Abstract
Negative symptoms are common in individuals at clinical high-risk (CHR) for psychosis and are associated with worse functional outcomes. Inflammation may be one mechanism underlying negative symptoms. Inflammatory markers are altered in individuals at CHR and are associated with negative symptoms in patients with schizophrenia. We thus hypothesized that baseline inflammatory markers would predict the development of negative symptoms in individuals at CHR for psychosis. Thirty seven individuals from the North American Prodromal Longitudinal Study who met CHR criteria were included in the study. Inflammatory cytokines, including interferon (IFN)-λ, Interleukin (IL)-1β, IL-1 receptor antagonist (IL-1RA), IL-4, IL-6, IL-8, IL-10, and tumor necrosis factor (TNF) were measured at baseline. Negative symptoms as measured by the Scale of Prodromal Symptoms, were measured at baseline and six and twelve months. Associations between inflammatory markers and the trajectory of negative symptoms (slope) over the first year of follow-up, were assessed using linear regression models controlling for age, sex, race and depressive symptom severity (as assessed by the Calgary Depression Scale for Schizophrenia). Baseline TNF (beta = 0.361, p = 0.007) and IL-6 (beta = −0.306, p = 0.026) predicted negative symptoms slopes, along with depressive symptom severity at baseline (beta = −0.596, p = 0.000). These findings demonstrate that inflammatory cytokines may underlie the development of negative symptoms in some individuals at CHR for psychosis. TNF predicted the development of negative symptoms independent of baseline depression. Given the heterogeneity of the CHR population, the comorbidity of negative symptoms and depression in this population, and the particular challenges in treating negative symptoms, immune markers could represent potential biomarkers that underlie the development of negative symptoms, representing a potential treatment target.
Keywords: Clinical High Risk, Negative Symptoms, Inflammation, Cytokines, Schizophrenia, Psychosis
1. Introduction
Recent attempts to understand the pathophysiologic mechanisms underlying symptoms of schizophrenia have focused on studying clinical high-risk (CHR) populations (Cannon et al., 2016; Fusar-Poli et al., 2013). Criteria to identify individuals at CHR rely on the presence of positive symptoms, similar to diagnostic criteria for schizophrenia itself. Despite this, negative symptoms, including blunted affect, alogia, asociality, anhedonia, and avolition are common in CHR individuals and are often the presenting symptoms (i.e., social isolation and changes to role function) that lead to seeking help and precede the onset of psychosis (positive symptoms) (Kirkpatrick et al., 2006; Lencz et al., 2004; Piskulic et al., 2012). In one sample from the North American Prodrome Longitudinal Study (NAPLS), 82% of CHR individuals had at least one negative symptom in the moderately severe range and 38% had at least one negative symptom in the severe range at baseline. Moreover, 54% continued to have moderately severe negative symptoms at one year follow up (Piskulic et al., 2012). The most common negative symptoms at baseline and at follow up were deterioration in role functioning, avolition, as well as social isolation and withdrawal.
Importantly, negative symptoms have been shown to be predictive of conversion to psychosis (Demjaha et al., 2012; Kwapil, 1998; Mason et al., 2004; Piskulic et al., 2012; Valmaggia et al., 2013). These symptoms have also been shown to significantly effect quality of life and functional outcomes in both CHR individuals and those with schizophrenia, adding to the illness burden (Bechdolf et al., 2005; Dominguez-Martinez et al., 2015; Harvey, 2013; Nelson et al., 2013). Antipsychotic medications show limited benefit for negative symptoms (Davis et al., 2014), and as such, there is a significant need for treatment options to target negative symptoms in CHR individuals (Carpenter and Schiffman, 2015; Devoe et al., 2017; Stafford et al., 2013). Moreover, understanding mechanisms that underlie these symptoms may provide further insight into the developmental trajectories of negative symptoms of schizophrenia.
Inflammation could be one such mechanism for some individuals at CHR for psychosis. The immune system has been posited to underlie neurodevelopmental abnormalities in patients with schizophrenia (Meyer, 2013; Miller and Goldsmith, 2017), as evidenced by epidemiologic findings of increased risk secondary to prenatal immune-related and other stress-related factors (Brown and Derkits, 2010), translational models of maternal immune activation leading to phenotypes similar to schizophrenia (Meyer, 2014), genome wide association studies implicating the Major Histocompatibility Complex region on chromosome 6 (2014; Stefansson et al., 2009), alterations in inflammatory markers (Goldsmith et al., 2016; Miller et al., 2011; Potvin et al., 2008; Upthegrove et al., 2014), and recent data implicating the innate immune system and complement system in disordered synaptic pruning (Sekar et al., 2016). Indeed, concentrations of immune markers, including inflammatory cytokines, have also been shown to be altered in individuals at clinic high risk for schizophrenia (Karanikas et al., 2017; Lizano et al., 2016; Metcalf et al., 2017; Smesny et al., 2017; Zeni-Graiff et al., 2016). In fact, inflammatory cytokines and other immune-relevant biomarkers make up the majority of the 15 blood analytes shown to be predictive of conversion to psychosis in the NAPLS CHR cohort (Perkins et al., 2015), and have been shown to be related to cortical grey matter volume loss as well (Cannon et al., 2015).
There is a growing literature demonstrating relationships between inflammatory cytokines and negative symptoms of schizophrenia, though many of these studies measure just one or a small panel of inflammatory cytokines (Asevedo et al., 2014; El Kissi et al., 2015; Garcia-Rizo et al., 2012; Goldsmith et al., 2018; Goldsmith et al., 2016; Liu et al., 2012; Noto et al., 2015; Stojanovic et al., 2014; Xiu et al., 2014). Though there have been studies that measure inflammatory marker concentrations in individuals at clinic high risk (Karanikas et al., 2017; Lizano et al., 2016; Metcalf et al., 2017; Perkins et al., 2015; Smesny et al., 2017; Zeni-Graiff et al., 2016), only one to our knowledge has investigated associations with negative symptoms, finding positive associations between IL-6 and negative symptoms at baseline assessment (Stojanovic et al., 2014). To date, the relation of inflammatory markers with longitudinal changes in negative symptom severity has not been examined in CHR individuals.
Given that negative symptoms are common in individuals at CHR, contribute to significant impairments, and may be predictive of conversion to psychosis, we examined a panel of inflammatory markers at baseline in a cohort of individuals who met CHR criteria and assessed negative symptoms at baseline and subsequent follow up visits. Due to the limited number of studies of inflammatory markers in the CHR population, we included markers that have been shown to be elevated in a previous metaanalysis of individuals at first episode of psychosis: Interferon (IFN)-λ, IL-1β, IL-1 receptor antagonist (IL-1RA), IL-4, IL-6, IL-8, IL-10, and TNF (Goldsmith et al., 2016). First episode patients were also chosen as they are less likely to have been exposed to antipsychotics for significant lengths of time that could alter inflammatory processes. All inflammatory cytokines included have been previously assessed in studies of CHR individuals. We hypothesized that inflammatory cytokines measured at baseline would predict negative symptom severity over the first 12 months of the study. Based on our previous work demonstrating relationships between TNF, IL-6, and negative symptoms in schizophrenia (Goldsmith et al., 2018) and relationships between inflammatory cytokines and symptoms such as anhedonia and motivational deficits (Felger and Treadway, 2017), the present study examined the relation of TNF and IL-6 (among other cytokines) with both the baseline severity and the longitudinal course of negative symptoms over one year in a CHR sample.
2.1. Methods and Materials
2.1. Subjects
Subjects were drawn from the North American Prodrome Longitudinal Study (NAPLS 2) cohort, whose aims and methods have been previously described. (Addington et al., 2012) Briefly, NAPLS 2 studies the predictors and mechanisms of conversion to psychosis in 765 CHR individuals (meeting the Criteria of Prodromal States (COPS)) (Miller et al., 2002) and 280 healthy controls recruited across the eight NAPLS study sites. No healthy controls were included in the present analysis as the range of negative symptom assessments were too narrow and would limit meaningful analyses (mean baseline total negative symptoms was 1.23 and half of controls had a total score of zero). In addition to the low negative symptom scores in controls, the lack of an association between schizophrenia genetic risk and inflammatory markers might also support the exclusion of healthy controls. Associations were tested between family history of psychosis in controls (n = 35) and the inflammatory markers, and no inflammatory markers were significantly correlated with family history of psychosis. As such, we chose to exclude healthy controls from all analyses. Ages of subjects ranged between 12 and 35. The Institutional Review Board from each site approved the study, and each subject provided written informed consent or assent, with a parent or guardian also providing consent for subjects who were minors. The present sample was 37 subjects who had complete data on negative symptoms at baseline, 6 month, and 12-month follow up.
2.2. Assessments
Clinical assessments were performed at baseline and at subsequent six month follow up visits for up to two years. Screening for prodromal symptoms was conducted using the Structured Interview for Prodromal Syndromes (SIPS) and ratings were made using the Scale of Prodromal Symptoms (SOPS). The SOPS is the diagnostic component of the SIPS and includes 19 items divided into positive, negative, disorganization, and general symptom subscales (Miller et al., 2003). The six negative symptom items from the SOPS were included in the analysis: social isolation or withdrawal (N1), avolition (N2), decreased expression of emotion (N3), decreased experience of emotions and self (N4), decreased ideational richness (N5), and deterioration in role functioning (N6). Each item was scored on a scale from 0 to 6, with 0 reflecting absent symptoms and 6 reflecting extreme symptoms. A total negative symptom score was also calculated by summing the individual negative symptoms items such that the total score range could be from 0 to 36. The following prodromal syndromes were considered: attenuated psychotic symptoms, brief intermittent psychotic symptoms, substantial functional decline combined with a first-degree relative with a psychotic disorder, or schizotypal personality disorder in individuals younger than 18 years (Miller et al., 2002). The Structured Clinical Interview for DSM IV (First MB, 2002) was used to determine psychiatric diagnoses. Depressive symptoms were assessed with the Calgary Depression Scale for Schizophrenia (CDSS)(Addington et al., 1993; Addington et al., 2014).
2.3. Plasma Collection and Assay
Details of sample collection and assay have been described previously (Perkins et al., 2015). Briefly, blood samples were collected at baseline visit in Becton Dickenson P100 blood collection tubes and all samples were processed within 120 minutes (mean time to freezer = 28 minutes, SD = 2 minutes) and stored at-80°C until analysis. Samples were sent on dry ice to Myriad Rules Based Medicine, and samples were analyzed with a Human Discovery Map assay, a Luminex bead-based multiplex immunoassay. 185 analytes were assayed as part of the larger study. Technicians were blind to clinical status of the subjects.
2.4. Data Analyses
Statistical analyses were conducted with SPSS 24.0. Descriptive statistics including means and standard deviations for the independent and dependent variables were calculated and are provided in Table 1. Inflammatory marker concentrations were standardized (z score) to the average and SD values of the unaffected comparison subjects in the parent study so that results of multiple blood analytes could be viewed on the same scale (Perkins et al., 2015). The mean baseline standardized inflammatory marker concentrations are provided in Table 2.
Table 1:
Sociodemographic, inflammatory marker, and clinical characteristics of the study sample reported as mean (standard deviation) unless where reported as a percentage (n=37).
| Baseline | 6-month follow up | 12-month follow up | |
|---|---|---|---|
| Sex (% male) | 62.2% | ||
| Age (years) | 19.76 (4.69) | ||
| Race (% white) | 56.8% | ||
| SOPS Negative Symptom Total Score | 10.70 (6.11) | 8.49 (5.40) | 7.86 (5.28) |
| N1: Social Isolation or Withdrawal | 1.95 (1.62) | 1.73 (1.54) | 1.73 (1.22) |
| N2: Avolition | 2.03 (1.59) | 1.57 (1.24) | 1.68 (1.53) |
| N3: Decreased Expression of Emotion | 1.30 (1.37) | 1.24 (1.50) | 0.95 (1.15) |
| N4: Decreased Experience of Emotions and Self | 1.81 (1.70) | 1.14 (1.42) | 0.97 (1.36) |
| N5: Decreased Ideational Richness | 1.11 (1.33) | 1.03 (1.32) | 0.73 (0.99) |
| N6: Deterioration in Role Functioning | 2.51 (1.94) | 1.78 (1.81) | 1.81 (2.12) |
| CDSS | 5.14 (4.50) | 3.67 (3.42)* | 3.27 (3.24) |
| IL-1beta | 0.0363 (1.05) | ||
| IL-6 | 0.6062 (1.26) | ||
| TNF | −0.1653 (0.35) | ||
| IFN-gamma** | 0.1248 (0.95) | ||
| IL-10* | 0.0714 (0.86) | ||
| IL-1RA | 0.5665 (0.95) | ||
| IL-8* | 0.1626 (0.99) | ||
| IL-4 | 0.9865 (1.81) |
CDSS: Calgary Depression Scale for Schizophrenia; IL-1beta: interleukin 1 beta; IL-6: interleukin 6; TNF: tumor necrosis factor; IFN-gamma: interferon gamma; IL-10: interleukin 10; IL-1RA: interleukin 1 receptor antagonist; IL-8: interleukin 8; IL-4: interleukin 4.
n=36
n=35
To determine which inflammatory markers predicted negative symptoms at baseline and at follow up visits, stepwise, backward linear regression models were used to determine the relationship between inflammatory markers and individual negative symptom items, including total negative symptoms. We chose to investigate individual negative symptoms to test relationships between inflammatory markers and specific negative symptom domains that have been shown to be associated with inflammatory cytokines (Felger and Treadway, 2017). Relevant covariates were also included in the models, including age, sex, race (modeled as white, black, other), weight, and baseline negative symptoms. In addition, due to the potential overlap of negative symptoms with depression, baseline CDSS scores were also included as a covariate. Due to the possible collinearity of peripheral inflammatory markers, the variable inflation factor (VIF) was calculated for each variable in the regression models. In order to control for multiple comparisons, a Benjamini and Hochberg approach was employed with a false discovery rate (FDR) set at 0.01 (Hochberg and Benjamini, 1990). This approach provides better control of type I error rates when conducting multiple hypothesis tests as compared to more conservative approaches (Benjamini and Hochberg, 1995; Holm, 1979).
In order to assess the impact of inflammatory cytokines on the trajectory of negative symptoms over time, slopes of negative symptoms were calculated from baseline to 6 and 12 months. The skewness and kurtosis statistics were −0.353 (standard error = 0.388) and 0.137 (standard error = 0.0759), respectively, suggesting that the slope scores were normally distributed. As such parametic tests were used. Linear regression models were performed with slope as the dependent variable and inflammatory cytokines, above demographic variables, and baseline CDSS score as the independent variables. The potential contribution of slope of CDSS scores was also explored as a covariate in a separate model.
3. Results
3.1. Sociodemographic, clinical, and inflammatory characteristics of the sample
Sociodemographic, clinical, and immune data for the sample are shown in Tables 1. Of note, many inflammatory markers were inter-correlated, however, the VIF for all the inflammatory predictors in the regression models were <3.0, indicating that multi-collinearity among inflammatory predictors did not bias the results.
3.2. Relationship between Inflammatory markers and negative symptoms at 6 and 12 months
Inflammatory markers were only associated with one negative symptom domains (N5: decreased ideational richness) at baseline, and these associations did not remain significant after correcting for multiple comparisons. Multiple baseline inflammatory markers were found to be significantly associated with negative symptom domains in linear regression models at 6 and 12 months, though only the association between N4 (decreased experience of emotions and self) with TNF (beta = 0.418, p = 0.006) and IL-6 (beta = −0.393, p = 0.01) survived correction for multiple comparisons. Results from baseline, 6 months, and 12 months are included in Supplementary Table 1.
3.3. Inflammatory markers and negative symptom slopes
Linear regression models showed that TNF (beta = 0.361, p = 0.007), IL-6 (beta = −0.306, p = 0.026), and CDSS (beta = −0.596, p = 0.000) significantly predicted negative symptom slopes. Figure 1a shows the partial regression plots for the associations between negative symptom slopes and TNF and IL-6. As shown, higher baseline TNF was associated with a greater increase in negative symptoms between baseline and subsequent follow-ups. In contrast, higher baseline IL-6 predicted a greater decrease in negative symptoms over time. Baseline negative symptoms and baseline CDSS scores were highly correlated (r = 0.546, p = 0.000) as were negative symptom slopes and CDSS slopes (r = 0.562, p = 0.000). To further explore the effect of depression on the association between inflammatory markers and negative symptoms, CDSS slope was included in the model instead of baseline CDSS score. In this model, TNF (beta = 0.296, p = 0.034), IL-6 (beta = −0.348, p = 0.023), and CDSS slope (beta = 0.530, p = 0.001) significantly predicted negative symptom slopes. Previous analyses using data from the NAPLS cohort have created summed standardized scores for pro- and anti-inflammatory cytokines (Cannon et al., 2015), which represents one approach to reduce the data given the number of inflammatory markers assessed. Though there was some overlap in the inflammatory cytokines included in the current analysis, there were some differences. We created similar summed standardized scores and found no relationship with the negative symptom slopes.
Figure 1.
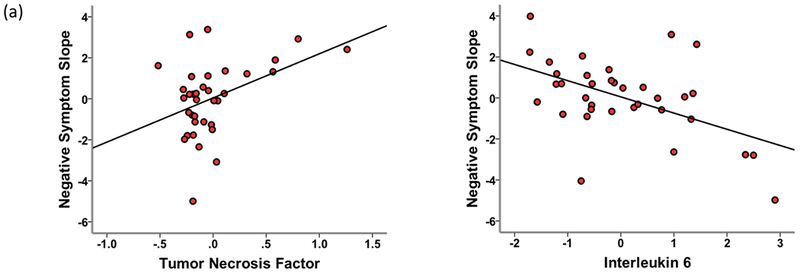
Partial regression plots of the relationship between negative symptom slopes and tumor necrosis factor and interleukin 6 in linear regression models that included baseline Calgary Depression Scale for Schizophrenia Score.
4. Discussion
In this study, we found that baseline TNF and IL-6 predicted negative symptoms trajectories in linear regression models. The association between inflammatory cytokines and negative symptoms were found to be independent of baseline depression or depression trajectory. TNF and IL-6 also predicted the specific negative symptom subscale, decreased experience of emotions and self, which includes descriptions of anhedonia, apathy, and loss of interest. Of note, inflammatory markers have been demonstrated to be associated with anhedonia and decreased connectivity in reward circuitry in individuals with depression (Felger et al., 2016; Felger and Treadway, 2017).
The association between TNF and negative symptoms in this CHR cohort is consistent with previous work demonstrating TNF to be one of the most consistently altered inflammatory cytokines in psychiatric illness (Goldsmith et al., 2016), including schizophrenia. A recent study showed that patients with deficit schizophrenia, who have primary and enduring negative symptoms, had increased TNF concentrations compared to non-deficit patients and healthy controls, and TNF concentrations predicted negative symptom severity in linear regression models, controlling for antipsychotic dose among other clinical and demographic variables (43). This is also consistent with a growing literature of studies demonstrating relationships between inflammatory cytokines and negative symptom severity in schizophrenia (Asevedo et al., 2014; El Kissi et al., 2015; Garcia-Rizo et al., 2012; Liu et al., 2012; Noto et al., 2015; Stojanovic et al., 2014; Xiu et al., 2014).
In our analyses, TNF predicted negative symptoms trajectories independent of baseline depression. Interestingly, baseline negative symptom and baseline CDSS scores were highly correlated as were their respective slope trajectories. As such, we also controlled for CDSS trajectory slope, and found that TNF and IL-6 continued to predict negative symptoms. Depression is common in individuals at CFIR, with recent data from a large meta-analysis (Fusar-Poli et al., 2014) demonstrating that 41% of CHR individuals met DSM-IV criteria for a depressive disorder. In the NAPLS-2 cohort, 60% of individuals were found to have a current or lifetime diagnosis of a depressive disorder. Moreover, those individuals with either current or historic symptoms of depression were found to have more severe negative symptoms (Kline et al., 2018). Patients with schizophrenia who have comorbid depression have been shown to have worse outcomes (Buckley et al., 2009). Thus, given the high prevalence of both negative symptoms and depression in CFIR populations, finding biomarkers that are related to and potentially differentiate these symptoms could be of utility. Our findings suggest that TNF may differentiate these symptoms as they predict worsening negative symptoms independent of depression.
The finding of IL-6 negatively predicting negative symptom slopes is intriguing, given that patients with schizophrenia typically have increased concentrations of IL-6 relative to healthy controls (Goldsmith et al., 2016). IL-6 is thought to be a pleiotropic cytokine with various roles in the immune response (Del Giudice and Gangestad, 2018; Rose-John et al., 2017). In fact, a recent study of inflammatory markers in individuals with PTSD found negative relationships between IL-6 and symptom severity, but positive relationships between IL-6 and markers of resilience (Bruenig et al., 2017). Moreover, IL-6 is also thought to have anti-inflammatory properties through its classical signaling pathway via membrane-bound IL-6 receptors and the signal transducing β-subunit glycoprotein 130 (gpl30). This is in contrast to the pro-inflammatory properties of IL-6 that involve trans-signaling through the soluble IL-6 receptor (Scheller et al., 2011; Wolf et al., 2014). The direction of relationship between IL-6 and negative symptoms in this analysis may reflect an anti-inflammatory effect that could possibly work to counter the pro-inflammatory effects of TNF and other cytokines.
A particular challenge in studying individuals at CHR is that the population is heterogenous in respect to both multiple putative CHR subgroups as well as later psychiatric diagnoses (Fusar-Poli, 2017). Despite this heterogeneity, previous studies have shown differences in inflammatory cytokines between individuals at CHR relative to controls, as well as between converters and non-converters to psychosis (Focking et al., 2016; Khoury and Nasrallah, 2018). Previous studies have found higher concentrations of IL-6 to be associated with negative symptoms in CHR individuals compared to controls (but not between CHR and individuals diagnosed with a psychotic disorder) (Stojanovic et al., 2014) whereas others have found no difference in IL-6 concentration between CHR converters and nonconverters (Smesny et al., 2017). Similarly, a number of inflammatory markers predicted conversion to psychosis in the NAPLS dataset, (Perkins et al., 2015) though not TNF or IL-6. None of the subjects included in our analyses converted to psychosis during the study period, though it is possible that some individuals who were considered non-converters may have gone on to convert to a psychotic disorder after the two-year follow up. It is possible that inflammatory markers may be less relevant for conversion risk but may instead be associated with specific symptoms domains, such as negative symptoms.
It is also interesting that there were few associations between baseline inflammatory cytokines and baseline negative symptoms. The association between negative symptom slopes and inflammatory markers suggests that these markers may predict negative symptom trajectory overtime. Most individuals who meet CHR criteria show improvements in their symptoms over time (Addington et al., 2015), though here we describe a subgroup of patients whose negative symptoms worsen from baseline, which appears to be associated with inflammatory markers. This is consistent with other findings in the CHR population such as recent data from the NAPLS group (Cannon et al., 2015) that showed no difference in baseline cortical gray matter thickness in converters compared to non-converters, but significantly greater cortical gray matter volume loss at follow-up in the subgroup of subjects who did go on to convert to psychosis. Furthermore, while inflammatory markers did not differ between converters, non-converters, and control subjects at baseline, they were associated with gray matter volume loss. The observation of putative neuropathological processes that distinguish between CHR and control subjects over time, but not at baseline, raises questions about factors that might act in concert to trigger this process and contribute to symptom exacerbation. Inflammation has been posited as one mechanism in which synergistic genetic and environmental influences may lead to neurodevelopmental changes, such as microglial activation and cortical gray matter loss, that may eventually lead to the development and exacerbation of symptoms (Howes and McCutcheon, 2017). As such, inflammation at baseline may be relevant for negative symptoms later in the course of illness progression, especially in a CHR cohort.
Not having longitudinal inflammatory markers is a primary weakness of previous studies as well as the current study. Longitudinal assessments of inflammatory markers could allow for more accurate determinations of the nature of relationships between inflammatory markers and negative symptoms. Given that the balance of pro- and anti-inflammatory effects of some cytokines, such as IL-6, can change over time, longitudinal measurement of cytokines may reveal changing relationships with symptom severity. The study is also limited by a small sample size, which in addition to the number of inflammatory markers studied likely accounts for the fact that none of the inflammatory markers survived correction for multiple comparisons in the individual models at each time point.
The lack of body mass index (BMI) was an additional limitation. Despite not having BMI, we included weight as a covariate and the results were largely unchanged. Though socioeconomic status (SES) is often an important covariate in analyses of inflammatory markers, SES is difficult to measure in a largely adolescent CHR population, who are not likely to be employed or financially independent of their family of origin. As a proxy, we conducted an exploratory analysis using maternal and paternal education level as covariates in the model. When added to the model, we found significant associations between negative symptom slope and IL-6 (beta = −0.293, p = 0.043), TNF (beta = 0.299, p = 0.032), and baseline CDSS (beta = −0.614, p = 0.000). The primary findings of associations between negative symptom slopes and baseline TNF and IL-6 were upheld.
In conclusion, individuals at CHR for psychosis showed relationships between baseline TNF and IL-6 and the course of negative symptoms over time, independent of depression. These relationships need to be further clarified and future work would measure markers in larger samples at various time points to consider relationships between markers and symptoms across time to establish the role of inflammation in the development of negative symptoms in individuals at CHR for psychosis. It will also be important to disentangle the association between inflammatory cytokines, negative symptoms, and depression. Furthermore, future work may investigate the role of anti-inflammatory treatments to target negative symptoms in this population.
Supplementary Material
Highlights.
Negative symptoms are common in individuals at clinical high risk for psychosis.
Inflammatory markers have been shown to be altered in CHR individuals.
Baseline TNF predicted 12-month negative symptoms that included anhedonia.
Baseline TNF and IL-6 predicted negative symptom trajectories in CHR individuals.
TNF predicted negative symptom development independent of baseline depression.
Acknowledgments
Funding Sources
Dr. Goldsmith has received research support from an NIMH K23 MH114037 as well as from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR002378 and KL2TR002381. This study has also been funded by a collaborative U01 award from the National Institute of Mental Health at the National Institutes of Health (MH081902 to TDC and CB; MH081857 to BAC; MH081988 to EW; MH081928 to LS; MH082004 to DP; MH081944 to KC; MH081984 to JA; MH082022 to SWW; and MH076989 TO DM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington D, Addington J, Maticka-Tyndale E, 1993. Assessing depression in schizophrenia: the Calgary Depression Scale. The British journal of psychiatry. Supplement, 39–44. [PubMed] [Google Scholar]
- Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Addington JA, Cannon TD, 2012. North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophrenia research 142, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Liu L, Buchy L, Cadenhead KS, Cannon TD, Cornblatt BA, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Bearden CE, Mathalon DH, McGlashan TH, 2015. North American Prodrome Longitudinal Study (NAPLS 2): The Prodromal Symptoms. The Journal of nervous and mental disease 203, 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Shah H, Liu L, Addington D, 2014. Reliability and validity of the Calgary Depression Scale for Schizophrenia (CDSS) in youth at clinical high risk for psychosis. Schizophrenia research 153, 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asevedo E, Rizzo LB, Gadelha A, Mansur RB, Ota VK, Berberian AA, Scarpato BS, Teixeira AL, Bressan RA, Brietzke E, 2014. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiology & behavior 129, 194–198. [DOI] [PubMed] [Google Scholar]
- Bechdolf A, Pukrop R, Kohn D, Tschinkel S, Veith V, Schultze-Lutter F, Ruhrmann S, Geyer C, Pohlmann B, Klosterkotter J, 2005. Subjective quality of life in subjects at risk for a first episode of psychosis: a comparison with first episode schizophrenia patients and healthy controls. Schizophrenia research 79, 137–143. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300. [Google Scholar]
- Brown AS, Derkits EJ, 2010. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. The American journal of psychiatry 167, 261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenig D, Mehta D, Morris CP, Lawford B, Harvey W, Mc DYR, Voisey J, 2017. Correlation between interferon gamma and interleukin 6 with PTSD and resilience. Psychiatry research 260, 193–198. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ, 2009. Psychiatric comorbidities and schizophrenia. Schizophrenia bulletin 35, 383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Jeffries C, Seidman LJ, Tsuang M, Walker E, Woods SW, Heinssen R, 2015. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biological psychiatry 77, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, Heinssen R, Jeffries CD, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Kattan MW, 2016. An Individualized Risk Calculator for Research in Prodromal Psychosis. The American journal of psychiatry 173, 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter WT, Schiffman J, 2015. Diagnostic Concepts in the Context of Clinical High Risk/Attenuated Psychosis Syndrome. Schizophrenia bulletin 41, 1001–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Horan WP, Marder SR, 2014. Psychopharmacology of the negative symptoms: current status and prospects for progress. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 24, 788–799. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Gangestad SW, 2018. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain, behavior, and immunity 70, 61–75. [DOI] [PubMed] [Google Scholar]
- Demjaha A, Valmaggia L, Stahl D, Byrne M, McGuire P, 2012. Disorganization/cognitive and negative symptom dimensions in the at-risk mental state predict subsequent transition to psychosis. Schizophrenia bulletin 38, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoe DJ, Peterson A, Addington J, 2017. Negative Symptom Interventions in Youth at Risk of Psychosis: A Systematic Review and Network Meta-analysis. Schizophrenia bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Martinez T, Kwapil TR, Barrantes-Vidal N, 2015. Subjective quality of life in At-Risk Mental State for psychosis patients: relationship with symptom severity and functional impairment. Early intervention in psychiatry 9, 292–299. [DOI] [PubMed] [Google Scholar]
- El Kissi Y, Samoud S, Mtiraoui A, Letaief L, Hannachi N, Ayachi M, Ali BBH, Boukadida J, 2015. Increased Interleukin-17 and decreased BAFF serum levels in drug-free acute schizophrenia. Psychiatry research 225, 58–63. [DOI] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH, 2016. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular psychiatry 21, 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Treadway MT, 2017. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42, 216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB SR, Givvon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV TR Axis I Disorder, Non-patient Edition (SCID-I NP). Biometrics Research, New York State Psychiatric Institute, New York, NY. [Google Scholar]
- Focking M, Dicker P, Lopez LM, Cannon M, Schafer MR, McGorry PD, Smesny S, Cotter DR, Amminger GP, 2016. Differential expression of the inflammation marker IL12p40 in the at-risk mental state for psychosis: a predictor of transition to psychotic disorder? BMC psychiatry 16, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, 2017. The Clinical High-Risk State for Psychosis (CHR-P), Version II. Schizophrenia bulletin 43, 44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rossler A, Schultze-Lutter F, Keshavan M, Wood S, Ruhrmann S, Seidman LJ, Valmaggia L, Cannon T, Velthorst E, De Haan L, Cornblatt B, Bonoldi I, Birchwood M, McGlashan T, Carpenter W, McGorry P, Klosterkotter J, McGuire P, Yung A, 2013. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA psychiatry 70, 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK, 2014. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophrenia bulletin 40, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rizo C, Fernandez-Egea E, Oliveira C, Justicia A, Bernardo M, Kirkpatrick B, 2012. Inflammatory markers in antipsychotic-naive patients with nonaffective psychosis and deficit vs. nondeficit features. Psychiatry research 198, 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Haroon E, Miller AH, Strauss GP, Buckley PF, Miller BJ, 2018. TNF-alpha and IL-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophrenia research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ, 2016. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Molecular psychiatry 21, 1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, 2013. Assessment of everyday functioning in schizophrenia: implications for treatments aimed at negative symptoms. Schizophrenia research 150, 353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y, 1990. More powerful procedures for multiple significance testing. Statistics in medicine 9, 811–818. [DOI] [PubMed] [Google Scholar]
- Holm S, 1979. A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics 6, 65–70. [Google Scholar]
- Howes OD, McCutcheon R, 2017. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Translational psychiatry 7, e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikas E, Ntouros E, Oikonomou D, Floros G, Griveas I, Garyfallos G, 2017. Evidence for Hypothalamus-Pituitary-Adrenal Axis and Immune Alterations at Prodrome of Psychosis in Males. Psychiatry investigation 14, 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury R, Nasrallah HA, 2018. Inflammatory biomarkers in individuals at clinical high risk for psychosis (CHR-P): State or trait? Schizophrenia research. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT Jr., Marder SR, 2006. The NIMH-MATRICS consensus statement on negative symptoms. Schizophrenia bulletin 32, 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline ER, Seidman LJ, Cornblatt BA, Woodberry KA, Bryant C, Bearden CE, Cadenhead KS, Cannon TD, Mathalon DH, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Addington J, 2018. Depression and clinical high-risk states: Baseline presentation of depressed vs. non-depressed participants in the NAPLS-2 cohort. Schizophrenia research 192, 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, 1998. Social anhedonia as a predictor of the development of schizophreniaspectrum disorders. Journal of abnormal psychology 107, 558–565. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, Auther A, Correll CU, Cornblatt B, 2004. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophrenia research 68, 37–48. [DOI] [PubMed] [Google Scholar]
- Liu H, Kang Y, Liang J, Li C, Xiu M, Chen D, Yang F, Wang F, Wu G, Haile CN, Kosten TA, Kosten TR, Zhang XY, 2012. Lower serum interleukin-2 levels in schizophrenic patients with tardive dyskinesia. Psychiatry research 198, 329–331. [DOI] [PubMed] [Google Scholar]
- Lizano PL, Keshavan MS, Tandon N, Mathew IT, Mothi SS, Montrose DM, Yao JK, 2016. Angiogenic and immune signatures in plasma of young relatives at familial high-risk for psychosis and first-episode patients: A preliminary study. Schizophrenia research 170, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason O, Startup M, Halpin S, Schall U, Conrad A, Carr V, 2004. Risk factors for transition to first episode psychosis among individuals with ‘at-risk mental states’. Schizophrenia research 71, 227–237. [DOI] [PubMed] [Google Scholar]
- Metcalf SA, Jones PB, Nordstrom T, Timonen M, Maki P, Miettunen J, Jaaskelainen E, Jarvelin MR, Stochl J, Murray GK, Veijola J, Khandaker GM, 2017. Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: A prospective birth cohort study. Brain, behavior, and immunity 59, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, 2013. Developmental neuroinflammation and schizophrenia. Progress in neuropsychopharmacology & biological psychiatry 42, 20–34. [DOI] [PubMed] [Google Scholar]
- Meyer U, 2014. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biological psychiatry 75, 307–315. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B, 2011. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biological psychiatry 70, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Goldsmith DR, 2017. Towards an Immunophenotype of Schizophrenia: Progress, Potential Mechanisms, and Future Directions. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42, 299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW, 2003. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophrenia bulletin 29, 703–715. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW, 2002. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. The American journal of psychiatry 159, 863–865. [DOI] [PubMed] [Google Scholar]
- Nelson B, Yuen HP, Wood SJ, Lin A, Spiliotacopoulos D, Bruxner A, Broussard C, Simmons M, Foley DL, Brewer WJ, Francey SM, Amminger GP, Thompson A, McGorry PD, Yung AR, 2013. Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA psychiatry 70, 793–802. [DOI] [PubMed] [Google Scholar]
- Noto C, Maes M, Ota VK, Teixeira AL, Bressan RA, Gadelha A, Brietzke E, 2015. High predictive value of immune-inflammatory biomarkers for schizophrenia diagnosis and association with treatment resistance. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry, 1–8. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Cornblatt BA, Mathalon DH, McGlashan TH, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Heinssen R, 2015. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophrenia bulletin 41, 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskulic D, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, McGlashan TH, 2012. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry research 196, 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E, 2008. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biological psychiatry 63, 801–808. [DOI] [PubMed] [Google Scholar]
- Rose-John S, Winthrop K, Calabrese L, 2017. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nature reviews. Rheumatology 13, 399–409. [DOI] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S, 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et biophysica acta 1813, 878–888. [DOI] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Daly MJ, Carroll MC, Stevens B, McCarroll SA, 2016. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smesny S, Milleit B, Schaefer MR, Hesse J, Schlogelhofer M, Langbein K, Hipler UC, Berger M, Cotter DR, Sauer H, McGorry PD, Amminger GP, 2017. Effects of omega-3 PUFA on immune markers in adolescent individuals at ultra-high risk for psychosis - Results of the randomized controlled Vienna omega-3 study. Schizophrenia research 188, 110–117. [DOI] [PubMed] [Google Scholar]
- Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T, 2013. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ (Clinical research ed.) 346, f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA, 2009. Common variants conferring risk of schizophrenia. Nature 460, 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E, Labad J, 2014. Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology 41, 23–32. [DOI] [PubMed] [Google Scholar]
- Upthegrove R, Manzanares-Teson N, Barnes NM, 2014. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophrenia research 155, 101–108. [DOI] [PubMed] [Google Scholar]
- Valmaggia LR, Stahl D, Yung AR, Nelson B, Fusar-Poli P, McGorry PD, McGuire PK, 2013. Negative psychotic symptoms and impaired role functioning predict transition outcomes in the at-risk mental state: a latent class cluster analysis study. Psychological medicine 43, 2311–2325. [DOI] [PubMed] [Google Scholar]
- Wolf J, Rose-John S, Garbers C, 2014. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine 70, 11–20. [DOI] [PubMed] [Google Scholar]
- Xiu MH, Yang GG, Tan YL, Chen DC, Tan SP, Wang ZR, Yang FD, Okusaga O, Soares JC, Zhang XY, 2014. Decreased interleukin-10 serum levels in first-episode drug-naive schizophrenia: relationship to psychopathology. Schizophrenia research 156, 9–14. [DOI] [PubMed] [Google Scholar]
- Zeni-Graiff M, Rizzo LB, Mansur RB, Maurya PK, Sethi S, Cunha GR, Asevedo E, Pan P, Zugman A, Yamagata AS, Higuchi C, Bressan RA, Gadelha A, Brietzke E, 2016. Peripheral immuno-inflammatory abnormalities in ultra-high risk of developing psychosis. Schizophrenia research 176, 191–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


