In a retrospective study of 501 neonates with potential in utero substance exposure, the drug detection performance of a commercially available umbilical cord tissue toxicology test was evaluated against a commercially available gold-standard meconium toxicology test. Drugs detected in paired MEC and UCT samples were often discordant.
According to the 2016 National Survey on Drug Use and Health, nearly 20% of pregnant women aged 15–44 in the United States are estimated to have used alcohol, tobacco, or other illicit drugs during their pregnancy (1). Accurate assessment of substance exposure has implications for both the infant and mother (2–5). The American College of Obstetrics and Gynecology and the American Academy of Pediatrics recommend that screening of substance use in pregnancy be universal to avoid bias and utilize a standardized screening tool (eg, 4Ps or CRAFFT) (5,6).
Suspected substance use in pregnancy is commonly confirmed using toxicology tests on specimens from the infant (7,8). Meconium, the stool produced by the neonate during gestation, has a long window of drug detection and is often regarded as the gold standard test (7–10); however, collections can be challenging and MEC is not always available in sufficient quantities (11–13). Umbilical cord tissue (UCT) has been proposed as an alternative to MEC because it is readily available in large quantities at the time of birth (14–17); however, the window of drug detection in UCT is generally accepted to be shorter than that of MEC (7,9). Most studies assessing the comparability of UCT and MEC relied on small cohorts of paired samples where few subjects tested positive for drugs (15,18–21) or on large cohorts where samples were not paired (17).Larger cohorts with paired samples have been examined (9,14,22). Due to a combination of biochemical differences in drug or metabolite deposition and accumulation in UCT and MEC, cohort size, frequency of drug abuse in the test population, variation in drugs and metabolites included in the test panel and in test methodology, the existing literature is conflicted as to whether UCT is equivalent to MEC for confirming in utero substance exposure, and both tests are used in practice.
To evaluate the equivalence of the UCT and MEC toxicology tests in use at our institution for confirmation of in utero substance exposure, we examined results from a cohort of 501 neonates that had both UCT and MEC toxicology testing performed by a national reference laboratory.
Methods
This retrospective cohort study included a convenience sample of 501 infants cared for at the Monroe Carell Jr Children’s Hospital at Vanderbilt between October 1, 2013 and February 1, 2016, who underwent screening for drug exposure using both MEC and UCT. Subjects were excluded from analysis for a specific drug group if toxicology results were not available in both UCT and MEC. This study was reviewed and approved by the Vanderbilt University Medical Center Institutional Review Board (IRB# 172002, 160434, 150839). The study population includes partial data from 217 subjects that was previously reported (22) and an additional 284 subjects.
Toxicology testing
All provider-ordered toxicology testing was performed by a national reference laboratory (Associated Regional and University Pathologists [ARUP], Salt Lake City, UT) using a combination of immunoassay and chromatography-mass spectrometry techniques (18,23–27). Both UCT and MEC tests were commercially available but were performed using different methodologies and had different limits of drug detection. In addition, the UCT and MEC tests detected different drugs, and for the purposes of this study, drugs that were only available in either the UCT or the MEC panel were excluded from analysis. The drugs analyzed in this study and the limits of detection in UCT and MEC can be found in Table 1.
Table 1.
Drug group analytes with screening limits of detection in meconium (MEC) and umbilical cord tissue (UCT).
| Limit of detection (ng/g) | ||
|---|---|---|
| MEC | UCT | |
| Screen | Screen | |
| Amphetamines | 30 | 8 |
| amphetamine | 30 | 8 |
| methamphetamine | 30 | 8 |
| MDMA | 30 | 8 |
| MDA | 30 | 8 |
| MDEA | 30 | 8 |
| Barbiturates | ||
| butalbital | 75 | 75 |
| phenobarbital | 75 | 75 |
| secobarbital | 75 | 75 |
| Benzodiazepines | ||
| alprazolam | 75 | 5 |
| alpha-OH-alprazolam | 75 | 5 |
| clonazepam | 75 | 5 |
| 7-aminoclonazepam | 75 | 5 |
| diazepam | 75 | 5 |
| desalkylflurazepam | 75 | 10 |
| alpha-OH-ethylflurazepam | 75 | 10 |
| lorazepam | 75 | 5 |
| midazolam | 75 | 5 |
| nordiazepam | 75 | 5 |
| oxazepam | 75 | 5 |
| temazepam | 75 | 5 |
| alpha-OH-triazolam | 75 | 5 |
| Buprenorphine | ||
| buprenorphine | 40 | 1 |
| norbuprenorphine | 40 | 0.5 |
| Cannabinoids | ||
| THC-COOH | 30 | 1501 |
| Cocaine | ||
| cocaine | 30 | 8 |
| benzoylecgonine (BE) | 30 | 8 |
| meta-OH-BE | 30 | 8 |
| Methadone | ||
| methadone | 40 | 10 |
| EDDP | 40 | 10 |
| Other Opioids | ||
| 6-monoacetylmorphine | 30 | 4 |
| codeine | 30 | 6 |
| heroin | 30 | 2 |
| hydrocodone | 30 | 6 |
| hydromorphone | 30 | 4 |
| morphine | 30 | 4 |
| oxycodone | 30 | 4 |
| oxymorphone | 30 | 4 |
| propoxyphene | 75 | 10 |
The limit of detection for this analyte changed to 1 ng/g on 11/16/15.
Data collection
Results for UCT and MEC toxicology testing were retrospectively collected from the laboratory information system (Cerner Millennium). Subject demographics and length of stay were retrospectively collected from the laboratory billing system (McKesson HealthQuest). Additional clinical information was collected through medical record review.
Statistical analyses
We included 38 individual drugs from 8 groups in our analysis (Table 1). MEC results were defined as the gold standard for drug presence and measures of diagnostic utility were calculated for each drug group in UCT. Sensitivity, specificity, agreement, positive predictive value (PPV), negative predictive value (NPV), prevalence, and Cohen κ (κ = po–pe/1–pe, where po is the observed proportionate agreement and pe is the probability of random agreement) were calculated using EP Evaluator software (Data Innovations).
A κ of 100% indicates perfect agreement, and a value of 0% indicates random agreement. For the purposes of this analysis, a κ of ≥75% was deemed acceptable (22). 95% confidence intervals were calculated using the Wilson Score method (EP Evaluator).
Results
A total of 501 subjects were included in this single-center study. The study population was predominantly Caucasian (69.9%). More than 37% of study subjects had a neonatal intensive care unit (NICU) stay with a median length of stay of 15 days. The results of each subject’s UCT and MEC toxicology tests were compared by drug group (Table 1 and Table 2). Although overall prevalence of drugs in UCT and MEC was similar for many drug groups, UCT had slightly higher prevalence of amphetamines, barbiturates, and benzodiazepines. The overall agreement between UCT and MEC ranged from 80–100%. Cohen’s κ, which accounts for agreement due to chance, ranged from 40–88%. Only two drug groups, amphetamines and methadone, had acceptable κ values of ≥75%. The sensitivity of UCT ranged from 41% for cannabinoids (95% CI 33.3 – 49.6%) to 100% for barbiturates (95% CI 34.2 – 100.0%). The sensitivity of UCT for opioids ranged from 53% for other opioids (95% CI 43.9 – 62.3%) to 75% for methadone (95% CI 50.5 – 89.8%; Table 2).
Table 2.
Comparison of paired umbilical cord tissue (UCT) and meconium (MEC) results by drug group.
| Amphetamines | |||||
| 95% CI | |||||
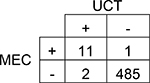 |
Agreement | 99% | 98.2 – 99.8% | Positive Predictive Value | 85% |
| Cohen’s κ | 88% | 73.8 – 101.6% | Negative Predictive Value | 100% | |
| Sensitivity | 92% | 64.6 – 98.5% | Prevalence in UCT | 3% | |
| Specificity | 100% | 98.5 – 99.9% | Prevalence in MEC | 2% | |
| Barbiturates | |||||
| 95% CI | |||||
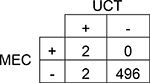 |
Agreement | 100% | 98.6 – 99.9% | Positive Predictive Value | 50% |
| Cohen’s κ | 67% | 20.1 – 112.8% | Negative Predictive Value | 100% | |
| Sensitivity | 100% | 34.2 – 100.0% | Prevalence in UCT | 1% | |
| Specificity | 100% | 98.6 – 99.9% | Prevalence in MEC | 0% | |
| Ben zodiazepines | |||||
| 95% CI | |||||
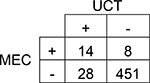 |
Agreement | 93% | 90.2 – 94.8% | Positive Predictive Value | 33% |
| Cohen’s κ | 40% | 21.5 – 59.1% | Negative Predictive Value | 98% | |
| Sensitivity | 64% | 43.0 – 80.3% | Prevalence in UCT | 8% | |
| Specificity | 94% | 91.7 – 95.9% | Prevalence in MEC | 4% | |
| Buprenorphine | |||||
| 95% CI | |||||
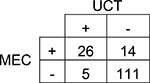 |
Agreement | 88% | 81.8 – 92.1% | Positive Predictive Value | 84% |
| Cohen’s κ | 66% | 51.0 – 80.0% | Negative Predictive Value | 89% | |
| Sensitivity | 65% | 49.5 – 77.9% | Prevalence in UCT | 20% | |
| Specificity | 96% | 90.3 – 98.1% | Prevalence in MEC | 26% | |
| Cannabinoids | |||||
| 95% CI | |||||
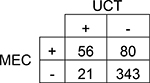 |
Agreement | 80% | 76.1 – 83.1% | Positive Predictive Value | 73% |
| Cohen’s κ | 41% | 30.7 – 51.3% | Negative Predictive Value | 81% | |
| Sensitivity | 41% | 33.3 – 49.6% | Prevalence in UCT | 15% | |
| Specificity | 94% | 91.3 – 96.2% | Prevalence in MEC | 27% | |
| Cocaine | |||||
| 95% CI | |||||
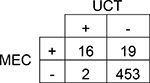 |
Agreement | 96% | 93.5 – 97.2% | Positive Predictive Value | 89% |
| Cohen’s κ | 58% | 40.9 – 75.8% | Negative Predictive Value | 96% | |
| Sensitivity | 46% | 30.5 – 61.8% | Prevalence in UCT | 4% | |
| Specificity | 100% | 98.4 – 99.9% | Prevalence in MEC | 7% | |
| Methadone | |||||
| 95% CI | |||||
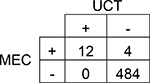 |
Agreement | 99% | 98.0 – 99.7% | Positive Predictive Value | 100% |
| Cohen’s κ | 85% | 71.0 – 99.6% | Negative Predictive Value | 99% | |
| Sensitivity | 75% | 50.5 – 89.8% | Prevalence in UCT | 2% | |
| Specificity | 100% | 99.2 – 100.0% | Prevalence in MEC | 3% | |
| Other Opioids | |||||
| 95% CI | |||||
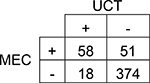 |
Agreement | 86% | 82.9 – 89.0% | Positive Predictive Value | 76% |
| Cohen’s κ | 55% | 44.6 – 64.5% | Negative Predictive Value | 88% | |
| Sensitivity | 53% | 43.9 – 62.3% | Prevalence in UCT | 15% | |
| Specificity | 95% | 92.9 – 97.1% | Prevalence in MEC | 22% | |
Discussion
Our study builds upon several smaller studies which previously used paired samples to assess confirmation of in utero substance exposure using UCT and MEC testing (14,15,18,22). Our results demonstrate that detection of drugs is variable between paired UCT and MEC samples as evidenced by lack of concordance and relatively low κ values (Table 2). Our findings support other studies which demonstrated that paired UCT and MEC results are often discordant (9,14,22). Differences in analytical detection limits of the tests may play a role in some of the discrepancies, as may administration of drugs close to or during labor. In addition, commercially available UCT testing may not include drug metabolites that preferentially accumulate in UCT, thus may contribute to false negative UCT results.
Our study does have limitations that merit mentioning. The drugs detected in study subjects are representative of use patterns observed in our setting; they may not be generalizable to other settings with different drug prevalence. The UCT and MEC tests rely on different but related analytical principles. Both tests underwent improvements during the study period. Because there is no FDA-approved toxicology test for MEC or UCT, each laboratory’s test will be different, and the tests used in this study may not be equivalent to tests performed by other laboratories. Both tests we used are commercially available, offer analytes and detection limits similar to tests offered by other commercial laboratories, and have been used in a number of previous publications (15,17,22). The choice to order MEC or UCT testing should be based on a number of factors, some of which are scientific (e.g. window of detection) and some of which are practical (e.g. specimen availability). Both UCT and MEC have a place in confirmation of in utero substance exposure, provided that those interpreting the results are aware that UCT and MEC may not produce equivalent results.
Acknowledgements:
We thank William O. Cooper, MD, MPH for his contributions to the manuscript
Supported by the National Institute on Drug Abuse of the National Institutes of Health (K23DA038720 [to S.P.]). The content is solely the responsibility of the authors and does not necessarily represent the official views of any funding organizations. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Substance Abuse and Mental Health Services Administration. 2016. National Survey on Drug Use and Health: Detailed Tables. 2017. September 7 [cited 2018 Mar 5]; Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf
- 2.Gunn JKL, Rosales CB, Center KE, Nuñez A, Gibson SJ, Christ C, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016. April;6:e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behnke M, Smith VC, Committee on Substance Abuse and Prevention, Committee on Fetus and Newborn. Prenatal Substance Abuse: Short- and Long-term Effects on the Exposed Fetus. PEDIATRICS. 2013. March 1;131:e1009–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Substance Use During Pregnancy [Internet]. Guttmacher Institute. 2016. [cited 2017 Nov 21]. Available from: https://www.guttmacher.org/statepolicy/explore/substance-use-during-pregnancy
- 5.Patrick SW, Schiff DM, Committee on Substance Abuse and Prevention. A Public Health Response to Opioid Use in Pregnancy. Pediatrics. 2017. March;139:e20164070. [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists. Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. Obstet Gynecol. 2017;130:e81–94. [DOI] [PubMed] [Google Scholar]
- 7.Gray T, Huestis M. Bioanalytical procedures for monitoring in utero drug exposure. Anal Bioanal Chem. 2007. July 5;388:1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wabuyele SL, Colby JM, McMillin GA. Detection of Drug-Exposed Newborns. Ther Drug Monit. 2018;40:166–85. [DOI] [PubMed] [Google Scholar]
- 9.Concheiro M, Lendoiro E, de Castro A, Gónzalez-Colmenero E, Concheiro-Guisan A, Peñas-Silva P, et al. Bioanalysis for cocaine, opiates, methadone, and amphetamines exposure detection during pregnancy: Bioanalysis for cocaine, opiates, methadone and amphetamines exposure detection during pregnancy. Drug Test Anal. 2017. June;9:898–904. [DOI] [PubMed] [Google Scholar]
- 10.Concheiro M, Huestis MA. Drug exposure during pregnancy: analytical methods and toxicological findings. Bioanalysis. 2018. March 21;10:587–606. [DOI] [PubMed] [Google Scholar]
- 11.Gourley GR, Kreamer B, Arend R. Excremental studies in human neonates. Identification of zinc coproporphyrin as a marker for meconium. Gastroenterology. 1990. December;99:1705–9. [DOI] [PubMed] [Google Scholar]
- 12.Verma A, Dhanireddy R. Time of first stool in extremely low birth weight (< or = 1000 grams) infants. J Pediatr. 1993. April;122:626–9. [DOI] [PubMed] [Google Scholar]
- 13.Ostrea EM, Brady M, Gause S, Raymundo AL, Stevens M. Drug screening of newborns by meconium analysis: a large-scale, prospective, epidemiologic study. Pediatrics. 1992. January;89:107–13. [PubMed] [Google Scholar]
- 14.Labardee RM, Swartzwelder JR, Gebhardt KE, Pardi JA, Dawsey AC, Brent Dixon R, et al. Method performance and clinical workflow outcomes associated with meconium and umbilical cord toxicology testing. Clin Biochem. 2017. September;50:1093–7. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery D, Plate C, Alder SC, Jones M, Jones J, Christensen RD. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. J Perinatol Off J Calif Perinat Assoc. 2006. January 1;26:11–4. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery DP, Plate CA, Jones M, Jones J, Rios R, Lambert DK, et al. Using umbilical cord tissue to detect fetal exposure to illicit drugs: a multicentered study in Utah and New Jersey. J Perinatol. 2008;28:750–753. [DOI] [PubMed] [Google Scholar]
- 17.Palmer KL, Wood KE, Krasowski MD. Evaluating a switch from meconium to umbilical cord tissue for newborn drug testing: A retrospective study at an academic medical center. Clin Biochem. 2017. April;50:255–61. [DOI] [PubMed] [Google Scholar]
- 18.Marin SJ, Metcalf A, Krasowski MD, Linert BS, Clark CJ, Strathmann FG, et al. Detection of neonatal drug exposure using umbilical cord tissue and liquid chromatography time-of-flight mass spectrometry. Ther Drug Monit. 2014;36:119–124. [DOI] [PubMed] [Google Scholar]
- 19.de Castro A, Jones HE, Johnson RE, Gray TR, Shakleya DM, Huestis MA. Methadone, cocaine, opiates, and metabolite disposition in umbilical cord and correlations to maternal methadone dose and neonatal outcomes. Ther Drug Monit. 2011. August;33:443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Concheiro M, González-Colmenero E, Lendoiro E, Concheiro-Guisán A, de Castro A, Cruz-Landeira A, et al. Alternative matrices for cocaine, heroin, and methadone in utero drug exposure detection. Ther Drug Monit. 2013;35:502–509. [DOI] [PubMed] [Google Scholar]
- 21.Concheiro M, Jones HE, Johnson RE, Choo R, Shakleya DM, Huestis MA. Umbilical cord monitoring of in utero drug exposure to buprenorphine and correlation with maternal dose and neonatal outcomes. J Anal Toxicol. 2010;34:498–505. [DOI] [PubMed] [Google Scholar]
- 22.Colby JM. Comparison of umbilical cord tissue and meconium for the confirmation of in utero drug exposure. Clin Biochem. 2017. September;50:784–90. [DOI] [PubMed] [Google Scholar]
- 23.Marin SJ, Keith L, Merrell M, McMillin GA. Comparison of Drugs of Abuse Detection in Meconium by EMIT® II and ELISA. J Anal Toxicol. 2009;33:148–154. [DOI] [PubMed] [Google Scholar]
- 24.Marin SJ, Merrell M, McMillin GA. Drugs of abuse detection in meconium: a comparison between ELISA and biochip microarray. J Anal Toxicol. 2011;35:40–45. [DOI] [PubMed] [Google Scholar]
- 25.Chittamma A, Marin SJ, Williams JA, Clark C, McMillin GA. Detection of In Utero Marijuana Exposure by GC-MS, Ultra-Sensitive ELISA and LC-TOF-MS Using Umbilical Cord Tissue. J Anal Toxicol. 2013. September 1;37:391–4. [DOI] [PubMed] [Google Scholar]
- 26.Wood KE, Krasowski MD, Strathmann FG, McMillin GA. Meconium Drug Testing in Multiple Births in the USA. J Anal Toxicol. 2014. September;38:397–403. [DOI] [PubMed] [Google Scholar]
- 27.Coles R, Kushnir MM, Nelson GJ, McMillin GA, Urry FM. Simultaneous determination of codeine, morphine, hydrocodone, hydromorphone, oxycodone, and 6-acetylmorphine in urine, serum, plasma, whole blood, and meconium by LCMS-MS. J Anal Toxicol. 2007. February;31:1–14. [DOI] [PubMed] [Google Scholar]


