Abstract
Objective
To examine the effect of increasing physical therapy staff in a cardiovascular ICU (CVICU) on temporal measures of physical therapy interventions and on outcomes important to patients and hospitals.
Design
Retrospective pre/post subgroup analysis from a quality improvement initiative.
Setting
Academic medical center.
Participants
Cardiovascular patients in either a baseline (N=52) or quality improvement period (N=62) with a CVICU length of stay (LOS) ≥ 7 days and use of any one of the following: mechanical ventilation, continuous renal replacement therapy, or mechanical circulatory support.
Interventions
The six-month quality improvement initiative increased CVICU-dedicated physical therapy staff from two to four.
Main Outcome Measures
Changes in physical therapy delivery were examined using the frequency and daily duration of physical therapy intervention. Post-CVICU LOS was the primary outcome. CVICU LOS, mobility change, and discharge level of care were secondary outcomes. A secondary analysis of hospital survivors was also conducted.
Results
Compared to those in the baseline period, cardiovascular patients in the quality improvement period participated in physical therapy for an additional 9.6 minutes (95% confidence interval [CI]: 1.9, 17.2) per day for all patients and 15.1 minutes (95% CI: 7.6, 22.6) for survivors. Post-CVICU LOS decreased 2.2 (95% CI: −6.0, 1.0) days for all patients and 2.6 days (95% CI: −5.3, 0.0) for survivors. CVICU LOS decreased 3.6 days (95% CI: −6.4, −0.8) for all patients and 3.1 days (95% CI: −6.4, −0.9) for survivors. Differences in mobility change and discharge level of care were not significant.
Conclusions
Additional CVICU-dedicated physical therapy staff was associated with increased physical therapy treatment and reductions in CVICU and post-CVICU LOS. The effects of each were greatest for hospital survivors.
Keywords: Health Services, Administration, Critical Care, Rehabilitation
Growing evidence supports the use of early mobility interventions, most often delivered by a physical therapist, to negate the deleterious effects of immobility associated with an intensive care unit (ICU) stay.1–15 The findings of these studies have been determined primarily from patients admitted to a medical or surgical ICU, a rather heterogeneous group. Such heterogeneity likely contributes to the equivocal results published in recent systematic reviews.16,17 Moreover, these reviews suggest that critically ill cardiovascular patient populations are underrepresented in previous studies. Yet, the treatment effects of early mobility interventions may actually be clearer in patients admitted to a cardiovascular ICU (CVICU) because of their relative homogeneity.
Early mobility is feasible and safe in critically ill populations.11,18,19 However, many barriers contribute to its inconsistent application in practice. These include limited staffing resources, staff culture, and a concern for patient safety due to both tenuous clinical states and a lack of training of clinical staff.20–25 Adequate and consistent staff may address some of these barriers by enhancing expertise and facilitating an increase in appropriate patient-centered mobility interventions.
To address staffing barriers, we implemented a quality improvement (QI) initiative that increased the number of physical therapy (PT) staff dedicated to the cardiovascular ICU (CVICU). The primary aim of this study was to investigate if changes in PT delivery and patient outcomes occurred for patients with prolonged cardiovascular critical illness as a result of the QI initiative. Changes in the frequency and mean daily duration of PT treatment were examined in addition to patient-relevant quality outcomes.
METHODS
This was a retrospective observational study of a patient subgroup admitted to the CVICU in a single academic medical center during a larger clinical quality improvement (QI) initiative. The Institutional Review Board at our institution approved this study under IRB_00084463.
Patient Population
Any patient with a CVICU admission longer than 24 hours occurring at any point during either a baseline (September 8, 2014 through March 8, 2015) or QI period (September 8, 2015 through March 8, 2016) was considered for the overall QI study. For the present study, patients defined as having prolonged critical illness were identified from the larger cohort. Inclusion criteria were CVICU length of stay (LOS) of at least seven days plus use of any one of the following: mechanical ventilation (MV) greater than 24 hours, continuous renal replacement therapy (CRRT), or mechanical circulatory support (MCS). MCS was defined as the use of extracorporeal membrane oxygenation (ECMO) or a temporary external ventricular assistance device (VAD).
Intervention
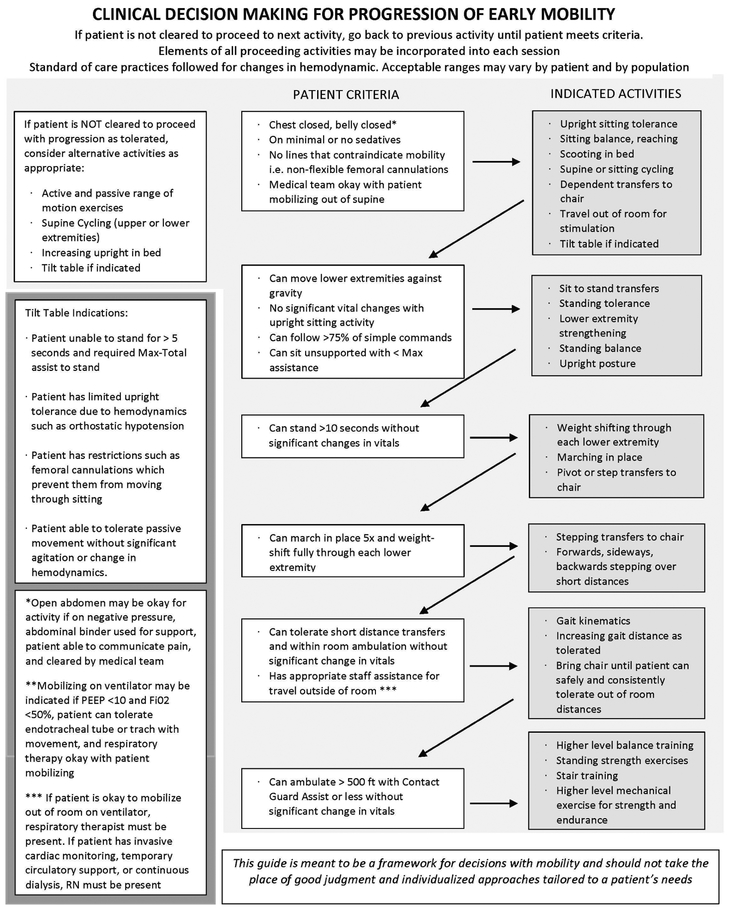
The primary intervention for the QI initiative was to increase the number of physical therapists providing care in the CVICU from two to four and fix these therapists in that unit. This facilitated the presence of two to three therapists in CVICU each day of the week, including weekends. They collectively managed 14–16 patients during their 10-hour day in the 16-bed unit. No specific treatment protocols were established. Rather, therapists were encouraged to use clinical judgment to provide the mode, intensity, and duration of intervention appropriate for each patient. Daily PT treatment, as clinically indicated, was the goal for each patient. Figure 1, developed post-hoc, describes the typical pattern of clinical decision-making. Patients were progressed through mobility activities as quickly as they could tolerate. As exemplified in Figure 2, the increased staff during the QI period gave therapists greater flexibility to maximize patients’ participation in physical activity while considering their prior level of function.
Figure 1:
Clinical decision-making flowsheet representative of physical therapist treatment decisions in the CVICU
Figure 2:
With assistance of a physical therapist and CVICU nurse, an 18-year old male, active in high-level athletics prior to his critical illness, is playing basketball while receiving veno-venous extracorporeal membrane oxygenation. (All those pictured gave written consent to photograph and disseminate the photograph.)
Data Extraction
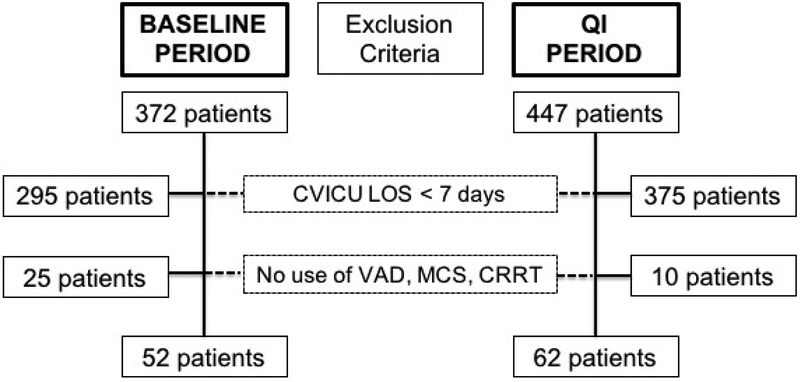
Data were extracted from our health system’s Enterprise Data Warehouse (EDW), which combines administrative and clinical data, for all patients with a CVICU LOS of at least seven days during the time periods of interest. Patient-level data pertaining to the utilization of MV, MCS, or CRRT is not available from the EDW. Therefore, cases were matched using data from a manually maintained ICU database, described elsewhere.26 This final dataset was used to determine the cohort of patients with prolonged cardiovascular critical illness, as summarized in Figure 3.
Figure 3:
Cohort flow diagram for patients admitted to CVICU during baseline or QI period
Assessment and Outcome Measures
Group assignment—whether in the baseline or QI period—was the primary predictor variable for all analyses. Other variables included age; sex; body mass index (BMI); use of MV, MCS, or CRRT; the duration of MV; and indicators of comorbidity burden and diagnostic severity, including the Acute Physiology and Chronic Health Evaluation version two (APACHE II)27, Charlson Comorbidity Index (CCI)28, Medicare Severity Diagnosis Related Group (MS-DRG) weight, and the patient’s initial physical function score as measured by the Activity Measure for Post-acute Care (AM-PAC)29,30
Temporal measures of PT interventions—frequency and mean daily treatment duration—were compared between groups in order to examine the extent to which increased staffing contributed to a change in the delivery of PT in the CVICU during the QI period. PT treatment frequency was calculated as the total number of a patient’s PT treatment sessions (as indicated by the number of unique treatment notes in the EDW) while in the CVICU divided by his or her CVICU LOS, in days. The duration of each unique PT treatment session was identified from the EDW and the mean PT treatment duration per day was calculated for each patient. We also observed whether adverse events were recorded in association with any PT treatment session.
The primary quality outcome was post-CVICU LOS, calculated as the duration spent in a non-ICU hospital ward following the patient’s final transfer out of the CVICU. Secondary outcomes included CVICU LOS—calculated as total days in the CVICU during the hospital visit—change in patient function, and discharge level of care.
Physical function was assessed using the AM-PAC inpatient basic mobility short form (AM-PAC-Mobility), a clinician-scored instrument previously validated for use among hospitalized patients.30 All scores were converted to t-scores.31 Higher scores indicate greater functional independence. The change between initial and final scores was calculated for the CVICU and hospital admission periods separately. For both calculations, the first AM-PAC-Mobility score recorded while the patient was in CVICU was used as the initial score. The last score recorded while in CVICU and the last score recorded prior to hospital discharge were used to assess mobility change in the CVICU and in the hospital, respectively.
Hospital discharge disposition was dichotomized (high vs low) based on the care requirement in the post-acute setting. Discharge to a skilled nursing facility (SNF), long-term acute care hospital (LTACH), or to another acute care hospital was considered a discharge to higher level of care. Since such dispositions are associated with stagnant or declining function, patients who died during their hospital admission were also categorized in this group. Discharge to home—with or without home health services—or to an acute rehabilitation facility were considered a discharge to lower level of care.
Data Analysis
Patient characteristics were described using means (standard deviation [SD]), medians (interquartile range [IQR]), or proportions. Continuous characteristics were compared using an independent samples t-test or a Wilcoxon-Mann-Whitney test. Categorical characteristics were compared using chi-square tests. For all analyses, group assignment was the primary independent variable. For each outcome, all patient characteristic variables were included as covariates in an initial regression model of the appropriate type. Backward variable selection with a conservative significance threshold (p=0.20) was used to identify meaningful predictors, which were retained to derive a final statistical model.32
To compare PT treatment frequency and mean daily duration between groups, we performed multiple linear regression. The adjusted association between group and post-CVICU LOS, was tested using generalized gamma regression, which is a generalized linear model with a log link and gamma family.33
CVICU LOS data was also modeled using gamma regression. Multiple linear regression was used to test the association between group and change in AM-PAC-Mobility. Discharge level of care was analyzed using multiple logistic regression. Since the study population included those patients with prolonged critical illness, we conducted secondary analyses for each outcome including only patients that survived their hospitalization. All analyses were completed using Stata version 14.1 (StataCorp. College Station, Texas, USA).
RESULTS
A total of 114 cardiovascular patients (52 in the baseline period and 62 in the QI period) met the criteria for prolonged critical illness. The sample included 93 patients (81.6%) admitted to the cardiac surgery service. Other patient characteristics and clinical markers, shown in Table 1, were similar between groups with the exception of the CCI; the mean comorbidity burden was higher among patients in the baseline group. Table 2 summarizes the adjusted outcomes of interest for the primary analysis.
Table 1.
Patient demographic and clinical characteristics
| Variable | Primary Analysis (All Patients) | Secondary Analysis (Survivors) | ||||
|---|---|---|---|---|---|---|
| Baseline | Ql | P | Baseline | Ql | P | |
| Total sample size | 52 | 62 | - | 43 | 48 | - |
| Male (n / %) | 37 / 71.2 | 44 / 71.0 | 0.98 | 33 / 76.7 | 34 / 70.8 | 0.52 |
| Age, years (mean, SD) | 56.5, 14.9 | 59.1, 16.5 | 0.40 | 56.5, 14.3 | 56.6, 13.8 | 0.96 |
| First AM-PAC t-score (median [IQR]) | 23.6 [23.6, 29.6] | 23.6 [23.6, 28.6] | 0.33 | 23.6 [23.6, 30.6] | 23.6 [23.6, 28.6] | 0.42 |
| APACHE II score (mean, SD) | 19.7, 7.3 | 18.3, 5.2 | 0.26 | 18.7, 6.6 | 17.5, 5.3 | 0.32 |
| Charlson comorbidity index (median [IQR]) | 5 [3, 7] | 1 [0, 4] | <0.01 | 5 [3, 7] | 1 [0, 3] | <0.01 |
| BMI, kg/m2 (mean,SD) | 29.1, 6.4 | 31.0, 7.6 | 0.15 | 29.1, 5.8 | 31.2, 8.3 | 0.17 |
| MS-DRG weight (median [IQR]) | 13.1 [7.7, 25.4] | 15.3 [7.4, 26.2] | 0.26 | 9.5 [7.7, 25.4] | 16.2 [7.4, 26.2] | 0.20 |
| Markers of critical illness (n / %) | ||||||
| Mechanical ventilator >24 hours | 46 / 88.5 | 56 / 90.3 | 0.75 | 37 / 86.1 | 43 / 89.6 | 0.61 |
| Hours on mechanical ventilator (median [IQR]) | 175.4 [72.2, 317.7] | 153.0 [63.5, 299.5] | 0.59 | 111.2 [65.2, 215.6] | 110.0 [47.9, 205.3] | 0.43 |
| MCS* for any time | 30 / 57.7 | 26 / 41.9 | 0.09 | 25 / 58.1 | 19 / 39.6 | 0.08 |
| CRRT for anytime | 10 / 19.2 | 22 / 35.5 | 0.05 | 6 / 14.0 | 12 / 25.0 | 0.19 |
Includes ECMO or temporary VAD
Table 2.
Unadjusted and adjusted outcomes for the primary analysis (all patients), grouped by time period
| Outcome | Unadjusted | Adjusted (Regression coefficient [95% CI]) | ||
|---|---|---|---|---|
| Baseline Period | Ql Period | Baseline Period | Ql Period | |
| Mean daily treatment time on days of treatment in ICU, minutes (mean, SD) | 51.7, 12.9 | 59.4, 25.5 | REF | 9.56 [1.90, 17.22] |
| Frequency of PT treatment (Total treatments per ICU day) (mean, SD) | 0.59, 0.21 | 0.76, 0.35 | REF | 0.16 [0.06, 0.27] |
| CVICU length of stay, days (median [IQR]) | 14.8 [10.5, 21.8] | 11.4 [8.6, 20.1] | REF | −3.60 [−6.36, −0.84] |
| Post-CVICU hospital length of stay, days (median [IQR]) | 5.0 [0.0, 7.7] | 2.0 [0.0, 6.5] | REF | −2.21 [−6.03, 1.60] |
| AM-PAC change in the ICU, t-score (mean, SD) | 0.8, 7.6 | 2.8, 6.6 | REF | 0.89 [−1.10, 2.89] |
| Overall AM-PAC change, t-score (mean, SD) | 6.5, 12.5 | 5.3, 9.5 | REF | -3.10 [−7.32, 1.12] |
| Unadjusted (n / %) | Adjusted (Odds ratio [95% Cl]) | |||
| Discharge to lower level of care* | 28 / 53.9 | 33 / 53.2 | REF | 1.32 [0.58, 3.04] |
Discharge settings associated with a lower level of care include an acute rehabilitation facility, home with home health services, or home without services.
Physical Therapy Delivery
The mean (±SD) daily PT treatment duration increased for each patient from 51.7 (±12.9) minutes in the baseline period to 59.4 (±25.5) minutes in the QI period. The adjusted mean difference (95% CI) was 9.6 (1.9, 17.2) additional minutes of PT per day in the QI period relative to the baseline period. The covariates in the final model included the patient’s age and use of CRRT.
Similarly, mean PT treatment frequency (SD) in CVICU increased for each patient from 0.59 (±0.21) to 0.76 (±0.35) treatments per ICU day. APACHE II scores were a significant covariate. Holding them constant, the mean difference (95% CI) was 0.16 (0.06, 0.27) more treatments per ICU day per patient. Together, the per-patient increase in both treatment frequency and daily duration accounted for an increase in the total treatment time from 39,730 minutes in the baseline period to 69,862 minutes in the QI period. There were no adverse events recorded in association with PT treatment in either time period.
Length of Stay
The median (IQR) post-CVICU LOS in the baseline period was 5.0 (0.0, 7.7) days compared to 2.0 (0.0, 6.5) days in the QI period. The final model included adjustment for age, duration of MV, and the use of CRRT. CCI, though different between groups, was not a significant covariate in this model so was dropped. The adjusted change in post-CVICU LOS was a decrease of 2.2 (95% CI: −6.0, 1.6) days in the QI period. A high proportion of patients in the QI period were discharged from the hospital directly from CVICU (43.5% compared to 28.8% in the baseline period) likely influencing the observed post-CVICU LOS. Since discharging patients earlier, but to a setting higher level of care could appreciably bias this finding, we conducted a post-hoc analysis to determine the discharge disposition for these patients. The results of the post-hoc analysis are included with the discussion of discharge level of care below.
The median (IQR) LOS in the CVICU during the baseline period was 14.8 (10.5, 21.8) days and decreased to 11.4 (8.6, 20.1) days in the QI period. After adjusting for MS-DRG weight, total time on MV, and use of CRRT, the adjusted difference (95% CI) was a decrease of 3.6 (−6.4, −0.8) days spent in the CVICU in the QI period.
Change in Physical Function
There were non-significant differences observed in physical function change between the baseline and QI period, for both the CVICU and overall hospital stay. The mean change in AM-PAC-Mobility in the CVICU was 2.0 points greater in the QI period (2.8 ± 6.6) compared to the baseline period (0.8 ± 7.6). However, after adjusting for the patient’s age, sex, initial AM-PAC-Mobility score, CCI, and use of CRRT—all significant covariates in the initial model—the mean difference was improvement of only 0.9 (95% CI: −1.1, 2.9) more points in the QI period. For the entire hospital stay, the observed change in physical function was greater in the baseline period (6.5 ± 12.5) than in the QI period (5.3 ± 9.5) in unadjusted analyses. Sex, age, initial AM-PAC-Mobility score, APACHE II, CCI, use of MV, and use of CRRT were the covariates in the final model. Holding them constant, the mean difference (95% CI) in AM-PAC-Mobility change in the hospital was a decrease of 3.10 (−7.32, 1.12) more points in the QI period compared to the baseline period.
Discharge Level of Care
An equal proportion of patients were discharged from the hospital to a lower level of care in the QI period (53.2%) as in the baseline period (53.9%). The adjusted odds ratio (95% CI) for being discharged to a lower level of care in the QI period compared to the baseline period was 1.32 (0.58, 3.04). For this analysis, age, APACHE II, time on MV, and use of CRRT were included as covariates.
A post-hoc analysis was conducted to determine the discharge disposition location for 15 out of 52 patients (28.8%) in the baseline period and 27 out of 62 patients (43.5%) in the QI period who were discharged out of the hospital directly from the CVICU (see Supplemental Table 1). Chi-square analysis showed that, for all patients (p=0.90) and for survivors only (p=1.00) discharged directly from CVICU, there was no difference in discharge disposition proportion between the baseline and QI periods.
Analysis of Survivors Only
The effect of the QI initiative on each outcome for those patients that survived their hospitalization was examined in secondary analyses. These findings are summarized in Table 3. For each patient in this population, the adjusted mean (95% CI) PT duration per day increased by 15.1 (7.6, 22.6) minutes while the frequency of PT treatment increased by 0.20 (0.1, 0.3) treatments per ICU day. The adjusted mean post-CVICU LOS decreased by 2.6 (95% CI: −5.3, 0) days in the QI period. Additionally, the adjusted mean difference in CVICU LOS was 3.1 (95% CI: −6.4, −0.9) fewer days in the QI period while patients improved their physical function in the CVICU by 1.9 (95% CI: 0.1, 3.8) greater points on the AM-PAC-Mobility.
Table 3.
Unadjusted and adjusted outcomes for the secondary analysis (survivors), grouped by time period
| Outcome | Unadjusted | Adjusted (Regression coefficient [95% CI]) | ||
|---|---|---|---|---|
| Baseline Period | Ql Period | Baseline Period | Ql Period | |
| Mean daily treatment time on days of treatment in ICU, minutes (mean, SD) | 53.6, 11.9 | 67.4, 22.7 | REF | 15.10; 7.64, 22.56 |
| Frequency of PT treatment (Total treatments per ICU day) (mean, SD) | 0.62, 0.21 | 0.85, 0.33 | REF | 0.20; 0.08, 0.32 |
| CVICU length of stay, days (median [IQR]) | 13.7 [9.6, 20.6] | 11.0 [8.5, 19.8] | REF | −3.08; −6.36, −0.883 |
| Post-CVICU hospital length of stay, days (median [IQR]) | 5.3 [4.0, 8.7] | 3.2 [0, 7.3] | REF | −2.64; −5.26, −0.01 |
| AM-PAC change in the ICU, t-score (mean, SD) | 1.1, 8.2 | 4.1, 6.6 | REF | 1.95; 0.11, 3.79 |
| Overall AM-PAC change, t-score (mean, SD) | 8.0, 13.1 | 7.2, 9.8 | REF | −4.59, −9.39, 0.22 |
| Unadjusted (n / %) | Adjusted (Odds ratio [95% Cl]) | |||
| Discharge to lower level of care* | 28 / 65.1 | 33 / 68.8 | REF | 1.31; 0.53, 3.23 |
Note:
Discharge settings associated with a lower level of care include an inpatient rehabilitation facility, home with home health services, or home without services.
DISCUSSION
This observational study examined, for a homogenous sample of patients admitted to the CVICU, whether increased PT staff contributed to more frequent and longer PT treatment and improved patient outcomes. Our findings indicate that an increase in PT staff during the QI initiative did contribute to an increase in PT treatment frequency and daily duration that had no effect on patient safety beyond interventions associated with usual care. These changes were associated with a decrease in post-CVICU LOS that was not statistically significant. For survivors only, the magnitude of these differences was greater and showed statistical significance for all three outcomes. Considering that increased PT treatment frequency and duration during the QI period was greatest among survivors, this could indicate a relationship between an increased volume of PT interventions and shorter hospital LOS for patients surviving prolonged cardiovascular critical illness. Well-designed prospective studies with this patient population are necessary to better examine this relationship.
Increasing CVICU-dedicated PT staff is consistent with literature describing quality care in two important ways. First, it has been shown that increased volume in a particular intervention is associated with greater expertise in that intervention and better patient outcomes.34,35 Second, Hodgson and colleagues22 note that adequate resources and dedicated staffing facilitate increased implementation of early mobility interventions.
A fortuitous benefit associated with an increase in PT staffing, of interest to both patients and hospitals, included a shorter CVICU LOS during the QI initiative, despite patients’ characteristics suggesting equivalent severity of illness between groups. Again, a greater magnitude of differences was observed when analyzing survivors only. Given the complex medical nature of the critical illness of these patients, it was surprising to see this independent association whereas the CVICU LOS requirement of these patients is typically driven by their medical need.
As an outcome important to patients, change in physical function during both the CVICU stay alone and the overall hospital stay was not statistically or, based on the minimal detectable change for the AM-PAC-Mobility (4.72 points)30, clinically different between the groups in the primary analysis. However, again among survivors, greater functional change in the CVICU was observed in the QI period relative to the baseline period, but the adjusted difference of 1.95 points may not be clinically relevant. One explanation for this may be due to limited sensitivity of the AM-PAC-Mobility to capture small, but meaningful, functional change in a critically ill population. The fact that 43.5% of the patients in the QI period discharged from the hospital directly from the CVICU may partially explain why those in the QI period had smaller improvement in physical function during hospitalization compared to those in the baseline period. Other potential factors contributing to this finding warrant exploration.
These findings have cost implications that can be estimated with the assumption that additional average costs to implement this intervention are $120,000 per therapist annually, depending on the particular market. These costs may be offset by reductions in both CVICU and post-CVICU LOS observed in this analysis. Kahn and colleagues36 note that changes in hospital LOS affect only marginal direct-variable costs. Further, they state that reducing the ICU LOS but not the hospital LOS overall does not significantly decrease overall hospital costs. From their study, they estimated that the marginal direct-variable cost of an ICU day was $649 and a non-ICU hospital day was $531. Thus, the reduction of CVICU LOS by 3.4 days observed in our study would equate to CVICU cost-savings of $136,809 over six months, or $273,618 annually. For this sample, the reduction of post-CVICU LOS by 3.0 days would equate to $98,766 over six months, or $197,532 annually. Combined, the annual savings would be $471,150 for the 62 patients included.
Additional savings may come from other sources. First, these 62 patients represent only 13.8% of the 447 treated by CVICU physical therapists during the QI period. Any LOS reduction for those patients not included in this analysis will further contribute to savings. Second, the costs over the entire episode of care may be decreased if discharge disposition was shifted to less costly settings. While there was no difference observed in discharge disposition in this sample of patients, modifying disposition was not a focus of the intervention. The observed decrease in hospital LOS and improvement in physical function, however, indicate that it may be possible to consider such modification. Formal cost-effectiveness study methods should be used to determine the reality of these potential implications.
Limitations
As an observational study, limitations to these findings should be considered. First, the QI initiative and present study pertain to a single ICU in an academic medical center so generalizability of the methods and findings is limited. Generalizability of the findings may be further limited by an underrepresentation of women in the overall sample given that cardiovascular disease risk and response to exercise are known to vary by sex.37,38 Second, despite the relative consistency in clinical decision-making by the expanded PT staff, there was no standardized change in the delivery of PT interventions in the QI period. Thus, the ability to test the relationship between PT delivery and the outcomes of interest is limited to what was observed regarding PT treatment duration and frequency. Third, occupational therapy interventions and nurse-led mobility interventions were not measured during the QI period, so their effect on the observed outcomes is not clear. Other potentially important factors, not accounted for in these analyses, may have also contributed to the observed effects.
CONCLUSIONS
This study provides preliminary evidence that increasing PT staff in a CVICU increases the volume of PT treatment for cardiovascular patients with prolonged critical illness. Doing so may facilitate shorter CVICU and post-CVICU stays and improved physical function, particularly for those patients that survive their critical illness. These are positive short-term outcomes for both the patient and the hospital that should be confirmed in similar, larger patient populations. Determining the cost implications of the intervention and the long-term patient outcomes associated with similar interventions should be considered in future research.
Supplementary Material
Supplemental Table 1.Post-hoc analysis of discharge disposition location among patients discharged directly from CVICU
Acknowledgements:
In addition to authors B. Lohse and H.A. Bento, Jennifer Chung-Peck and Jennifer Underdown delivered the physical therapy interventions described in this report and were instrumental to the execution of the QI project. We offer appreciation to the staff of the SICU/CVICU Surgery Database for their assistance with data collection.
Role of Funding Sources:
This work was supported, in part, by the Foundation for Physical Therapy and the University of Utah Population Health Research (PHR) Foundation, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067–05 (formerly 8UL1TR000105 and UL1RR025764). Neither funding agency played any role in the conduct or report of this study.
List of Abbreviations
- AM-PAC
Activity Measure for Post-acute Care
- APACHE
Acute Physiology and Chronic Health Evaluation
- BMI
body mass index
- CCI
Charlson comorbidity index
- CRRT
continuous renal replacement therapy
- CVICU
Cardiovascular Intensive Care Unit
- ECMO
extracorporeal membrane oxygenation
- EDW
enterprise data warehouse
- ICU
intensive care unit
- LOS
length of stay
- LTACH
long-term acute care hospital
- MCS
mechanical circulatory support
- MS-DRG
Medicare Severity Diagnosis Related Group
- MV
mechanical ventilation
- PT
physical therapy
- SNF
skilled nursing facility
- QI
quality improvement
- VAD
ventricular assistance device
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior presentation:
This work was presented, in part, at the 2017 Combined Sections Meeting of the American Physical Therapy Association, February 17, 2017, San Antonio, TX.
Disclosures:
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Cameron S, Ball I, Cepinskas G, et al. Early mobilization in the critical care unit: A review of adult and pediatric literature. J Crit Care. 2015;30(4):664–672. [DOI] [PubMed] [Google Scholar]
- 2.Fan E Critical illness neuromyopathy and the role of physical therapy and rehabilitation in critically ill patients. Respir Care. 2012;57(6):933–944; discussion 944–936. [DOI] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. [DOI] [PubMed] [Google Scholar]
- 4.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med. 2013;41(6):1543–1554. [DOI] [PubMed] [Google Scholar]
- 5.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370(17):1626–1635. [DOI] [PubMed] [Google Scholar]
- 6.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor ED, Walsham J. Should we mobilise critically ill patients? A review. Crit Care Resusc. 2009;11(4):290–300. [PubMed] [Google Scholar]
- 9.Hodgson CL, Berney S, Harrold M, Saxena M, Bellomo R. Clinical review: early patient mobilization in the ICU. Crit Care. 2013;17(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zomorodi M, Topley D, McAnaw M. Developing a mobility protocol for early mobilization of patients in a surgical/trauma ICU. Crit Care Res Pract. 2012;2012:964547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brummel NE, Girard TD, Ely EW, et al. Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the Activity and Cognitive Therapy in ICU (ACT-ICU) trial. Intensive Care Med. 2014;40(3):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashem MD, Parker AM, Needham DM. Early Mobilization and Rehabilitation of Patients Who Are Critically Ill. Chest. 2016;150(3):722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopkins RO, Mitchell L, Thomsen GE, Schafer M, Link M, Brown SM. Implementing a Mobility Program to Minimize Post-Intensive Care Syndrome. AACN Adv Crit Care. 2016;27(2):187–203. [DOI] [PubMed] [Google Scholar]
- 14.Joseph B, Jehan FS. The Mobility and Impact of Frailty in the Intensive Care Unit. Surg Clin North Am. 2017;97(6):1199–1213. [DOI] [PubMed] [Google Scholar]
- 15.Sigler M, Nugent K, Alalawi R, et al. Making of a Successful Early Mobilization Program for a Medical Intensive Care Unit. South Med J. 2016;109(6):342–345. [DOI] [PubMed] [Google Scholar]
- 16.Castro-Avila AC, Seron P, Fan E, Gaete M, Mickan S. Effect of Early Rehabilitation during Intensive Care Unit Stay on Functional Status: Systematic Review and Meta-Analysis. PLoS One. 2015;10(7):e0130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43(2):171–183. [DOI] [PubMed] [Google Scholar]
- 18.Hodgson CL, Stiller K, Needham DM, et al. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care. 2014;18(6):658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sricharoenchai T, Parker AM, Zanni JM, Nelliot A, Dinglas VD, Needham DM. Safety of physical therapy interventions in critically ill patients: a single-center prospective evaluation of 1110 intensive care unit admissions. J Crit Care. 2014;29(3):395–400. [DOI] [PubMed] [Google Scholar]
- 20.Costa DK, White MR, Ginier E, et al. Identifying Barriers to Delivering the Awakening and Breathing Coordination, Delirium, and Early Exercise/Mobility Bundle to Minimize Adverse Outcomes for Mechanically Ventilated Patients: A Systematic Review. Chest. 2017;152(2):304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgin KE, Nordon-Craft A, McFann KK, Mealer ML, Moss M. Physical therapy utilization in intensive care units: results from a national survey. Crit Care Med. 2009;37(2):561–566; quiz 566–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodgson CL, Capell E, Tipping CJ. Early Mobilization of Patients in Intensive Care: Organization, Communication and Safety Factors that Influence Translation into Clinical Practice. Crit Care. 2018;22(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94(4):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malone D, Ridgeway K, Nordon-Craft A, Moss P, Schenkman M, Moss M. Physical Therapist Practice in the Intensive Care Unit: Results of a National Survey. Phys Ther. 2015;95(10):1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koo KK, Choong K, Cook DJ, et al. Early mobilization of critically ill adults: a survey of knowledge, perceptions and practices of Canadian physicians and physiotherapists. CMAJ Open. 2016;4(3):E448–E454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lonardo NW, Mone MC, Nirula R, et al. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. Am J Respir Crit Care Med. 2014;189(11):1383–1394. [DOI] [PubMed] [Google Scholar]
- 27.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 29.Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):I49–61. [DOI] [PubMed] [Google Scholar]
- 30.Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379–391. [DOI] [PubMed] [Google Scholar]
- 31.Jette AM, Tao W, Norweg A, Haley S. Interpreting rehabilitation outcome measurements. J Rehabil Med. 2007;39(8):585–590. [DOI] [PubMed] [Google Scholar]
- 32.Budtz-Jorgensen E, Keiding N, Grandjean P, Weihe P. Confounder selection in environmental epidemiology: assessment of health effects of prenatal mercury exposure. Ann Epidemiol. 2007;17(1):27–35. [DOI] [PubMed] [Google Scholar]
- 33.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data In. Harris School Working Paper Series.: University of Chicago; 2003. [DOI] [PubMed] [Google Scholar]
- 34.Hayanga JW, Lira A, Aboagye JK, Hayanga HK, D’Cunha J. Extracorporeal membrane oxygenation as a bridge to lung transplantation: what lessons might we learn from volume and expertise? Interact Cardiovasc Thorac Surg. 2016;22(4):406–410. [DOI] [PubMed] [Google Scholar]
- 35.Joynt KE, Orav EJ, Jha AK. Physician volume, specialty, and outcomes of care for patients with heart failure. Circ Heart Fail. 2013;6(5):890–897. [DOI] [PubMed] [Google Scholar]
- 36.Kahn JM, Rubenfeld GD, Rohrbach J, Fuchs BD. Cost savings attributable to reductions in intensive care unit length of stay for mechanically ventilated patients. Med Care. 2008;46(12):1226–1233. [DOI] [PubMed] [Google Scholar]
- 37.Leening MJ, Ferket BS, Steyerberg EW, et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ. 2014;349:g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheatley CM, Snyder EM, Johnson BD, Olson TP. Sex differences in cardiovascular function during submaximal exercise in humans. Springerplus. 2014;3:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1.Post-hoc analysis of discharge disposition location among patients discharged directly from CVICU