Abstract
Lysophosphatidic acid (LPA) is an extracellular lipid mediator involved in many physiological functions by signaling through six known G-protein-coupled receptors (LPA1-LPA6). In the central nervous system (CNS), LPA mediates a wide range of effects, including neural progenitor cell physiology, astrocyte and microglia activation, neuronal cell death, axonal retraction, and contributions to pain, schizophrenia and hydrocephalus. We recently reported that LPA-LPA1 signaling mediates functional deficits and myelin loss after spinal cord injury (SCI). Here, we provide clear evidence on the deleterious contribution of another LPA receptor, LPA2, to myelin loss after SCI. We found that LPA2 is constitutively expressed in the spinal cord parenchyma and its transcripts were up-regulated after contusion injury, in part, by microglial cells. We also found that the demyelinating lesion triggered by intraspinal injection of LPA into the undamaged spinal cord was markedly reduced in the lack of LPA2. Similarly, LPA2 deficient mice showed enhanced motor skills and myelin sparing after SCI. To gain insights into the detrimental actions of LPA2 in spinal cord we performed cell culture studies. These experiments revealed that, similar to LPA1, activation of microglia LPA2 led to oligodendrocyte cell death. Moreover, we also found that the cytotoxic effects underlaying microglial LPA-LPA2 axis were mediated by the release of purines by microglia and the activation of P2X7 receptor on oligodendrocytes. Overall, this study provides new mechanistic insights into how LPA contributes to SCI physiopathology, and suggest that targeting LPA2 could be a novel therapeutic approach for the treatment of acute SCI.
Keywords: Demyelination, Lysophosphatidic Acid, Inflammation, Neurodegeneration, Microglia, Spinal Cord Injury
INTRODUCTION
Traumatic spinal cord injury (SCI) is a major cause of disability that results in a functional decline due to disruption of axonal pathways and death of neurons and oligodendrocytes (David et al., 2012; Hilton et al., 2017). There are two waves of tissue degeneration after SCI which are known as a primary and secondary injury. The first is caused by the mechanical damage to the spinal cord parenchyma followed by the activation of several cellular and molecular events that take place in the spinal cord tissue from hours to weeks after trauma (David et al., 2012; Hilton et al., 2017). Primary injury cannot be avoided, however, the mechanisms participating in secondary injury can be substantially abrogated or even blocked, providing some options to minimize secondary degeneration and functional loss.
LPA is an extracellular lipid mediator with a wide range of biological functions. LPA receptors are expressed in almost all cells of the nervous system (Choi and Chun, 2013; Choi et al., 2010; Yung et al., 2015). The different responses of LPA in neural cells include neuronal cell death (Holtsberg et al., 1998), axonal retraction (Tigyi et al., 1996), inhibition of oligodendrocyte maturation (Dawson et al., 2003), and proliferation of astrocytes and mouse microglial cells (Shano et al., 2008; Sorensen et al., 2003). Moreover, LPA has been related with some nervous system pathologies such as fetal hydrocephalus (Yung et al., 2011; Yung et al., 2014), psychiatric diseases (Harrison et al., 2003; Roberts et al., 2005), neuropathic pain (Halder et al., 2013; Inoue et al., 2004; Ma et al., 2009), and tissue damage after trauma to the spinal cord (Goldshmit et al., 2012; Santos-Nogueira et al., 2015)and brain (Crack et al., 2014). We recently reported that LPA triggers demyelination after SCI by signaling, in part, via microglia LPA1(Santos-Nogueira et al., 2015). However, considering the pleiotropic effects of LPA and the variety of receptors with which it interacts, it is possible that LPA could exert beneficial or detrimental actions in SCI depending on the receptor it signals through, as previously observed with other lipid mediators, such as prostaglandins (Kawano et al., 2006; Kerr et al., 2008; Liang et al., 2011; Redensek et al., 2011).
In the present work we provide evidence for the first time that the LPA-LPA2 axis contributes to the physiopathology of SCI. In particular, we found that mice lacking LPA2 are protected against demyelination triggered by intraspinal injection of LPA and by SCI. Our in vitro work also reveals that LPA2 stimulation in oligodendrocytes does not cause cell death. However, activation of microglia LPA2 leads to release of purines that mediate cytotoxic effects on oligodendrocyte via P2X7.
MATERIAL AND METHODS
Animal genotyping
PCR analysis was used to detect the presence of WT and lpar2 mutated allele. For tissue sampling, the tip of the tail was cut at the time of weaning the mice. Genomic DNA extraction was carried out by using the ArchivePure DNA Purification System (5PRIME), as described by the manufacturer. Allele amplification was performed by PCR reaction, using the Taq DNA Polymerase kit (Invitrogen), and PCR products were analyzed by standard electrophoresis in 1% agarose gels. Primers sequences were the following: LPA2 forward 5′- AGTGTGCTGGTATTGCTGACCA −3´, LPA2 reverse 5′- CTCTCGGTAGCGGGGATGG −3´, LPA2 mutated 5′- CAGCTGGGGCTCGACTAGAGGAT −3′. Product sizes for WT and targeted alleles were 576 bp and 328 bp, respectively.
Surgical procedure
All surgical procedures were approved by the Universitat Autònoma de Barcelona Animal Care Committee and followed the guidelines of the European Commission on Animal Care. Adult female (8–10 weeks old) LPA2 deficient mice (C57BL/6J X129X1/ SvJ background; provided by Dr Jerold Chun) or wildtype littermates (WT) were anesthetized with an intramuscular injection of a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg). After performing a laminectomy at the 11th thoracic vertebrae, the exposed spinal cord was either intraspinally injected or contused.
Intraspinal injections were performed using a glass needle (30 μm internal diameter, Eppendorf, Hamburg, Germany) coupled to a 10 ml Hamilton syringe (Hamilton #701, Hamilton Co, Reno, NV, USA). 1 μl of saline containing 5 nmoles of 18:1 LPA (Avantis Polar Lipids, AL, USA) or sterile saline alone was injected in the dorsal funiculi of intact spinal cords. Injections were made at a perfusion speed of 1 μl/min controlled by an automatic injector (KDS 310 Plus, Kd Scientific, Holliston, MA, USA), and the tip of the needle was maintained inside the cord tissue 3 min after each injection to avoid liquid reflux. The surgeon was blinded to the injection groups.
Spinal cord contusion injuries were performed using the Infinite Horizon Impactor device (Precision Scientific Instrumentation) (Sheff et al., 2003), using a force of 50 kdynes and tissue displacement ranging between 400 and 600 μm (Francos-Quijorna et al., 2017). The surgeon was blinded to the experimental groups.
Functional assessment
Locomotor recovery was evaluated at 1, 3, 5, 7, 10, 14, 21 and 28 dpi in an open-field test using the nine-point Basso Mouse Scale (BMS) (Basso et al., 2006). The BMS analyses hindlimb movements and coordination was performed by two independent assessors who were blinded to the experimental groups and the consensus score taken.
The highest locomotion speed of the mice was also tested at day 28 post-injury. Animals were placed on a belt of a motorized treadmill (DigiGait™ Imaging System, Mouse Specifics, Boston, MA). Each mouse was allowed to explore the treadmill compartment, with the motor speed set to zero, for 5min. Then speed was gradually increased from 0 up to 35 cm/s and stopped at the maximum speed at which each mouse was able to run. Maximum speed was that in which animals were able to perform for at least 5 seconds (Coll-Miro et al., 2016; Francos-Quijorna et al., 2017). Mice that refuse to locomote in the treadmill were excluded.
Electrophysiological analysis
Electrophysiological tests were performed to assess spared motor central pathways after SCI. Motor evoked potentials (MEPs) were recorded from the tibialis anterior and gastrocnemius muscles with microneedle electrodes after transcranial electrical stimulation of the motor cortex by single rectangular pulses of 0.1 ms duration (Grass S88). Pulses were delivered through needle electrodes inserted subcutaneously, the cathode over the skull, overlaying the sensoriomotor cortex, and the anode at the nose (Garcia-Alias et al., 2003).
Histology
Mice were deeply anaesthetized using Dolethal (pentobarbital sodium; Vetoquinol E. V. S. A.) and perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB) at 4 days post-injection or at 28 days post-contusion. Seven mm length of spinal cord containing the injection or the lesion site centered was harvested, post-fixed for 1 hour in 4% paraformaldehyde in 0.1 M PB and cryoprotected with 30% sucrose in 0.1 M PB at 4°C, for a minimum of 48h. Spinal cords were fast-frozen at −60°C in cryoembedding compound (Tissue-Tek® OCT, Sakura) and cut on a cryostat (Leica). Ten series of 10 μm-thick transverse sections were picked up on glass slides, so adjacent sections on the same slide were 100 μm apart. For assessing demyelination after intraspinal injection, tissue sections were gradually dehydrated and placed in a 1 mg/ml Luxol fast blue (LFB) solution in 95% ethanol and 0.05% acetic acid overnight at 37°C. Then, sections were placed into a solution of 0.5 mg/ml Li2CO3 in distilled water for 1 min at RT, washed, dehydrated and mounted in DPX mounting media (Sigma).
For histological analysis after spinal cord injury, sections were stained using FluoroMyelin Green fluorescent myelin stain (Invitrogen) for assessing myelin loss. Briefly, tissue sections were rehydrated in PBS and incubated with FluoroMyelin 1:300 in PBS for 20 min at room temperature (RT). Then sections were washed and mounted in Mowiol mounting media containing DAPI (1 μg/ml; Sigma). Tissue sections were viewed with Olympus BX51 microscope and images were captured using an Olympus DP50 digital camera attached to it and using the Cell^A Image acquisition software.
The epicenter of the injection or contusion injury impact was determined for each mouse spinal cord by localizing the tissue section with the greatest demyelination (Santos-Nogueira et al., 2015). The NIH ImageJ software was used to quantify the histological parameters.
Flow cytometry
Fluorescent-activated cell sorting protocol (FACS)analysis were done as described previously (Amo-Aparicio et al., 2018) to quantify different immune cell populations in the injured spinal cord). Briefly, at 7 days post-injury, WT and LPA2 null mice were perfused with ice-cold HBSS without Ca2+/Mg2 (Gifco) to eliminate blood. A piece of 1 cm of spinal cord with the lesion site centered was harvested, cut in small pieces and enzymatically digested for 30 minutes at 37°C in an tube containing 1ml of 0.1% collagenase (Sigma) and 0.1% DNase (Roche) in HBSS without Ca2+/Mg2+, and then, passed through a 70 μm cell strainer (BD Bioscience Discovery Labware). The cell suspension obtained was centrifuged at 500×g for 10 min at 4 °C. Samples were then divided and incubated with a combination of antibodies against several extracellular immune cell markers or isotype-matched controls. Isotype control antibodies were purchased from eBioscience and included phycoerythrin– cyanine 7 (PE–Cy7)-labeled rat IgG2b, adenomatous polyposis coli (APC)-labeled rat IgG2b, peridinin chlorophyll-a protein cyanine 5.5 (PerCP–Cy5.5)-conjugated rat IgG2b, phycoerythrin-conjugated (PE) rat IgM (1:100). The specific antibodies were also purchased from eBioscience and included: CD45–PerCP–Cy5.5, CD11b–PE–Cy7, F4/80–APC, Ly6G-PE (1:100). After 1 h of incubation with combinations of antibodies at 4 °C, samples were washed and fixed in 1% paraformaldehyde. Microglial cells were identified as CD45low and CD11b+cells. Peripheral macrophages were identified as CD45high, CD11b+, F4/80+, whereas neutrophils were identified as CD45high, CD11b+, F4/80-, Ly6Ghigh (Francos Quijorna et al., 2017; Amo-Aparicio et al., 2018). FACS samples contained at least 3000 CD45+ cells per tube were analyzed using FlowJo software on a FACSCanto flow cytometer (BD Biosciences). Four mice per group were used in FACS analysis.
Cell sorting microglia from adult CNS tissue.
Briefly, spinal cord and brain from adult C57/Bl6 mice (8–10 weeks old) were removed and enzymatically digested with a collagenase B 0.2% (Roche Diagnostics GmbH) and trypsin-EDTA 0.2% at 37°C for 30 min, and then passed through a cell strainer of 40 μm (BD falcon), and cell suspension centrifuged twice at 300g for 10 minutes at 4°C. Microglia were isolated by magnetic sorting using a CD11b antibody (MiltenyiBiotec) and then stained with PerCP-Cy5.5-conjugated CD45 and PE-Cy7-conjugated CD11b antibodies for further purification on cell sorter (FACSARIATM III, BD Bioscience). Microglia cells were assessed on a flow cytometer (FACSCalibur; BD Biosciences), and only samples showing population >90% purity were used for gene expression analysis.
Cell Cultures
Oligodendrocyte primary cultures
Primary oligodendrocyte progenitor cells (OPCs) were isolated from the cerebral cortices of WT littermate of LPA2 deficient mice at 2–4 post-natal days (P2–4) as described previously (O’Meara et al., 2011; Santos-Nogueira et al., 2015). Briefly, after removal of the meninges, cortical tissue was minced and dissociated by incubating in a solution of 1.54 mg/ml papain (Worthington Biochemical), 400 μg/ml L-cystein (Sigma) and 1 mg/ml DNase I (Roche) in MEM (Gibco) for 20 min at 37°C. Mixed glial culture media (MGCM; 10% inactivated and filtered fetal bovine serum (FBS, Sigma), 0.33% penicillin-streptomycin (P/S, Sigma) and 1% Glutamax 100x (Gibco) in DMEM (Gibco)) was added to stop papain and DNase I activity. Cells were plated into T25 tissue culture flasks coated with 10 μg/ml poly-D-lysine (Sigma) for 1h at 37°C and cultured at 37°C in an humidified incubator with 5% CO2 supplementation. Three hours after plating, the floating cells were discarded by replacing the medium. Two thirds of the MGCM was replaced every 3 days with new MGCM supplemented with 5 μg/ml insulin (Seralab). Nine days later, OPCs were harvested by shaking at 37°C at 220 rpm overnight. The collected cells were plated into 10 μg/ml poly-D-lysine coated 24 well plates with DMEM (Gibco) supplemented with 1% Glutamax 100x, 2% B27 (Gibco), 0.5% FBS, 50 pg/ml recombinant mouse ciliary-neurotrophic factor (CNTF, BioTrend) and 1% OL supplement (10 μl/ml N-2 supplement 100x (Gibco), 10 mg/ml bovine serum albumin (BSA, Sigma) and 40 μg/ml 3,3’,5-triiodo-L-thyronine (Sigma)). OPC maturation in oligodendrocytes was achieved after 7 days in vitro (DIV) and for maintenance of the culture half of the medium was changed every 2–3 days.
Microglial cells
Primary microglial cells were isolated from LPA2 null P4 mice or wildtype littermate cerebral cortices as described previously (Santos-Nogueira et al., 2015; Saura et al., 2003). Tissue dissociation and cell isolation was performed as in OPC cultures. However, mixed glial cultures were prepared into T25 tissue culture flasks with no coating. Cells were seeded at a density of 300.000 cells/ml in DMEM-F12 (Gibco) with 10% FBS and 5% P/S (MG medium), and cultured at 37°C in a humidified incubator with 5% CO2 supplementation. Medium was replaced every 4–5 days and confluency was achieved after 10–12 DIV. At this point, mixed cultures were incubated with 0.25% trypsin-EDTA (Gibco) diluted 1:4 in DMEM-F12 for 30 min at 37°C. This mild trypsinization resulted in the detachment of the upper layer of cells in one piece. The remaining adhered microglial cells were cultured for 24h before starting experiments.
For the generation of microglial conditioned medium, WT and LPA2 null microglial cells were incubated for 24h in new medium with DMSO (control) and 1 μM LPA (Avantis Polar Lipids)). Then, this culture medium was replaced with new oligodendrocyte culture medium, leading microglial cells to continue releasing factors induced by the treatment for 24 hours. Afterwards, the microglia conditioned medium (MCM) was centrifuged and frozen and applied to oligodendrocytes for assessment of cell death
Assessment of oligodendrocyte cell death
To assess whether oligodendrocyte LPA2 signaling leads to cell death, WT and LPA2 null mice oligodendrocytes were exposed to 1 μM of LPA for 24h. Oligodendrocytes were fixed in 4% paraformaldehyde for 20 min washed in 0.1 M PBS and incubated overnight with rat anti-MBP antibody (1:200, Abcam) diluted in BB. After several washes in PBST, cells were incubated with anti-rat Alexa 594-conjugated secondary antibody diluted in BB for 1h at RT, and then mounted in Mowiol mounting media containing DAPI. Oligodendrocyte survival was determined by counting the number of nucleated MBP+ cells.
To study the involvement of microglia LPA2 on oligodendrocyte cell death, oligodendrocytes culture from WT mice were exposed for 24h to MCM obtained from WT and LPA2 null microglia stimulated with LPA. The MCM was supplemented with 30 μM CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) (Alomone Labs, Jerusalem, Israel) or 50 nM BBG (Brillian Blue G) (Sigma Aldrich) to block the AMPA/Kainate and P2X7 receptors in oligodendrocytes, respectively.
Assessment of cytokine and nitrate levels
Conditioned media from non-stimulated and LPA-stimulated microglia were collected as described above. Cytokine protein levels in the supernatants were then analyzed using the Milliplex MAP Mouse Cytokine/Chemokine magnetic bead panel (Millipore) on a Luminex (Millipore) as per manufacturers’ protocol to assess the levels of 20 cytokines (FGF, GM-CSF, IFN gamma, IL-1 alpha, IL-1 beta, IL-10, IL-12, IL-13, IL-17, IL-2, IL-4, IL-5, IL-6, IP-10, KC, MCP-1, MIG, MIP-1 alpha, TNF alpha, VEGF. For assessment of nitric oxide metabolites in MCM, samples were measured by Nitrate/Nitrite Colorimetric assay kit (Cayman Chemical, Ann Arbor, MI) as per manufacturers’ protocol
RNA isolation, reverse transcription and real-time PCR
For spinal cord mRNA extraction, mice were perfused with sterile saline and 5mm length of uninjured or injured spinal cord centered at the impact site was harvested at 1, 3, 7, 14, 21 and 28 dpi. Tissue was homogenized with QIAzol lysis reagent (Qiagen) and RNA extracted using RNeasy Lipid Tissue kit (Qiagen), according to the manufacturer’s protocol. mRNA from cultured and in vivo sorted microglia was extracted using RNeasy Mini Tissue kit (Qiagen), according to the manufacturer’s protocol. RNA was treated with DNaseI (Qiagen) to eliminate genomic DNA contamination. 1 μg of obtained RNA was primed with random hexamers (Promega) and reverse transcribed using Omniscript RT kit (Qiagen). RNase inhibitor (Roche) was added (1 U/μl final concentration) to avoid RNA degradation. Real Time (RT)-PCR analysis was performed using a MyiQ Single-Color Real-Time PCR Detection System (BIO RAD). The GAPDH housekeeping gene was selected as the reference gene. RT-PCR reactions were performed using the Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies) according to the manufacturer’s instructions. Primer sequences included the following: LPA2 forward 5’- CTCACTGGTCAATGCAGTGGTATAT-3’, LPA2 reverse 5’- GAAGGCGGCGGAAGGT-3’. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene: GAPDH forward 5’- TCAACAGCAACTCCCACTCTTCCA-3’,GAPDH reverse 5’- ACCCTGTTGCTGTAGCCGTATTCA-3’. The amount of cDNA was calculated based on the threshold cycle (CT) value, and was standardized by the amount of housekeeping gene using the 2-ΔΔCT method (Livak and Schmittgen, 2001).
Statistical analysis
All analyses were conducted through IBM SPSS Statistics v19. Two-tailed Student’s t test was used for the single comparison between two groups. Functional follow-ups for BMS score and histopathological data was analyzed using two-way repeated measure (RM)-ANOVA with post hoc Bonferroni’s test. The rest of the data were analyzed using one-way ANOVA with post hoc Bonferroni’s test. P values for multiple comparisons were adjusted using Bonferroni’s correction. Results are expressed as mean ± SEM. Differences were considered significant at P < 0.05.
RESULTS
Expression of LPA2 in the injured and uninjured mouse spinal cord
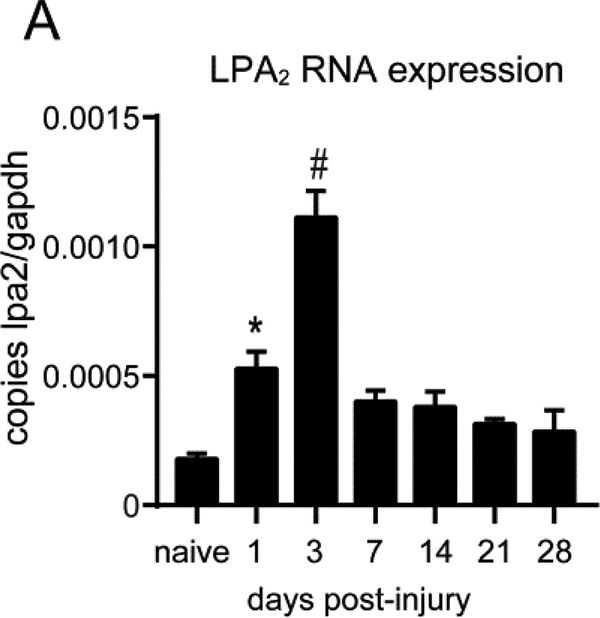
We first assessed the changes in mRNA levels of LPA2 in the spinal cord parenchyma after contusion injury by using real time PCR. We found that LPA2 was constitutively expressed in the spinal cord and its levels were notably up-regulated for the first 3 days following injury, reaching the peak expression at day 3 (one-way ANOVA p=0.022 at day 1 and p<0.001 at day 3 vs naive; n=3 per group) (Fig. 1A).
Figure 1. LPA2 mRNA expression is up-regulated in the injured spinal cord.
Real Time-PCR quantification of LPA2 mRNA levels from spinal cords harvested at different time points after contusion injury. (*p<0.05, #p<0.01; n=3 per time point). Error bars indicate SEM.
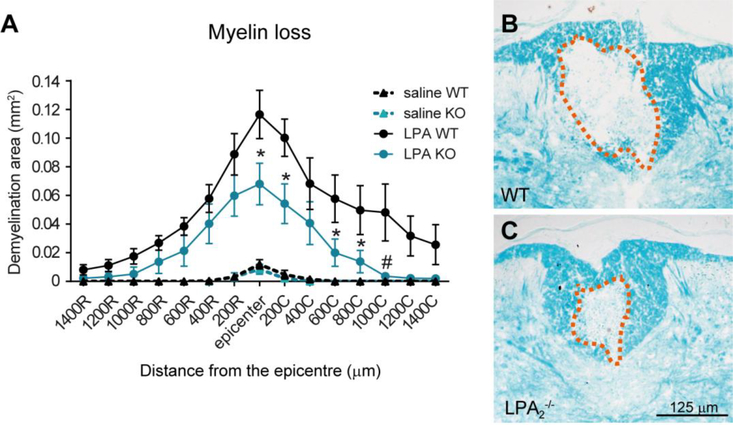
We previously reported that increasing the local levels of LPA in the intact spinal cord resulted in demyelination which was partially, but not totally, depending on LPA1 signaling (Santos-Nogueira et al., 2015). We therefore tested whether LPA-LPA2 axis also evoked a demyelinating lesion. To carry out these experiments we injected 5 nmoles of LPA or sterile saline (control) into the dorsal column of intact spinal cords of WT and LPA2 deficient mice. These experiments revealed that the demyelinating lesion caused by LPA in the spinal cord was substantially reduced in the lack of LPA2, indicating that, similar to LPA1, LPA2 activation by LPA also contributes to myelin loss in the adult intact spinal cord (two-way RM ANOVA; p<0.05; n=4 WT + saline, n=4 LPA2 null + saline, n=8 WT + LPA, n=7 LPA2 null + LPA) (Fig 2A-C). Post-hoc Bonferroni’s test analysis revealed that LPA-mediated demyelination was reduced in LPA2 null mice at the injection site, as well as, at 200, 600, 800 and 1000 μm caudal to the lesion epicenter as compared to WT mice.
Figure 2. Lack of LPA2 reduces LPA-triggered demyelination.
(A) Quantification of myelin loss at various distances rostral and caudal to the injury epicenter. (B, C) Representative micrographs showing myelin loss at the injury epicenter in WT (B) and LPA2 deficient (C) mice spinal cords injected with 5 nmoles of LPA. (*p<0.05, #p<0.01; n=4 WT + saline, n=4 LPA2 null + saline, n=8 WT + LPA, n=7 LPA2 null + LPA). Error bars indicate SEM. Scale bar=125 μm.
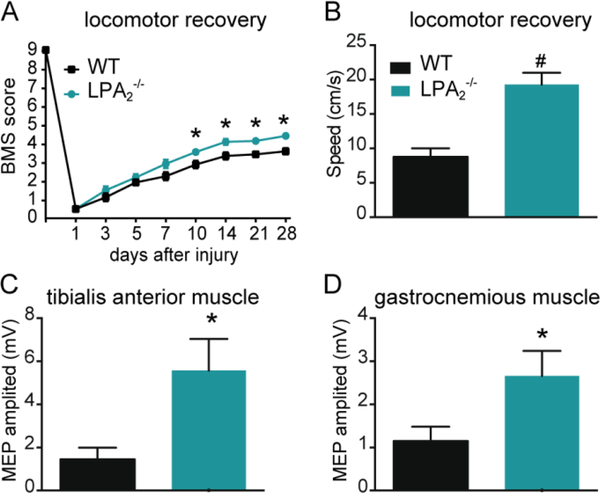
Myelin loss is a pathological hallmark that contributes to functional deficits after SCI. We therefore examined whether LPA2 activation after SCI leads to locomotor impairments and demyelination. For this purpose, we performed spinal cord contusion injuries in mice lacking LPA2 and WT littermates. These experiments revealed that the lack of LPA2 conferred protection against neurological impairments as revealed by locomotion assessment in an open field using the BMS score (p<0.05; two-way RM-ANOVA; n=12 for WT and 11 for LPA2 deficient mice). Post-hoc Bonferroni’s test analysis revealed that BMS scores were significantly enhanced in LPA2 null mice starting from day 10 post-injury until the end of the follow up (day 28 post-injury) (Fig. 3A). At this time point, all WT mice performed plantar paw placement and only 50% showed occasional stepping. In contrast, all LPA2 null mice performed occasional stepping and 55% of them displayed frequent stepping without coordination. LPA2 null mice were able to locomote at significant faster speeds than WT littermate mice upon forced locomotion on a treadmill (p<0.001; t test; n=8 per group) (Fig 3B). In line with the locomotor skills, electrophysiological assessment revealed higher MEP amplitudes for the tibialis anterior and gastrocnemius muscles in LPA2 null mice as compared to WT littermates (p=0.01 and 0.02, respectively; t test; n=12 for WT and 11 for LPA2 deficient mice) (Fig 3C-D), suggesting greater preservation of motor descending pathways.
Figure 3. Lack of LPA2 promotes functional recovery after SCI.
(A) Locomotor recovery after spinal cord contusion injury in WT and LPA2 null mice using the 9-point Basso Mouse Scale. The lack of LPA2 led to significant improvement in locomotor performance compared to WT mice (*p<0.05; n=12 for WT and 11 for LPA2 deficient mice). (B) The ability to run at a constant speed was evaluated at 28 dpi by placing mice onto the belt of a motorized treadmill. Note that mice lacking LPA2 were able to run at faster speeds. (#p<0.01; n=8 per group). (C, D) Preservation of descending axonal tracts after SCI was evaluated by registering the motor evoked potentials (MEPs) from tibialis anterior and gastrocnemius muscles. Mice lacking LPA2 showed higher MEP amplitudes for both muscles. (*p<0.05; n=12 for WT and 11 for LPA2 deficient mice). Error bars indicate SEM.
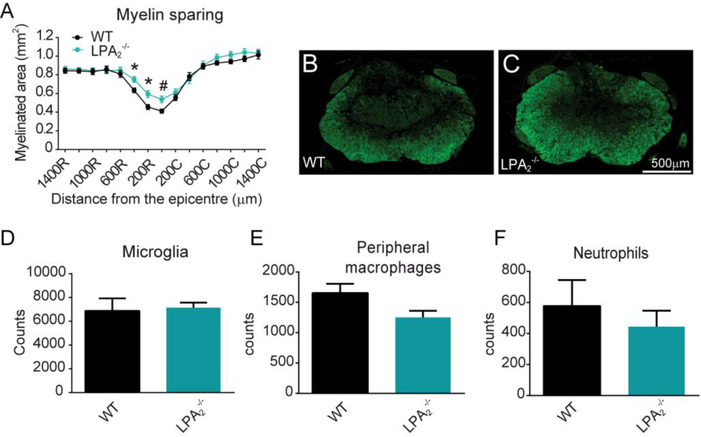
We then assessed whether enhancement in motor skills observed in mice lacking LPA2 was associated with amelioration of demyelination. Histological sections spinal cords stained against fluoromyelin revealed that LPA2 null mice had greater myelin preservation at the injury epicenter and in adjacent rostral regions than WT littermates (p<0.001; two-way ANOVA; n=12 for WT and 11 for LPA2 deficient mice) (Fig 4A-C).
Figure 4. The lack of LPA2 results in reduced secondary damage after SCI.
(A) Quantification of myelin sparing at various distances rostral and caudal to the injury epicenter. (B, C) Representative micrographs of spinal cord tissue at the injury showing myelin sparing at the injury epicenter in WT (B) and LPA2 deficient (C) mice. (*p<0.05, #p<0.01 vs WT; n=12 for WT and 11 for LPA2 deficient mice). Error bars indicate SEM. Scale bar=500 μm. (D-E) Quantification of microglia, peripheral macrophages and neutrophils in the contused spinal cord of WT and LPA2 deficient mice at day 7 post-injury. (n=4 per group)
We finally studied whether LPA2 signaling modulates immune cell counts in the injured spinal cord. FACS analysis of contused spinal cord harvested at 7 days postinjury, revealed that similar to LPA1 (Santos-Nogueira et al., 2015) the counts of microglia, macrophages from peripheral origin and neutrophils were not affected by the lack of LPA2 signaling (Fig 4D-F). Overall, these experiments provide clear evidence that LPA-LPA2 axis leads to myelin loss and functional deficits after SCI without modulating immune cell counts.
Microglia LPA2 induces oligodendrocyte cell death in vitro
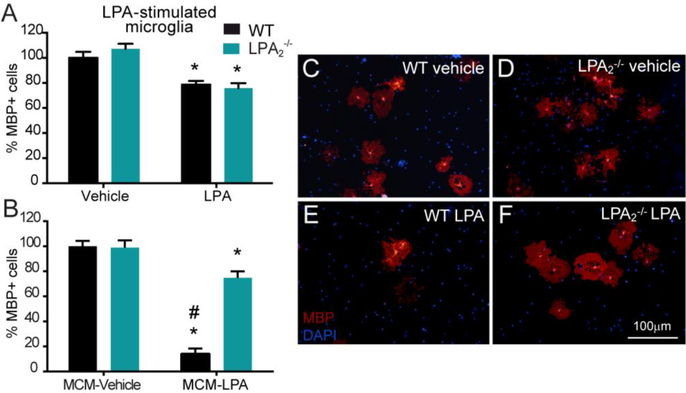
To gain insights into the mechanisms underlying the effects of LPA2 signaling on demyelination, we conducted cell culture studies. We previously reported that LPA exerted cytotoxic effects on oligodendrocytes, which was not mediated by direct actions of LPA in oligodendrocytes but in microglia (Santos-Nogueira et al., 2015). Since previous reports revealed that mouse and human oligodendrocytes expresses LPA2 (Zhang et al., 2014; Zhang et al., 2016) we assessed whether LPA2 activation in oligodendrocytes leads to cell death. We observed that the lack of LPA2 in oligodendrocytes did not confer protection against the cytotoxic effects evoked by LPA in this glial cell, indicating that activation of LPA2 in oligodendrocytes is unlikely to trigger demyelination (t-test; n=8 for vehicle and n=7 for LPA) (Fig 5A). We therefore studied whether microglial LPA2 was responsible for oligodendrocyte cell death. With this purpose, we first examined whether microglial cells express LPA2. Real time PCR experiments revealed the presence of LPA2 in cultured microglia, as well as, in cell sorted microglia from the adult CNS at similar levels (ratio lpa2/gapdh was 0.00134±0.00014 and 0.00146±0.0009 in cultured microglia and adult sorted microglia, respectively; n=4 per group). This data is in line with previous RNAseq data obtained from human and mouse microglia (Zhang et al., 2014; Zhang et al., 2016). Knowing that microglia expresses LPA2, we then stimulated WT or LPA2 null microglia with LPA and applied the conditioned medium to oligodendrocytes. These experiments revealed that LPA-stimulated conditioned medium of WT microglia led to marked reduction in the number of MBP+ cells (p<0.001; two-way ANOVA; n=8 per group) (Fig 5B, E), confirming our previous data (Santos-Nogueira et al., 2015). Interestingly, the conditioned medium of LPA-stimulated microglia harvested from LPA2 deficient mice exerted minor cytotoxic effects (two-way ANOVA post hoc Bonferroni’s p<0.001 vs. WT LPA-MCM; n=8 per group) (Fig 5B, F). This data provides clear evidence that activation of microglial LPA2 induces the release of some unknown factors that are harmful for oligodendrocytes.
Figure 5. LPA induced oligodendrocyte cell death in vitro through LPA2 signaling on microglial cells.
(A) Quantification of MBP+ cells on primary cultured WT and LPA2 null oligodendrocytes exposed to 1 μM LPA for 24h. (*p<0.05; n=8 biological replicates for vehicle and n=7 biological replicates for LPA in both groups). (B) Quantification of MBP+ cells on primary cultured WT oligodendrocytes exposed for 24h to LPA-stimulated microglial-conditioned medium (MCM) from WT or LPA2 deficient mice. (*p<0.05, #p< 0.01 vs. control; n=8 biological replicates per group). (C-F) Representative micrographs showing MBP+ cells exposed to WT (C, E) or LPA2 null (D, F) MCM in control (C, D) or LPA (E, F) conditions. Error bars indicate SEM. Scale bar=100 μm
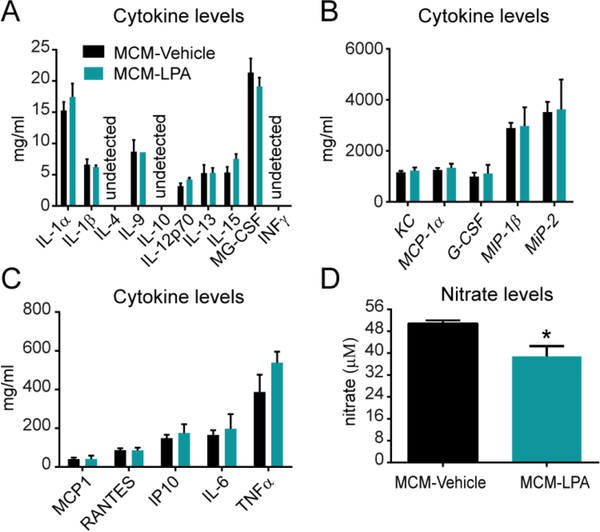
Since microglia is one of the main sources of cytokines, which can cause oligodendrocyte cell death, we examined the protein levels of 20 cytokines in the supernatants of microglial cells activated with LPA. However, Luminex assay revealed that LPA did not increase the amounts of any of these cytokines, suggesting that cytokines are unlikely to be the toxic factors released by microglia upon LPA2 stimulation, at least the twenty we assessed (t-test p>0.05; n=4 per group) (Fig. 6A-C).
Figure 6. Cytokine and nitrate levels in LPA-stimulated microglia conditioned medium.
Measurement of protein levels for various cytokines. (n=4 biological replicates per group). (A, B, C) as well as nitrates (D) in the supernatant LPA-stimulated microglia conditioned medium (*p<0.05; n=3 per group). Error bars indicate SEM.
It is also known that microglia cells release nitric oxide (NO) when they become activated. This NO leads to the formation of toxic peroxynitrites, among other products, that trigger cell death. We therefore assessed whether microglia increase the release of NO upon LPA stimulation by measuring the nitrite/nitrate levels in the supernatants. However, these results revealed that nitrate levels were indeed reduced after LPA stimulation, highlighting that the cell death triggered by LPA-activated microglia is independent to NO formation (t-test p>0.05; n=3 per group) (Fig 6D).
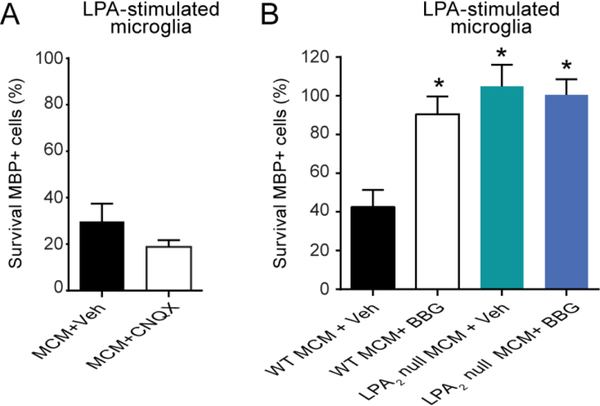
We then assessed whether glutamate, which is released by activated microglial cells and mediates cytotoxicity to oligodendrocytes, was one of the harmful mediators released by LPA-stimulated microglia. With this purpose, we exposed oligodendrocytes to conditioned medium of LPA-stimulated microglia in the presence of CNQX, an AMPA/kainate receptor antagonist. These experiments revealed that CNQX failed to rescue oligodendrocyte cell death, indicating that glutamate is also unlikely to be the harmful factor released by microglial cells upon LPA2 stimulation (one-way ANOVA post hoc Bonferroni’s p<0.05; n=7 per group) (Fig. 7A).
Figure 7. Effects of CNQX and BGG in oligodendrocyte survival after incubation with LPA-stimulated microglial conditioned medium.
(A) Quantification of MBP+ cells in oligodendrocyte cell cultures incubated with LPA-stimulated microglial conditioned medium. Note ~35% of oligodendrocyte survived after incubation with the conditioned medium of LPA-activated microglia, and that treatment of oligodendrocytes with the AMPA/Kainate receptor antagonist CNQX did not rescue cell death. (n=7 biological replicates per group). (B) Assessment of MBP+ cells after incubation of oligodendrocytes with LPA-stimulated WT or LPA2 null microglial conditioned in the presence of BBG. This P2X7 antagonist prevented oligodendrocyte death induced by LPA-stimulated WT microglia to similar levels to that triggered by the conditioned medium of LPA2 null microglia stimulated with LPA. However, BBG did not show additive effects on the LPA-stimulated LPA2 microglial conditioned medium. (*p<0.05 vs. Control; n=4 biological replicates per group). Error bars indicate SEM.
Previous work indicates that LPA induces the release of ATP in microglial cells (Fujita et al., 2008). Since ATP exerts potent excitotoxic effects to oligodendrocytes by signaling via P2X7 present on these myelinating cells (Matute et al., 2007), we investigated whether purines were the cytotoxic factors produced by LPA2 stimulated microglia. We therefore exposed oligodendrocytes to the conditioned medium of LPA-stimulated microglia in the presence of BBG, a selective P2X7 antagonist. These experiments revealed that the treatment of oligodendrocytes with BBG rescued oligodendrocyte cell death induced by the conditioned medium of LPA-stimulated microglia (one-way ANOVA post hoc Bonferroni’s p<0.05; n=4 per group) (Fig 7B). Indeed, treatment with BBG reduced oligodendrocyte cell death to similar levels to that observed after incubation of oligodendrocytes with the supernatants of LPA-stimulated microglia lacking LPA2. Interestingly, BBG did not exert additional protective effects to oligodendrocyte when it was added to conditioned medium of LPA2 null microglia stimulated with LPA. Therefore, these data reveal that LPA-LPA2 signaling in microglia promotes the release of purines, which in turn, mediates oligodendrocyte cell death by signaling via P2X7.
DISCUSSION
In the present work, we studied whether LPA2 signaling contributes to the physiopathology of SCI. We show that mRNA levels of LPA2 are increased in the spinal cord after contusion injury and that LPA2 activation leads to demyelination and functional deficits after SCI. We demonstrate, by doing cell culture work, that LPA2 signaling does not mediate direct harmful effects on oligodendrocytes, but evokes microglial cells to release purines, which in turn, mediates cytotoxic cell death of oligodendrocytes via P2X7. Overall, this study provides new mechanistic insights into how LPA contributes to SCI physiopathology.
SCI is frequently companied by necrosis and apoptosis of oligodendrocytes, resulting in demyelination of axonal pathways (Brosius Lutz and Barres, 2014; Mekhail et al., 2012). This occurs during the firsts days following SCI (Mekhail et al., 2012) and it is induced by several events that take place in the spinal cord parenchyma following lesion, including the formation of reactive oxygen or NO species, a myriad of inflammatory components, increase in Ca2+ influx, excitotoxicity, and hypoxia, among others (Brosius Lutz and Barres, 2014; David et al., 2012; Dumont et al., 2001; Mekhail et al., 2012).
LPA has emerged as a novel lipid mediator involved in CNS pathologies (Choi and Chun, 2013; Yung et al., 2015). LPA levels are increased at the lesion site after spinal cord injury or brain trauma which leads to secondary damage and neurological deficits (Crack et al., 2014; Goldshmit et al., 2012; Santos-Nogueira et al., 2015). In particular, we found that LPA triggers demyelination by signaling, in part, via LPA1 (Santos-Nogueira et al., 2015). However, the demyelinating effects of LPA were not completely prevented in mice lacking LPA1 or after pharmacological blockade of LPA1 suggesting that activation of other LPA receptors are likely to contribute to myelin loss. Here, we revealed that LPA2 signaling, similar to LPA1, also leads to demyelination.
LPA2, together with LPA1 and LPA3, belong to the endothelial differentiation gene LPA receptor family (Choi and Chun, 2013; Choi et al., 2010). LPA2 shows high homology to LPA1 since it shares 60% amino acid similarity and couples with the same three types of Gα proteins as does LPA1: Gi/o, Gq/11, and G12/13 (Choi and Chun, 2013; Choi et al., 2010). Little is known about the physiological functions of LPA2, but its signaling has been generally associated with cell survival and cell migration (Choi and Chun, 2013; Choi et al., 2010; Yung et al., 2015). Indeed, LPA2 is involved with the invasion and metastasis of several type of cancer cells (Lee and Yun, 2010; Lopane et al., 2017; Park et al., 2018).
LPA2 transcripts are expressed at high levels in the testis and leukocytes, whereas some other tissues, including the central nervous system, display much lower levels (An et al., 1998; Choi and Chun, 2013; Santos-Nogueira et al., 2015). Previous RNAseq analysis done in isolated neural cells also show that LPA2 is expressed in neurons, oligodendrocytes, microglia and peripheral macrophages (Zhang et al., 2014; Zhang et al., 2016). Here, we show that LPA2 are markedly upregulated in the spinal cord for the first 3 days after contusion injury, and that LPA-LPA2 axis triggers demyelination. Microglia and peripheral macrophages are likely to be the main cell source of LPA2 in the injured spinal cord since these cells increase in number after SCI and because of the marked cell death of neurons and oligodendrocytes.
Similar to LPA1, LPA2 is found on oligodendrocytes and Schwann cells and its expression appears shortly before maturation/myelination (Garcia-Diaz et al., 2014; Weiner et al., 2001). Activation of LPA2 in myelinating cells seems to play a key role in myelin formation, since it leads to synthesis of myelin P0 protein in cultured Schwann cells (Weiner et al., 2001).
We recently showed that LPA exerts mild toxicity in mature oligodendrocytes at doses ≥ 1μM, but not in oligodendrocyte precursor cells. This deleterious effect of LPA on oligodendrocytes is not mediated via LPA1, since pharmacological blockade of LPA1 did not prevent cell death upon LPA exposure (Santos-Nogueira et al., 2015). Here we show that this effect is also unlikely to be triggered by LPA2 signaling, since the lack of LPA2 did not enhance oligodendrocyte survival after LPA exposure. These observations suggest that LPA leads to demyelination by activation of LPA2 in other cell types, rather than to direct activation of LPA2 in oligodendrocytes.
Microglial cells become cytotoxic to oligodendrocytes when stimulated with LPA, in part, via microglial LPA1 (Santos-Nogueira et al., 2015). Here, we show that microglial cells express LPA2 in vitro and in vivo. Interestingly, we reveal that the conditioned medium of LPA2 null microglia stimulated with LPA does not mediate oligodendrocyte cell death. This data suggests that LPA2 activation by LPA in microglia induces the release of some unknown factors that exert potent cytotoxic actions to oligodendrocytes. Although we previously showed that microglia LPA1 also contributes to oligodendrocyte cell death (Santos-Nogueira et al., 2015), our novel observations demonstrate that the lack of LPA2 in microglia cells is sufficient to abrogate its detrimental actions on oligodendrocytes upon LPA stimulation, despite of the presence of microglial LPA1. This may suggest that LPA1 signaling may converge with some of the intracellular pathways triggered by LPA2 that are responsible to evoke the cytotoxic effects. Indeed, LPA2 couples with the same three types of Gα proteins as does LPA1: Gi/o, Gq/11, and G12/13 (Choi and Chun, 2013; Choi et al., 2010).
There are multiple mediators released by microglial cells that are potent inducers of cell death. However, these factors are unlikely to be cytokines or nitric oxide since their levels were not increased in the conditioned medium of LPA-stimulated microglia. Other works demonstrate that activated microglia release glutamate to the extracellular milieu which triggers oligodendrocyte cell death via AMPA/Kainate receptor signaling (Garcia-Barcina and Matute, 1998; McDonald et al., 1998). However, addition of CNQX, an AMPA/Kainate antagonist, in the conditioned medium of LPA-stimulated microglia did not rescue oligodendrocyte cell death, suggesting that glutamate is not the harmful factor released by LPA-stimulated microglia. Another study reveals that microglia releases ATP upon exposure of LPA (Fujita et al., 2008). Interestingly, oligodendrocytes express the purinergic receptor P2X7 which leads to cell death upon stimulation with ATP (Matute et al., 2007; Peng et al., 2009). Besides, inhibition of the purinergic receptor P2X7 confers protection against experimental autoimmune encephalomyelitis (EAE) (Matute et al., 2007) and SCI (Peng et al., 2009). In line with these observations, we found that treatment with BBG, a potent P2X7 antagonist, fully rescues oligodendrocyte cell death induced by the conditioned medium of LPA-activated microglia, but it does not have any effect on oligodendrocyte survival when added in the conditioned medium of LPA2 null microglia exposed to LPA. These findings therefore suggest that activation of microglia LPA2 evokes the release of purines to the extracellular milieu, which in turn, activate P2X7 in oligodendrocytes, provoking cell death, at least in cell culture conditions. Further studies are therefore needed to elucidate whether this mechanism also occurs in vivo. We do not exclude that other factors released by microglial cells upon LPA2 activation could be also triggering oligodendrocytes cell death, although to a lesser extent than purines.
Despite our data reveals that LPA2 signaling contributes to myelin loss after SCI, these findings cannot be extrapolated to all demyelinating disorders. Indeed, a recent report demonstrated that LPA2 conferred protection against experimental autoimmune encephalomyelitis (EAE), the mouse model of multiple sclerosis (Schmitz et al., 2017). The beneficial effects of LPA-LPA2 in EAE was not mediated in the CNS but in the periphery. In particular, this work demonstrated that T cells express LPA2, and that its loss facilitated lymphocyte mobilization, which may account for the faster onset and the greater clinical signs of this autoimmune disease in mice lacking LPA2 (Schmitz et al., 2017).
Overall, the results provide clear and novel evidence that LPA2 contributes to secondary damage in SCI and reveal the mechanisms underlying the cytotoxic actions of LPA. Our data also suggest that selective blockade of LPA2 could lead to a novel approach to reduce functional deficits in patients with acute SCI.
HIGHLIGHTS.
LPA2 is upregulated in the spinal cord parenchyma after traumatic injury
The lack of LPA2 results in reduced myelin loss after intraspinal injection of LPA and after spinal cord injury
Microglial cells become cytotoxic to oligodendrocytes after LPA stimulation.
Microglia express LPA2 in the adult CNS, but also in cell culture conditions.
LPA2 signaling in microglia triggers oligodendrocyte cell death.
LPA2 activation induces the release of purines in microglial cells that trigger oligodendrocyte cell death via P2X7 activation.
ACKNOWLEDGMENTS
This work has been supported by grants from the Spanish Ministry of Economy and Competitiveness (SAF2010–17851; SAF2013–48431-R), Marie-Curie International Reintegration Program Grant (MC IRG 249274), Wings for Life Foundation International Foundation for Research in Paraplegia and by funds from the Fondo de Investigación Sanitaria of Spain (TERCEL and CIBERNED) to R.L-V. and NIH NS084398 to JC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amo-Aparicio J, Martinez-Muriana A, Sanchez-Fernandez A, Lopez-Vales R, 2018. Neuroinflammation quantification for spinal cord injury. Curr. Protoc. Immunol 123:e57. [DOI] [PubMed] [Google Scholar]
- An S, Bleu T, Hallmark OG, Goetzl EJ, 1998. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J. Biol. Chem 273, 7906–7910. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG, 2006. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 23, 635–659. [DOI] [PubMed] [Google Scholar]
- Brosius Lutz A, Barres BA, 2014. Contrasting the glial response to axon injury in the central and peripheral nervous systems. Dev. Cell 28, 7–17. [DOI] [PubMed] [Google Scholar]
- Choi JW, Chun J, 2013. Lysophospholipids and their receptors in the central nervous system. Biochimica et biophysica acta 1831, 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J, 2010. LPA receptors: subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol 50, 157–186. [DOI] [PubMed] [Google Scholar]
- Coll-Miro M, Francos-Quijorna I, Santos-Nogueira E, Torres-Espin A, Bufler P, Dinarello CA, Lopez-Vales R, 2016. Beneficial effects of IL-37 after spinal cord injury in mice. Proc. Natl. Acad. Sci. U.S.A 113, 1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack PJ, Zhang M, Morganti-Kossmann MC, Morris AJ, Wojciak JM, Fleming JK, Karve I, Wright D, Sashindranath M, Goldshmit Y, Conquest A, Daglas M, Johnston LA, Medcalf RL, Sabbadini RA, Pebay A, 2014. Anti-lysophosphatidic acid antibodies improve traumatic brain injury outcomes. J Neuroinflammation 11, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Lopez-Vales R, Wee Yong V, 2012. Harmful and beneficial effects of inflammation after spinal cord injury: potential therapeutic implications. Handb. Clin. Neurol 109, 485–502. [DOI] [PubMed] [Google Scholar]
- Dawson J, Hotchin N, Lax S, Rumsby M, 2003. Lysophosphatidic acid induces process retraction in CG-4 line oligodendrocytes and oligodendrocyte precursor cells but not in differentiated oligodendrocytes. J. Neurochemistry 87, 947–957. [DOI] [PubMed] [Google Scholar]
- Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS, 2001. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin. Neuropharmacol 24, 254–264. [DOI] [PubMed] [Google Scholar]
- Francos-Quijorna I, Santos-Nogueira E, Gronert K, Sullivan AB, Kopp MA, Brommer B, David S, Schwab JM, Lopez-Vales R, 2017. Maresin 1 Promotes Inflammatory Resolution, Neuroprotection, and Functional Neurological Recovery After Spinal Cord Injury. J. Neurosci. Offic. J. Soc. Neurosci 37, 11731–11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita R, Ma Y, Ueda H, 2008. Lysophosphatidic acid-induced membrane ruffling and brain-derived neurotrophic factor gene expression are mediated by ATP release in primary microglia. J. Neurochemistry 107, 152–160. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Verdu E, Fores J, Lopez-Vales R, Navarro X, 2003. Functional and electrophysiological characterization of photochemical graded spinal cord injury in the rat. J. Neurotrauma 20, 501–510. [DOI] [PubMed] [Google Scholar]
- Garcia-Barcina JM, Matute C, 1998. AMPA-selective glutamate receptor subunits in glial cells of the adult bovine white matter. Brain research. Molecular brain research 53, 270–276. [DOI] [PubMed] [Google Scholar]
- Garcia-Diaz B, Riquelme R, Varela-Nieto I, Jimenez AJ, de Diego I, Gomez-Conde AL, Matas-Rico E, Aguirre JA, Chun J, Pedraza C, Santin LJ, Fernandez O, Rodriguez de Fonseca F, Estivill-Torrus G, 2014. Loss of lysophosphatidic acid receptor LPA alters oligodendrocyte differentiation and myelination in the mouse cerebral cortex. Brain structure & function. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Matteo R, Sztal T, Ellett F, Frisca F, Moreno K, Crombie D, Lieschke GJ, Currie PD, Sabbadini RA, Pebay A, 2012. Blockage of lysophosphatidic acid signaling improves spinal cord injury outcomes. Am. J. Pathol 181, 978–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder SK, Yano R, Chun J, Ueda H, 2013. Involvement of LPA1 receptor signaling in cerebral ischemia-induced neuropathic pain. Neuroscience 235, 10–15. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Reavill C, Brown G, Brown JT, Cluderay JE, Crook B, Davies CH, Dawson LA, Grau E, Heidbreder C, Hemmati P, Hervieu G, Howarth A, Hughes ZA, Hunter AJ, Latcham J, Pickering S, Pugh P, Rogers DC, Shilliam CS, Maycox PR, 2003. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol. Cell. Neurosci 24, 1170–1179. [DOI] [PubMed] [Google Scholar]
- Hilton BJ, Moulson AJ, Tetzlaff W, 2017. Neuroprotection and secondary damage following spinal cord injury: concepts and methods. Neuroscience letters 652, 3–10. [DOI] [PubMed] [Google Scholar]
- Holtsberg FW, Steiner MR, Keller JN, Mark RJ, Mattson MP, Steiner SM, 1998. Lysophosphatidic acid induces necrosis and apoptosis in hippocampal neurons. J. Neurochemistry 70, 66–76. [DOI] [PubMed] [Google Scholar]
- Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H, 2004. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nature Medicine 10, 712–718. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C, 2006. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nature Medicine 12, 225–229. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Girolami EI, Ghasemlou N, Jeong SY, David S, 2008. The protective effects of 15-deoxy-delta-(12,14)-prostaglandin J2 in spinal cord injury. Glia 56, 436–448. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Yun CC, 2010. Colorectal cancer cells - Proliferation, survival and invasion by lysophosphatidic acid. Int. J. Biochem. Cell. Biol 42, 1907–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Lin L, Woodling NS, Wang Q, Anacker C, Pan T, Merchant M, Andreasson K, 2011. Signaling via the prostaglandin E(2) receptor EP4 exerts neuronal and vascular protection in a mouse model of cerebral ischemia. J. Clin. Invest 121, 4362–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lopane C, Agosti P, Gigante I, Sabba C, Mazzocca A, 2017. Implications of the lysophosphatidic acid signaling axis in liver cancer. Biochimica et biophysica acta 1868, 277–282. [DOI] [PubMed] [Google Scholar]
- Ma L, Uchida H, Nagai J, Inoue M, Chun J, Aoki J, Ueda H, 2009. Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid: an initiator of nerve injury-induced neuropathic pain. Mol. Pain 5, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Torre I, Perez-Cerda F, Perez-Samartin A, Alberdi E, Etxebarria E, Arranz AM, Ravid R, Rodriguez-Antiguedad A, Sanchez-Gomez M, Domercq M, 2007. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. Offic. J. Soc. Neurosci 27, 9525–9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP, 1998. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nature Medicine 4, 291–297. [DOI] [PubMed] [Google Scholar]
- Mekhail M, Almazan G, Tabrizian M, 2012. Oligodendrocyte-protection and remyelination post-spinal cord injuries: a review. Prog. Neurobiol 96, 322–339. [DOI] [PubMed] [Google Scholar]
- O’Meara RW, Ryan SD, Colognato H, Kothary R, 2011. Derivation of enriched oligodendrocyte cultures and oligodendrocyte/neuron myelinating co-cultures from post-natal murine tissues. J. Vis. Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Jang JH, Oh S, Kim M, Shin C, Jeong M, Heo K, Park JB, Kim SR, Oh YS, 2018. LPA-induced migration of ovarian cancer cells requires activation of ERM proteins via LPA1 and LPA2. Cellular signalling 44, 138–147. [DOI] [PubMed] [Google Scholar]
- Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M, 2009. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc. Natl. Acad. Sci. U.S.A 106, 12489–12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redensek A, Rathore KI, Berard JL, Lopez-Vales R, Swayne LA, Bennett SA, Mohri I, Taniike M, Urade Y, David S, 2011. Expression and detrimental role of hematopoietic prostaglandin D synthase in spinal cord contusion injury. Glia 59, 603–614. [DOI] [PubMed] [Google Scholar]
- Roberts C, Winter P, Shilliam CS, Hughes ZA, Langmead C, Maycox PR, Dawson LA, 2005. Neurochemical changes in LPA1 receptor deficient mice--a putative model of schizophrenia. Neurochem. Res 30, 371–377. [DOI] [PubMed] [Google Scholar]
- Santos-Nogueira E, Lopez-Serrano C, Hernandez J, Lago N, Astudillo AM, Balsinde J, Estivill-Torrus G, de Fonseca FR, Chun J, Lopez-Vales R, 2015. Activation of Lysophosphatidic Acid Receptor Type 1 Contributes to Pathophysiology of Spinal Cord Injury. J. Neurosci. Offic. J. Soc. Neurosci 35, 10224–10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J, Tusell JM, Serratosa J, 2003. High-yield isolation of murine microglia by mild trypsinization. Glia 44, 183–189. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., 2003. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma 20: 179–193 [DOI] [PubMed] [Google Scholar]
- Schmitz K, Brunkhorst R, de Bruin N, Mayer CA, Haussler A, Ferreiros N, Schiffmann S, Parnham MJ, Tunaru S, Chun J, Offermanns S, Foerch C, Scholich K, Vogt J, Wicker S, Lotsch J, Geisslinger G, Tegeder I, 2017. Dysregulation of lysophosphatidic acids in multiple sclerosis and autoimmune encephalomyelitis. Acta neuropathologica communications 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shano S, Moriyama R, Chun J, Fukushima N, 2008. Lysophosphatidic acid stimulates astrocyte proliferation through LPA1. Neurochem. Int 52, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SD, Nicole O, Peavy RD, Montoya LM, Lee CJ, Murphy TJ, Traynelis SF, Hepler JR, 2003. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol. Pharmacol 64, 1199–1209. [DOI] [PubMed] [Google Scholar]
- Tigyi G, Fischer DJ, Sebok A, Marshall F, Dyer DL, Miledi R, 1996. Lysophosphatidic acid-induced neurite retraction in PC12 cells: neurite-protective effects of cyclic AMP signaling. J. Neurochemistry 66, 549–558. [DOI] [PubMed] [Google Scholar]
- Weiner JA, Fukushima N, Contos JJ, Scherer SS, Chun J, 2001. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J. Neurosci. Offic. J. Soc. Neurosci 21, 7069–7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung YC, Mutoh T, Lin ME, Noguchi K, Rivera RR, Choi JW, Kingsbury MA, Chun J, 2011. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci. Transl. Med 3, 99ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung YC, Stoddard NC, Chun J, 2014. Thematic Review Series: Lysophospholipids and their Receptors LPA receptor signaling: pharmacology, physiology, and pathophysiology. J. Lipid Res 55, 1192–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung YC, Stoddard NC, Mirendil H, Chun J, 2015. Lysophosphatidic Acid signaling in the nervous system. Neuron 85, 669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ, 2014. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. Offic. J. Soc. Neurosci 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA, 2016. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 89, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]