Abstract
To determine whether conditional depletion of Progesterone Receptor Membrane Component (PGRMC) 1 and PGRMC2 affected ovarian follicle development, follicle distribution was assessed in ovaries of young (≈ 3 month-old) and middle-aged (≈6 month-old) control (Pgrmc1/2fl/fl) and double conditional PGRMC1/2 knockout (Pgrmc1/2d/d) mice. This study revealed that the distribution of primary, preantral and antral follicles was not altered in Pgrmc1/2d/d mice, regardless of the age. Although the number of primordial follicles was similar at ≈ 3 months of age, their numbers were reduced by ≈ 80% in 6-month old Pgrmc1/2d/d mice compared to age-matched Pgrmc1/2fl/fl mice. The Pgrmc1/2d/d mice were generated using Pgr-cre mice, so ablation of Pgrmc1 and Pgrmc2 in the ovary was restricted to peri-ovulatory follicles and subsequent corpora lutea (CL). In addition, the vascularization of CL was attenuated in Pgrmc1/2d/d mice, although mRNA levels of Vascular Endothelial Growth Factor A (Vegfa) were elevated. Moreover, depletion of Pgrmc1 and Pgrmc2 altered the gene expression profile in the non-luteal component of the ovary such that Vegfa expression, a stimulator of primordial follicle growth, was elevated; Kit Ligand expression, another stimulator of primordial follicle growth, was suppressed and Anti-Mullerian Hormone, an inhibitor of primordial follicle growth, was enhanced compared to Pgrmc1/2fl/fl mice. These data reveal that luteal cell depletion of Pgrmc1 and 2 alters the expression of growth factors within the non-luteal component of the ovary, which could account for the premature demise of the adult population of primordial follicles.
Keywords: Premature ovarian failure, Aging, Corpus luteum, Follicular development, Ovary
Introduction
Female fertility depends on primordial follicles developing into preovulatory follicles that are capable of ovulating fertilizable oocytes. While some primordial follicles transition into growing primary follicles, most remain in a non-growing state for long periods of time (Kerr et al. 2013, Monniaux et al. 2014). Whether through growth or atresia, the number of primordial follicles gradually decreases with age. When their numbers become limiting, follicular development does not proceed to the ovulatory stage, menstrual cycles become irregular and eventually cease, and women enter menopause (Kerr et al. 2013, Monniaux et al. 2014). Typically, women enter menopause around 52 years of age. However, in about 1% of women, primordial follicles are reduced prematurely and these women enter menopause before 40 years of age due to primary ovarian insufficiency (POI) (Maclaran & Panay 2015, Daan et al. 2016).
Understanding the mechanism that results in POI therefore centers around the factors that control the growth and survival of the primordial follicles. One aspect of this mechanism relates to when primordial follicles are formed. The work from the Capel lab (Mork et al. 2012) reveals that pregranulosa cells derived from the surface epithelium of the developing gonads of a female fetus on embryonic day 11.5 interact with germ cells to form primordial follicles that ultimately move toward the center of the developing ovary. Primordial follicles that form during early embryonic development are often referred to as first wave follicles, because they grow and account for the follicles that develop throughout the neonatal and prepubertal periods. These follicles undergo atresia and form the interstitial component of the ovary. Importantly in mice, these first wave primordial follicles are virtually depleted by 3 months of age (Zheng et al. 2014a, Zheng et al. 2014b). However, there exists another population of primordial follicles that are formed around the time of birth that are also derived from surface epithelial cells (Mork et al. 2012). This second wave of primordial follicles remain within the ovarian cortex and this population is often referred as to the adult population of primordial follicles. The concept of two types of primordial follicles has been confirmed and expanded by a series of elegant studies published by Zheng et al (Zheng et al. 2014a, Zheng et al. 2014b). Thus, the adult population of primordial follicles gives rise to all the follicles that develop throughout the reproductive lifespan.
How the fate of the primordial follicles within the adult ovary is controlled remains somewhat of a mystery but likely involves changes in the hormonal/growth factor environment within the ovary. It is likely that the ovarian environment is influenced in part by genetic alterations in that mutations and/or changes in the expression of numerous genes have been proposed to promote POI (Pelosi et al. 2015a). Some of these genes are encoded by sequences within the X-chromosome, (Pelosi et al. 2015a, Pelosi et al. 2015b). One of these genes is Progesterone Receptor Membrane Component 1 (Pgrmc1) (Mansouri et al. 2008, Qin et al. 2015). Interestingly, progesterone (P4) signaling through PGRMC1 inhibits the formation of primordial follicles in cultured neonatal mouse ovaries without reducing the total number of oocytes (Guo et al. 2016). In addition, deletion mutations of Pgrmc1 have been detected in women with POI. A point mutation in Pgrmc1 has also been observed in women with POI which converts the histidine residue at amino acid 165 to arginine and this renders PGRMC1 less effective in mediating P4’s anti-apoptotic action (Mansouri et al. 2008). A second point mutation in Pgrmc1 was observed in Chinese women but this point mutation did not alter the structure of PGRMC1 (Wang et al. 2014). In addition, decreased expression of PGRMC2 has been observed in the granulosa cells of young women (< 35 years of age) with diminished ovarian reserve (DOR) compared to age matched controls (Skiadas et al. 2012).
The decreased expression PGRMC1 and PGRMC2 in some women with POI and DOR, respectively, suggests that these two proteins play a role in regulating the reproductive lifespan of women. To address this issue, we developed several unique transgenic mouse models in which Progesterone Receptor (Pgr)-cre driver mice were used to conditionally deplete Pgrmc1 and/or Pgrmc2 (Clark et al. 2017). Pgrmc1d/d, Pgrmc2d/d and Pgrmc1/2d/d mice experience a reduction in fertility and premature reproductive senescence. Each group of mutant mice had a 50% decrease in litter size by the third or fourth parity (i.e., 4–5 months of age) with litter sizes declining more rapidly than in control mice with increasing age (Clark et al. 2017). The progressive decline in fertility was hypothesized to be due to changes in the uterus due to the presence of endometrial glandular cysts, but changes in ovarian function were not explored.
To determine whether the ovary played a role in the premature loss in fertility of the Pgrmc1/2d/d mice, the present study was designed to assess age-related changes follicle distribution. This study demonstrated that an ≈ 80% decrease in the number of primordial follicles occurred between 3 and 6 months of age, indicating that PGRMC1/2 plays an essential role in maintaining the “second wave” or adult population of primordial follicles. Importantly, the loss of the primordial follicle population starts shortly after puberty, which coincides with the initiation of estrous cycles and the formation of CL. Because Pgr-cre expression in the ovary is restricted to the CL (Soyal et al. 2005), these findings suggested that the adult population of primordial follicles is maintained by PGRMC1/2-directed signals originating from the CL. To assess this possibility, a second series of studies was conducted using pseudopregnant mouse model as the CL of pseudopregnant mice are functional and fully developed by day 4 (DOPP4) and then enter into the initial stages of regression around day 9 of pseudopregnancy (DOPP9) (Critser et al. 1982, Clark et al. 2017). Using the pseudopregnant mouse model, luteal morphology and the expression of various ovarian growth factors that could account for reduction in the primordial follicle populations were monitored in Pgrmc1/2d/d mice and compared to that of Pgrmc1/2fl/fl control mice.
Methods and Materials
Animals and Treatments
The generation of the Pgrmc1/2fl/fl and Pgrmc1/2d/d mice has been previously described (McCallum et al. 2016, Clark et al. 2017). All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Washington State University. Briefly, Pgrmc1/2 floxed (Pgrmc1/2fl/fl) founder mice were mated with Pgrcre/+mice (Soyal et al. 2005) to generate Pgrmc1/2d/d mice. In the initial study three sexually mature control (Pgrmc1/2fl/fl) and four Pgrmc1/2d/d mice autopsied at approximately 3 months of age and three control and four Pgrmc1/2d/d mice were autopsied at approximately 6 months of age. The ovaries were removed, trimmed of fat, fixed in 4% paraformaldehyde and stored in 70% ethanol until processed for histological examination.
Since Pgrcre/+mice were used to generate Pgrmc1/2d/d mice, subsequent studied focused on the CL. This is because CL are the only ovarian component that express cre recombinase in the Pgrcre/+mice (Soyal et al. 2005). For the second series of studies, pseudopregnancy mice were used as they have functional CL which are fully mature by the 4th day of pseudopregnancy and are in the initial stages of regression by the 9th day of pseudopregnancy (Critser et al. 1982, Clark et al. 2017). Pseudopregnancy was induced by mating 6 to 8 week old female Pgrmc1/2fl/fl (control) and Pgrmc1/2d/d mice with a vasectomized male (van der Heijden et al. 2005). Four control and five Pgrmc1/2d/d mice and three control and four Pgrmc1/2d/d mice were autopsied on day of pseudopregnancy (DOPP) 4 and 9, respectively. Blood samples were obtained and assayed for serum P4 at the University of Virginia School of Medicine Ligand Assay and Analysis Core (Clark et al. 2017). Ovaries were collected and ether fixed for histological and histochemical analysis or used for RNA isolation. In a separate experiment, CL were dissected from the ovaries of three control and three Pgrmc1/2d/d mice on DOPP4 and RNA isolated from the CL. In the final experiment, CL were isolated from the ovaries of four control and four Pgrmc1/2d/d mice on DOPP9. For this study RNA was isolated from both the luteal and non-luteal components of the pseudopregnant ovaries.
While the CL of pseudopregnant mice are functional, histological analysis of the CL of pseudopregnant mice is complicated because CL from the current cycle as well as previous cycles are present. The CL derived in previous cycles are approximately 40 to 50% smaller than those formed during the current cycle, possess numerous necrotic areas and found in the medullary region (Sato et al. 2014). CL possessing these characteristics were not assessed and only fully developed CL as indicated by having diameters greater than 300 μm and without necrotic areas were analyzed. Note that some of these CL possessed areas with vacuolated luteal cells but these were not excluded as vacuoles often reflect an increase in steroid synthesis (Westwood 2008).
RNA Isolation and Real Time PCR Measurements
Total RNA was isolated from the ovarian tissue using Tri Reagent (Sigma-Aldrich) according to the manufacturer’s specifications and the amount of RNA in each sample was quantified using a Nanodrop spectrophotometer. For each sample, 1 ng of RNA was converted to cDNA as described before (Sueldo et al. 2015). All real-time PCR reactions were run in the CFX96 real-time PCR system. Gene expression was evaluated using the 2−ΔΔCT method provided by Bio-Rad CFX96 software. The mRNA level for each gene of interest was expressed relative to actin. The primer sequences and probes used for the real-time PCR assays are shown in Table 1. A negative control (no reverse transcription) was included in each PCR reaction to confirm the absence of genomic DNA.
Table 1.
Primers and probes used to assess mRNA levels by real-time PCR.
| Gene Identifier1 | Sequence (5’−3’) | Probe (5’−3’) |
|---|---|---|
| Pgrmc1_F | CTTTGCCGGAAGAGATGCA | FAM-CCTTGCCACATTTTGCCTGGACAA-BHQ-1 |
| Pgrmc1_R | GAGGTCAGAAAGGTCGTCATAC | |
| Pgrmc2_F | TGCCTGGATAAGGATGCACTTAG | FAM-ATGACGACCTCTCAGATTTGAACGCA-BHQ-1 |
| Pgrmc2_R | CCCATTCTCGAACACTCTCCATT | |
| Vegfa_F | GCTGCACCCACGACAGAA | FAM-AGAGCAGAAGTCCCATGAAAGTGATCA-BHQ1 |
| Vegfa_R | TACCTACAGATGGTCGCTTCG | |
| Mmp9_F | GGGCTACGTGACCTACGA | FAM-CCTCCTGCAGTGCCCTTGAACCTA-BHQ-1 |
| Mmp9_R | TGCACGGTTGAAGCAAAGAAG | |
| Timp1_F | GTCCCAGAACCGCAGTGAAG | FAM-AGTTTCTCATCACGGGCCGCCTA-BHQ-1 |
| Timp1_R | GTACGCCAGGGAACCAAGAAG | |
| Lnc2_F | CACCCGCTTTGCCAAGTCT | FAM-TGACCTTCAGTCTCGGCAAGTAGA-BHQ-1 |
| Lcn2_R | CACACTCACCACCCATTCAGT | |
| Kitl_F | GGTGTGGGCTTAGGAGTGATC | FAM-CAACAAGGGACAAGACC-BHQ-1 |
| Kitl_R | GGAGATGGCAGTTGTGCATTTAC | |
| Fgf2_F | CCAACCGGTACCTTGCTATGAAG | FAM-AAGATGGACGGCTGCTGGCTTC-BHQ-1 |
| Fgf2_R | TCCGTGACCGGTAAGTATTGTAG | |
| Lif_F | CTGCGTCAGGCTCATCTCA | FAM-ACTGGCTGCCCTTCTCCAAGCT-BHQ-1 |
| Lif_R | CCAGGCTGTGGCATCATCT | |
| Bmp4_F | CTGGCTGATCACCTCAACTCAA | FAM-CAACCATGCCATTGTGCAGACCC-BHQ-1 |
| Bmp4_R | CACAACAGGCCTTAGGGATACTAG | |
| Amh_F | CGGGCAAGCTGCTCATCAG | FAM-CCTGTCCGAGGAGCGCATCCA-BHQ-1 |
| Amh_R | GCACTCGGTGGCTACCAT | |
| ß-Actin_F | TCACAGGATGCAGAAGGAGAT | CAL FLUOR GOLD-ACTGCTCTGGCTCCTAGCACCAT-BHQ-1 |
| ß-Actin_R | GCGCTCAGGAGGAGCAAT |
The column contains the gene identifier follow by either a _F (Forward Primer) or _R (Reverse Primer) with the corresponding sequence presented in the Sequence column. All primers and probes were designed and synthesized by LGC Biosearch Technologies, Petaluma, CA.
Histological and Immunohistochemical Analysis
The ovaries were collected from the nulliparous young (≈3 months of age) and middle-aged (≈ 6-month-old) mice and embedded in paraffin, serially sectioned at 5 μm and stained with picric acid stain (Clark et al. 2017). Every fifth section of each ovary was examined and the number of primordial, primary, preantral and antral follicle counted. Follicles were classified as described by Johnson et al (Johnson et al. 2004).
Ovaries were collected from the ovaries of pseudopregnant mice that were 6–8 weeks of age and sectioned at 5 μm. Some sections were stained with hematoxylin and eosin to assess histological characteristics of the pseudopregnant ovary. Other sections were used to identify vascular endothelial cells by detecting CD31 by immunohistochemistry. Briefly, ovarian sections were deparaffinized, incubated with sodium citrate buffer at 95°C for 20 min and incubated in 3% hydrogen peroxide in PBS for 10 min at room temperature to quench endogenous peroxidase activity. The sections were stained for the presence of CD31 using the anti-CD31 antibody at a 1:100 dilution (Cell Signaling Technology, Catalog Number 77699). The slides were then incubated with ImmPRESS peroxidase reagent (Vector Laboratories) for 30 min at room temperature and washed in PBS. The slides were developed using a DAB-peroxidase substrate for 5 min. Finally, the slides were counter stained with hematoxylin for 10 sec, rinsed in distilled water, dehydrated, cleared and mounted. The CD31 was revealed by the presence of a brown stain. Negative controls for each immunohistochemical reaction were conducted by excluding the primary antibody. The percentage of the area occupied by vascular endothelial cells was assessed in 20 CL from 5 Pgrmc1/2fl/fl and 15 CL from 5 Pgrmc1/2d/d pseudopregnant mice as follows. The total area of each CL and the area stained for CD31 was determined using IVision imaging software. The percentage of the CL stained for CD31 was calculated and use to estimate the degree of luteal vascularization.
Statistical Analysis
The data from all the experiments was expressed as a mean ± one standard error. Differences between the means of two groups was assessed by a Student’s t test, while comparisons of means of 3 or more groups was assessed by a one or two-way ANOVA followed by Fisher LSD post hoc test. The statistical analysis was performed using GraphPad Prism 6.0 (San Diego, CA). Regardless of the statistical test used, p values of < 0.05 were considered to be significantly different.
Results
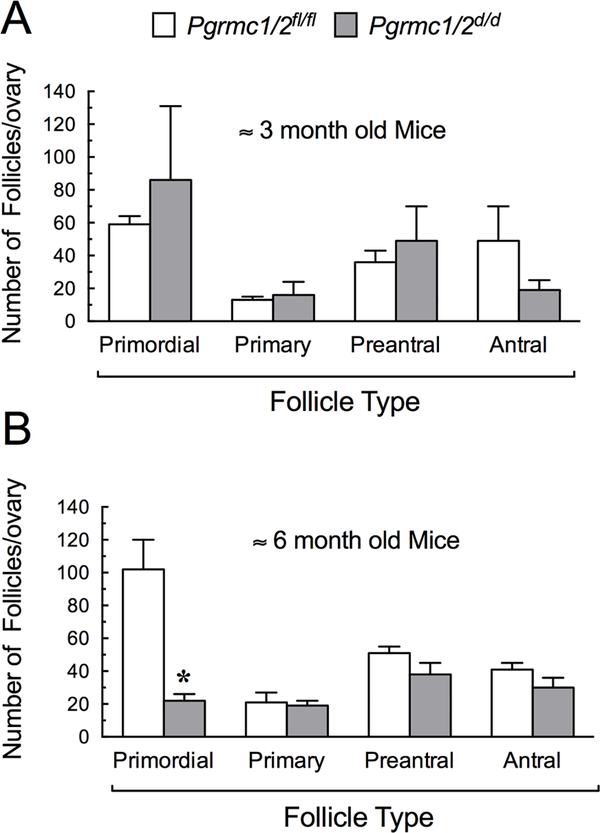
The ovaries of young sexually mature Pgrmc1/2d/d mice of approximately 3 months of age possessed follicles ranging in size from primordial to antral follicles. The distribution of follicles was not statistically different than that observed in control Pgrmc1/2fl/fl mice (Figure 1A). By around 6 months of age, the ovaries of Pgrmc1/2d/d mice possessed a similar number of primary, preantral and antral follicles compared to in the aged-matched controls (Pgrmc1/2fl/fl) (Figure 1B). Roughly equal numbers of CL were also observed in both control and Pgrmc1/2d/d mice indicating that the Pgrmc1/2d/d mice were ovulating and undergoing oestrous cycles. However, at ≈ 6 months of age, the number of primordial follicles was reduced by approximately 80% in Pgrmc1/2d/d mice compared to aged-match controls (Figure 1B). This observation indicated that the population of primordial follicles was essentially depleted within a three-month period.
Figure 1.
The distribution of ovarian follicles in Pgrmc1/2fl/fl control and Pgrmc1/2d/d mice at ≈ 3 months of age (A) and 6 months of age (B). In this and all subsequent graphs, values are shown as a mean ± one standard error with an * indicating a value that is different from the Pgrmc1/2fl/fl control group (p < 0.05).
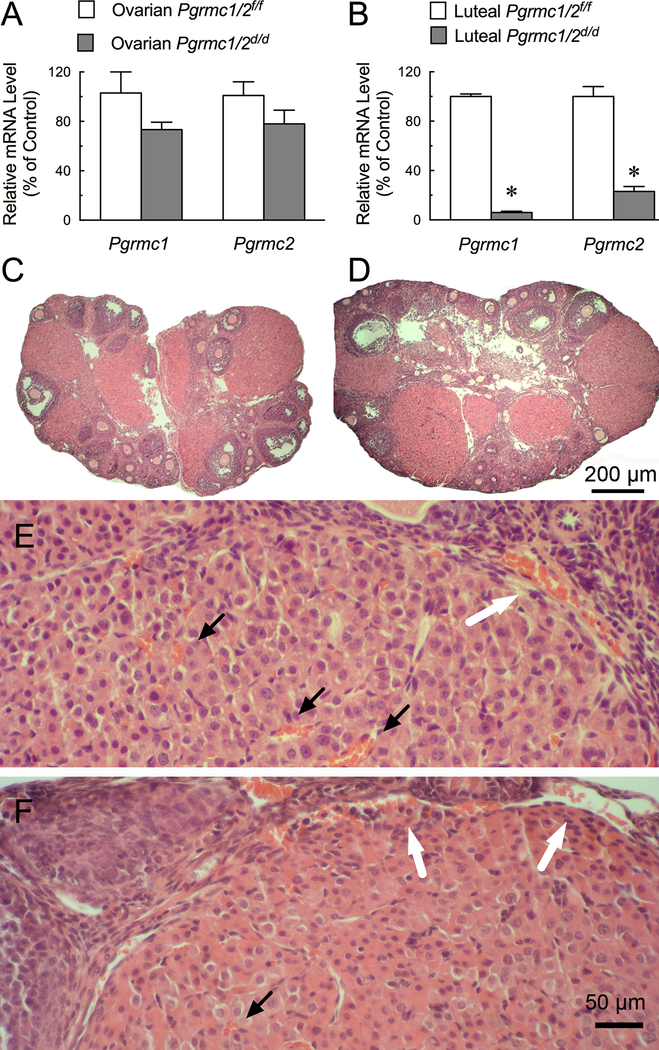
Since Pgr-directed cre recombinase expression in the ovary is restricted to the CL (Soyal et al. 2005), it is possible that signals from the CL are involved in maintaining the adult population of primordial follicles. To test this hypothesis, a pseudopregnant mouse model was used as the CL of pseudopregnant mice are functional and fully developed by day 4 (DOPP4) and then enter into the initial stages of regression around day 9 of pseudopregnancy (DOPP9) (Critser et al. 1982, Clark et al. 2017). The CL of pseudopregnant Pgrmc1/2d/d mice had Pgrmc1 and Pgrmc2 mRNAs that were reduced by ≥ 80%. Pgrmc1 and Pgrmc2 mRNAs were only slightly reduced in the RNA isolated from whole ovaries of DOPP4 Pgrmc1/2d/d mice (compare Figure 2A with 2B). In spite of the reduced expression of Pgrmc1 and Pgrmc2, the average size of the CL (487±19 μm for control vs 460± 31 μm for Pgrmc1/2d/d mice; p > 0.05) and histological characteristics of the luteal cells of Pgrmc1/2d/d pseudopregnant mice were similar to that of controls (compare Figure 2C, E with 2D, F). However, there appeared to be fewer red blood cells among the luteal cells of the Pgrmc1/2d/d mice compared to controls (compare Figure 2E with 2F). There were also “pools” of red blood cells in the vascular network at the basement membrane of the CL of Pgrmc1/2d/d mice. These two observations implied that the vascularization of the CL was adversely affected by Pgrmc1/2 depletion (compare Figure 2E with 2F). To more precisely examine the vascularization of the ovaries, the vascular endothelial cells were detected using a immunohistochemical protocol using a CD31 antibody. The specificity of this approach was demonstrated by failing to detect CD31 stained cells throughout the CL when the CD31 antibody was not included in the staining protocol (compare Supplemental Figure 1A,C with 1B,D). That the CL of Pgrmc1/2d/d mice were not completely vascularized was further evidenced by a 30% reduction in the area occupied by CD31-stained vascular endothelial cells within the CL of Pgrmc1/2d/d mice (7.9 ± 0.5% for controls vs 5.5 ± 0.6% for Pgrmc1/2d/d mice; p < 0.05) (compare Figure 3A with 3B). Note that in Pgrmc1/2d/d mice CD31-stained vascular endothelial cells were mainly observed at the periphery of the CL. These changes in histology and the percentage of CD-31 stained vascular endothelial cells were characteristic of the CL of Pgrmc1/2d/d mice at DOPP4 and 9. Finally, the vasculature in the non-luteal component of the Pgrmc1/2d/d ovary as judged by CD-31 staining was not different than that observed in the Pgrmc1//2fl/fl ovary (compare Figure 3A with 3B). Even with these structural alterations in the CL of pseudopregnant Pgrmc1/2d/d mice, serum P4 levels on DOPP4 (23 ±3 ng/ml) were similar to that of controls (29 ± 1 ng/ml; p > 0.05) with serum P4 levels decreasing on DOPP9 in both Pgrmc1/2d/d (11 ± 5 ng/m) and Pgrmc1//2fl/fl (9 ±2 ng/ml; p> 0.05).
Figure 2.
The levels of Pgrmc1 and Pgrmc2 mRNA in the ovary (A) and CL (B) of Pgrmc1/2fl/fl and Pgrmc1/2 d/d mice on DOPP4. Ovarian morphology of control Pgrmc1/2fl/f (C,E) and Pgrmc1/2d/d (D,F) mice. Note the red blood cells (orange; marked by black arrows) among the luteal cells of controls (E) compared to Pgrmc1/2d/d knockout mice (F). Some red blood cells in vessels outside the CL are marked by white arrows in E and F.
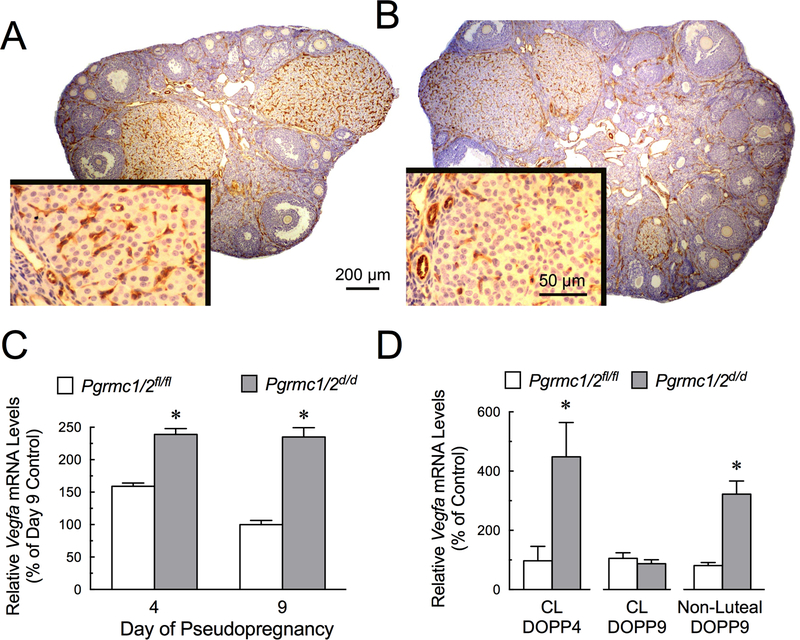
Figure 3.
Changes in the ovarian vasculature of control (A) and Pgrmc1/2d/d (B) mice as revealed by CD31 staining (brown stain), which is a marker for vascular endothelial cells. Insets are higher magnifications of CL. An image of negative control and positively stained section are shown in Supplemental Figure 1). Alterations in the levels of Vegfa mRNA in the ovary of DOPP4 and DOPP9 mice (C) and the luteal and non-luteal components of Pgrmc1/2fl/fl and Pgrmc1/2 d/d mice (D).
One factor that regulates vascularization of the CL is Vascular Endothelial Growth Factor A (VEGFA) (Lee et al. 2007). Vegfa mRNA levels were increased in the ovaries of DOPP4 and 9 by ≈ 2-fold in Pgrmc1/2 d/d mice (Figure 3C). Interestingly, Vegfa mRNA levels in the CL on DOPP4 increased by ≥ 4-fold, accounting in part for the increase in ovarian Vegfa mRNA on DOPP4 (Figure 3D). However, on DOPP9 the CL levels of Vegfa mRNA in in Pgrmc1/2 d/d mice were similar to control levels, while Vegfa expression in the non-luteal component of Pgrmc1/2 d/d mice increased by ≈ 3-fold (Figure 3D).
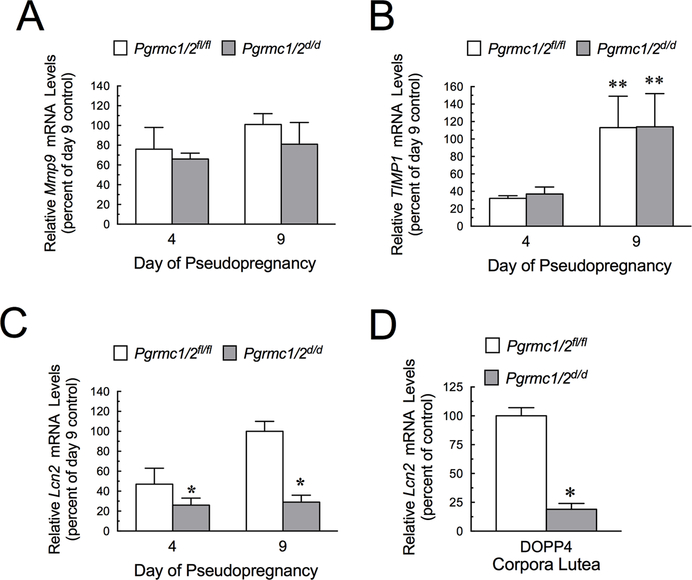
The reduced vasculature observed in the CL of Pgrmc1/2d/d mice occurred in the presence of elevated Vegfa mRNA levels. This suggests that there were other essential factors that act in conjunction with VEGFA to promote CL vascularization with their expression being dependent on the presence of PGRMC1 and/or PGRMC2. Potential factors include Matrix Metalloproteinase-9 (MMP9), Tissue Inhibitor of Metalloproteinases 1(TIMP1) and Lipocalin 2 (LCN2). Real-time PCR analysis revealed that the ovarian mRNA levels of Mmp9 (Figure 4A) and Timp1 (Figure 4B) of pseudopregnant Pgrmc1/2 d/d mice did not differ from those of pseudopregnant Pgrmc1/2 fl/fl mice. However, Lcn2 mRNA levels were reduced in the ovaries of pseudopregnant in Pgrmc1/2 d/d mice. (Figure 4C). Although ovarian levels were not dramatically reduced on DOPP4, Lcn2 mRNA levels were reduced to 26 ± 7% in the CL of Pgrmc1/2 d/d mice compared to the CL of Pgrmc1/2 fl/fl mice (Figure 4D).
Figure 4.
Effect of depleting Pgrmc1 and Pgrmc2 on the relative ovarian mRNA levels of Mmp9 (A) Timp1 (B) and Lcn2 (C) on DOPP4 and DOPP9. Effect of depleting Pgrmc1 and Pgrmc2 on the relative Lcn2 mRNA levels in CL on DOPP4 (D). In panel B the ** indicates that values for DOPP9 are greater than DOPP4 in both Pgrmc1/2fl/fl control and Pgrmc1/2d/d mice (p < 0.05).
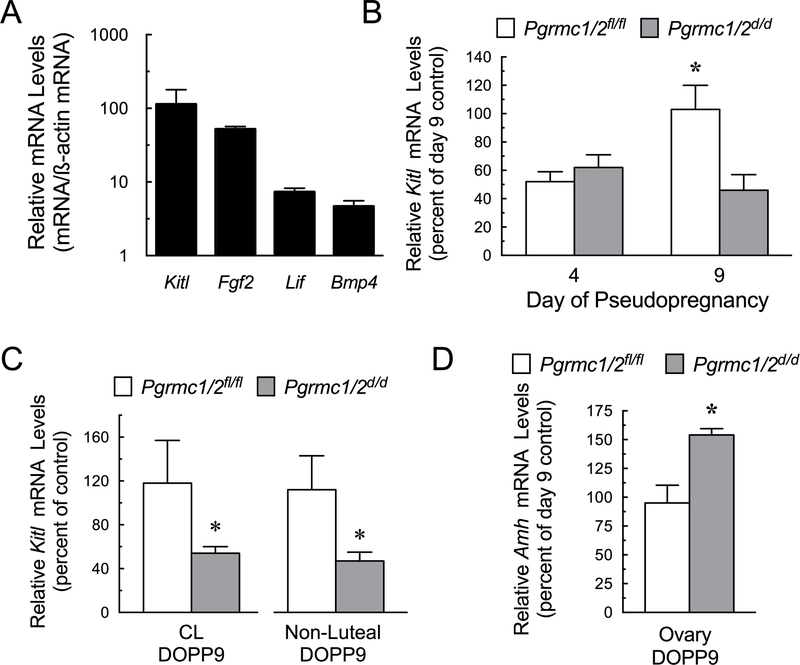
Numerous ovarian growth factors that influence primordial follicle growth and/or survival were detected in the pseudopregnant ovaries of Pgrmc1/2 fl/fl mice, including Kit Ligand (Kitl) (Zhang et al. 2014, Saatcioglu et al. 2016), Fibroblast Growth Factor 2 (Fgf2) (Chaves et al. 2012), Leukemia Inhibitory Factor (Lif) (Nilsson et al. 2002, da Nobrega et al. 2012) and Bone Morphogenic Protein 4 (Bmp4) (Skinner 2005) (Figure 5A). Kitl mRNA was most abundant and its expression was the only one of these growth factors that was altered in the ovaries of pseudopregnant Pgrmc1/2 d/d mice. Specifically, in Pgrmc1/2 fl/fl mice, ovarian mRNA levels of Kitl increased on DOPP9 but this increase was not observed in DOPP9 Pgrmc1/2 d/d mice (Figure 5B). The lower levels of ovarian Kitl mRNA were due to lower levels of Kitl mRNA in both the luteal and non-luteal components of DOPP9 ovary (Figure 5C). An increase in Anti-Mullerian Hormone (Amh) mRNA levels was observed in the ovaries of DOPP9 Pgrmc1/2 fl/fl mice (Figure 5D).
Figure 5.
Relative expression of growth factors that regulate primordial follicle growth and/or survival as expressed as growth factor mRNA level normalized to ß-actin mRNA (A). Effect of the depletion of Pgrmc1 and Pgrmc2 on Kitl mRNA levels within the whole ovary (B) as well as the luteal and non-luteal components of the DOPP9 ovary (C). Effect of the depletion of Pgrmc1 and Pgrmc2 on Amh mRNA levels within the whole ovary on DOPP9 (D).
Discussion
The present studies reveal that conditional depletion of Pgrmc1 and Pgrmc2 using the Pgr-cre driver results in the premature demise of primordial follicles, which coincides with the previously observed decrease in fertility (Clark et al. 2017). Because Pgr-cre driver mice were used to generate Pgrmc1/2 d/d mice, Pgrmc1 and Pgrmc2 were depleted in all cells that express PGR. Importantly, neuroanatomical mapping studies reveal that high levels of Pgrmc1, Pgrmc2 and Pgr are expressed in the same regions of the hypothalamus that control GnRH secretion (Intlekofer & Petersen 2011). Although GnRH neurons express PGR, P4’s does not act through PGR to suppress in vitro secretion of GnRH (Sleiter et al. 2009). Rather P4 acts through membrane progestin receptorα and/or ß (Sleiter et al. 2009) and/or PGRMC1 (Bashour & Wray 2012, Mittelman-Smith et al. 2017). It is possible then that in the Pgrmc1/2 d/d mice, Pgrmc1 and Pgrmc2 would be reduced in GnRH secreting neurons and this would attenuate P4’s ability to suppress GnRH secretion in vivo. This would likely result in an increase serum gonadotropin levels. However, for this to occur, the individual neurons that regulate GnRH would have to express Pgr and Pgrmc1 but this has not been demonstrated in vivo.
Regardless of whether serum gonadotropin levels are elevated, the present study demonstrates that in Pgrmc1/2 d/d mice, preovulatory follicles develop, ovulate and differentiate into functional CL capable of secreting P4 at the same level as the control mice. In addition, the age at which the mice obtain sexual maturity and the interval between pregnancies in Pgrmc1/2d/d mice is the same as controls (Clark et al. 2017). Therefore, the hypothalamic-pituitary-ovarian axis in Pgrmc1/2 d/d mice matures and results in normal ovarian function in the expected time frame. However, the mechanism that maintains the population of adult primordial follicle in Pgrmc1/2 d/d mice after puberty is altered.
The mechanism that governs the fate of primordial follicles in adult animals is not well defined but it is unlikely to involve gonadotropins, since primordial follicles are maintained and transition into primary follicles in the absence of the pituitary (Hirshfield 1991). We propose that the CL plays an essential but not necessarily an exclusive role in preserving the adult population of primordial follicles throughout adulthood. We further propose that the capacity of the CL to maintain the primordial follicle population is regulated by PGRMC1 and/or PGRMC2 and that these PGRMC family members do so by influencing two aspects of luteal function.
The first function involves the vascular development within the CL. This is evidenced by the reduction in the number of vascular endothelial cells within the CL of Pgrmc1/2 d/d mice. VEGFA is a well-known factor that regulates vascularization in the CL (Lee et al. 2007) and its expression in the CL is inhibited by Pgrmc1 and/or Pgrmc2 as demonstrated by Vegfa mRNA levels in the CL on DOPP4 in Pgrmc1/2 d/d mice increasing by ≥ 4-fold. The failure of the CL to vascularize fully in the presence of elevated levels of Vegfa mRNA suggests that there are other PGRMC1/2-dependent luteal-derived factors that act in conjunction with VEGFA to promote CL vascularization. One such factor is LCN2 as its expression in the CL is dependent on the presence of PGRMC1 and/or PGRMC2. That LCN2 expression in luteal cells is dependent on PGRMC1 is consistent with previous findings in breast cancer cells (Mir et al. 2012). LCN2 promotes vascular development by binding and activating MMP9 (Leng et al. 2009, Mir et al. 2012). MMP9 activity plays several roles in vascular development, one of which is to cleave the extracellular matrix thereby releasing VEGFA allowing it to be more biologically active (Hawinkels et al. 2008). Thus, the decrease in Lcn2 mRNA observed in the CL of Pgrmc1/2 d/d mice could result in reduced MMP9 activity and a subsequent reduction in VEGFA’s biological activity. This would in part account for the limited vascularization of the CL of Pgrmc1/2 d/d mice even in the presence of elevated Vegfa mRNA. This concept merits further consideration.
The reduced vasculature of CL of Pgrmc1/2 d/d mice would not be expected to affect P4 secretion as P4 is lipophilic and diffuses from the luteal cell into the extracellular space and eventually entering the circulation (Niswender & Nett 1988). Nor would P4 synthesis be expected to be reduced in Pgrmc1/2 d/d mice, since ablation of Pgrmc1 (McCallum et al. 2016) or Pgrmc2 (Clark et al. 2017) does not suppress P4 secretion. However, KITL is a luteal cell growth factor that is not lipophilic and regulates primordial follicle growth (Skinner 2005, Zheng et al. 2014a, Zheng et al. 2014b, Saatcioglu et al. 2016). The secretion of KITL could be attenuated due to poor vascularization of the CL.
The second function that luteal PGRMC1/2 affects is gene expression, as evidenced by the reduced levels of Kitl mRNA in DOPP9 CL of Pgrmc1/2 d/d mice. It is likely then that ablating Pgrmc1/2 in luteal cells suppresses both the expression and secretion of KITL. If so, this could alter the intraovarian environment in a manner that is not supportive of the adult population of primordial follicles and account at least in part to the premature demise of the adult population of primordial follicles.
Interestingly, ablation of luteal Pgrmc1/2 also alters the expression profile in the non-luteal component of the ovary on DOPP9, which could also contribute to the premature demise of the primordial follicles. For example, Vegfa mRNA levels are elevated, while Kitl mRNA levels are suppressed in the non-luteal component of the ovary. Both of these growth factors are known to stimulate primordial to primary follicle transition (Roberts et al. 2007, Yang & Fortune 2007, McFee et al. 2009, Saatcioglu et al. 2016). In addition, the ovarian Amh mRNA levels are also elevated in the DOPP9 ovary of Pgrmc1/2 d/d mice. Elevated levels of AMH are important in that AMH is expressed and secreted by developing follicle and acts to maintain primordial follicle viability but inhibits their transition into primary follicles (Durlinger et al. 1999, Kano et al. 2017). Thus, ablation of Pgrmc1/2 alters the non-luteal component of the ovary such that one intraovarian factor that promotes primordial follicle growth (Vegfa) is over expressed; the expression of another factor that promotes primordial follicle growth (Kitl) is suppressed; and a factor that suppresses primordial follicle growth (Amh) is elevated. It is possible then that this imbalance in growth promoting and suppressing factors within the non-luteal component of the ovary induces an incomplete or altered signal cascade that results in primordial follicle death as opposed to its transition into a primary follicle. Importantly, this imbalance in the non-luteal ovarian growth factor environment would be transient, only occurring during luteal regression. This would likely result in a gradual decrease in the number of primordial follicles starting with the initiation of the estrous cycles. While this hypothesis is consistent with our data, caution must be exercised in accepting it as it is based on mRNA measurements. Thus, this hypothesis must be confirmed by considerably more experimentation. Such future experiments include demonstrating that the proteins encoded by these mRNAs are changing in parallel with mRNA levels as well as generating knockout mice in which each growth factor is conditionally ablated.
Finally, the present data are consistent with the possibility that in normal cycling mice PGRMC1 and/or PGRMC2 direct changes in the gene expression profile during luteal regression that allow for an increase in ovarian Kitl mRNA and a suppression of Amh mRNA. As such this would be conductive to increasing the number of primordial follicles that transition into growth primary follicles. The expression of Amh is important because it inhibits the growth of primordial follicles (Durlinger et al. 1999, Kano et al. 2017) by suppressing the growth promoting actions of KITL (Nilsson et al. 2007). Since serum AMH levels are maintained at a relatively constant level in mature mice ≤ 8 months of age (Kevenaar et al. 2006), there must be a mechanism to override AMH’s growth inhibiting action. We propose that the transient 2-fold increase in Kitl mRNA which is observed throughout the ovary of control mice during the initial stages of luteal regression (i.e. DOPP9) could override AMH’s actions and stimulate a limited number of the primordial follicles to transition into growing primary follicles. Since the increase in ovarian Kitl mRNA occurs while the CL is regressing, the time that Kitl mRNA levels are elevated would be limited to the life of the CL. This CL-dependent increase in Kitl expression would account for the relatively constant number of primary follicles that start to grow in mature adult mice (Pedersen 1972). This proposed mechanism provides for the efficient and controlled utilization of the adult population of primordial follicles and a prolonged female reproductive lifespan and therefore merits further investigation.
Supplementary Material
Supplemental Figure 1. CD31 staining in the presence (A,C) or absence of the CD31 antibody. Sections were taken from the same control ovary. CD31 was detected as a brown stain and all sections lightly stained hematoxylin. Compare Panel A with Panel B and Panel C with Panel D. Images were taken under the same optical conditions.
Acknowledgments
Funding
The authors would like to thank the Department of Cell Biology at UCONN Health for providing financial support for this project as well as support from NIH grant R21OD016564 and R21RR030264.
Footnotes
Declaration of interest: The authors do not have any conflicts of interest.
References
- Bashour NM & Wray S 2012. Progesterone directly and rapidly inhibits GnRH neuronal activity via progesterone receptor membrane component 1. Endocrinology 153 4457–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves RN, de Matos MH, Buratini J Jr. & de Figueiredo JR 2012. The fibroblast growth factor family: involvement in the regulation of folliculogenesis. Reproduction, fertility, and development 24 905–915. [DOI] [PubMed] [Google Scholar]
- Clark NC, Pru CA, Yee SP, Lydon JP, Peluso JJ & Pru JK 2017. Conditional Ablation of Progesterone Receptor Membrane Component 2 Causes Female Premature Reproductive Senescence. Endocrinology 158 640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critser ES, Savage PJ, Rutledge JJ & French LR 1982. Plasma concentrations of progesterone and 13,14-dihydro-15-keto prostaglandin F-2 alpha in pregnant, pseudopregnant and hysterectomized pseudopregnant mice. Journal of reproduction and fertility 64 79–83. [DOI] [PubMed] [Google Scholar]
- da Nobrega JE Jr., Goncalves PB, Chaves RN, Magalhaes Dde M, Rossetto R, Lima-Verde IB, Pereira GR, Campello CC, Figueiredo JR et al. 2012. Leukemia inhibitory factor stimulates the transition of primordial to primary follicle and supports the goat primordial follicle viability in vitro. Zygote 20 73–78. [DOI] [PubMed] [Google Scholar]
- Daan NM, Hoek A, Corpeleijn E, Eijkemans MJ, Broekmans FJ, Fauser BC & Koster MP 2016. Reproductive characteristics of women diagnosed with premature ovarian insufficiency. Reproductive biomedicine online 32 225–232. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA & Themmen AP 1999. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 140 5789–5796. [DOI] [PubMed] [Google Scholar]
- Guo M, Zhang C, Wang Y, Feng L, Wang Z, Niu W, Du X, Tang W, Li Y, Wang C et al. 2016. Progesterone Receptor Membrane Component 1 Mediates Progesterone-Induced Suppression of Oocyte Meiotic Prophase I and Primordial Folliculogenesis. Scientific reports 6 36869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, Fontijn RD, David G, Hommes DW, Lamers CB et al. 2008 VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. European journal of cancer 44 1904–1913. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN 1991. Development of follicles in the mammalian ovary. Int Rev Cytol 124 43–101. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA & Petersen SL 2011. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience 172 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK & Tilly JL 2004. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428 145–150. [DOI] [PubMed] [Google Scholar]
- Kano M, Sosulski AE, Zhang L, Saatcioglu HD, Wang D, Nagykery N, Sabatini ME, Gao G, Donahoe PK & Pepin D 2017. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proceedings of the National Academy of Sciences of the United States of America 114 E1688–E1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB, Myers M & Anderson RA 2013. The dynamics of the primordial follicle reserve. Reproduction 146 R205–215. [DOI] [PubMed] [Google Scholar]
- Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, Themmen AP & Visser JA 2006. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 147 3228–3234. [DOI] [PubMed] [Google Scholar]
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP & Iruela-Arispe ML 2007. Autocrine VEGF signaling is required for vascular homeostasis. Cell 130 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng X, Ding T, Lin H, Wang Y, Hu L, Hu J, Feig B, Zhang W, Pusztai L, Symmans WF, et al. 2009. Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer research 69 8579–8584. [DOI] [PubMed] [Google Scholar]
- Maclaran K & Panay N 2015. Current concepts in premature ovarian insufficiency. Women’s health 11 169–182. [DOI] [PubMed] [Google Scholar]
- Mansouri MR, Schuster J, Badhai J, Stattin EL, Losel R, Wehling M, Carlsson B, Hovatta O, Karlstrom PO, Golovleva I, Toniolo D, Bione S, Peluso J & Dahl N 2008. Alterations in the expression, structure and function of progesterone receptor membrane component-1 (PGRMC1) in premature ovarian failure. Human molecular genetics 17 3776–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum ML, Pru CA, Niikura Y, Yee SP, Lydon JP, Peluso JJ & Pru JK 2016. Conditional Ablation of Progesterone Receptor Membrane Component 1 Results in Subfertility in the Female and Development of Endometrial Cysts. Endocrinology 157 3309–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFee RM, Artac RA, McFee RM, Clopton DT, Smith RA, Rozell TG & Cupp AS 2009. Inhibition of vascular endothelial growth factor receptor signal transduction blocks follicle progression but does not necessarily disrupt vascular development in perinatal rat ovaries. Biology of reproduction 81 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir SU, Jin L & Craven RJ 2012. Neutrophil gelatinase-associated lipocalin (NGAL) expression is dependent on the tumor-associated sigma-2 receptor S2RPgrmc1. The Journal of biological chemistry 287 14494–14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Rudolph LM, Mohr MA & Micevych PE 2017. Rodent Models of Non-classical Progesterone Action Regulating Ovulation. Front Endocrinol (Lausanne) 8 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monniaux D, Clement F, Dalbies-Tran R, Estienne A, Fabre S, Mansanet C & Monget P 2014. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link? Biology of reproduction 90 85. [DOI] [PubMed] [Google Scholar]
- Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP & Capel B 2012. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biology of reproduction 86 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E, Rogers N & Skinner MK 2007. Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction 134 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson EE, Kezele P & Skinner MK 2002. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Molecular and cellular endocrinology 188 65–73. [DOI] [PubMed] [Google Scholar]
- Niswender GD & Nett TM 1988. The Corpus Luteum and Its Control. In The Physiology of Reproduction, pp. 489–525. [Google Scholar]
- Pedersen T 1972. Follicle Growth in the Mouse Ovary. In Oogenesis, pp. 397–412. [Google Scholar]
- Pelosi E, Forabosco A & Schlessinger D 2015a. Genetics of Ovarian Reserve. Frontiers in Genetics 6 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi E, Simonsick E, Forabosco A, Garcia-Ortiz JE & Schlessinger D 2015b. Dynamics of the ovarian reserve and impact of genetic and epidemiological factors on age of menopause. Biology of reproduction 92 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Jiao X, Simpson JL & Chen ZJ 2015. Genetics of primary ovarian insufficiency: new developments and opportunities. Human reproduction update 21 787–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AE, Arbogast LK, Friedman CI, Cohn DE, Kaumaya PT & Danforth DR 2007. Neutralization of endogenous vascular endothelial growth factor depletes primordial follicles in the mouse ovary. Biology of reproduction 76 218–223. [DOI] [PubMed] [Google Scholar]
- Saatcioglu HD, Cuevas I & Castrillon DH 2016. Control of Oocyte Reawakening by Kit. PLoS genetics 12 e1006215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J, Hashimoto S, Doi T, Yamada N & Tsuchitani M 2014. Histological characteristics of the regression of corpora lutea in wistar hannover rats: the comparisons with sprague-dawley rats. J Toxicol Pathol 27 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiadas CC, Duan S, Correll M, Rubio R, Karaca N, Ginsburg ES, Quackenbush J & Racowsky C 2012. Ovarian reserve status in young women is associated with altered gene expression in membrana granulosa cells. Molecular human reproduction 18 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK 2005. Regulation of primordial follicle assembly and development. Human reproduction update 11 461–471. [DOI] [PubMed] [Google Scholar]
- Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P & Levine JE 2009. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology 150 3833–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ & Lydon JP 2005. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41 58–66. [DOI] [PubMed] [Google Scholar]
- Sueldo C, Liu X & Peluso JJ 2015. Progestin and AdipoQ Receptor 7, Progesterone Membrane Receptor Component 1 (PGRMC1), and PGRMC2 and Their Role in Regulating Progesterone’s Ability to Suppress Human Granulosa/Luteal Cells from Entering into the Cell Cycle. Biology of reproduction 93 63. [DOI] [PubMed] [Google Scholar]
- van der Heijden OW, Essers YP, Spaanderman ME, De Mey JG, van Eys GJ & Peeters LL 2005. Uterine artery remodeling in pseudopregnancy is comparable to that in early pregnancy. Biology of reproduction 73 1289–1293. [DOI] [PubMed] [Google Scholar]
- Wang JL, Li SL, Qin YY & Chen ZJ 2014. Analysis of progesterone receptor membrane component 1 mutation in Han Chinese women with premature ovarian failure. Reproductive biomedicine online 29 640–643. [DOI] [PubMed] [Google Scholar]
- Westwood FR 2008. The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol 36 375–384. [DOI] [PubMed] [Google Scholar]
- Yang MY & Fortune JE 2007. Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Molecular reproduction and development 74 1095–1104. [DOI] [PubMed] [Google Scholar]
- Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, Bosbach B, Brannstrom M & Liu K 2014. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Current biology : CB 24 2501–2508. [DOI] [PubMed] [Google Scholar]
- Zheng W, Zhang H, Gorre N, Risal S, Shen Y & Liu K 2014a. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Human molecular genetics 23 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhang H & Liu K 2014b. The two classes of primordial follicles in the mouse ovary: their development, physiological functions and implications for future research. Molecular human reproduction 20 286–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. CD31 staining in the presence (A,C) or absence of the CD31 antibody. Sections were taken from the same control ovary. CD31 was detected as a brown stain and all sections lightly stained hematoxylin. Compare Panel A with Panel B and Panel C with Panel D. Images were taken under the same optical conditions.