Abstract
Objective:
The average age at the time of spinal cord injury (SCI) has increased to 43 years old. Middle-aged mice (14 months old, MO) exhibit impaired recovery after SCI with age-dependent increases in reactive oxygen species (ROS) production through NADPH oxidase (NOX) along with pro-inflammatory macrophage activation. Despite these aging differences, clinical therapies are being examined in individuals regardless of age based upon preclinical data generated primarily using young animals (~4 MO). Our objective is to test the extent to which age affects SCI treatment efficacy. Specifically, we hypothesize that the effectiveness of apocynin, a NOX inhibitor, is age-dependent in SCI.
Methods:
Apocynin treatment (5 mg/kg) or vehicle was administered 1 and 6 hours after moderate T9 contusion SCI (50kdyn IH) and then daily for 1 week to 4 and 14 MO mice. Locomotor and anatomical recovery was evaluated for 28 days. Monocyte-derived macrophage (MDM) and microglial activation and ROS production was evaluated at 3 and 28 days post injury.
Results:
Apocynin improved functional and anatomical recovery in 14 but not 4 MO SCI mice. Apocynin-mediated recovery was coincident with significant reductions in MDM infiltration and MDM-ROS production in 14 MO SCI mice. Importantly, microglial activation was unaffected by treatment.
Conclusion:
These results indicate that apocynin exhibits age-dependent neuroprotective effects by blocking excessive neuroinflammation through NOX-mediated ROS production in MDMs. Further, these data identify age as a critical regulator for SCI treatment efficacy and indicate that pharmacologically reduced macrophage, but not microglia, activation and ROS production reverses age-associated neurological impairments.
Keywords: age, spinal cord injury, NADPH oxidase, apocynin, reactive oxygen species, neuroinflammation, microglia, monocyte, macrophage
Introduction
The average age at the time of spinal cord injury (SCI) has steadily increased since the mid-1970s with the current average age being 43-years-old (“Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance.,” 2016). Age exacerbates SCI tissue damage and reduces functional recovery after both clinical and experimental SCI (Fenn et al., 2014; Hooshmand et al., 2014; McKinley et al., 2003; Scivoletto et al., 2003; B. Zhang et al., 2015a). Reactive oxygen species (ROS) generation and oxidative damage are implicated as potential mechanisms underlying age-related pathology following traumatic SCI in rodents (Leden et al., 2017; B. Zhang et al., 2016). Moreover, several studies report that age-dependent increases in NOX (NADPH oxidases) activation may give rise to the enhanced ROS production observed in middle-aged and aged traumatic CNS injuries (Kumar et al., 2012; Qiu et al., 2016; Ritzel et al., 2018). Whether increased NOX activity impairs SCI recovery in middle-aged animals has not been directly evaluated.
SCI triggers a robust inflammatory response through activation of resident microglia and infiltrating monocyte-derived macrophages (MDMs). In response to injury, within minutes, microglia adopt an activated phenotype. MDM infiltrate into the injury site within days post injury (dpi) and peak at 7 dpi (Greenhalgh and David, 2014). Activated microglia and MDMs secrete a variety of inflammatory cytokines and produce ROS. Recently, we showed that microglia/MDMs are responsible for the majority of cellular ROS generation during the sub-acute phase of SCI (B. Zhang et al., 2016).
ROS such as superoxide anion (O2−•), hydroxyl radical (•OH), and hydrogen peroxide (H2O2), are produced during normal conditions as byproducts of mitochondria, NADPH oxidases (NOX), or other cellular activities. NADPH oxidase is a multicomponent enzyme that includes 7 isoforms (NOX1–5, and DUOX 1–2) (Nayernia et al., 2014) and growing evidence suggests that the NOX enzyme system is a major source of ROS generation in the traumatically injured CNS and in chronic neurodegenerative disorders (B. Y. Choi et al., 2015; Cooney et al., 2014; Hernandes and Britto, 2012; Kumar et al., 2012; Qin et al., 2013). Among these NOX isoforms, NOX2 is highly expressed in inflammatory phagocytic cells. We recently reported that NOX2 activation in activated microglia/macrophages is significantly upregulated in middle-aged SCI mice relative to young controls (B. Zhang et al., 2016).
Both inflammation and oxidative stress are important mediators of age-dependent secondary injury; however, clinical therapies are being examined in individuals regardless of age based upon data generated almost exclusively using young animals. These practices raise concerns about the translational potential of pre-clinical data specifically for immunomodulatory/neuroprotective therapies. To address this concern, in the current study, we tested the treatment effect of a potent NOX inhibitor with affinity for NOX2, apocynin (4-hydroxy3-methoxyacetophenone, APO), in middle-aged (14-month-old (MO)) vs. young (4 MO) SCI mice. We observed that APO significantly decreases MDM accumulation and macrophage ROS production coincident with improved locomotor recovery and tissue sparing in 14 MO SCI mice. We detected no treatment effect on microglia or in 4 MO animals. These data implicate MDM as key mediators of age-associated secondary injury processes and highlight NOX2 as an important mechanism of age-induced differences in neuroinflammation and functional recovery. Since age-dependent inflammation is noted across a variety of conditions our findings have broad implications for the translational development of interventions to treat age-related neuropathologies.
Materials and Methods
Animals
Mice (C57BL/6, female, 4- and 14-month-old) were obtained from National Institute of Aging. These ages model humans approximately 18- and 45-year-old respectively, and represent the demographic shift in the SCI population (Quinn, 2005; “Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance.,” 2016). A total of 85 mice were used in the current study. Three mice died after SCI due to anesthesia complications; 2 mice died because of ruptured bladders during bladder expression; and one mouse was excluded from the study based upon a priori exclusion criteria of an abnormal time versus force curve indicating a bone hit at the time of SCI. The n for each outcome measure is described in the figure legends. Animals were housed in a standard 12h:12h, light:dark environment with ad libitum access to food and water. All procedures were performed in accordance with the guidelines and protocols of the Office of Responsible Research Practices and with approval of the Institutional Animal Care and Use Committees at the University of Kentucky.
Experimental model of spinal cord injury (SCI)
A mild-to-moderate (50 kdyn displacement, no dwell time) mid-thoracic contusion SCI was performed using the Infinite Horizons injury device (Precision Systems and Instrumentation) as described previously (Orr et al., 2017; Scheff et al., 2003). Briefly, mice were anesthetized via intraperitoneal (i.p.) injections of ketamine (100 mg/kg) and xylazine (10 mg/kg), followed by a laminectomy to expose the T9 spinal cord. After the delivery of SCI, the muscle and skin incisions were closed using monofilament suture. Post-surgically, mice were immediately given one injection of buprenorphine-SR (subcutaneously, 1 mg/kg) and antibiotic (5 mg/kg, Enrofloxacin 2.27%: Norbook Inc, Lenexa, KS) dissolved in 2 mL of saline. Animals were returned to warm housing units (cages on warm pads) overnight to recover from the surgery before returning to regular home cages. Mice continued to receive antibiotic subcutaneously in 1 mL of saline for 5 days. Apocynin (5 mg/kg body weight) (Sigma-Aldrich, Cat# AC10242–0250) or vehicle (1% DMSO) was administered via intraperitoneal injection beginning at 1 and 6 hours post injury then daily for 1 week. Manual bladder expression was performed on injured mice twice daily until voiding function returned.
Behavioral analysis
Hindlimb movements of all experimental animals were graded using the Basso Mouse Scale (BMS) as previously described (Basso et al., 2006). Mice were assessed in an open field for 4 min pre-surgically and at 1, 3, 7, 14, 21, and 28 days post-injury (dpi) by two trained observers under blind conditions. On the day of IP injections of apocynin or vehicle (1, 3, and 7 dpi), BMS was conducted at least 5 hours after injection. The performance of each hindlimb was assessed separately based on movement (e.g., ankle placement and stepping), coordination, and trunk stability. Scores of both hindlimbs were averaged to generate BMS scores and subscores for each animal.
In situ ROS detection
Detection of ROS production in mice was conducted by using dihydroethidium (DHE, Thermo Fisher Scientific) as previously described (B. Zhang et al., 2016). Briefly, at designated time points (3 and 7 dpi), mice were injected with DHE (0.01 mg/g body weight, intraperitoneal injection) 4 h prior to sacrificing. Oxidized DHE emits a bright red fluorescence, which can be detected at the emission wavelength at 570 nm. The fluorescent signal was captured with a C2+ laser scanning confocal microscope (Nikon Instruments Inc, Melville, NY, USA) and the threshold-based area of DHE-positive staining was quantified using the MetaMorph program (Molecular Devices, Sunnyvale, CA, USA).
Tissue processing
Mice were anesthetized (ketamine (120 mg/kg) and xylazine (10 mg/kg)) and transcardially perfused with cold 0.1M PBS (pH 7.4) followed by cold, 4% paraformaldehyde (PFA) in PBS. Spinal cord tissues were dissected and post-fixed for another 2 h in 4% PFA. Fixed tissue was then rinsed and stored in phosphate buffer (0.2 M, pH 7.4) overnight at 4 °C, followed by cryoprotection in 30% sucrose for 3–4 days at 4°C. Spinal cords (8 mm in length centered on the lesion epicenter) were randomly distributed (to ensure that equal numbers of each group were present on every slide) into blocks in optimal cutting temperature (OCT) compound (Sakura Finetek USA, Inc., Torrance, CA) and rapidly frozen on dry ice before sectioning. Serial transverse sections (10 μm) were cut for each block, mounted on coated slides (Fisher Scientific, Waltham, MA), and then stored at −80°C prior to staining.
Immunohistochemistry
Spinal cord sections were dried at 37°C for 1 hour and rinsed in 0.1 M PBS before incubation with blocking buffer (0.1 M PBS containing 1% bovine serum albumin, Fisher Scientific, Cat# BP1605), 0.1% Triton X-100 (Sigma Aldrich, Cat# X-100), 0.1% fish gelatin (Sigma-Aldrich, Cat# G7765), and 5% normal goat or donkey serum (Sigma-Aldrich, Cat# G9203; D9663). After blocking for 1 hour, tissue sections were incubated with primary antibodies diluted in blocking buffer overnight at 4°C. On the following day, tissue sections were rinsed in 0.1 M PBS and then incubated with secondary antibodies at room temperature for 1 hour. For immunofluorescence staining, all the slides were coverslipped with Immu-Mount (Thermo Fisher Scientific) after washing in PBS. Fluorescent images were obtained using a C2+ laser scanning confocal microscope (Nikon Instruments Inc, Melville, NY, USA). The details of primary and secondary antibodies used in the current study are listed in Table 1.
Table 1:
Antibodies Used
| Antibodies | Host | Dilution | Vendor | Cat # |
|---|---|---|---|---|
| Immunohistochemistry-Primary Antibodies | ||||
| 1. P2Y12 (20: #5) | Rabbit | 1:1000 | Anaspec Inc | AS-55043A |
| 2. F4/80 (20: #6) | Rat | 1:1000 | AbD Serotec | MCA497 |
| 3. GFAP (20: #7) | Chicken | 1:500 | Aves Lab | GFAP |
| 4. Neurofilament (20: #7) | Chicken | 1:500 | Aves Lab | NFH |
| Immunohistochemistry-Secondary Antibodies | ||||
| 5. Alexa Fluor 488 anti-rabbit (IgG) | Goat | 1:1000 | ThermoFisher | A11008 |
| 6. Alexa Fluor 546 anti-rat (IgG) | Goat | 1:1000 | ThermoFisher | A11081 |
| 7. Biotinylated goat anti-chicken (IgY) | Goat | 1:1000 | Aves Lab | B-1005 |
| 8. Streptavidin, Alexa Fluor 633 conjugate (used for #3) | 1:1000 | ThermoFisher | S-21375 | |
Areas of spared and injured tissue were identified with eriochrome cyanine (myelin) and neurofilament (axons, EC/NF) labeling as previously reported (B. Zhang et al., 2015b). NF immunoreactivity was visualized by using a biotinylated secondary antibody (Table 1) and the Vectastain Elite ABC kit (Vector Laboratories, PK-6100), followed by color differentiation using a diaminobenzidine (DAB) substrate kit (Vector Laboratories, SK-4100). Brightfield images were captured with Aperio ScanScope (Leica Biosystems). Lesion volumes were calculated from spinal cord tissue sections spaced at 100 μm intervals using a Cavalieri estimator program (Stereo Investigator software, MBF Bioscience). Spared tissue areas were estimated on the sections with the greatest tissue damage (lesion epicenter). Microglia (P2Y12-positive), MDMs (F4/80-positive, P2Y12-negative) and ROS (DHE), were quantified using threshold-based measurements to identify positive fluorescent signals above background at the lesion epicenter with the MetaMorph analysis program (Molecular Devices, Sunnyvale, CA, USA). The “logical AND” arithmetic function in MetaMorph was used to identify double- and triple-labeled cells. A user, blinded to treatment and age groups, then verified double- and triple-labeled cells for quantification.
Experimental Design and Statistical Analysis
The data analyses and acquisition were performed by investigators blinded to both age and treatment groups. Statistical analyses were done using GraphPad Prism 6.0 (GraphPad Software). Unpaired t-test tests with Welch’s correction were applied to evaluate the treatment effect of apocynin on lesion volume, lesion length, percentage of spared tissue, and chronic macrophage accumulation between 4 and 14 MO mice. Two-way repeated (BMS and BMS subscore x time) measures ANOVA or two-way ANOVA followed by Sidak’s multiple comparisons were performed to analyze the remaining data. For each outcome measure n refers to a separate biological replicate, i.e. animal. Measurements and quantifications are reported as mean +/− SEM. Results were considered statistically significant at p<0.05.
Results
Apocynin improves functional and anatomical recovery in middle-aged but not young SCI mice
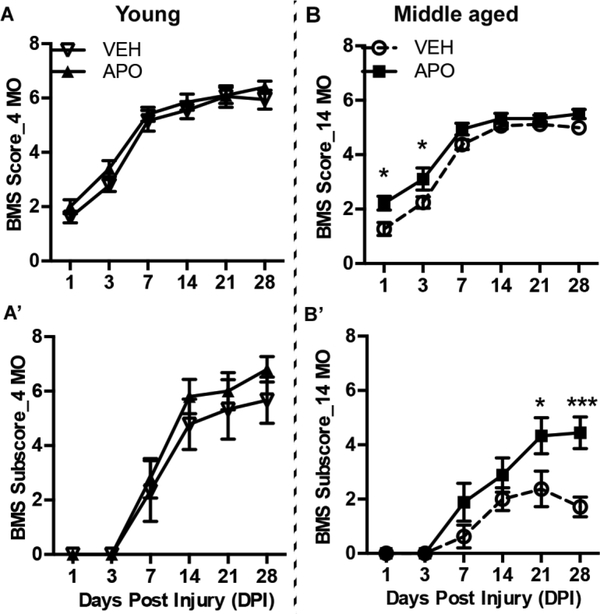
Previously, we observed significantly impaired functional recovery in middle - aged versus young SCI mice concomitant with potentiated pro-inflammatory macrophage activation and increased macrophage ROS production (Fenn et al., 2014; B. Zhang et al., 2016; 2015a). Specifically, we observed increased expression of the macrophage-specific ROS producing enzyme, NOX2, in middle-age SCI mice (B. Zhang et al., 2016). To determine the extent to which this age-dependent inflammation contributes to the efficacy of neuroprotective agents, we treated young and middle-aged SCI mice with apocynin (APO), a potent NOX inhibitor with affinity for NOX2. Adult (4 months) and middle-aged (14 months) mice were subjected to mild-to-moderate mid-thoracic (T9) spinal cord contusion injury and treated with APO (5mg/kg, i.p.) or vehicle at 1 and 6 hours post-injury then daily for 7 days. We then assessed hindlimb locomotor function (BMS locomotor test) over a 28-day period. As shown in Figure 1, 14 MO SCI mice treated with APO showed significant functional improvement (two-way repeated measures ANOVA main effect of treatment; BMS score: F(1,15)=5.58, p=0.032; BMS subscore: F(1, 15)=7.37, p=0.016)) with extensive ankle movement (BMS score: 2.2 ± 0.3) at 1 day post injury (dpi) and plantar paw placement (BMS score: 3.1 ± 0.4) at 3 dpi as compared to vehicle (VEH) treated 14 MO SCI mice. VEH treated mice demonstrated only slight ankle movement (BMS score: 1.3 ± 0.2) at 1 dpi and extensive ankle movement (BMS score: 2.2 ± 0.2) at 3 dpi. Moreover, at 28 dpi, APO treated 14 MO mice showed plantar stepping with some coordination (BMS score 5.5 ± 0.2 and subscore: 4.4+/−0.6); while VEH treated 14 MO animals exhibited only abnormal limb movement without consistent stepping or fore and hindlimb coordination (BMS score: 5.0 ± 0.0 and BMS subscore: 1.7 ± 0.4) (Fig. 1B-B’). The transitions of limb movement to stepping and from stepping to fore- and hindlimb coordination are recognized as important functional milestones in SCI recovery. Interestingly, we did not observe a treatment effect of APO on locomotor recovery in 4 MO SCI mice (BMS score: F(1, 17)=1.0, p=0.33; BMS subscore: F(1,17)=0.64, p=0.43) (Fig. 1A-A’).
Figure 1. Apocynin (APO) significantly improves locomotor functional recovery in middle-aged but not young mice after spinal cord injury (SCI).
Time course of locomotor functional recovery assessed by Basso Mouse Score (BMS)(A&B) and BMS subscore (A’&B’). Young (4 months old (MO)) and middle-aged female C57BL/6 mice (14 MO) were subjected to T9 contusion SCI then treated with vehicle (VEH) or APO (5mg/kg, i.p.) at 1 and 6 hours post-SCI then daily for seven days. A-A’) There was no significant effect of treatment in 4 MO SCI mice. B-B’) 14 MO SCI mice treated with APO show significant improvement in functional recovery at 1 and 3 dpi, as well as at 28 dpi, compared to VEH treated 14 MO animals. These BMS and BMS subscores correspond to extensive ankle movement (BMS ~ 2) at 1 day post injury (dpi) and plantar paw placement (BMS ~ 3) at 3 dpi as compared to vehicle (VEH) treated 14 MO mice with only slight ankle movement (BMS ~ 1) at 1 dpi and extensive ankle movement (BMS ~ 2) at 3 dpi. At 28 dpi, APO treated 14 MO mice showed plantar stepping with some coordination (BMS ~ 5–6 subscore ~ 4–5) while VEH treated 14 MO animals exhibited only abnormal limb movement without consistent stepping or fore and hindlimb coordination (BMS ~ 5.0 and subscore < 2). Results are mean+/−SEM, n=7–10/group. *p<0.05 or ***p<0.001.
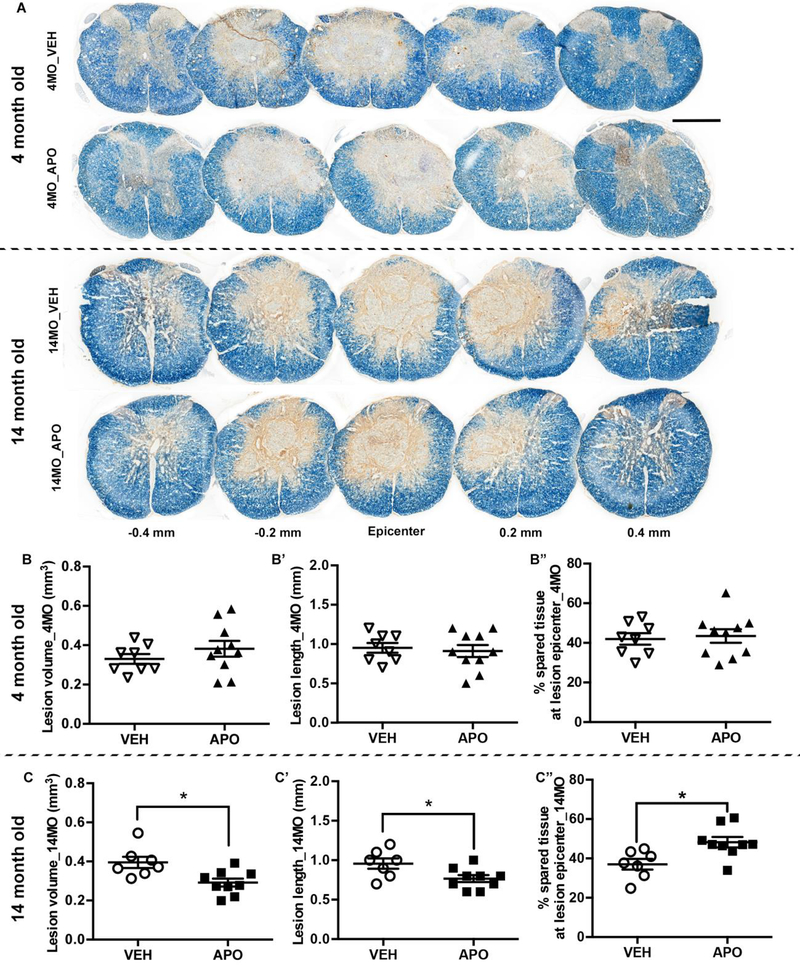
The degree of locomotor recovery after SCI is highly correlative with tissue pathology (Basso et al., 1996). Since APO improved functional recovery in 14 MO animals, we further investigated the age-dependent benefits of treatment on tissue preservation after SCI. Consistent with the behavioral results, APO treated 4 MO SCI mice exhibited no significant difference compared to VEH treated age-matched SCI mice in lesion volume (t=1.08, p=0.30), lesion length (t=0.404, p=0.70), or tissue sparing (t=0.34, p=0.74) (Fig. 2B-B”). In contrast, APO treatment significant decreased lesion volume (Fig. 2C, t=2.92, p=0.014) and lesion length (Fig. 2C’, t=2.43, p=0.041) in 14 MO SCI mice compared to vehicle-treated age-matched controls. Moreover, APO treatment significantly increased tissue sparing at the lesion epicenter (t=2.98, p=0.01) in 14 MO SCI animals (Fig. 2C”). These data indicate that age is a key determinant of therapy-mediated functional and anatomical recovery.
Figure 2. Apocynin treatment decreases lesion volume and lesion length and increases tissue sparing in midde-aged but not young spinal cord injured (SCI) mice.
Tissue analyses were conducted on tissue sections isolated 4 weeks after SCI. (A) Representative images of neurofilament/eriochrome cyanine (NF/EC) stained spinal cord cross-sections encompassing the rostral (−0.4 mm) to caudal (0.4 mm) extent of the injury from each treatment and age group. Scale bar = 500μm. There was no effect of APO on lesion volume, lesion length, or tissue sparing in 4 MO SCI mice (B-B”). In contrast, APO significantly decreased lesion volume (C) and lesion length (C’) in 14 MO animals following SCI. In addition, treatment of APO significantly improved the amount of spared tissue at the lesion epicenter (C”) in 14 MO SCI mice. Results are mean +/− SEM, n=7–10/group. *p<0.05 vs. VEH treated age-matched mice..
Apocynin reduces ROS production in middle-aged but not young mice
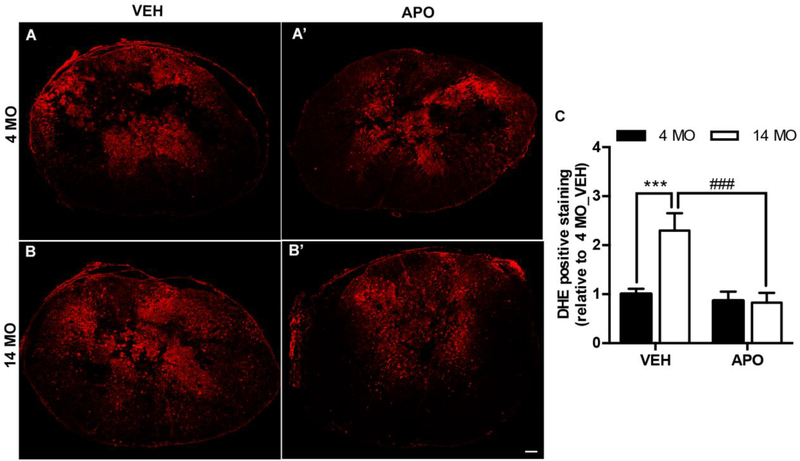
We recently identified ROS production mediated by the upregulation of NADPH oxidase (NOX2) enzyme activity as a potential regulator of enhanced tissue damage and impaired functional recovery in middle-aged mice after SCI (B. Zhang et al., 2016). Next, we investigated whether the beneficial effects of APO are due to age-dependent modulation of oxidative stress in the injured spinal cord. Since we observed treatment effects acutely after SCI (Fig. 1), we evaluated ROS production in the injured spinal cord at 3 dpi. Similar to previous observations (Leden et al., 2017; B. Zhang et al., 2016), SCI induced significantly higher ROS production at the lesion epicenter of vehicle treated 14 MO versus 4 MO mice (Fig. 3A-B, t=4.1, p=0.0005). Interestingly, APO treatment significantly decreased ROS production in the injured spinal cord of 14 MO mice (Fig. 3B-B’, t=4.6, p=0.0002); however, ROS production was unchanged with APO treatment in 4 MO animals (t=0.43, p=0.9). These data indicate ROS production disproportionately contributes to secondary injury in middle-aged versus young SCI.
Figure 3. Apocynin treatment reduces reactive oxygen species (ROS) production in middle-aged, but not young, mice after SCI.
Representative tissue sections 3 days post injury of DHE labeling ROS (red fluorescence) from 4 (A-A’) and 14 (B-B’) MO SCI mice treated with VEH or APO. Quantification of DHE fluorescence intensity at 3 days post injury reveals that APO treatment significantly decreases ROS generation in the lesion epicenter of 14 MO, but not 4 MO, SCI mice. Results are mean+/− SEM, n=8–9/group. ***p<0.001; ###p<0.001. Scale bar = 100μm.
Apocynin selectively affects the response of monocyte-derived macrophages acutely after SCI
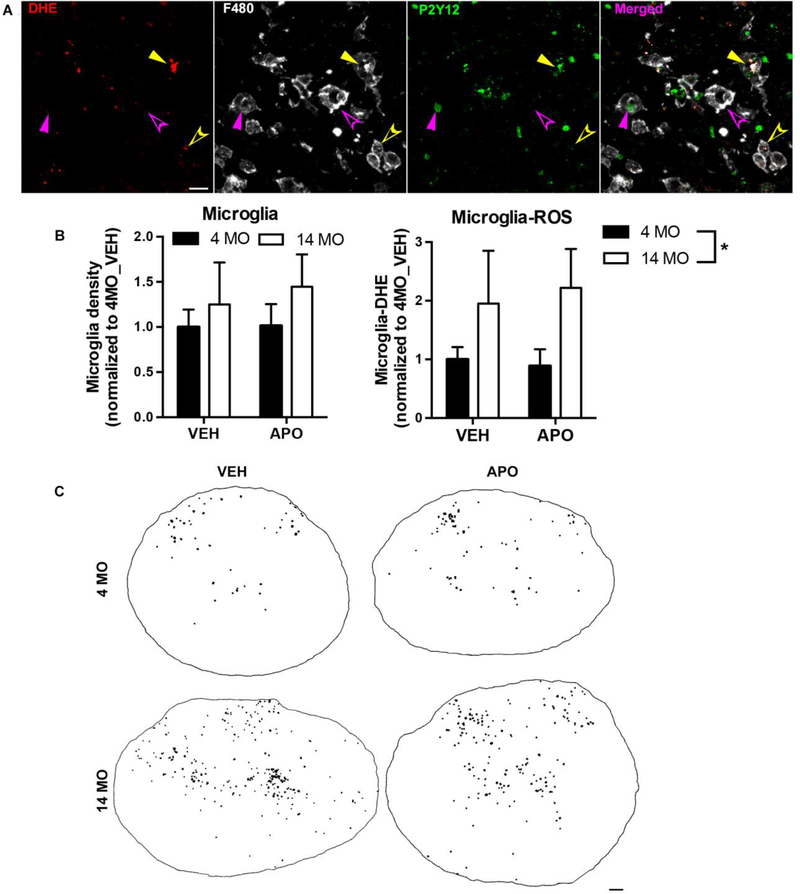
Resident microglia and infiltrated monocyte-derived macrophages (MDMs) play important roles in inflammatory cascades after SCI. Previously, we observed that macrophages contribute the majority of ROS production during the acute phase of SCI through the NOX2 activation (B. Zhang et al., 2016); however, we did not differentiate between activated microglia and MDMs. There is evidence that age modulates NOX-mediated ROS production in both microglia and MDMs (Qin et al., 2013; Stout and Suttles, 2005). To further determine how age differentially affects microglia and MDM populations after SCI, we stained the tissue with a microglia specific marker P2Y12 (Butovsky et al., 2014) and the MDM and microglia marker F4/80. We adopted techniques used in traumatic brain injury to identify activated microglia as cells double-stained with P2Y12 and F4/80 (Figure 4A filled arrowheads) and to identify infiltrated MDMs as F4/80-positive only (opened arrowheads in Fig. 4A) (Villapol et al., 2017). In the absence of injury, few F4/80pos/P2Y12neg MDMs were present in the spinal cord with no overt difference between 4 and 14 MO animals (Supplemental Figure 1). We detected ROS production in both microglia (DHE positive, yellow filled arrowhead in Fig. 4A) and MDMs (yellow opened arrowhead in Fig. 4A) in the injured spinal cord regardless of age.
Figure 4. Microglia reactive oxygen species (ROS) production is significantly increased in middle-aged SCI mice compared to young at 3 days post injury.
Analyses were performed at 3 days post injury on tissue sections from the lesion epicenter. (A) Microglia are identified as cells double-stained with P2Y12 and F480 (filled arrowheads) while infiltrated macrophages are F480-positive only (opened arrowheads). There are both ROS-producing (DHE positive, yellow arrowheads) and non-ROS-producing (magenta arrowheads) microglia and macrophages in the injured spinal cords. Scale bar=20 μm. (B) Quantification of P2Y12pos/F480pos immunofluorescence signal reveals that there is no significant effect of age or APO treatment in microglia activation. Quantification of triple positive P2Y12/F480/DHE immunofluorescence shows that age significantly enhances ROS production in activated microglia, while APO treatment has no effect in decreasing ROS production in either 4 or 14 MO SCI mice. Results are mean+/− SEM. N=8–9/group. *14 MO vs. 4 MO is significantly higher at p<0.05 regardless of treatment. (C) Representative images where each black dot represents ROS-producing microglia (B, P2Y12pos/F4/80pos/DHEpos) from 4 and 14 MO SCI mice treated with VEH or APO (scale bar= 100μm). Specifically, triple-labeled pixels have been dilated for illustrative purposes.
There were no significant effects of age or APO treatment in microglia activation (F480pos/P2Y12pos) after SCI (two-way ANOVA; overall age effect: F(1,30)=1.6, p=0.2; overall treatment effect: F(1, 30)=0.07 p=0.9; treatment x age interaction: F(1,30)=0.24 p=0.6) (Fig. 4B). In contrast, age significantly potentiated microglia ROS production, as indicated by an increased proportion of activated microglia co-labeled with DHE in the 14 MO versus 4 MO injured spinal cord (main effect of age: F(1,30)=4.6, p=0.04) (Fig. 4B-C). Interestingly, APO did not significantly decrease ROS production in activated microglia for either age group (two-way ANOVA main effect of treatment: F(1, 30)=0.0013 p=0.97; age x treatment interaction: F(1,30)=0.20 p=0.7) (Fig. 4B-C). Collectively, these data indicate that age potentiates microglia ROS production that is refractory to APO treatment.
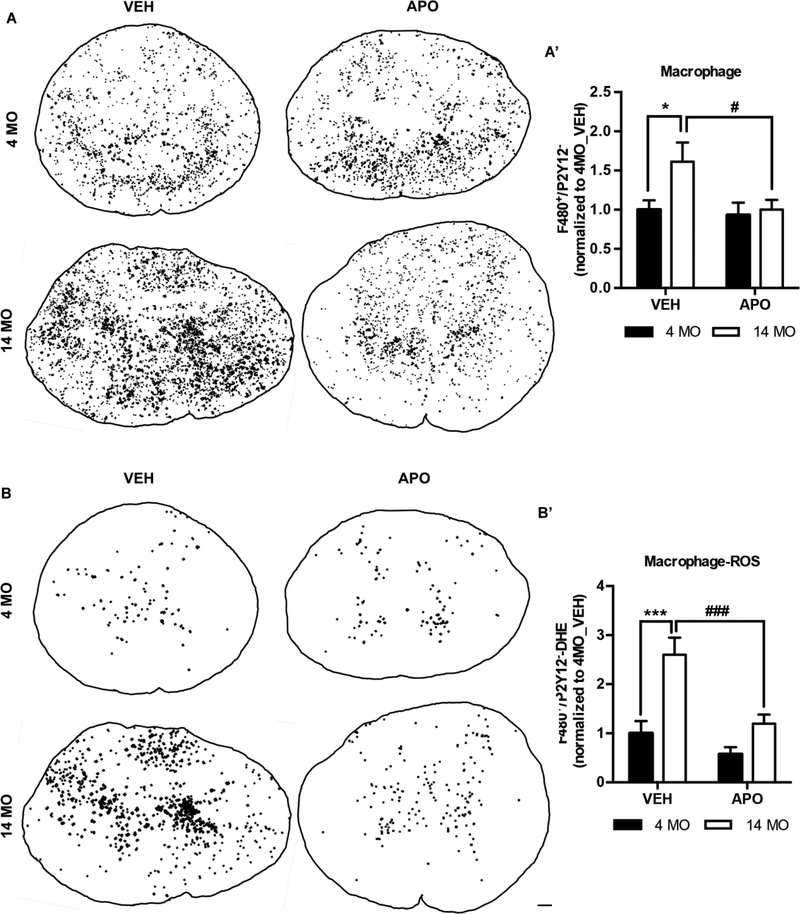
Next we examined the extent to which age and APO treatment alter MDM responses. We detected a significant increase in MDM (F480pos/P2Y12neg) accumulation in 14 MO vs. 4 MO SCI mice (main effect of age: F(1,30)=4.3 p=0.047) (Fig 5A-A’). APO decreased this age-dependent increase in MDM activation after SCI (main effect of treatment: F(1,30)=4.3 p<0.048) by significantly reducing MDM accumulation in 14 MO (VEH vs, APO, t= 2.6, p<0.032), but not 4 MO, SCI mice (VEH vs. APO, t=0.30, p=0.94) (Fig. 5A’). Interestingly, APO treatment was sufficient to reduce MDM accumulation in 14 MO SCI mice to that of 4 MO SCI mice (Fig. 5A’).
Figure 5. Apocynin treatment decreases the infiltration of monocyte-derived macrophages (MDMs) and reduces MDM ROS production in middle-aged but not young SCI mice.
Analyses were performed at 3 days post injury on tissue sections from the lesion epicenter using the identification technique presented in Figure 4. Representative images where each black dot represents MDM (A, P2Y12neg/F4/80pos) and ROS-producing MDMs (B, P2Y12neg/F4/80pos/DHEpos) from 4 and 14 MO SCI mice treated with VEH or APO (scale bar= 100μm). Specifically, in B, triple-labeled pixels have been dilated for illustrative purposes. Quantification of the P2Y12neg/F4/80pos MDMs and P2Y12neg/F4/80pos/DHEpos ROS producing MDMs signals (A’&B’, see open arrows in Fig. 4A for phenotypes) reveals a treatment effect of APO. Specifically APO significantly reduced overall MDM accumulation, as well as, the proportion of MDMs producing ROS in 14 MO but not 4 MO SCI mice. Results are mean+/− SEM. N=8–9/group. *p<0.05, ***p<0.001, #p<0.05, ###p<0.001.
We then quantified ROS production in MDMs. Similar to our observations with microglia, age significantly increases macrophage ROS production (main effect of age: (F1, 30)=20.2 p<0.0001) (Fig. 5B-B’). However, unlike for microglia, APO treatment significantly reduced MDM ROS production in an age-dependent manner (main effect of treatment: F(1, 30)=13.7 p=0.0009). Specifically, APO significantly reduced MDM ROS production in 14 MO (VEH vs. APO, t=3.9, p=0.001) but not 4 MO SCI mice (VEH vs. APO, t=1.3, p=0.4) (Fig. 5B-B’). Importantly, we quantified the relative proportion of MDMs expressing ROS. Therefore, our data show that APO treatment significantly decreases age-dependent MDM infiltration (Figure 5A) and reduced the proportion of infiltrated macrophages producing ROS in the aged injured spinal cord (Figure 5B).
APO reduces monocyte-derived macrophages in chronic SCI
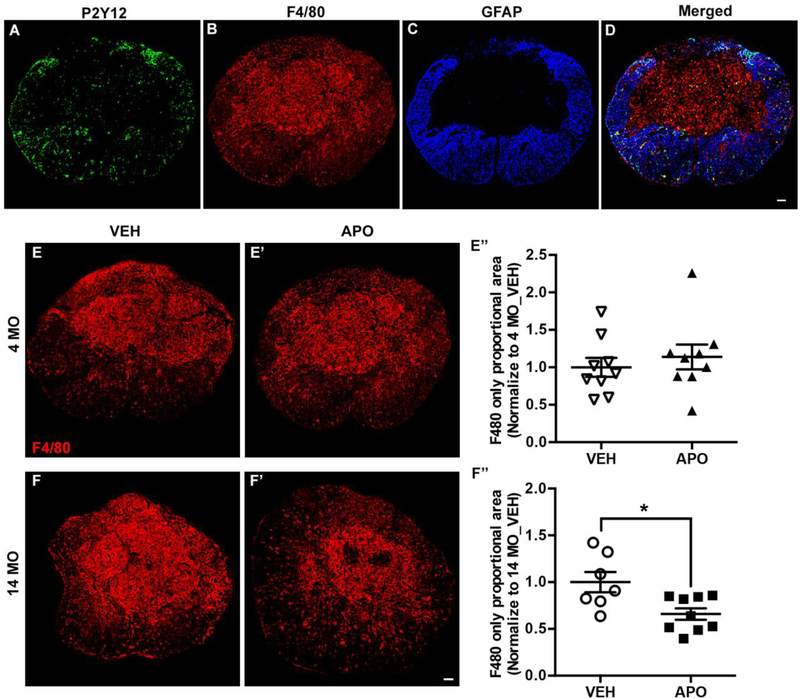
Macrophages infiltrate the injured spinal cord as early as 3 dpi and persist for several months. Chronically, monocyte-derived macrophages (MDMs) dominate the fibrotic lesion core while microglia persist in the astroglial scar region of the lesion penumbra (Mawhinney et al., 2012; Orr and Gensel, 2018). We observed similar lesion and penumbra distribution of MDMs and microglia respectively in both 4 and 14 MO SCI mice at 28 days post injury using the combination of P2Y12 and F4/80 labeling. As shown in Figure 6A-D, although there are sporadic P2Y12 and F4/80 double positive cells in the GFAP-negative lesion core, the majority of the core is comprised of monocyte-derived F4/80pos/P2Y12neg macrophages. P2Y12pos microglia are present mostly in the GFAP-positive glial scar area (Fig. 6A-D). To determine the long-term immunomodulatory effects of APO treatment, we quantified the density of MDMs (F4/80pos/P2Y12neg) and microglia (P2Y12pos) at 28dpi. We found that APO treatment significantly decreased the density of monocyte-derived macrophages at the lesion epicenter of 14 MO SCI mice compared to vehicle treated age-matched controls (Fig. 6F-F”, t=2.7, p=0.022). There was no effect of APO treatment on MDMs in 4 MO SCI mice (Fig. 6E-E”, t=0.67, p=0.5). We did not detect a treatment effect of APO on microglia (P2Y12pos) density for either 4 MO (t=0.28, p=0.8) or 14 MO (t=0.11, p=0.9) SCI mice. Collectively, the acute and chronic effects of APO treatment on MDM accumulation and ROS production in 14 MO SCI mice indicate that these cells may disproportionally contribute to age-related secondary injury and inflammation in SCI.
Figure 6. Apocynin treatment reduces chronic (4-weeks after SCI) monocyte-derived macrophages accumulation in 14 MO but not 4 MO SCI mice.
Tissue analyses were conducted on tissue sections of the lesion epicenter isolated 4 weeks after SCI. Representative images of the lesion epicenter immunolabeled with P2Y12 (A), F4/80 (B), and GFAP (C). The majority of F4/80+/P2Y12- MDMs are presented at the fibrotic scar area, devoid of astrocyte (GFAP) immunoreactivity, while P2Y12+ microglia cells are preferentially localized in GFAP+ areas (D). Quantification of the density of MDM immunoreactivity reveals that APO has no effect in 4 MO SCI mice (E-E”), while APO significantly decreases the proportion of F4/80+/P2Y12- macrophages at the lesion epicenter of 14 MO SCI mice (F-F”). Scale bar=100 μm. Results are mean+/− SEM, n=7–10/group. *p<0.05 vs. VEH treated age-matched mice.
Discussion
In the current study, we made a number of novel observations: 1) apocynin (APO) selectively improves functional recovery and reduces tissue damage in 14 month old (MO), but not 4 MO, SCI mice; 2) age potentiates ROS production in macrophages and microglia after SCI; 3) APO specifically reduces monocyte-derived macrophage (MDM) ROS production coincident with functional recovery in 14 MO SCI mice; and 4) APO treatment decreases MDM accumulation in middle-aged SCI mice. To our knowledge, this is the first study to report the effect of age on an immunomodulatory therapeutic intervention using a clinical relevant SCI model and to report that age may be a key regulator of treatment efficacy. Importantly, these data highlight a disproportionate role for MDMs vs. microglia in age-related neuropathologies.
Previously, we reported that excessive age-related ROS production in microglia/macrophages is specifically associated with NOX2 activation in middle-aged SCI mice (B. Zhang et al., 2016). Increased levels of ROS production are a major cause of secondary injury and lesion progression after SCI (Hall et al., 2016; Khayrullina et al., 2015). In the current study, we utilized APO as a tool to investigate whether inhibition of NOX activity modifies ROS production and consequently changes pathological and behavioral functions following SCI. APO prevents the translocation and assembly of cytosolic p47phox to NOX membrane components, thereby inhibiting NOX2 activation and concomitant ROS production (Stefanska and Pawliczak, 2008). The effects of APO have been extensively studied in animal models of neuroinflammation and neurodegeneration. By inhibiting NOX2 activity, APO is capable of reducing pro-inflammatory responses and oxidative stress and prevents microglia/macrophage activation in vivo and in vitro (S.-H. Choi et al., 2012; Ghosh et al., 2012; Harraz et al., 2008; Mander et al., 2006). APO also reduces inflammation and secondary injury after spinal cord trauma (Impellizzeri et al., 2011; Johnstone et al., 2013).
Due to its recognized action as an NADPH oxidase inhibitor, APO is widely used in in vivo studies. However, the treatment effects of APO vary. In experimental models of traumatic brain injury (TBI), pre- (20 min prior to controlled cortical impact injury (CCI)) or post-administration (2 hours after CCI) with a single injection of APO (4 mg/kg, i.p.) markedly inhibits microglial activation and oxidative damage (Q.-G. Zhang et al., 2012). In addition, APO treatment (5 mg/kg, i.p.) beginning at 23 h and continued daily for 4 consecutive days after CCI reduces oxidative damage and enhances neuronal survival, as well as, significantly reduces lesion size at 4 dpi (J. Wang et al., 2017). In contrast, another study using the CCI injury model reported that although APO treatment (5mg/kg, 30 min post CCI, i.p. injection) significantly reduces lesion volume and improves motor function in mice, it has no effect on cognitive function measured by the Morris Water Maze test (Loane et al., 2013). Moreover, in the moderate lateral fluid percussion TBI model, subcutaneous administration of APO (5 mg/kg) at 30 min and 24hr post injury in mice had no effect on brain edema or motor function but significantly improved performance on the novel object recognition task and reduced lesion volume (Ferreira et al., 2013). These conflicting results across studies may be due to variations in TBI models, treatment paradigms (i.e. pre vs. post-injury administration; i.p. vs. s.c. injection), or locations of the primary injury.
There are fewer studies investigating APO in pre-clinical SCI models. Following extradural spinal compression (1 min) using an aneurysm clip, APO (5 mg/kg, i.p.) effectively reduced nitrotyrosine formation, neutrophil infiltration, and pro-inflammatory cytokine production in the injured spinal cord coincident with improved locomotor recovery (Impellizzeri et al., 2011). In contrast, after contusive SCI, APO treatment (10 mg/kg, i.p.) only modestly improved functional recovery and did not significantly reduce lesion volume despite significantly decreasing oligodendrocyte death (Johnstone et al., 2013). Similar to the observations of Johnstone et al., here, in our contusion model, we did not detect modified ROS production or functional improvement in 4 MO mice with APO treatment (5 mg/kg i.p.). However, we observed that this dose of APO significantly decreased overall ROS production and improved hindlimb function in 14 MO SCI mice. It is highly possible that the effective dose of APO varies in different SCI models and injury severities. It is also worth noting that age might be an important factor regulating NOX activity and the response to APO treatment. For example, a study using newborn mice reported that in contrast to findings in the adult, pharmacological inhibition of NOX activity with APO or genetic deletion of NOX2 did not protect against perinatal brain injury induced by hypoxia-ischemia (Doverhag et al., 2008). Collectively, these data indicate that injury models, dosage, route of administration, and physiology factors such as age potentially affect APO treatment efficacy.
There is some debate that APO may attenuate ROS through antioxidant properties rather than NOX inhibition (Heumüller et al., 2008). While this may have contributed in the current study, it is unlikely that the treatment effects of APO here are entirely dependent on antioxidant activity. We did not detect a decrease in ROS in APO-treated 4 MO SCI mice nor decreased microglia ROS production with APO treatment in either age group suggesting an absence of antioxidant effects. However, we cannot rule out the antioxidant activity of APO.
APO inhibits NOX activity by preventing the binding of cellular p47phox to the membrane components of the enzyme complex. While APO is a general NOX inhibitor, it is likely that the NOX2 isoform is most responsive to treatment in the current study. NOX2 is present in all cell types in the injured spinal cord, however, NOX3 and NOX4 are only found in neurons and glial cells, respectively (Cooney et al., 2014) and likely do not require the assembly of cellular and membrane components for activity (Ueno et al., 2005). Both microglia and MDMs express NOX2. Interestingly, microglia also express NOX1 (Chéret et al., 2008) while F4/80-positive macrophages exclusively express NOX2 (De Logu et al., 2017). In the current study, we found that age increases ROS production in both microglia and MDMs but APO only reduces ROS in infiltrated, F4/80-positive MDMs. Since aging also results in higher MDM infiltration, it is possible that with current treatment paradigm, APO effectively blocks NOX2 activity without reducing NOX1 activation in microglia. Indeed, in the absence of NOX2, microglia produce ROS in response to inflammatory stimuli through NOX1 (Chéret et al., 2008). Collectively, these observations indicate that APO-mediated inhibition of NOX2 underlie the reduction of age-dependent SCI MDM activation in the current study. Further studies combining NOX-deficient mice and APO treatment may better clarify the relative contributions of different NOX isoforms to age-related SCI pathophysiology.
Although NOX2 inhibition likely contributes to the therapeutic effects we observed in middle-aged animals, our results in young animals are inconsistent with previously observations. Indeed, in a presumed young mouse model of contusive SCI, NOX2 inhibition with the selective antagonist gp91ds-tat, delivered intrathecally, is sufficient to reduce inflammation and facilitate functional improvements (Khayrullina et al., 2015). In addition, systemic delivery of gp91ds-tat initiated 15 minutes post contusion SCI is sufficient to reduce markers of oxidative damage in young rats (Cooney et al., 2014). Differences in timing (15 minutes vs. 1 hour) and route (intrathecal vs. systemic) likely contribute to the discrepancies between the current work and these previous observations. Our observation of age-dependent treatment efficacy with APO does not indicate that NOX2 inhibition cannot be protective in young animals.
Previous observations of age-dependent macrophage ROS generation after SCI did not discriminate between microglia and MDMs (Cooney et al., 2014; Khayrullina et al., 2015; B. Zhang et al., 2016). In the current study, we differentiated between microglia vs. infiltrated MDM ROS production using anti-P2Y12 and anti-F4/80 antibodies (Villapol et al., 2017) and observed different responses between the two cell populations. In an Alzheimer’s disease model, infiltrated MDMs are only detectable with extreme age in the mouse brain, whereas activated microglia are observable with both normal aging and Aβ pathology (Martin et al., 2017). Microglia and infiltrated MDMs exhibit unique spatial distribution within the glial and fibrotic scar and exhibit distinct functions on scar formation and wound resolution after SCI (Orr and Gensel, 2018; Shechter et al., 2011; X. Wang et al., 2015; Zhu et al., 2014). Microglia phagocytize damaged tissue efficiently within 24 hours of SCI while infiltrated MDMs are responsible for phagocytosis after 3 dpi but are less efficient in processing CNS debris (Greenhalgh and David, 2014). Additionally, arginase-1, a well-characterized anti-inflammatory modulator of wound healing, is exclusively expressed in MDMs but not microglia in animal models of SCI and EAE (Greenhalgh et al., 2016). Given that we previously detected age-dependent decreases in arginase-1 after SCI (Fenn et al., 2014), it is likely that MDMs may be selectively prone to age-related changes in function. Collectively, these observations indicate that microglia vs. infiltrated MDMs have distinctive phenotypes in SCI and their activation might be differentially regulated by aging and/or disease and injury status.
After SCI, circulating blood monocytes increase at 24 hours and remain high at least up to 96 hours post-injury (Stirling and Yong, 2008). At the injury site, monocytes invade at 2–3 days and differentiate into macrophages then activated macrophages remain for months to years after trauma (Fleming et al., 2006). In the current study, we treated SCI mice with APO systemically and observed decreased MDM infiltration and ROS production in 14 MO animals. It is unclear whether the effects of APO were mediated through changes in the injury microenvironment or through modification of circulating monocytes prior to migration into the injury site. Since APO is blood-brain barrier (BBB) permeable, it is highly possible that APO treatment affected both compartments. Interestingly, mitochondrial ROS production increases with age in the spinal cord in the absence of injury (Yonutas et al., 2014). Our preliminary observations indicate increased ROS production in middle age vs. young, sham-injured mice (data not shown). Therefore, it is possible that APO-mediated decreases in age-dependent ROS production within the spinal cord, prior to MDM infiltration, contributed to the neuroprotection observed in the current study. In contrast, a recent study reported that NOX2 from circulating immune cells vs. NOX2 in the CNS disproportionately contributes to ischemic brain injury (Tang et al., 2011), highlighting the importance of NOX2 activity in peripheral immune cells. Indeed, APO can inhibit superoxide production in peripherally activated neutrophils and monocytes, as well as prevent the expression of TNF-α and cyclooxygenase-2 (COX-2) on circulating monocytes (Barbieri et al., 2004; Mattsson et al., 1996; Stolk et al., 1994). We detected decreased MDMs in the chronically injured aged spinal cord in response to APO treatment. Since the lifespan of a macrophage is 2 months, it is possible that APO-mediated decreases in MDMs at 28 dpi in the injured spinal cords, observed in the current study, are due to reduced recruitment acutely after injury. However, we cannot rule out the possibility that chemotactic gradients specific for MDMs persist and recur periodically, allowing sustained recruitment of new monocytes to the injury site. Since APO has been shown to reduce chemokine production (Bhatt et al., 2017), it may decrease MDM infiltration by modifying chemokine/cytokine production in the aged injured spinal cords.
In summary, the main finding of the current work is that 7 day constitutive treatment with the NOX2 inhibitor, APO, ameliorates age-related exaggerations of SCI tissue damage and functional deficits with concomitant decreases in MDM activation and MDM-ROS. The age-dependent treatment effect of APO likely acts through excessive ROS production mediated by NOX2 specifically on infiltrated macrophages. The importance of age is increasingly implicated in CNS disease and injury progression. Following stroke, accelerated glial reactivity, increased neutrophil infiltration and higher MMP-9 and ROS production are associated with increased hemorrhagic transformation and poor functional outcomes in aged mice (Badan et al., 2003; Ritzel et al., 2018). Age alters inflammatory processes closely associated with oxidative stress and repair in SCI (Fenn et al., 2014; Hooshmand et al., 2014; Khayrullina et al., 2015; B. Zhang et al., 2016; 2015a), thus manipulating SCI inflammation and ROS production may provide insights in developing therapeutic interventions to repair aged and injured CNS. Indeed, a recent report demonstrated that bone marrow rejuvenation can reduce the severity of ischemic stroke in aged mice (Ritzel et al., 2018). Our results suggest that age is a key determinant of SCI therapeutic efficacy, and pharmacologically reducing macrophage activation and ROS production is sufficient to reverse the age-associated impairments. Given the prevalence of microglia and monocyte activation in neuropathologies along with increased focus on the pathophysiology of the aged CNS, these data provide an innovative cellular perspective on understanding and treating age-related neurological disorders.
Supplementary Material
Highlights.
Apocynin selectively improves recovery in middle-aged, not young, SCI mice
Apocynin reduces monocyte-derived macrophage (MDM) ROS in middle-aged SCI mice
Apocynin decreases MDM accumulation in middle-aged injured spinal cord tissue
Monocyte-derived macrophages, not microglia, underlie age-dependent pathophysiology
Apocynin selectively improves recovery in middle-aged SCI mice;
Age potentiates ROS production in macrophages and microglia after SCI;
Acknowledgments:
We would like to thank Katelyn McFarlane for technical support.
Funding: This study was supported by the University of Kentucky and the Spinal Cord and Brain Injury Research Center, the Craig H. Neilsen Foundation (Postdoctoral fellowship to BZ), and NIH NINDS R01NS091582 (JCG).
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A, 2003. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab 23, 845–854. doi: 10.1097/01.WCB.0000071883.63724.A7 [DOI] [PubMed] [Google Scholar]
- Barbieri SS, Cavalca V, Eligini S, Brambilla M, Caiani A, Tremoli E, Colli S, 2004. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic Biol Med 37, 156–165. doi: 10.1016/j.freeradbiomed.2004.04.020 [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC, 1996. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139, 244–256. doi: 10.1006/exnr.1996.0098 [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, Mctigue DM, Popovich PG, 2006. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23, 635–659. doi: 10.1089/neu.2006.23.635 [DOI] [PubMed] [Google Scholar]
- Bhatt NP, Park J-Y, Lee HJ, Kim S-S, Kwon Y-S, Chun W, 2017. Apocynin protects mesangial cells from lipopolysaccharide-induced inflammation by exerting heme oxygenase 1-mediated monocyte chemoattractant protein-1 suppression. Int. J. Mol. Med 40, 1294–1301. doi: 10.3892/ijmm.2017.3090 [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL, 2014. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci 17, 131–143. doi: 10.1038/nn.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chéret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause K-H, Mallat M, 2008. Neurotoxic activation of microglia is promoted by a nox1dependent NADPH oxidase. Journal of Neuroscience 28, 12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BY, Kim J-H, Kho AR, Kim IY, Lee SH, Lee BE, Choi E, Sohn M, Stevenson M, Chung TN, Kauppinen TM, Suh SW, 2015. Inhibition of NADPH oxidase activation reduces EAE-induced white matter damage in mice. J Neuroinflammation 12, 104. doi: 10.1186/s12974-015-0325-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S-H, Aid S, Kim H-W, Jackson SH, Bosetti F, 2012. Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation. J Neurochem 120, 292–301. doi: 10.1111/j.1471-4159.2011.07572.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney SJ, Zhao Y, Byrnes KR, 2014. Characterization of the expression and inflammatory activity of NADPH oxidase after spinal cord injury. Free Radic. Res 48, 929–939. doi: 10.3109/10715762.2014.927578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Logu F, Nassini R, Materazzi S, Carvalho Gonçalves M, Nosi D, Rossi Degl’Innocenti D, Marone IM, Ferreira J, Li Puma S, Benemei S, Trevisan G, Souza Monteiro de Araújo D, Patacchini R, Bunnett NW, Geppetti P, 2017. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat Commun 8, 1887. doi: 10.1038/s41467-017-01739-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doverhag C, Keller M, Karlsson A, Hedtjarn M, Nilsson U, Kapeller E, Sarkozy G, Klimaschewski L, Humpel C, Hagberg H, Simbruner G, Gressens P, Sävman K, 2008. Pharmacological and genetic inhibition of NADPH oxidase does not reduce brain damage in different models of perinatal brain injury in newborn mice. Neurobiol Dis 31, 133–144. doi: 10.1016/j.nbd.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Fenn AM, Hall JCE, Gensel JC, Popovich PG, Godbout JP, 2014. IL-4 signaling drives a unique arginase+/IL-1β+ microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Rα after traumatic spinal cord injury. Journal of Neuroscience 34, 8904–8917. doi: 10.1523/JNEUROSCI.114614.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira APO, Rodrigues FS, Della-Pace ID, Mota BC, Oliveira SM, Velho Gewehr C de C, Bobinski F, de Oliveira CV, Brum JS, Oliveira MS, Furian AF, de Barros CSL, Ferreira J, Santos ARSD, Fighera MR, Royes LFF, 2013. The effect of NADPH-oxidase inhibitor apocynin on cognitive impairment induced by moderate lateral fluid percussion injury: role of inflammatory and oxidative brain damage. Neurochemistry International 63, 583–593. doi: 10.1016/j.neuint.2013.09.012 [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC, 2006. The cellular inflammatory response in human spinal cords after injury. Brain 129, 3249–3269. doi: 10.1093/brain/awl296 [DOI] [PubMed] [Google Scholar]
- Ghosh A, Kanthasamy A, Joseph J, Anantharam V, Srivastava P, Dranka BP, Kalyanaraman B, Kanthasamy AG, 2012. Anti-inflammatory and neuroprotective effects of an orally active apocynin derivative in pre-clinical models of Parkinson’s disease. J Neuroinflammation 9, 241. doi: 10.1186/1742-2094-9-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh AD, David S, 2014. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. Journal of Neuroscience 34, 6316–6322. doi: 10.1523/JNEUROSCI.4912-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh AD, Passos Dos Santos R, Zarruk JG, Salmon CK, Kroner A, David S, 2016. Arginase-1 is expressed exclusively by infiltrating myeloid cells in CNS injury and disease. Brain Behav Immun 56, 61–67. doi: 10.1016/j.bbi.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Hall ED, Wang JA, Bosken JM, Singh IN, 2016. Lipid peroxidation in brain or spinal cord mitochondria after injury. J. Bioenerg. Biomembr 48, 169–174. doi: 10.1007/s10863-0159600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schöneich C, Engelhardt JF, 2008. SOD1 mutations disrupt redoxsensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest 118, 659–670. doi: 10.1172/JCI34060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandes MS, Britto LRG, 2012. NADPH oxidase and neurodegeneration. Curr Neuropharmacol 10, 321–327. doi: 10.2174/157015912804143540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HHHW, Busse R, Schröder K, Brandes RP, 2008. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51, 211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214 [DOI] [PubMed] [Google Scholar]
- Hooshmand MJ, Galvan MD, Partida E, Anderson AJ, 2014. Characterization of recovery, repair, and inflammatory processes following contusion spinal cord injury in old female rats: is age a limitation? Immun Ageing 11, 15. doi: 10.1186/1742-4933-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impellizzeri D, Mazzon E, Esposito E, Paterniti I, Bramanti P, Cuzzocrea S, 2011. Effect of Apocynin, an inhibitor of NADPH oxidase, in the inflammatory process induced by an experimental model of spinal cord injury. Free Radic. Res 45, 221–236. doi: 10.3109/10715762.2010.526604 [DOI] [PubMed] [Google Scholar]
- Johnstone JT, Morton PD, Jayakumar AR, Johnstone AL, Gao H, Bracchi-Ricard V, Pearse DD, Norenberg MD, Bethea JR, 2013. Inhibition of NADPH oxidase activation in oligodendrocytes reduces cytotoxicity following trauma. PLoS ONE 8, e80975. doi: 10.1371/journal.pone.0080975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayrullina G, Bermudez S, Byrnes KR, 2015. Inhibition of NOX2 reduces locomotor impairment, inflammation, and oxidative stress after spinal cord injury. J Neuroinflammation 12, 172. doi: 10.1186/s12974-015-0391-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ, 2012. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging doi: 10.1016/j.neurobiolaging.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leden, von RE, Khayrullina G, Moritz KE, Byrnes KR, 2017. Age exacerbates microglial activation, oxidative stress, inflammatory and NOX2 gene expression, and delays functional recovery in a middle-aged rodent model of spinal cord injury. J Neuroinflammation 14, 161. doi: 10.1186/s12974-017-0933-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Stoica BA, Byrnes KR, Jeong W, Faden AI, 2013. Activation of mGluR5 and inhibition of NADPH oxidase improves functional recovery after traumatic brain injury. J Neurotrauma 30, 403–412. doi: 10.1089/neu.2012.2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander PK, Jekabsone A, Brown GC, 2006. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J Immunol 176, 1046–1052. [DOI] [PubMed] [Google Scholar]
- Martin E, Boucher C, Fontaine B, Delarasse C, 2017. Distinct inflammatory phenotypes of microglia and monocyte-derived macrophages in Alzheimer’s disease models: effects of aging and amyloid pathology. Aging Cell 16, 27–38. doi: 10.1111/acel.12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson E, Van Dijk H, Van Kessel K, Verhoef J, Fleer A, Rollof J, 1996. Intracellular pathways involved in tumor necrosis factor-alpha release by human monocytes on stimulation with lipopolysaccharide or staphylococcal peptidoglycan are partly similar. J. Infect. Dis 173, 212–218. [DOI] [PubMed] [Google Scholar]
- Mawhinney LA, Thawer SG, Lu W-Y, Rooijen NV, Weaver LC, Brown A, Dekaban GA, 2012. Differential detection and distribution of microglial and hematogenous macrophage populations in the injured spinal cord of lys-EGFP-ki transgenic mice. J Neuropathol Exp Neurol 71, 180–197. doi: 10.1097/NEN.0b013e3182479b41 [DOI] [PubMed] [Google Scholar]
- McKinley W, Cifu D, Seel R, Huang M, Kreutzer J, Drake D, Meade M, 2003. Age-related outcomes in persons with spinal cord injury: a summary paper. NeuroRehabilitation 18, 83–90. [PubMed] [Google Scholar]
- Nayernia Z, Jaquet V, Krause K-H, 2014. New insights on NOX enzymes in the central nervous system. Antioxid. Redox Signal 20, 2815–2837. doi: 10.1089/ars.2013.5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MB, Gensel JC, 2018. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics 15, 541–553. doi: 10.1007/s13311-018-0631-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MB, Simkin J, Bailey WM, Kadambi NS, McVicar AL, Veldhorst AK, Gensel JC, 2017. Compression Decreases Anatomical and Functional Recovery and Alters Inflammation after Contusive Spinal Cord Injury. J Neurotrauma 34, 2342–2352. doi: 10.1089/neu.2016.4915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Hong J-S, Crews FT, 2013. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia 61, 855–868. doi: 10.1002/glia.22479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L-L, Ji M-H, Zhang H, Yang J-J, Sun X-R, Tang H, Wang J, Liu W-X, Yang JJ, 2016. NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav Immun 51, 109–118. doi: 10.1016/j.bbi.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Quinn R, 2005. Comparing rat”s to human”s age: how old is my rat in people years? Nutrition 21, 775–777. doi: 10.1016/j.nut.2005.04.002 [DOI] [PubMed] [Google Scholar]
- Ritzel RM, Lai Y-J, Crapser JD, Patel AR, Schrecengost A, Grenier JM, Mancini NS, Patrizz A, Jellison ER, Morales-Scheihing D, Venna VR, Kofler JK, Liu F, Verma R, McCullough LD, 2018. Aging alters the immunological response to ischemic stroke. Acta Neuropathol. doi: 10.1007/s00401-018-1859-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE, 2003. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma 20, 179–193. doi: 10.1089/08977150360547099 [DOI] [PubMed] [Google Scholar]
- Scivoletto G, Morganti B, Ditunno P, Ditunno JF, Molinari M, 2003. Effects on age on spinal cord lesion patients’ rehabilitation. Spinal Cord 41, 457–464. doi: 10.1038/sj.sc.3101489 [DOI] [PubMed] [Google Scholar]
- Shechter R, Raposo C, London A, Sagi I, Schwartz M, 2011. The glial scar-monocyte interplay: a pivotal resolution phase in spinal cord repair. PLoS ONE 6, e27969. doi: 10.1371/journal.pone.0027969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance, 2016. Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance. J Spinal Cord Med 39, 493–494. doi: 10.1080/10790268.2016.1210925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanska J, Pawliczak R, 2008. Apocynin: molecular aptitudes. Mediators Inflamm. 2008, 106507. doi: 10.1155/2008/106507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling DP, Yong VW, 2008. Dynamics of the inflammatory response after murine spinal cord injury revealed by flow cytometry. J Neurosci Res 86, 1944–1958. doi: 10.1002/jnr.21659 [DOI] [PubMed] [Google Scholar]
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ, 1994. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol 11, 95–102. doi: 10.1165/ajrcmb.11.1.8018341 [DOI] [PubMed] [Google Scholar]
- Stout RD, Suttles J, 2005. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol. Rev 205, 60–71. doi: 10.1111/j.0105-2896.2005.00260.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XN, Zheng Z, Giffard RG, Yenari MA, 2011. Significance of marrow-derived nicotinamide adenine dinucleotide phosphate oxidase in experimental ischemic stroke. Ann Neurol 70, 606–615. doi: 10.1002/ana.22476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, Loane DJ, Burns MP, 2017. Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 65, 1423–1438. doi: 10.1002/glia.23171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ma MW, Dhandapani KM, Brann DW, 2017. Regulatory role of NADPH oxidase 2 in the polarization dynamics and neurotoxicity of microglia/macrophages after traumatic brain injury. Free Radic Biol Med 113, 119–131. doi: 10.1016/j.freeradbiomed.2017.09.017 [DOI] [PubMed] [Google Scholar]
- Wang X, Cao K, Sun X, Chen Y, Duan Z, Sun L, Guo L, Bai P, Sun D, Fan J, He X, Young W, Ren Y, 2015. Macrophages in spinal cord injury: phenotypic and functional change from exposure to myelin debris. Glia 63, 635–651. doi: 10.1002/glia.22774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonutas HM, Pandya JD, Sullivan PG, 2014. Changes in mitochondrial bioenergetics in the brain versus spinal cord become more apparent with age. J. Bioenerg. Biomembr doi: 10.1007/s10863-014-9593-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Bailey WM, Braun KJ, Gensel JC, 2015a. Age decreases macrophage IL-10 expression: Implications for functional recovery and tissue repair in spinal cord injury. Exp Neurol 273, 83–91. doi: 10.1016/j.expneurol.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Bailey WM, Kopper TJ, Orr MB, Feola DJ, Gensel JC, 2015b. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflammation 12, 218. doi: 10.1186/s12974-015-0440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Bailey WM, McVicar AL, Gensel JC, 2016. Age increases reactive oxygen species production in macrophages and potentiates oxidative damage after spinal cord injury. Neurobiol. Aging 47, 157–167. doi: 10.1016/j.neurobiolaging.2016.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q-G, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, Brann DW, 2012. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS ONE 7, e34504. doi: 10.1371/journal.pone.0034504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Soderblom C, Krishnan V, Ashbaugh J, Bethea JR, Lee JK, 2014. Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol Dis 74C, 114–125. doi: 10.1016/j.nbd.2014.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.