Abstract
Background:
While use of prescription opioids and medication assisted therapy (MAT) for opioid use disorder in pregnancy, as well as the prevalence of neonatal opioid withdrawal syndrome (NOWS) continue to rise, little is known about outcomes for children with NOWS beyond the newborn period.
Methods:
We examined 1) prenatal MAT exposure vs. unexposed healthy controls [HC]; and 2) treatment for NOWS and NOWS severity on infant neurodevelopmental and behavioral outcomes at 5–8 months of age in 78 maternal-infant pairs from the ENRICH prospective cohort study. Data were obtained from 3 study visits: prenatal, delivery, and neurodevelopmental evaluation at 5–8 months of age. Neurodevelopmental outcomes included the Bayley Scales of Infant Development [BSID-III], caregiver questionnaires (Parenting Stress Index [PSI-SF], Infant Behavior Questionnaire [IBQ-R], Sensory Profile), and the experimental Still-Face Paradigm (SFP).
Results:
No differences in the BSID-III, PSI-SF, or IBQ-R scores were observed between MAT groups and HC; however, MAT-exposed and HC infants differed with respect to SFP self-regulation (β=−18.9; p=0.01) and Sensory Profile sensation seeking (OR=4.87; 95% CI: 1.55; 15.30) after adjusting for covariates. No significant differences between Treated-for-NOWS vs. not-Treated-for-NOWS were observed. Shorter timing to NOWS treatment initiation was associated with higher Total Stress (β=−9.08; p=0.035), while longer hospitalization was associated with higher Parent-child dysfunction (p=0.018) on PSI-SF.
Conclusions:
Our results provide additional evidence of little-to-no effect of MAT and pharmacological treatment of NOWS on infant neurodevelopmental and behavioral outcomes at 5–8 months of age. However, prolonged hospitalization might increase family psychosocial stress and requires further examination.
Keywords: Opioids, substance use disorder, pregnancy, medication-assisted treatment, parental stress, infant neurodevelopment
INTRODUCTION
As the opioid crisis in the United States continues to grow, the number of pregnancies affected increases accordingly. Recent estimates show that nearly one-fourth of pregnant women in the U.S. fill a prescription for opioids [1], while gestational opioid use has increased five-fold from 1999 to 2013 [2]. For pregnant women with opioid use disorder (OUD), medication assisted therapy (MAT) is associated with improved perinatal outcomes, decreased risk of relapse, and lower rates of maternal criminal involvement [3, 4]. While adverse outcomes are lower among women on MAT, many infants with prenatal opioid exposure (POE) − whether illicit, prescription, or MAT – have neonatal opioid withdrawal syndrome (NOWS), which is now estimated to affect as many as 2% of all births [5].
Traditionally, newborns at risk for NOWS are observed in a hospital setting for at least four days for signs of withdrawal and need for treatment. Non-pharmacologic treatments, such as skin-to-skin contact, gentle handling, swaddling, demand feeding, and avoiding unnecessary awakening of the infant, are recommended as first-line therapy for NOWS [6]. More recently, maternal-infant rooming-in, reduced environmental stimuli, and breastfeeding have been identified as protective factors associated with shorter hospital stays, and are promoted as a first-line approach to minimize NOWS [7]. If withdrawal symptoms are moderate or severe, therapy is indicated for the newborn. The most common opioid agent used to treat NOWS is morphine, followed by methadone [6]; infants with severe or prolonged NOWS without an adequate response to opioid analgesics occasionally require clonidine or phenobarbital as additional agents. The opioids are slowly weaned over days to weeks; the average length of stay (LOS) for infants diagnosed with NOWS is 15–17 days. This prolonged hospitalization is recognized to be difficult for families, and bonding between primary caregivers and their infants may be impaired [8].
A limited number of preclinical studies using both rat and mice models have examined the effects of POE. Decreased neuronal counts were obtained in studies using both subcutaneous and intraperitoneal injection during the 2nd trimester equivalent age [9, 10]. Further studies indicated that therapeutic equivalent doses of subcutaneously-administered buprenorphine and methadone during 2nd and 3rd trimester equivalents led to increased myelination and alterations in myelin structure in adolescent rats [11]. While a primary limitation of rodent models is the known differences in metabolism rate relative to humans, consistency of results was obtained in preclinical studies despite differences in animal model and opioid form and dose, suggesting alterations in brain development due to POE.
Clinical studies have shown lower numeracy, literacy, IQ, and cognitive functions in school-aged children with POE or diagnosis of NOWS [12–15]. Additionally, a few studies demonstrated motor delays in children with POE [14]. Major confounders of these studies include postnatal environment, prenatal polysubstance use, suboptimal follow-up, and reliance on maternal reporting of substance use, precluding unequivocal conclusions about the effect of POE and NOWS on long-term outcomes. Despite the growing epidemic of maternal opioid use and multiple studies assessing the effects of POE on neonatal outcomes, little attention has been paid to the effects of neonatal opioid exposure on early childhood development. A recent follow-up study of the MOTHER trial’s participants was the first to do so. Results indicated minimal differences between infants treated and nontreated for NOWS [16].
The objectives of our study were two-fold: 1) To examine the effect of MAT relative to healthy controls (HC) on infant neurodevelopmental and behavioral (NDB) outcomes as well as quality of caregiver-infant interaction at 5–8 months of age; and 2) Among children prenatally exposed to MAT, to examine the effect of NOWS requiring pharmacological treatment and NOWS severity measures on infant NDB outcomes, as well as quality of caregiver-infant interaction, at 5–8 months of age as compared to infants with mild/no NOWS not necessitating pharmacological treatment. We hypothesized that there would be significant differences between MAT and HC subjects with respect to evaluated outcomes, but these would be largely due to differences in pre-and postnatal confounders. We hypothesized no differences between Treated-for-NOWS vs. not-Treated-forNOWS subjects with respect to outcomes at 5–8 months of age.
METHODS
Study design and recruitment:
The data for this study were prospectively obtained at the University of New Mexico (UNM) through the Ethanol, NeuRodevelopment, Infant and Child Health (ENRICH) birth cohort. All study activities were approved by the UNM Human Research Review Committee. All participants gave informed, written consent. The ENRICH study, designed to longitudinally assess the effects of prenatal substance use, was initiated in 2013 and consists of four visits: 1) baseline prenatal (visit 1), 2) maternal and newborn evaluations at delivery/birth (visit 2), 3) 5–8 month postpartum assessment (visit 3), and 4) 20-month postpartum assessment (visit 4). Based on data obtained at baseline, participants are enrolled into the following groups: 1) unexposed Healthy Controls (HC), 2) patients with OUD receiving MAT, 3) patients with prenatal alcohol exposure (PAE), and 4) MAT+PAE co-exposure. For purposes of this study, subjects in the PAE group were excluded from analyses.
The ENRICH study methodology has been described elsewhere [4, 17, 18]. Briefly, patients receiving prenatal care at UNM-affiliated clinics in the Albuquerque, NM metropolitan area are invited to participate. Women receiving MAT are recruited from a UNM comprehensive perinatal program for women with substance use disorders (SUD). Interested patients are screened for the following baseline eligibility criteria: 1) ultrasoundconfirmed singleton pregnancy, 2) no major fetal anomalies, 3) estimated gestational age (EGA) between 12 and 35 weeks, 4) planning to deliver at UNM Hospital and remain in the Albuquerque area for up to two years, and 5) no greater than ‘monthly’ use of stimulants (cocaine, methamphetamines, or MDMA) in pregnancy in the first trimester (per self-report or >1 positive urine drug screen) and no use in the second or third trimesters. Co-exposure with nicotine products, marijuana, and non-stimulant drugs were allowed in all groups except HC; use of tobacco or alcohol after the LMP would result in disqualification from the HC group.
Study sample, assessment of prenatal substance use, and other risk factors:
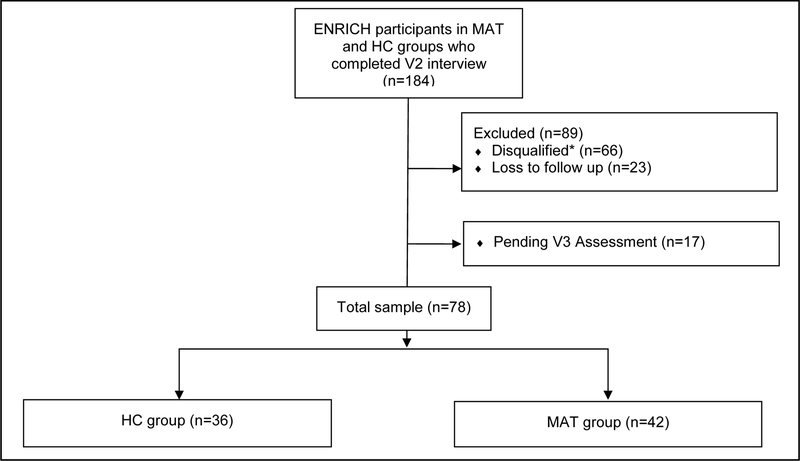
Based on the outlined inclusion/exclusion criteria, the sample size was limited to 78 maternal-infant pairs who completed visit 3 neurodevelopmental assessments as of March 2018 (Figure 1). Among 184 subjects in the MAT and HC groups who completed visit 2 assessments, 66 were disqualified (mostly due to disqualifying drug use among MAT groups and being positive for an ethanol biomarker among HC). Among the remaining 118 eligible subjects, 23 were lost to follow up, 17 had not yet aged into the window, and 78 completed visit 3 evaluations (77.2% participation rate: 78 out of 101 eligible and in-window subjects).
Figure 1: Flowchart of ENRICH Study Participants Included in the Analysis.
*The most common reasons for disqualifications include disqualifying drug use among any study groups and being positive for alcohol biomarkers among HC group.
All MAT group participants were patients with OUD receiving methadone or buprenorphine with or without exposure to other opioids. MAT, opioid use, and exposure to other substances of abuse were captured through: 1) repeated prospective Timeline Follow-Back [TLFB] interviews [19]; 2) a study-specific 7-panel urine drug test (amphetamines, barbiturates, benzodiazepines, cocaine, opiates, PCP, cannabinoids/THC) prenatally and at delivery, with confirmatory testing by GC-MS/MS (for all except benzodiazepines, which uses LC-MS/MS) [20]; and 3) abstraction of urine drug screen results each trimester from medical records. Additionally, a comprehensive panel of ethanol biomarkers (gamma-glutamyl transpeptidase [GGT], carbohydrate deficient transferrin [CDT], phosphatidylethanol [PEth], urine ethyl glucuronide and ethyl sulfate [uEtG/uEtS], and PEth in a newborn dry blood spot card [PEth-DBS]), and a nicotine metabolites panel (nicotine, cotinine, 3hydroxycotinine, nornicotine, anabasine) were administered, as described [18, 21]. PAE data obtained from TLFB interviews were summarized as absolute ounces of alcohol per day (AA/day) and per drinking day (AA/drinking day) [22].
Structured maternal interviews captured socio-demographic characteristics (maternal age, marital status, Barratt Simplified Measure of Social Status, race, ethnicity, education), medical and reproductive health (gravidity, parity, pregnancy complications), and perinatal/birth outcomes (infant sex, anthropometric measures, complications in the newborn period). At visit 3, postnatal risk factors were assessed, including maternal depressive symptoms (Beck Depression Inventory [BDI]), household income, and utilization of social services.
NOWS measures:
Infants who received pharmacological treatment for NOWS were classified into the Treated-for-NOWS group, while infants born to women on MAT who did not receive pharmacological treatment were categorized into the not-Treated-for-NOWS group. Severity of NOWS was characterized using hospital LOS (number of days from birth to discharge), time to initiation of the first morphine/methadone dose, cumulative dose of morphine or methadone received (converted into morphine equivalent), and use of adjunctive therapy (i.e. clonidine, phenobarbital). Conversion of methadone dose to morphine equivalent (conversion ratio of 3:1) was based on a previous publication [23].
Neurodevelopmental Outcomes:
An assessment battery for visit 3 (5–8 months of age, adjusted for prematurity) of neurodevelopmental outcomes were grouped into 3 categories: 1) standardized assessments administered by a pediatric developmental diagnostician (JL), who was blinded to the exposure status; 2) caregiver-administered questionnaires; and 3) the Still-Face Paradigm (SFP). All are described in our earlier publications [4, 17, 18].
Bayley Scales of Infant and Toddler Development (BSID-III) [24]: The BSID-III is the most widely-used standardized scale of infant and toddler development. The Cognitive, Language, and Motor scales were administered to each child with parents present. The composite score was used in analyses, which has a population mean of 100 and standard deviation of 15.
The Parenting Stress Index-Short Form (PSI-SF) [25] is a parent-completed questionnaire. Percentile scores for the following scales were used in analyses: Parent stress, Parent-child dysfunctional interaction, Difficult child, and Total Stress.
The Infant Behavior Questionnaire-Revised (IBQ-R) [26] is a parent-completed questionnaire measuring infant temperament in the areas of Negative affect, Surgency, and Effortful control. The raw score for each scale was used in analyses.
The Infant/Toddler Sensory Profile (ITSP) [27] is a parent-completed questionnaire measuring infant sensation seeking, sensory sensitive and sensory avoidance behaviors. The raw score for each subscale was converted into an ordinal scale with the following categories: “less than others”, “typical performance”, or “more than others”.
The Still Face Paradigm (SFP) was administered and videotaped with the mother-child dyad, as described [28], and included five episodes: 1) baseline play; 2) the first ‘still-face’ episode, (mother maintains a neutral expression while refraining from making eye contact or responding to her child; 3) a reunion or play episode (mother resumes typical interaction); 4) a second ‘still-face’ episode; and 5) a second reunion or play episode. Infant affect was coded as follows: −3 (rhythmic crying for ≥3 seconds), −2 (shorter cry in duration, a protest or yell), −1 (mild fuss/frown), 0 (baby is neutral), +1 (corners of the mouth straight, soft coo), +2 (corners of the mouth go up, cheeks raised, chuckle or small giggle), +3 (laugh for ≥2 s). The percentage of time the infant displayed positive or negative affect over the duration of each 120-second episode was calculated. Infant positive affect was defined as a score greater than 0, and negative affect was a score below 0. Self-regulation was coded as behaviors that were self-comforting, such as child mouthing their hands or an object, or clasping or bracing their hands or feet. All assessments were coded offline by trained members of the study team who were blinded to participant exposure status.
Statistical analyses:
T-tests and Chi-square tests (or equivalently Wilcoxon rank sum and Fisher exact tests) were performed to compare the difference between study groups for continuous and categorical variables, respectively. Univariate comparisons were made between HC and MAT groups, followed by Treated-for-NOWS vs. not-Treated-forNOWS groups for all behavioral outcomes (BSID-III, PSI, IBQ-R, ITSP and SFP). For multivariable analysis of SFP, linear mixed effect model with repeated measures (5 episodes of the SFP) was used to assess the effect of the study groups (Model 1: MAT vs HC; Model 2: Treated-for-NOWS vs. not-Treated-for-NOWS). Compound symmetry covariance structure was selected because of its comparatively lower number of parameters (2 vs. 30 for covariance structure with 5 repeated measures) and lower AIC indicating a better fit. Similarly, logistic regression was used to estimate the effect of the study group (Model 1: MAT vs HC; Model 2: Treated-for-NOWS vs. not-Treated-for-NOWS) on ITSP sensation seeking behavior. The original ordinal variable (more than others; typical behavior; less than others) for sensation seeking was converted into a binary variable (typical vs. more than others) since no subjects were categorized into a “less than others” group based on the ITSP questionnaire. Covariates which were differentially distributed between study groups were selected for inclusion in the full multivariable models, consisting of birth weight, maternal education, marital status, BDI score, SES, gravidity, and parity for Model 1, and gestational age, preterm status, MAT regimen, maternal tobacco use, and maternal race for Model 2. Backward elimination methods (based on model fit using AIC) were used to create parsimonious models which included birth weight and SES for Model 1, and gestational age, MAT regimen, maternal tobacco use, and maternal race for Model 2. Lastly, the association between NOWS severity measures (LOS, cumulative morphine equivalent dose, time to treatment initiation) and behavioral outcomes were assessed using linear regression analysis.
Power calculations were conducted in PASS [29] a priori for the comparison of MAT and HC groups. Sample size of 37 subjects per group was sufficient to achieve >80% power to detect a 10-point difference in the BSIDIII scores with SD=15 (available sample sizes of 36 and 42 in the HC and MAT groups, respectively, resulted in 83% power). For behavioral outcomes, given limited information available in the field, previously observed differences on the Test of Sensory Functions in Infants (TSFI) between MAT and HC (35.2 and 40.6, respectively) were used to estimate expected effect size for power calculations, even though TSFI does not directly translate into ITSP. A sample size of 28 subjects per group achieved 81% power to detect a similar effect size with a significance level of 0.05 using a two-sided two-sample equal-variance t-test.
RESULTS
No differences in maternal age, gestational age at enrollment, race, ethnicity, parity, gestational age at delivery, preterm delivery, infant sex, or infant age at visit 3 were observed between subjects enrolled into the two MAT groups and HC group (all p’s >0.05; Table 1). However, MAT group participants had lower family SES (MAT: 28.5±7.7 vs. HC: 35.2±12.2), higher BDI (10.6±9.8 vs. 5.8±4.7), were more likely to be single/separated/divorced (61.9% vs. 27.8%), have less than high school education level (42.9% vs. 13.9%), and fewer were primigravida (11.9% vs. 38.9%) compared to HC (all p’s <0.05). Additionally, MAT group infants had lower birth weight compared to HC (2,948±632 vs. 3,328±528 grams, respectively, p<0.05), and a higher proportion were small-for-gestational age (SGA; birth weight <10th percentile at given gestational age) relative to HC (p=0.05; data not shown). No differences between the two MAT groups were observed except a slightly higher proportion of Hispanic/Latina subjects in the Treated-for-NOWS compared to not-Treated-for-NOWS (75.0% vs, 65.4%; p<0.05).
Table 1.
Description of the Study Population
| HC (n=36) |
MAT (n=42) |
Treated-for- NOWSa (n=16) |
Not-Treated-for- NOWSa (n=26) |
|
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Maternal age at enrollment (yrs) | 26.8 ± 5.7 | 28.3 ± 5.1 | 29.4 ± 5.3 | 27.5 ± 5.0 |
| Gestational age at enrollment (wks) | 25.5 ± 7.7 | 23.0 ± 6.9 | 22.1 ± 5.6 | 23.5 ± 7.6 |
| Gestational age at delivery (wks) | 39.2 ± 1.3 | 38.7 ± 2.2 | 38.4 ± 3.0 | 38.9 ± 1.5 |
| Infant birth weight (grams) | 3328 ± 528 | 2948 ± 632ǂǂ | 2956 ± 817 | 2944 ± 504 |
| Infant age at assessment (mos.)a | 6.9 ± 1.1 | 6.8 ± 1.2 | 7.0 ± 1.4 | 6.7 ± 1.1 |
| Maternal BDI score | 5.8 ± 4.7 | 10.6 ± 9.8ǂǂ | 8.1 ± 6.9 | 11.9 ± 10.9 |
| Family SES score | 35.2 ± 12.2 | 28.5 ± 7.7ǂǂ | 27.1 ± 7.0 | 29.4 ± 8.2 |
| n (%*) | n (%*) | n (%*) | n (%*) | |
| Family household income (at V3) | ||||
| Under $20,000 | 8 (22.2) | 25 (61.0)ǂǂ | 8 (50.0) | 17 (68.0)§ |
| $20,000 – $39,999 | 8 (22.2) | 12 (29.3) | 5 (31.3) | 7 (28.0) |
| $40,000 – $59,999 | 7 (19.4) | 3 (7.3) | 2 (12.5) | 1 (4.0) |
| $60,000 or over | 13 (36.1) | 1 (2.4) | 1 (6.3) | 0 (0.0) |
| Employed (at enrollment) | 19 (52.8) | 6 (14.3)ǂ | 1 (6.3) | 5 (19.2) |
| Preterm delivery | 3 (8.3) | 5 (11.9) | 4 (25.0) | 1 (3.85) |
| Ethnicity: Hispanic/Latina | 23 (63.9) | 29 (69.1) | 12 (75.0) | 17 (65.4)§ |
| Race: | ||||
| White | 36 (100) | 36 (85.7) | 12 (75.0) | 24 (92.3) |
| African American | 0 (0.0) | 2 (4.8) | 2 (12.5) | 0 (0.0) |
| American Indian | 0 (0.0) | 2 (4.8) | 0 (0.0) | 2 (7.7) |
| Other | 0 (0.0) | 2 (4.8) | 2 (12.5) | 0 (0.0) |
| Marital/cohabiting status: | ||||
| Single/separated/divorced | 10 (27.8) | 26 (61.9)ǂǂ | 9 (56.2) | 17 (65.4) |
| Married/cohabitating | 26 (72.2) | 16 (38.1) | 7 (43.8) | 9 (34.6) |
| Education Level: | ||||
| Less than high school | 5 (13.9) | 18 (42.9)ǂǂ | 8 (50.0) | 10 (38.5) |
| High school to some college | 22 (61.1) | 23 (54.8) | 7 (43.8) | 16 (61.5) |
| College/professional degree | 9 (25.0) | 1 (2.4) | 1 (6.2) | 0 (0.0) |
| Gravidity: primigravida | 14 (38.9) | 5 (11.9)ǂǂ | 0 (0.0) | 5 (19.2) |
| Parity: nulliparous | 15 (41.7) | 9 (21.4) | 3 (18.8) | 6 (23.1) |
| Infant’s gender: male | 19 (52.8) | 22 (52.4) | 8 (50.0) | 14 (53.9) |
p<0.05;
p<0.01 for comparison between two MAT and HC groups
p<0.05;
p<0.01 for comparison between TREATED-FOR-NOWS and Not-Treated-for-NOWS groups
Adjusted for preterm birth
BDI, Beck Depression Inventory; SES, Barratt family socio-economic status.
Column percentages
As shown in Table 2, no statistically significant differences in prenatal use of alcohol, MAT, other opioids, marijuana, methamphetamines, or tobacco were observed between the two MAT groups (all p’s >0.05; Table 2). A higher proportion of Treated-for-NOWS participants were on methadone (50%) compared to not-Treatedfor-NOWS (34.6%); however, differences did not reach statistical significance (p=0.283). Hospital LOS was substantially greater in infants Treated-for-NOWS (median; IQR: 15.9; 11.0–33.9 days) compared to notTreated-for-NOWS (median; IQR: 4.5; 4.2–5.3 days). Cumulative morphine equivalent dose administered to Treated-for-NOWS infants was 35.3±68.9 mg (median 13.6; IQR: 3.7–23.5 mg; data not shown). Only one subject in the Treated-for-NOWS group used adjunctive (clonidine) therapy.
Table 2.
MAT regimen, NOWS Severity Measures, and Co-exposures among MAT Participants
| Treated-for- NOWSa (n=16) |
Not-Treated-for- NOWSa (n=26) |
p | |
|---|---|---|---|
| Median (Q1, Q3) | Median (Q1, Q3) | ||
| Length of Stay (days) | 15.9 (11.0; 33.9) | 4.5 (4.2; 5.3) | <0.001 |
| Mean ± SD | Mean ± SD | ||
| Cumulative morphine equivalent dose (mg) | 35.3 ± 68.9 | NA | NA |
| n (%) | n (%) | ||
| Cumulative Alcohol use across pregnancy & periconceptional period: | |||
| AA/day | 0.4 ± 0.8 | 0.4 ± 2.0 | 0.886 |
| AA/drinking day | 3.2 ± 2.7 | 6.1 ± 8.7 | 0.364 |
| n (%) | n (%) | ||
| Positive for ≥1 biomarker (V1 or V2)b | 6 (37.5) | 6 (23.1) | 0.483 |
| MAT regimen: | |||
| Methadone only | 8 (50.0) | 9 (34.6) | 0.283c |
| Buprenorphine only | 7 (43.8) | 16 (61.5) | |
| Methadone and buprenorphine | 1 (6.3) | 1 (3.8) | |
| Other substances: | |||
| Other opioids (heroin or opioid analgesicsd) | 10 (62.5) | 13 (50.0) | 0.530 |
| Marijuana | 2 (12.5) | 9 (34.6) | 0.158 |
| Methamphetaminese | 0 (0.00) | 3 (11.5) | 0.275 |
| Any tobacco use | 13 (81.3) | 22 (84.6) | 1.000 |
NOWS requiring pharmacological treatment
Either positive urine drug panel at V1 or V2 or self-reported anytime in pregnancy.
The Chi-square test excludes two individuals who had exposure to both MAT regimens during pregnancy
Used either as prescribed or recreational use
occasional use around LMP, no use during pregnancy
AA, absolute ounces of alcohol (1.0 oz AA = 2 standard drinks)
No differences in scores on the BSID-III, PSI-SF, or IBQ-R were observed between MAT groups and HC or between Treated-for-NOWS vs. not-Treated-for-NOWS groups (all p’s >0.05; Table 3). The two MAT groups demonstrated higher infant negative affect and lower infant self-regulation during some of the episodes of SFP compared to HC (p<0.05). Additionally, significantly fewer MAT-exposed infants (those in the Treated-forNOWS (40%) or not-Treated-for-NOWS (41.7%) groups) demonstrated ‘typical performance’ on the ITSP sensation seeking scale compared to HC (77.1%; p<0.05). No significant differences between Treated-for-NOWS vs. not-Treated-for-NOWS were observed on any evaluated outcome.
Table 3.
Neurodevelopmental and Behavioral Outcomes at 5–8 Months of Age among Healthy Controls, Treatedfor-NOWS, and Not-Treated-for-NOWS Subjects (n=78a)
| HC (n=36) |
Treated-for- NOWSb (n=16) |
Not-Treated-for- NOWSb (n=26) |
|
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Bayley Scales of Infant Development (BSID-III) | |||
| BSID-III: Cognitive | 100.8 ± 9.4 | 103.4 ± 9.1 | 100 ± 7.7 |
| BSID-III: Language | 98.6 ± 7 | 101.2 ± 7.1 | 101.3 ± 6.6 |
| BSID-III: Motor | 95.6 ± 12.9 | 95.6 ± 10.4 | 96.2 ± 9.4 |
| Parental Stress Index (PSI-SF) | |||
| Parental Distress | 33.2 ± 31.1 | 28.1 ± 18.5 | 39.5 ± 31.2 |
| Parent-Child Dysfunctional Interaction | 26.4 ± 22 | 30.6 ± 22.1 | 24.4 ± 20 |
| Difficult Child | 20.4 ± 19.5 | 26.9 ± 23.2 | 20.9 ± 21 |
| Total Stress | 22.5 ± 21.1 | 25.9 ± 22.7 | 23.2 ± 22.2 |
| Infant Behavioral Questionnaire (IBQ-R) | |||
| Surgency | 5.1 ± 0.9 | 5.6 ± 0.5 | 5.4 ± 0.8 |
| Negative Affect | 4 ± 1.1 | 4.3 ± 1.2 | 4.2 ± 1.1 |
| Effortful Control | 5.6 ± 0.8 | 5.7 ± 0.7 | 5.8 ± 0.7 |
| Still-Face Paradigm (SFP) | |||
| Positive Infant Affect | |||
| Episode 1 | 26.6 ± 23.1 | 25.7 ± 22.5 | 27.7 ± 25.6 |
| Episode 2 | 2.1 ± 7 | 0.4 ± 0.8 | 0.5 ± 1.1 |
| Episode 3 | 34.1 ± 23.2 | 34 ± 28.2 | 32.8 ± 28.3 |
| Episode 4 | 1.1 ± 5.7 | 0.2 ± 0.7 | 0.2 ± 0.6 |
| Episode 5 | 32.8 ± 26.4 | 31.8 ± 29.7 | 29.5 ± 23.8 |
| Negative Infant Affect | |||
| Episode 1 | 4.9 ± 10.9 | 6.1 ± 9.4 | 4.5 ± 10.8 |
| Episode 2ǂǂ | 22.6 ± 18.5 | 37.5 ± 30.7 | 39.9 ± 34.9 |
| Episode 3 | 12.3 ± 21.5 | 21.8 ± 26.6 | 17.4 ± 28.6 |
| Episode 4 | 40.5 ± 31.9 | 49.9 ± 40.4 | 41.7 ± 35.1 |
| Episode 5 | 21 ± 28.5 | 25.8 ± 36.5 | 24.4 ± 29.4 |
| Infant Self-Regulation | |||
| Episode 1 | 45.8 ± 33.9 | 34 ± 41.3 | 30.3 ± 29.7 |
| Episode 2 | 63.5 ± 31.3 | 40.3 ± 41.4 | 52 ± 36.6 |
| Episode 3 | 49.1 ± 34.2 | 24.1 ± 30.1 | 40.9 ± 36.4 |
| Episode 4 ǂ | 70.0 ± 29.4 | 49.4 ± 34.9 | 56.3 ± 38.5 |
| Episode 5 | 45.6 ± 36.5 | 42.3 ± 36.3 | 43.4 ± 37.4 |
| Infant/Toddler Sensory Profile | |||
| Low Registration | n (%) | n (%) | n (%) |
| Less than others | 9 (25.7) | 3 (20.0) | 8 (33.3) |
| Typical performance | 16 (45.7) | 9 (60.0) | 12 (50.0) |
| More than others | 10 (28.6) | 3 (20.0) | 4 (16.7) |
| Sensation Seeking ǂǂ | |||
| Less than others | 0 (0) | 0 (0) | 0 (0) |
| Typical performance | 27 (77.1) | 6 (40.0) | 10 (41.7) |
| More than others | 8 (22.9) | 9 (60.0) | 14 (58.3) |
| Sensory Sensitivity | |||
| Less than others | 2 (5.7) | 1 (6.7) | 0 (0) |
| Typical performance | 27 (77.1) | 9 (60.0) | 14 (58.3) |
| More than others | 6 (17.1) | 5 (33.3) | 10 (41.7) |
| Sensation Avoiding | |||
| Less than others | 2 (5.7) | 0 (0) | 0 (0) |
| Typical performance | 25 (71.4) | 10 (66.7) | 21 (87.5) |
| More than others | 8 (22.9) | 5 (33.3) | 3 (12.5) |
Sample size for the SFP assessment is limited to 75 maternal-infant pairs.
NOWS requiring pharmacological treatment
p<0.05;
p<0.01 for comparison between two MAT and HC groups
No significant differences were detected between TREATED-FOR-NOWS and Not-Treated-for-NOWS group
Repeated measures analyses across five SFP episodes demonstrated a significant association between MAT grouping vs. HC with respect to infant self-regulation (β=−13.7; p=0.047; Table 4). Additionally, there was an association between study grouping (MAT vs HC) and sensation seeking behavior (OR=4.85; 95% CI: 1.76; 13.38 for “more than others” vs. “typical” performance). These associations remained significant for selfregulation (β=−18.9; p=0.01) and sensation seeking (OR=4.87; 95% CI: 1.55; 15.30) after adjusting for covariates. No association was observed between MAT groups vs. HC with respect to repeated measures of negative affect in unadjusted (β=7.2; p=0.098) or adjusted (β=4.5; p=0.359) analyses. No statistically significant differences in these outcomes were observed between Treated-for-NOWS vs. not-Treated-for-NOWS groups in multivariable analysis (all p’s >0.05; Table 4).
Table 4.
Associations between MAT Exposure (Model 1) and Treatment for NOWS (Model 2) on Neurodevelopmental Outcomes: Results of Multivariable Analyses
| Neurodevelopmental outcomes | Unadjusted results | Adjusted results | ||
|---|---|---|---|---|
| Model 1 (Both MAT groups vs. HC) | ||||
| β | p | β | p | |
| Infant self-regulation (repeated measures)a | −13.7 | 0.047 | −18.9 | 0.015 |
| Negative infant affect (repeated measures)a | 7.2 | 0.098 | 4.5 | 0.359 |
| Sensation Seeking: | OR (95% CI) | p | OR (95% CI) | p |
| More than others vs. Typical | 4.85 (1.76 , 13.38) | 0.002 | 4.87 (1.55, 15.3) | 0.007 |
| Model 2 (Treated-for-NOWS vs. Not-Treated-for-NOWS) | ||||
| β | p | β | p | |
| Infant self-regulation (repeated measures)a | −6.6 | 0.532 | −0.61 | 0.961 |
| Negative infant affect (repeated measures)a | 2.0 | 0.788 | 4.9 | 0.594 |
| Sensation Seeking: | OR (95% CI) | p | OR (95% CI) | p |
| More than others vs. Typical | 1.07 (0.29, 3.99) | 0.918 | 0.74 (0.15, 3.63) | 0.683 |
Mixed effects modelling for repeated measures across 5 SFP episodes
Model 1: a parsimonious model included birth weight, and family SES predictors. Other covariates initially examined that did not make it into the final model were maternal education, marital status, BDI score, gravidity, and parity.
Model 2: a parsimonious model included gestational age at delivery, type of MAT (buprenorphine vs. methadone), prenatal tobacco use, and maternal race. An additional covariate, preterm status, was examined but was not selected in the final model.
NDB outcomes differentially distributed among the study groups at p<0.10 were examined further against severity measures of NOWS. As shown in Appendix A.1, shorter timing to treatment initiation (an indicator of more severe NOWS) was associated with higher Total Stress on the PSI-SF (β=−9.08; p=0.035). Higher cumulative morphine equivalent dose was associated with higher negative affect at episode 3 of the SFP (p=0.015). Similarly, higher LOS was associated with higher negative affect at episode 3 of the SFP (p=0.019); associations with negative affect at episodes 4 and 5 were of borderline statistical significance (p=0.059 and 0.057, respectively). In mixed effect modeling, which incorporates repeated measures across 5 SFP episodes, no association was observed between cumulative morphine equivalent dose and negative affect (β=0.14; p=0.110), or LOS and negative affect (β=0.63; p=0.107; data not shown). Higher LOS was associated with higher Parentchild Dysfunctional Interaction (p=0.018).
DISCUSSION
The results of this prospective cohort study are generally reassuring with respect to the effects of MAT and NOWS on infant neurodevelopment. Among 17 evaluated outcomes, multivariable analyses demonstrated differences between MAT-exposed and unexposed subjects only for infant self-regulation and sensation seeking behaviors. Difference in negative affect observed in isolated SFP episodes became non-significant in mixedeffect modelling and after adjustment for covariates. No differences between Treated-for-NOWS and notTreated-for-NOWS groups with respect to evaluated outcomes were observed, and BSID-III scores were within normal range for all groups. It should be noted that NOWS severity measures were associated with higher parenting stress scores indicating that prolonged hospitalization and treatment for NOWS might affect parentchild interaction and increase family overall psychosocial stress even months after discharge.
Studies of non-pharmacologic care modalities for NOWS treatment, and the effect of treatment regimens on NOWS severity and immediate infant outcomes on long-term development remain scarce [7]. One of the earliest reports assessing 6-month outcomes in infants prenatally exposed to methadone [30] found no differences between infants treated for NOWS and not treated (or treated with different regimens). Emerging results from the MOTHER trial [16], which evaluated outcomes in 96 MAT-exposed infants aged 3–36 months old, also indicate no differences between Treated-for-NOWS and not-Treated-for-NOWS groups on BSID-III scores. However, in the MOTHER trial, higher scores in the IBQ-R Distress to Limitation scale and lower scores on ITSP sensation seeking across 3–36 months (but not at 6-months assessment) were found in the Treated-for-NOWS group compared to not-Treated-for-NOWS. In the ENRICH cohort, we also observed no difference in sensation seeking between Treated-for-NOWS and not-Treated-for-NOWS groups at 5–8 months of age, but did find a significant difference between MAT-exposed infants relative to controls (unexposed controls were not included in MOTHER).
In our study, a higher proportion of children in both MAT groups scored in the ‘more than others’ category on sensation seeking, meaning they are more likely to actively look for additional sensory information such as through touching or biting/mouthing objects. A prospective study among 81 opioid-exposed and 26 unexposed infants in Scotland reported lower scores on the Griffiths Mental Development Scales at 6 months among those treated for NOWS [31]; however, it was not clear whether the differences were due to NOWS, treatment, LOS, co-exposures, or other factors.
Earlier studies reported some neurodevelopmental differences between HC and MAT subjects, but social environment and other risk factors were often not adequately controlled for [6], making it difficult to draw unequivocal conclusions. A Scandinavian cohort study evaluated differences in NDB outcomes between 38 children exposed to MAT and 36 HC, and found significant differences on a sensory integration test at 6 months, although both groups scored within the normal range [32]. In that study, maternal interaction style had a much greater effect on infant outcomes than prenatal MAT. At a 2.5 year follow-up, group differences in perceived problems in toddlerhood were reported, but maternal distress remained a major predictor [33]. At a 4 year follow-up, a significant effect of prenatal MAT and maternal interaction on cognitive development (i.e., behavioral inhibition, sensorimotor function, short-term memory) was found [34]. In another cohort study of children aged 1 to 8.5 years (POE/polysubstance exposed versus unexposed controls), sex-specific outcomes were found, with decreased cognitive scores in exposed boys at age 3, and lower functioning among exposed girls emerging over time [13]. However, the study had numerous limitations, including recruitment from a social service institution for high-risk children where most of exposed children did not live with biological parents, reliance on medical records and maternal report (when available) to obtain information on prenatal exposures, and non-blinded assessments of the outcomes [13, 35]. Attempts to compare the effects of different prenatal MAT regimens (methadone vs. buprenorphine and dose levels) on neurodevelopment have yielded inconsistent results. For example, the MOTHER study [16] and a retrospective cohort in Massachusetts [36] found no association, while a prospective cohort in Australia found differences in visual evoked potentials between methadone- vs. buprenorphine-exposed infants at 4 months of age, which did not persist to a 36months evaluation [37]. While there are some indications that brain function may be altered by prenatal exposure to methadone vs. buprenorphine in infancy, the small number of studies to examine this have also reported normalization of the assessed sensory function between 6 and 36 months of age [35, 37]. It remains unclear whether these early sensory deficits lead to downstream effects on brain development.
Our study findings should be viewed in light of the limitations. First, the sample is relatively small, especially for the Treated-for-NOWS group. Power analysis conducted for MAT/HC comparisons demonstrated sufficient sample size to achieve 80% power. It should be noted that the sample size of the Treated-for-NOWS group did not allow for examination of other factors which could have contributed to parental stress. The sample size also did not allow for stratification by infant sex or MAT regimen. Second, polysubstance use is a common confounder in the field, making it challenging to separate the results of MAT from co-exposures. Of note, methamphetamine or cocaine co-exposures were study disqualifiers (other than occasional use prior to pregnancy recognition), while the effect of other substances was monitored by prospective repeated interviews and urine drug tests. No differences in the prevalence of co-exposures were observed between Treated-forNOWS and not-Treated-for-NOWS groups; however, when comparing HC vs. MAT, we could not adjust for alcohol, tobacco, or marijuana, which were exclusionary criteria for the HC group. While co-exposure with alcohol was not an exclusionary criterion for this study, the level of alcohol consumption was low among MAT subjects (<0.4 AA/day) and similar among Treated-for-NOWS and not-Treated-for-NOWS groups. Additionally, to maximize generalizability of the study findings, we allowed co-exposure with other opioids in the MAT group since only 40–60% of patients strictly adhere to a prescribed regimen [38]. Prevalence of heroin and/or recreational opioid analgesics was similar between Treated-for-NOWS and not-Treated-for-NOWS groups.
Third, common NOWS severity measures [39] might be affected by hospital practices and confounders. For example, time to treatment initiation for NOWS might be affected by type of maternal MAT, with longer halflife of methadone compared to buprenorphine. Fourth, this report is limited to neurodevelopmental outcomes evaluated only at 5–8-month postnatal visit given that infants are still progressing through the 20-month visit due to the longitudinal nature of the study. Future reports will examine changes in neurodevelopmental outcomes during the first two years of life. Finally, while we extensively examined the effect of prenatal and postnatal environmental factors (birth weight, education, marital status, BDI score, SES score, gravidity, gestational age, preterm status, MAT regimen, maternal tobacco use, and maternal race) on evaluated outcomes, differences in self-regulation and sensation seeking between MAT and HC infants could result from residual confounding not captured in the ENRICH cohort and requires careful examination in future studies.
The unique strengths of this study included the prospective cohort design with repeated prospective evaluation of substance use across pregnancy, as well as environmental risk and resilience factors. While there were some differences in socio-demographic characteristics between MAT and HC subjects, it is important to note that New Mexico has one of the highest poverty rates in the U.S. [40]; thus, many HC participants have a social environment typically observed in high-risk populations, allowing us to minimize the effect of these confounders. Another unique strength of the ENRICH cohort is ethnic diversity (66.7% Hispanic/Latina), as prior studies have included mostly non-Hispanic White subjects. Additionally, no differences in demographic characteristics were observed between participants who were lost to follow up and those who completed visit 3 assessments (data not shown), minimizing the potential effect of selection bias. Finally, a unique strength of this study is the inclusion of experimental SFP to evaluate infant stress reactivity, in addition to standardized neurodevelopmental tests and caregiver-reported outcomes. The focus on gross and fine motor skills, cognition, language, temperament, sensory processing, positive and negative affect, self-regulation, and quality of parentchild interaction allowed for comprehensive evaluation of infant NDB outcomes and parent-child interaction.
Our results provide tentative reassurance regarding effects of MAT and pharmacological treatment of NOWS on infant neurodevelopment. Differences in self-regulation and sensory profile between MAT-exposed and unexposed subjects, as well as the effect of severe NOWS and prolonged hospitalization on parent-child bonding and interaction require careful examination in larger studies with longer follow-up. Future studies with larger cohorts are needed to examine subgroups based on specific medications used in MAT and NOWS treatment and to assess the potential role of maternal polysubstance use. Longerterm studies including measurements of executive function, school performance, and behavioral assessments in home and school environments are needed; however, distinguishing the effects of MAT, NOWS, and environmental factors presents a challenging barrier.
The “Eat-Sleep-Console” (ESC) model deserves special mention. There is decreased need for pharmacological treatment of NOWS and reduced LOS when neonates are able to “room-in” with their mothers in a low-stimulus environment, have extended skin-to- skin contact, breastfeeding, and use NOWS treatment protocols that are based primarily on the newborn’s ability to ESC [41]. Given these emerging data, hospital administrators should focus on helping families bond with their newborn during NOWS treatment. Future studies should also focus on neurodevelopmental outcomes of infants with POE that do not develop NOWS or are treated without pharmacological agents.
Supplementary Material
Highlights.
No developmental delays were observed in opioid-exposed infants treated for NOWS
No developmental delays were observed in opioid-exposed infants relative to control
Shorter time to NOWS treatment initiation was associated with higher parental stress
Longer hospital stay of MAT-exposed infants led to greater parent-child dysfunction
ACKNOWLEDGEMENTS
We are thankful to Rebecca Rieger, Natalia Moss, Sonnie Williams, Alyssa Ortega, Danielle Kabella, Lucinda Flynn, and Crystal Aragon for recruitment of study subjects, data collection, and data management, and to Laura Garrison for editorial assistance and assistance with IRB approvals and compliance. We are in debt to William Rayburn, MD, MBA for assisting with the research design, and especially to ENRICH research subjects for volunteering their time to the study.
Financial disclosure: This work has been supported by a research grant from NIAAA/NIH (1R01AA021771). For statistical support, we acknowledge the University of New Mexico Clinical & Translational Science Center (UL1TR001449) and the Mountain West Clinical Translational Research Infrastructure Network (5 U54 GM104944) from the National Institute of General Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None declared.
References
- [1].Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF, Increase in Prescription Opioid Use During Pregnancy Among Medicaid-Enrolled Women, Obstet Gynecol 123 (2014) 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ko JY, Patrick SW, Tong VT, Patel R, Lind JN, Barfield WD, Incidence of Neonatal Abstinence Syndrome - 28 States, 1999–2013, MMWR. Morbidity and mortality weekly report. 65 (2016) 799–802. [DOI] [PubMed] [Google Scholar]
- [3].Kaltenbach K, Berghella V, Finnegan L, Opioid dependence during pregnancy. Effects and management, Obstetrics and gynecology clinics of North America. 25 (1998) 139–51. [DOI] [PubMed] [Google Scholar]
- [4].Lowe J, Qeadan F, Leeman L, Shrestha S, Stephen JM, Bakhireva LN, The effect of prenatal substance use and maternal contingent responsiveness on infant affect, Early human development. 115 (2017) 51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Milliren CE, Gupta M, Graham DA, Melvin P, Jorina M, Ozonoff A, Hospital Variation in Neonatal Abstinence Syndrome Incidence, Treatment Modalities, Resource Use, and Costs Across Pediatric Hospitals in the United States, 2013 to 2016, Hospital pediatrics. 8 (2018) 15–20. [DOI] [PubMed] [Google Scholar]
- [6].Kocherlakota P, Neonatal abstinence syndrome, Pediatrics. 134 (2014) e547–61. [DOI] [PubMed] [Google Scholar]
- [7].Wachman EM, Schiff DM, Silverstein M, Neonatal Abstinence Syndrome: Advances in Diagnosis and Treatment, Jama. 319 (2018) 1362–74. [DOI] [PubMed] [Google Scholar]
- [8].Boucher AM, Nonopioid Management of Neonatal Abstinence Syndrome, Advances in neonatal care : official journal of the National Association of Neonatal Nurses. 17 (2017) 84–90. [DOI] [PubMed] [Google Scholar]
- [9].Harlan RE, Song DD, Prenatal morphine treatment and the development of the striatum, Regul Pept 54 (1994) 117–8. [Google Scholar]
- [10].Wu CC, Hung CJ, Shen CH, Chen WY, Chang CY, Pan HC, et al. , Prenatal buprenorphine exposure decreases neurogenesis in rats, Toxicology letters. 225 (2014) 92–101. [DOI] [PubMed] [Google Scholar]
- [11].Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato-Bigbee C, The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination, Dev Neurosci 36 (2014) 409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oei JL, Melhuish E, Uebel H, Azzam N, Breen C, Burns L, et al. , Neonatal Abstinence Syndrome and High School Performance, Pediatrics. 139 (2017) e20162651. [DOI] [PubMed] [Google Scholar]
- [13].Nygaard E, Moe V, Slinning K, Walhovd KB, Longitudinal cognitive development of children born to mothers with opioid and polysubstance use, Pediatric research. 78 (2015) 330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Maguire DJ, Taylor S, Armstrong K, Shaffer-Hudkins E, Germain AM, Brooks SS, et al. , LongTerm Outcomes of Infants with Neonatal Abstinence Syndrome, Neonatal network : NN 35 (2016) 277–86. [DOI] [PubMed] [Google Scholar]
- [15].Konijnenberg C, Melinder A, Executive function in preschool children prenatally exposed to methadone or buprenorphine, Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 21 (2015) 570–85. [DOI] [PubMed] [Google Scholar]
- [16].Kaltenbach K, O’Grady KE, Heil SH, Salisbury AL, Coyle MG, Fischer G, et al. , Prenatal exposure to methadone or buprenorphine: Early childhood developmental outcomes, Drug and alcohol dependence. 185 (2018) 40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bakhireva LN, Lowe J, Garrison LM, Cano S, Leyva Y, Qeadan F, et al. , Role of caregiverreported outcomes in identification of children with prenatal alcohol exposure during the first year of life, Pediatric research May 16 (2018) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bakhireva LN, Lowe JR, Gutierrez HL, Stephen JM, Ethanol, Neurodevelopment, Infant and Child Health (ENRICH) prospective cohort: Study design considerations, Advances in pediatric research. 2 (2015) [Epub only]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL, Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome, Pediatrics. 109 (2002) 815–25. [DOI] [PubMed] [Google Scholar]
- [20].USDTL. Urine Screen: Report Results 2018. [Available from: http://www.usdtl.com/assets/Urine_Cutoffs.pdf. Accessed: 10/01/2018.
- [21].Bakhireva LN, Cano S, Rayburn WF, Savich RD, Leeman L, Anton RF, et al. , Advanced gestational age increases serum carbohydrate-deficient transferrin levels in abstinent pregnant women, Alcohol and alcoholism (Oxford, Oxfordshire). 47 (2012) 683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sobell LC, Sobell MB. Timeline follow-back. Measuring alcohol consumption: Springer; 1992. p. 41–72. [Google Scholar]
- [23].Von Korff M, Saunders K, Ray GT, Boudreau D, Campbell C, Merrill J, et al. , Defacto long-term opioid therapy for non-cancer pain, Clin J Pain 24 (2008) 521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio, TX: The Psychological Corporation 2006. [Google Scholar]
- [25].Abidin RR. Parenting Stress Index. 3rd ed. Lutz, Florida: Psychological Assessment Resources, Inc.; 1995. [Google Scholar]
- [26].Gartstein M, Rothbart M, Infant temperament via the Revised Infant Behavior Questionnaire, Infant Behav Dev. 26 (2003) 64–86. [Google Scholar]
- [27].Dunn W. Infant/toddler sensory profile: User’s manual. San Antonio, TX: Harcourt Assessment Inc; 2002. [Google Scholar]
- [28].Lowe JR, MacLean PC, Duncan AF, Aragón C, Schrader RM, Caprihan A, et al. , Association of maternal interaction with emotional regulation in 4-and 9-month infants during the Still Face Paradigm, Infant Behav Dev. 35 (2012) 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].PASS. PASS 14 Power Analysis and Sample Size Software. Kaysville, Utah, USA: NCSS, LLC; 2015. [Google Scholar]
- [30].Kaltenbach K, Finnegan LP, Neonatal abstinence syndrome, pharmacotherapy and developmental outcome, Neurobehavioral toxicology and teratology. 8 (1986) 353–5. [PubMed] [Google Scholar]
- [31].McGlone L, Mactier H, Infants of opioid-dependent mothers: neurodevelopment at six months, Early human development. 91 (2015) 19–21. [DOI] [PubMed] [Google Scholar]
- [32].Sarfi M, Smith L, Waal H, Sundet JM, Risks and realities: dyadic interaction between 6-month-old infants and their mothers in opioid maintenance treatment, Infant Behav Dev. 34 (2011) 578–89. [DOI] [PubMed] [Google Scholar]
- [33].Sarfi M, Sundet JM, Waal H, Maternal stress and behavioral adaptation in methadone- or buprenorphine-exposed toddlers, Infant Behav Dev. 36 (2013) 707–16. [DOI] [PubMed] [Google Scholar]
- [34].Konijnenberg C, Sarfi M, Melinder A, Mother-child interaction and cognitive development in children prenatally exposed to methadone or buprenorphine, Early human development. 101 (2016) 91–7. [DOI] [PubMed] [Google Scholar]
- [35].Paul JA, Logan BA, Krishnan R, Heller NA, Morrison DG, Pritham UA, et al. , Development of auditory event-related potentials in infants prenatally exposed to methadone, Developmental psychobiology. 56 (2014) 1119–28. [DOI] [PubMed] [Google Scholar]
- [36].Bier JB, Finger AS, Bier BA, Johnson TA, Coyle MG, Growth and developmental outcome of infants with in-utero exposure to methadone vs buprenorphine, Journal of perinatology : official journal of the California Perinatal Association. 35 (2015) 656–9. [DOI] [PubMed] [Google Scholar]
- [37].Whitham JN, Spurrier NJ, Baghurst PA, Weston P, Sawyer MG, Visual evoked potential latencies of three-year-old children prenatally exposed to buprenorphine or methadone compared with non-opioid exposed children: The results of a longitudinal study, Neurotoxicology and teratology. 52 (2015) 17–24. [DOI] [PubMed] [Google Scholar]
- [38].Connery HS, Medication-Assisted Treatment of Opioid Use Disorder: Review of the Evidence and Future Directions, Harv Rev Psychiatry 23 (2015) 63–75. [DOI] [PubMed] [Google Scholar]
- [39].Jansson LM, Velez M, Harrow C, The Opioid Exposed Newborn: Assessment and Pharmacologic Management, Journal of opioid management. 5 (2009) 47–55. [PMC free article] [PubMed] [Google Scholar]
- [40].Bishaw A, Brian G. Poverty: 2014 and 2015: U.S. Census Bureau; 2016. [Available from: https://www.census.gov/content/dam/Census/library/publications/2016/demo/acsbr15-01.pdf.
- [41].Wachman EM, Grossman M, Schiff DM, Philipp BL, Minear S, Hutton E, et al. , Quality improvement initiative to improve inpatient outcomes for Neonatal Abstinence Syndrome, Journal of perinatology : official journal of the California Perinatal Association. 00 (2018) [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.