. Thrombosis and haemostasis 92 (5):1099–1107. doi: 10.1267/THRO04051099 [DOI] [PubMed] [Google Scholar]
- 13.Edwards G, Niederste-Hollenberg A, Schneider J, Noack T, Weston AH (1994) Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br J Pharmacol 113 (4):1538–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhary G, Troncales F, Martin D, Harrington EO, Klinger J (2011) Bosentan Attenuates Right Ventricular Hypertrophy and Fibrosis in Normobaric Hypoxia Model of Pulmonary Hypertension. J Heart Lung Transplant 2011 Jul;30(7):827–33. doi: 10.1016/j.healun.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godinez RI, Longmore WJ (1973) Use of the isolated perfused rat lung in studies on lung lipid metabolism. Journal of lipid research 14 (2):138–144 [PubMed] [Google Scholar]
- 16.Watkins CA, Rannels DE (1979) In situ perfusion of rat lungs: stability and effects of oxygen tension. Journal of applied physiology: respiratory, environmental and exercise physiology 47 (2):325–329 [DOI] [PubMed] [Google Scholar]
- 17.Thebaud B, Michelakis E, Wu XC, Harry G, Hashimoto K, Archer SL (2002) Sildenafil reverses O2 constriction of the rabbit ductus arteriosus by inhibiting type 5 phosphodiesterase and activating BK(Ca) channels. Pediatric research 52 (1):19–24. doi: 10.1203/00006450-200207000-00006 [DOI] [PubMed] [Google Scholar]
- 18.Bentzen BH, Osadchii O, Jespersen T, Hansen RS, Olesen SP, Grunnet M (2009) Activation of big conductance Ca(2+)-activated K (+) channels (BK) protects the heart against ischemia-reperfusion injury. Pflugers Arch 457 (5):979–988. doi: 10.1007/s00424-008-0583-5 [DOI] [PubMed] [Google Scholar]
- 19.McKenzie C, MacDonald A, Shaw AM (2009) Mechanisms of U46619-induced contraction of rat pulmonary arteries in the presence and absence of the endothelium. Br J Pharmacol 157 (4):581–596. doi: 10.1111/j.1476-5381.2008.00084.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichhorn B, Dobrev D (2007) Vascular large conductance calcium-activated potassium channels: Functional role and therapeutic potential. Naunyn Schmiedebergs Arch Pharmacol 376 (3):145–155. doi: 10.1007/s00210-007-0193-3 [DOI] [PubMed] [Google Scholar]
- 21.Simon A, Harrington EO, Liu GX, Koren G, Choudhary G (2009) Mechanism of C-type natriuretic peptide-induced endothelial cell hyperpolarization. Am J Physiol-Lung C 296 (2):L248–L256 [DOI] [PubMed] [Google Scholar]
- 22.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M (2004) Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287 (4):L665–672. doi: 10.1152/ajplung.00050.2003 [DOI] [PubMed] [Google Scholar]
- 23.Archer S, Huang J, Reeve H, Hampl V, Tolarova S, Michelakis E, Weir E (1996) Differential Distribution of Electrophysiologically Distinct Myocytes in Conduit and Resistance Arteries Determines Their Response to Nitric Oxide and Hypoxia. Circ Res 78 (3):431. [DOI] [PubMed] [Google Scholar]
- 24.Félétou (2009) Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol. doi: 10.1111/j.1476-5381.2009.00052.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson AS, Lederer WJ, Knot HJ, Bonev AD, Nelson MT (1998) Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand 164 (4):577–587. doi: 10.1046/j.1365-201X.1998.00462.x [DOI] [PubMed] [Google Scholar]
- 26.Dubuis E, Potier M, Wang R, Vandier C (2005) Continuous inhalation of carbon monoxide attenuates hypoxic pulmonary hypertension development presumably through activation of BKCa channels. Cardiovasc Res 65 (3):751–761. doi: 10.1016/j.cardiores.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 27.Bonnet S, Dumas-de-La-Roque E, Bégueret H, Marthan R, Fayon M, Dos Santos P, Savineau J-P, Baulieu E-E (2003) Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA 100 (16):9488–9493. doi: 10.1073/pnas.1633724100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn YT, Kim YM, Adams E, Lyu SC, Alvira CM, Cornfield DN (2012) Hypoxia-inducible factor-1alpha regulates KCNMB1 expression in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 302 (3):L352–359. doi: 10.1152/ajplung.00302.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M (2004) Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43 (12 Suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029 [DOI] [PubMed] [Google Scholar]
- 30.Resta TC, Walker BR (1996) Chronic hypoxia selectively augments endothelium-dependent pulmonary arterial vasodilation. Am J Physiol 270 (3 Pt 2):H888–896 [DOI] [PubMed] [Google Scholar]
- 31.Jernigan NL, Walker BR, Resta TC (2004) Endothelium-derived reactive oxygen species and endothelin-1 attenuate NO-dependent pulmonary vasodilation following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287 (4):L801–808. doi: 10.1152/ajplung.00443.2003 [DOI] [PubMed] [Google Scholar]
- 32.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B (1999) Molecular diversity of K+ channels. Annals of the New York Academy of Sciences 868:233–285 [DOI] [PubMed] [Google Scholar]
- 33.Hulme JT, Coppock EA, Felipe A, Martens JR, Tamkun MM (1999) Oxygen sensitivity of cloned voltage-gated K(+) channels expressed in the pulmonary vasculature. Circ Res 85 (6):489–497 [DOI] [PubMed] [Google Scholar]