Abstract
Background & Aims:
T-helper-type 17 (Th17) cells are involved in autoimmune tissue damage. CD39 is an ectonucleotidase that catalyzes extracellular ATP/ADP hydrolysis, culminating in the generation of immunosuppressive adenosine. Functional CD39 expression confers immunosuppressive properties upon immune cells. As the proportion of CD39 lymphocytes is decreased in juvenile autoimmune liver disease (AILD), we have explored whether decreased CD39 expression is present on Th17 cells and whether this phenomenon is associated with heightened effector function and inflammation.
Methods:
Thirty-eight patients with juvenile AILD (22 autoimmune hepatitis and 16 autoimmune sclerosing cholangitis), 8 disease controls (DC) and 16 healthy subjects (HS) were studied. Peripheral blood cell phenotype was determined by flow cytometry; ability to suppress by inhibition of cell proliferation/effector cytokine production; ectoenzymatic activity by thin layer chromatography; expression of adenosine receptor, adenosine deaminase (ADA) and phosphodiesterases (PDE) by quantitative real-time PCR or by Western Blot.
Results:
CD39+ Th17 (Th17CD39+) cells from HS appear activated and contain high frequencies of lymphocytes producing regulatory cytokines. In AILD, however, Th17CD39+ cells are markedly diminished and fail to generate AMP/adenosine, thereby limiting control of both target cell proliferation and IL-17 production. When compared to HS, Th17 cells from AILD patients also show lower A2A adenosine receptor expression while displaying similar levels of PDE4A, PDE4B and ADA. Only rare Th17CD39+ cells are observed by liver immunohistochemistry.
Conclusions:
Th17CD39+ cells in juvenile AILD are both quantitatively decreased and qualitatively deficient. Low levels CD39 and A2A expression may contribute to the perpetuation of Th17 cell effector properties and unfettered inflammation in this disease.
Keywords: Th17 cells, CD39, adenosine, ATP, autoimmune liver disease
1. INTRODUCTION
T helper type 17 (Th17) cells are an effector subset that develops in parallel with Th1 and Th2 cell lineages. Th17 cells differentiate from CD4 cell progenitors upon exposure to IL-6, TGF-E and IL-21 in mice, and IL-6, TGF-β and IL-1 β in humans [1, 2]. Th17 cells are thought to develop under the influence of RORγt transcription factor, which induces transcription of the IL-17 gene in naïve T helper cells. RORγt acts in cooperation with other transcription factors, such as ROR-α, the signal transducer and activator of transcription 3 (STAT-3), interferon regulatory factor 4 (IRF-4) and runt-related transcription factor 1 (Runx1) to achieve comprehensive differentiation of naïve T-cell precursors into the Th17 lineage [3–6].
Th17 cells are involved in a number of inflammatory and autoimmune conditions in mice and humans, including rheumatoid arthritis [7], psoriasis [8], multiple sclerosis [9] and inflammatory bowel disease (IBD) [10]. Involvement of Th17 cells in the perpetration of tissue damage has been also reported in autoimmune liver disease (AILD), including autoimmune hepatitis (AIH), where increased levels of serum IL-17 and higher proportions of liver infiltrating IL-17 producing cells are observed [11]. Autoimmune sclerosing cholangitis (ASC) is a childhood autoimmune liver overlap condition in which features of classical AIH type 1, namely hypergammaglobulinaemia, positivity for anti-nuclear antibody (ANA) and/or anti-smooth muscle antibody (SMA) and interface hepatitis on histology, all co-exist with bile duct abnormalities noted on cholangiography or pathological examination [12]. In AISC there is a strong positive correlation between frequency of Th17 cells and gamma-glutamyl transferase (γGT) as well as alkaline phosphatase (ALP) levels [13].
In Th17 cells expression of CD39, an ectonucleotidase that initiates the major extracellular ATP/ADP hydrolysis cascade culminating in the generation of immunosuppressive adenosine [14, 15], is associated with the acquisition of regulatory properties. This pattern of CD39 expression appears mechanistic in controlling the pathogenic potential of these cells [16].
Distinct from the prototypic ‘effector’ Th17 cells, Th17 cells positive for CD39 (Th17CD39+) display regulatory properties such as expression of FOXP3 and suppression of responder cell effector function, while retaining phenotypic (i.e. IL-17 production and RORγt expression) and ectoenzymatic (i.e. generation of inosine from adenosine because of high levels of adenosine deaminase, ADA) features typical of effector cells [16]. The joint expression of regulatory and effector properties suggest that Th17CD39+ cells can act as either effectors or regulators depending on the milieu in which these cells are located. We have previously shown that patients with AIH have impaired CD39+ regulatory T-cells (T-regs), which are defective in suppressing IL-17 production by responder cells possibly as a result of low adenosine generation [17].
In this study, we have aimed to determine the proportion, phenotypic and functional properties of circulating Th17CD39+ cells in patients with ANA/SMA positive juvenile AILD and to investigate whether low levels of CD39 expression are associated with persistence of Th17 cells at an inflammatory state.
2. MATERIALS AND METHODS
2.1. Subjects
Thirty-eight patients with anti-nuclear (ANA) and/or anti-smooth muscle (SMA) positive AILD were studied. Seventeen patients were female. A liver biopsy performed at the time of or close to diagnosis showed histological features of interface hepatitis in all patients. All patients underwent cholangiography to assess bile duct integrity and intestinal endoscopic examination to exclude inflammatory bowel disease (IBD) at the time of or close to the diagnosis of AILD [12]. Sixteen of the thirty-eight patients had bile duct changes characteristic of sclerosing cholangitis on retrograde or magnetic resonance cholangiography and were diagnosed as having ASC [12]. Thirteen of the 22 AIH patients and 4 of the 16 ASC patients were females. Six AIH patients had IBD (5 ulcerative colitis [UC], 1 Crohn disease [CD]); 11 ASC patients had concomitant IBD (9 UC and 2 CD). When considered together, AIH and ASC are indicated as AILD. Twenty-six patients were studied during drug-induced remission (i.e. normal transaminase levels; [R] patients; 14 AIH and 12 ASC) while 12 patients had active disease [A] at the time of study. Seven of the 12 [A] patients were studied during an episode of relapse (3 AIH and 4 ASC); five AIH patients were studied at diagnosis. Patients’ demographics, clinical and laboratory data are summarized in Table 1. Patients were treated with prednisolone (2.5–5 mg daily at remission and 1–2 mg/kg/day at relapse) either alone or in combination with azathioprine (1–2 mg/kg/day). In two patients prednisolone was used in combination with mycophenolate mofetil (MMF, 40 mg/kg/day). In ASC, ursodeoxycholic acid (UDCA) at a dose of 15–20 mg/kg/day was added to the immunosuppressive regimen. Eight subjects with liver disorders of non-autoimmune and non-viral etiology served as disease controls (DC) (7 females, median age 15 years [range 6–25 years; 2 non-alcoholic fatty liver disease (NAFLD), 1 α−1 antitrypsin deficiency, 1 Gilbert syndrome, 1 Wilson disease, 1 congenital portosystemic shunt, 1 Alagille syndrome, 1 hepatic adenoma]. Sixteen healthy subjects (HS) (10 females, median age 29 years [range 23–46 years]) served as normal controls. Age differences between patients and HS were a consequence of ethical concern in obtaining blood from healthy children. Informed consent was obtained from all patients - or guardians if they were younger than 16 years of age - and from controls. The study was approved by the Ethics Committee of King’s College Hospital, London, United Kingdom.
Table 1.
Demographic and laboratory data
| Number of subjects | Sex (F/M): |
Age (years): |
AST (nv<50IU/1) |
GGT (nv<55IU/1) |
Bilirubin (nv<20μmo1/1) |
IgG (nv 6.5–17g/1) |
Autoantibody Titer* | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ANA | SMA | LKM-1 | ||||||||
| AILD | 38 | 17/21 | 0.1–34.1 (15.2) |
|||||||
| Active patients† | 12 | 5/7 | 3.6–30.2 (14.6) |
55–2462 (100) |
143 (27–883) |
12–257 (31) |
8.5–44.3 (19.1) |
0–160 (20) |
0–1280 (10) |
neg |
| Remission patients | 26 | 12/14 | 0.1–34.1 (15.6) |
11–49 (23)¶ |
7–223 (17.5)∞ |
3–26 (9)§ |
6.4–29.9 (12.6)‡ |
0–20 (0) |
0–80 (0) |
neg |
Data presented as range (median) unless noted otherwise; nv: normal value
AILD: autoimmune liver disease
: including five patients at disease presentation
AST: aspartate aminotransferase
GGT: gamma glutamyl transferase
IgG: immunoglobulin G
: Autoantibody titer shown as reciprocal
ANA: anti-nuclear antibody
SMA: smooth muscle antibody
LKM-1: liver kidney microsomal antibody type 1
P=0.006,
P=0.009,
P<0.001,
P=0.01 when comparing AST, GGT, bilirubin and IgG levels between patients with active disease and at remission
2.2. Cell separation
Peripheral blood mononuclear cells (PBMCs) were obtained as previously described [18]. Mononuclear cell viability exceeded 98% as determined by Trypan blue exclusion.
2.3. Flow cytometry
The frequency and phenotype of Th17 cells was determined by flow cytometry [16]. PBMCs were stained with allophycocyanin (APC)-cychrome (Cy)-7-conjugated anti-CD4 (clone # OKT3), phycoerythrin (PE)-Cy7-conjugated anti-CD39 (clone # A1) (BioLegend, London, UK), peridinin chlorophyll protein (PerCP)-Cy5.5 conjugated anti-CCR6 (clone # 11A9) (BD Bioscience Discovery Labware, Oxford, UK), PerCP-conjugated anti-IL-23R (clone # 218213) (R&D Systems, Abingdon, UK), PE-conjugated anti-CD69 (clone # FN50) (BD Bioscience), PE-conjugated anti-CD44 (clone # IM7) (BioLegend), APC or PE-Cy7-conjugated anti-CD25 (clone # M-A251) (BD Bioscience), APC-conjugated anti-CD73 (clone # AD2) (eBioscience, Hatfield, UK) and APC-conjugated anti-CD161 (clone # DX12) (BD Bioscience). Polarized Th17 cells (see below) were stained with APC-conjugated CD26 (clone # BA5b) monoclonal antibodies (BioLegend).
Cells were incubated at 4°C in the dark for 30 minutes, washed with phosphate buffered saline (PBS)/1% fetal calf serum (FCS), resuspended and analyzed by flow cytometry on a Becton Dickinson fluorescent activated cell sorter (FACS Canto II, Becton Dickinson Immunocytochemistry Systems, San Jose, CA); FACSDiva or FlowJo (TreeStar Inc) software were used for analysis. A minimum of 2×104 gated events was acquired for each sample.
Intracellular staining: the frequency of cells positive for RORC and FOXP3 - transcription factors of Th17 cells and T-regs - was determined by intracellular staining after cell fixation and permeabilization with Cytofix/Cytoperm (BD Bioscience) and counter-staining with APC-conjugated anti-RORC (clone # AFKJS-9) and FITC or APC-conjugated anti-FOXP3 (clone # 236A/E7) (clone PCH101) (both antibodies from eBioscience).
The frequency of IL-17A, IFNγ, TNF-α, IL-10 and TGF-β producing cells was assessed after exposure of PBMCs to phorbol-12-myristate 13-acetate (PMA) (10 ng/ml)/Ionomycin (500 ng/ml) (both from Sigma Aldrich Company Ltd, Gillingham, UK), incubation with Brefeldin A (10 μg/ml, Sigma Aldrich) for 5 hours and counterstaining with AlexaFluor 488 or PE-conjugated anti-IL-17A (clone # eBio64CAP17) (eBioscience), PE-conjugated anti-TNF-α (clone # MAb11) (BD Bioscience), PE or APC-conjugated anti-IFNγ (clone # 45–15) (IQ Products, Groningen, The Netherlands), APC-conjugated anti-IL-10 (clone # JES3–19F1) (BD Bioscience), and PerCP-conjugated anti-TGF-E (clone # 27232) (R&D Systems, Abingdon, UK) monoclonal antibodies. Flow cytometry was performed, as indicated above.
2.4. Cell purification
Th17 cells were purified using the IL-17 secretion assay-detection kit (Miltenyi Biotec, Bergisch-Gladback, Germany) according to the manufacturer’s instructions. Th17 cells were then further purified according to the expression of CD39. Briefly, Th17 cells were incubated with PE-conjugated anti-CD39 for 30 minutes, then with microbeads conjugated with monoclonal anti-PE antibodies (Miltenyi Biotec) for 15 minutes at 4°C. The Th17CD39+ and Th17CD39- cell populations were respectively purified by positive and negative selection using MS columns (Miltenyi Biotec) according to the manufacturer’s instructions. The purity of Th17CD39+ and Th17CD39- cells was consistently higher than 92%. CD4+ cells and CD4+CD25− cells to be used in cell polarization experiments (CD4 cells) and suppression assays as target cells (CD4+CD25− cells) were purified from PBMCs using immunomagnetic beads (Dynal Invitrogen, Oslo, Norway) as previously described [18, 19]. The purity of CD4+ and CD4+CD25− cells exceeded 95%.
2.5. Suppression assays
Th17CD39+ and Th17CD39- cells were added at ratios of 1/8 to CD4+CD25− cells [16]. Parallel cultures of CD4+CD25− cells alone were set up under identical conditions. Cells were co-cultured at 37°C and 5% CO2 for 5 days in the presence of anti-CD3/anti-CD28 T-cell expander (Dynal Invitrogen) (ratio bead/cell: 1/2) and IL-2 (30 U/ml). Suppression was assessed as both inhibition of target cell proliferation and decrease in pro-inflammatory cytokine (e.g. IL-17, IFNJ) levels. In the proliferation assay, experiments were performed in duplicate. For the last 18 hours of culture, cells were pulsed with 0.25 PCi/well 3H-thymidine and harvested using a multi-channel harvester. Percentage inhibition was calculated using the formula: [1 - count per minute (cpm) in the presence of Th17CD39+/Th17CD39- cells]. As previously shown [17], the 3H-thymidine incorporation method was chosen as we were dealing with low yield cell samples.
2.6. Cell polarization
Expression of A1, A2A, A2B, A3 adenosine receptors, PDE4A, PDE4B and adenosine deaminase (ADA) (see below) were determined on Th17 cells obtained from purified CD4+ cells following exposure to Th17 polarizing conditions. Culture conditions favoring the development of Th17 cells consisted of exposure to anti-CD3/anti-CD28 T-cell expander (ratio bead/cell: 1/50), recombinant human (rh) IL-23 (20 ng/ml), IL-6 (50 ng/ml), IL-1β (10 ng/ml) and TGF-β (3 ng/ml) (all recombinant cytokines were from R&D Systems) for 4 days.
2.7. Immunohistochemistry
Paraffin embedded liver sections, obtained from 7 biopsy specimens (6 from [A] patients, 1 from [R] patient) were processed as previously described [13], then incubated overnight at 4°C with anti-CD4 (mouse monoclonal, 4B12, Dako, Denmark) and anti-ENTPD1 (rabbit polyclonal, Sigma Aldrich) primary antibodies, used at 1/30 and 1/100 respectively. After endogenous peroxidase blocking with 3% H2O2, sections were incubated with horse anti-mouse and goat anti-rabbit secondary antibodies (Vector Laboratories, Burlingame, CA), used at 1/250 and 1/800 for 1 hour at room temperature. After treatment with Vectastain Elite ABC kit (Vector Laboratories), ImmPACT DAB (Vector Laboratories) was applied and sections examined by light microscopy.
For immunofluorescence staining, following blocking and incubation with primary antibodies, sections were probed with AlexaFluor 488 (Jackson ImmunoResearch Laboratories, West Grove, PA) and AlexaFluor 594 labeled secondary antibodies (Molecular Probes, Thermo-Scientific, Rockford, IL), used at 1/500 and 1/300 respectively. Hoechst staining was used to identify nuclei. Staining was then analyzed by Zeiss ApoTome Immunofluorescence microscope.
2.8. Quantitative real-time PCR
Expression of A1, A2A, A2B and A3 adenosine receptors and of PDE4A and PDE4B was determined by real-time PCR. Total RNA was extracted from 2–3×105 cells using TRIzol reagent (Invitrogen) and mRNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA), according to the manufacturer’s instructions. Primer sequences of adenosine receptors and PDE were as previously reported [16, 20]. Samples were run on an ABI-Prism 7000 Sequence Detection system (Applied Biosystems, Foster City, CA) and results were analyzed by matched software and expressed as relative quantification. Relative gene expression was determined by normalizing to human β-actin [16, 20].
2.9. Immunoblot analysis
3–5×105 cells were lysed in ice-cold RIPA buffer, containing 1% NP-40, 0.25% sodium deoxycolate, 50 mM Tris-HCl and 150 mM NaCl and supplemented with Complete Proteinase Inhibitor Cocktails (Roche Diagnostics, Indianapolis, IN) and Phosphatase Inhibitor Cocktails (Sigma-Aldrich). Following 30 minutes incubation on ice, samples were spun at 14,000g for 30 minutes. Supernatants, containing the total cell lysates, were collected and the total protein concentration determined using Bio-Rad Dc Protein assay reagent (Bio-Rad Laboratories) using bovine serum albumin as standard. Following protein denaturation with SDS, cell lysates were separated on a 4–12% Criterion XT Bis-Tris SDS-Page (Bio-Rad Laboratories). Then 10 μg of protein were loaded per lane. Gels were run for 20 minutes at 80V and then at 110V for additional 80 minutes. Proteins were transferred onto PVDF membranes (Immobilon-P, Millipore, Billerica, MA) by semi-dry electroblotting and subsequently incubated in blocking buffer containing 2.5% skimmed milk. Following 60 minutes, mouse anti-ADA antibody (Abcam, Cambridge, MA) was applied at 1μg/ml. Following overnight incubation membranes were incubated for 60 minutes with HRP-labeled goat anti-mouse (Thermo-Scientific) at 1/50,000. Bands were visualized using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo-Scientific) according to the manufacturer’s instructions. For immunoblot normalization, the same membranes were stripped (using a buffer containing 15g glycine, 1g SDS and 10ml Tween20), incubated in blocking buffer containing 5% BSA for 60 minutes and reprobed with mouse anti-human E-actin (Abcam) at 1/10,000 and subsequently with a HRP-labeled goat anti-mouse polyclonal antibody at 1/20,000. ADA and E-actin band density was determined using Image J densitometry software.
2.10. Analysis of ectonucleotidase activity
Thin layer chromatography (TLC) was performed as previously described [16, 21]. 3×105 Th17 cells were incubated with 2 mCi/ml [C14] ADP (GE Healthcare Life Sciences) in 10mM Ca2+ and 5mM Mg2+. 5μl aliquots, collected at 5, 10, 20, 40 and 60 minutes, were then analyzed for the presence of [C14] ADP hydrolysis products by TLC and applied onto silica gel matrix plates (Sigma-Aldrich). [C14] ADP and the radiolabeled derivatives were separated using an appropriate solvent mixture as previously described [16, 21]. Image J densitometry software was used to quantify TLC band intensity.
2.11. Statistical analysis
The normality of variable distribution was assessed by the Kolmogorov-Smirnov goodness-of-fit-test; once the hypothesis of normality was accepted (P>0.05), comparisons were performed by paired or unpaired Student t test as appropriate. A oneway analysis of variance, followed by Tukey’s multiple comparisons test, was used to compare means of multiple samples. P values <0.05 were considered significant. Data were analyzed using GraphPad Prism 5 software (GraphPad software Inc; San Diego, CA) and SPSS software (IBM; Hampshire, UK).
3. RESULTS
3.1. Expression of CD39 marks activated Th17 cells that display heightened FOXP3 levels and contain high proportions of lymphocytes producing regulatory cytokines
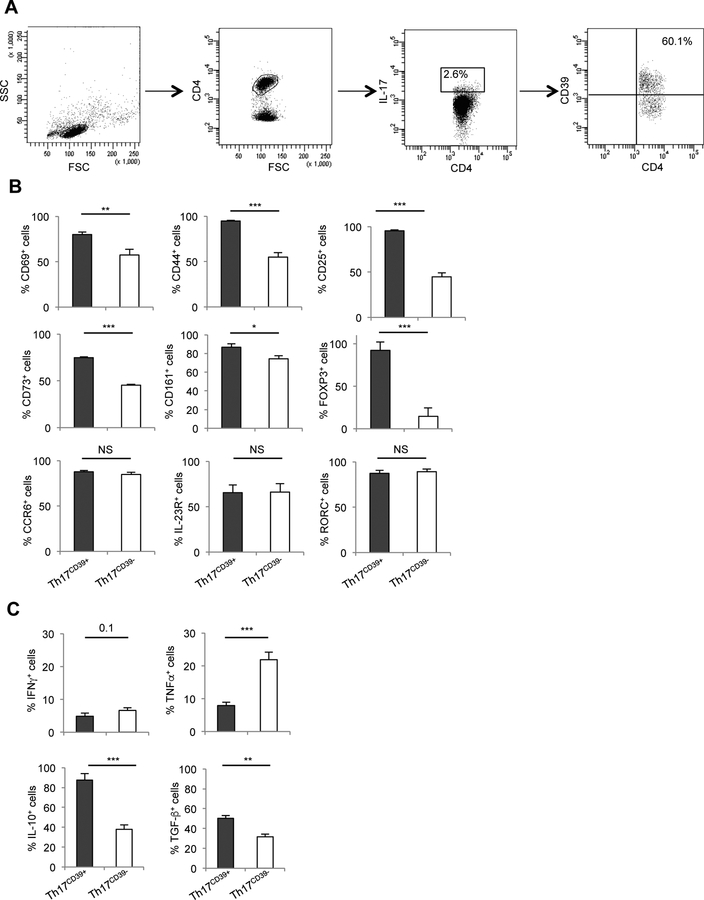
The phenotype of Th17CD39+ cells was initially assessed in PBMCs from HS. The gating strategy adopted for their identification is depicted in Fig. 1A. When compared to the Th17CD39- counterparts, Th17CD39+ cells contained higher proportions of cells positive for the activation markers CD69, CD44 and CD25, and higher frequencies of cells positive for CD73 (the ectoenzyme working in tandem with CD39 and responsible for hydrolyzing adenosine monophosphate [AMP] into immunosuppressive adenosine). A substantive proportion of these Th17CD39+ cells also were noted to express CD161 (a Th17 cell marker involved in the development of these cells) [22] and FOXP3 (Fig. 1B and Supplementary Fig. 1A).
Figure 1. Phenotype and cytokine profile of Th17CD39+ cells.
(A) Lymphocytes were gated based on their FSC and SSC patterns. CD4+ lymphocytes and IL-17+ cells within them were subsequently gated. CD39+ cells within CD4+IL-17+ lymphocytes are also shown. Representative plots from one HS. (B) Mean+SEM frequency of circulating Th17CD39+ and Th17CD39- cells positive for CD69, CD44, CD25, CD73, CD161, FOXP3, CCR6, IL-23R and RORC (n=16 HS). (C) Mean+SEM frequency of IFNγ, TNF-α, IL-10 and TGF-β producing cells amongst Th17CD39+ and Th17CD39- cells (n=11 HS). *: P≤0.05; **: P≤0.01; ***: P≤0.001.
Th17CD39- and Th17CD39+ cells had similar expression of CCR6, IL-23R and RORC, standard Th17 cell markers (Fig. 1B and Supplementary Fig. 1A). Analysis of the cytokine profile showed that compared to their Th17CD39- counterparts, Th17CD39+ cells contained higher proportions of IL-10+ and TGF-β+ cells, lower frequencies of TNF-α producing lymphocytes and tended to have lower proportions of IFNγ+ cells (Fig. 1C and Supplementary Fig. 1B).
Therefore, expression of CD39 defines Th17 cells with an activated phenotype that contain increased proportions of lymphocytes positive for FOXP3 and immunomodulatory cytokines.
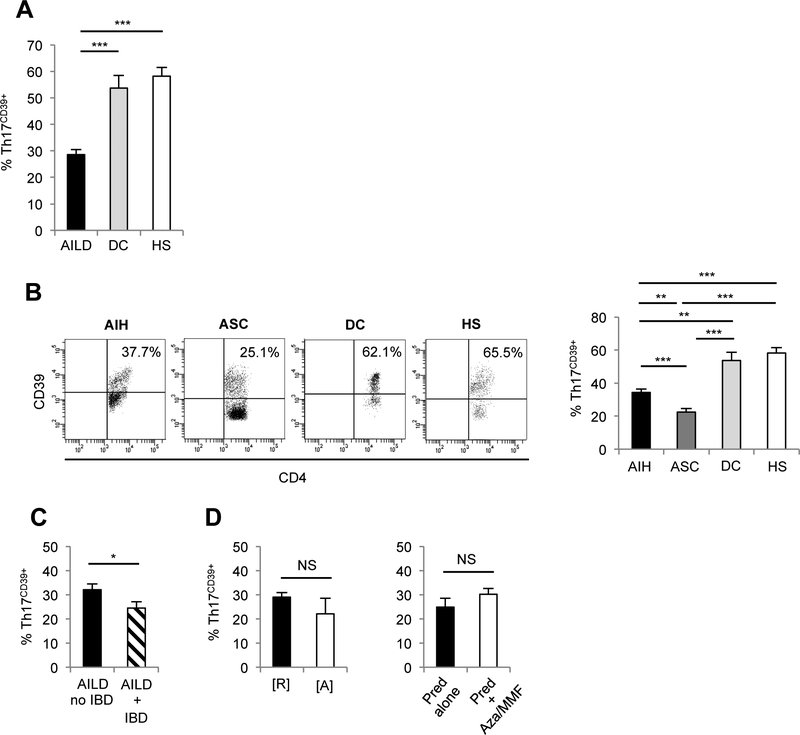
3.2. Th17CD39+ cells from AILD patients are decreased, less activated and contain lower proportions of FOXP3+ and IL-10 producing lymphocytes than in health
The proportion of circulating Th17CD39+ cells was decreased amongst PBMCs from AILD patients compared to DC and HS (Fig. 2A), with ASC patients displaying the lowest frequency (Fig. 2B). Frequencies of Th17CD39+ cells were lower in AILD patients with concomitant IBD than in those without IBD (Fig. 2C). No differences in Th17CD39+ percentages were noted between patients with and without IBD within the AIH and ASC subgroups (AIH: 34.5±4.9 vs 34.3±2.4, P=NS; ASC: 19.9±2.3 vs 27.2±4.8, P=NS). Th17CD39+ cell frequencies were not significantly different in AILD patients at remission or during relapse (Fig. 2D) and between patients treated with prednisolone alone or with prednisolone in association with azathioprine or MMF (Fig. 2D).
Figure 2. In AILD Th17CD39+ cells are numerically impaired.
(A) Mean+SEM frequency of Th17CD39+ cells in AILD (n=34), DC (n=8) and HS (n=16). (B) Th17CD39+ cells in one AIH, one ASC, one DC and one HS. Mean+SEM frequency of Th17CD39+ cells in AIH (n=18), ASC (n=16), DC (n=8) and HS (n=16); (C) AILD with (n=11) and without (n=5) IBD; (D) AILD at remission [R] (n=26) and active disease [A] (n=8), patients on prednisolone (n=11) or prednisolone+azathioprine/MMF (n=23); *: P≤0.05; **: P≤0.01; ***: P≤0.001.
In order to evaluate whether in AILD the decreased frequency of blood Th17CD39+ cells resulted from aberrant homing of these cells to the liver, we investigated the presence of CD4+ and CD39+ cells in 7 liver biopsy samples. In all biopsies, whether taken during active or controlled disease, there were likewise low numbers of CD4+CD39+ cells (Supplementary Fig. 2A,B).
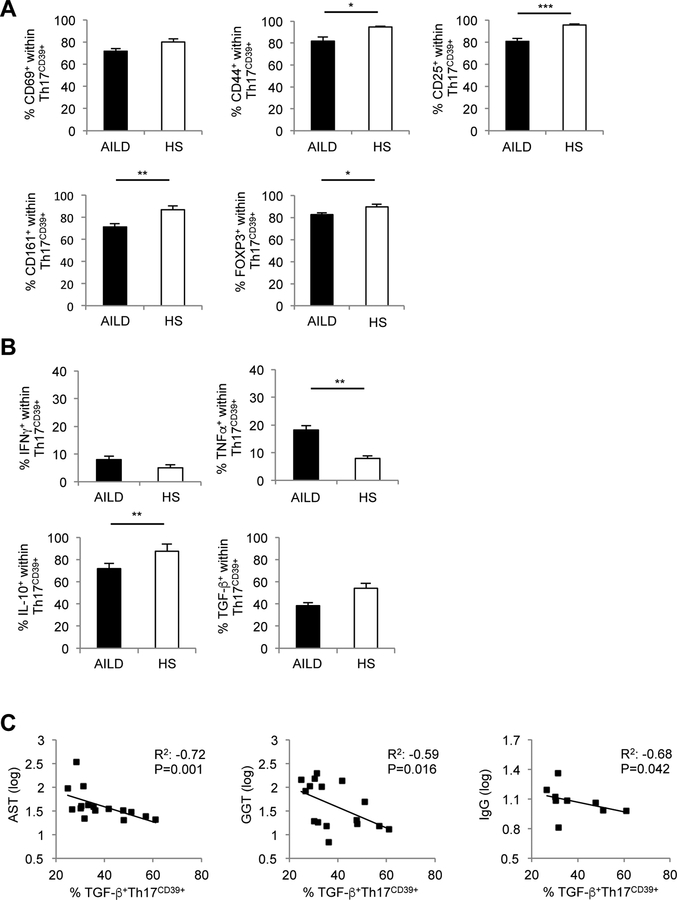
Given that DC and HS displayed comparable frequencies of circulating Th17CD39+ cells, only cells from HS were used as control for the subsequent experiments. Compared to HS, Th17CD39+ cells from AILD patients had lower frequencies of CD44+, CD25+, CD161+ and FOXP3+ cells (Fig. 3A), higher proportions of TNF-α+ and lower frequencies of IL-10+ lymphocytes (Fig. 3B). The proportion of TGF-β producing Th17CD39+ cells was negatively correlated with AST, γGT and IgG levels (Fig. 3C).
Figure 3. Th17CD39+ cells from AILD patients display a less activated profile and contain lower proportions of FOXP3+ and IL-10+ lymphocytes.
(A) Mean+SEM frequency of Th17CD39+ cells expressing CD69, CD44, CD25, CD161 and FOXP3 in AILD (n=34) and HS (n=16). (B) Mean+SEM frequency of IFNγ, TNF-α, IL-10 and TGF-β producing CD39+ Th17 cells in AILD (n=34) and HS (n=16). *: P≤0.05; **: P≤0.01; ***: P≤0.001. (C) Inverse correlation between frequency of TGF-β producing Th17CD39+ cells and levels of AST, GGT and IgG.
3.3. In AILD Th17CD39+ cells show impaired ectoenzymatic activity
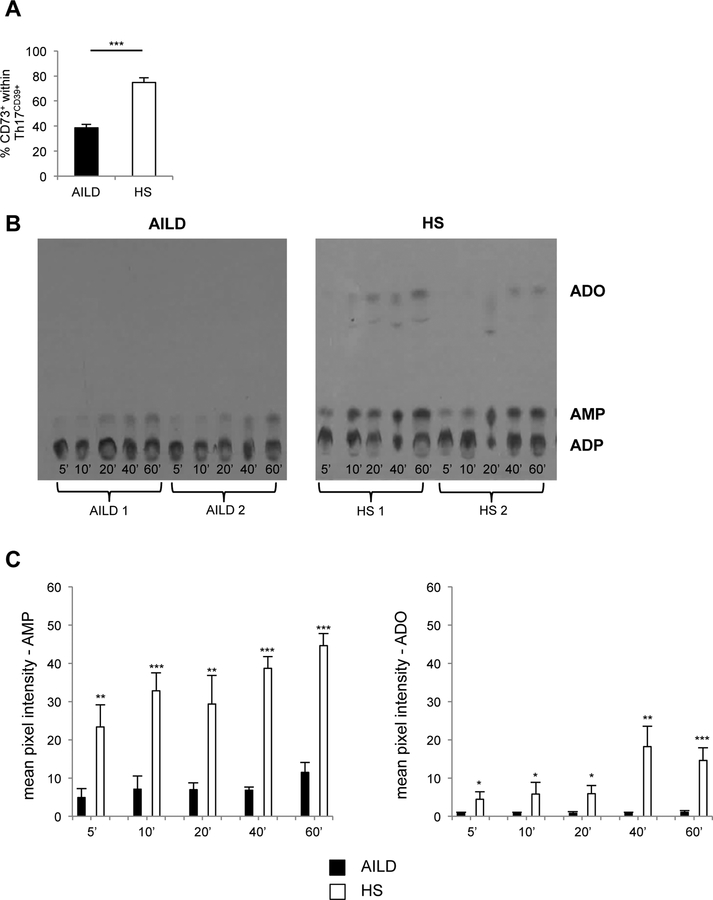
We next determined whether Th17CD39+ cells exhibited ectoenzymatic activity. We initially assessed the proportion of cells positive for CD73 - the ectoenzyme converting AMP into adenosine - within the Th17CD39+ subset. As shown in Fig. 4A, there is a lower proportion of Th17CD39+ cells expressing CD73 in AILD patients compared to HS.
Figure 4. Th17CD39+ cells from AILD patients display lower levels of ectoenzymatic activity.
(A) Mean+SEM frequency of CD73+ cells within Th17CD39+ cells in AILD (n=34) and HS (n=16). (B) CD39 ADPase enzymatic activity was assessed by TLC following incubation of Th17 cells with [14C] radiolabeled ADP substrates at different time points. Results from 2 representative AILD patients and 2 representative HS are shown. (C) Mean+SEM AMP and adenosine pixel intensity in AILD (n=4) and HS (n=4). *: P≤0.05; **: P≤0.01; ***: P≤0.001.
The ectoenzymatic activity of Th17CD39+ cells was then assessed by TLC. As depicted in Fig. 4B,C, Th17CD39+ cells from AILD patients displayed defective ADP hydrolysis, generating lower AMP and adenosine levels, at short and long reaction times.
3.4. Reduced Th17CD39+ cell suppressive properties in AILD patients
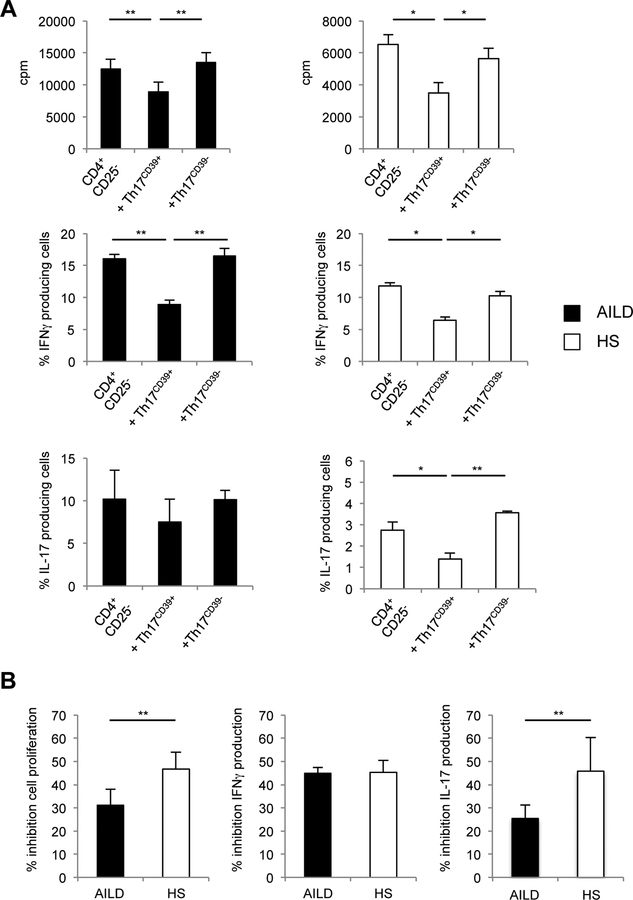
Compared to Th17CD39-, Th17CD39+ cells were more effective at suppressing CD4+CD25− responder cell proliferation, IFNγ and IL-17 production (determined as proportion of IFNJ and IL-17 producing lymphocytes) (Fig. 5A, B). Inhibition of responder cell proliferation and IL-17 production following addition of Th17CD39+ cells was markedly lower in AILD than HS (Fig. 5B). In both HS and AILD no control of responder cell proliferation and effector cytokine production was observed following addition of Th17CD39- cells.
Figure 5. Th17CD39+ cells from AILD patients fail to suppress effector cell proliferation and IL-17 production.
(A) Mean+SEM cpm and frequency of IFNγ/IL-17 producing cells within responder CD4+CD25− cells in the absence and presence of Th17CD39+ or Th17CD39- cells in AILD (n=8) and HS (n=4). (B) Mean+SEM % inhibition of cell proliferation, IFNγ and IL-17 production by Th17CD39+ cells in AILD (n=8) and HS (n=4). *: P≤0.05; **: P≤0.01.
3.5. Th17CD39+ cells from AILD patients show decreased A2A adenosine receptor expression
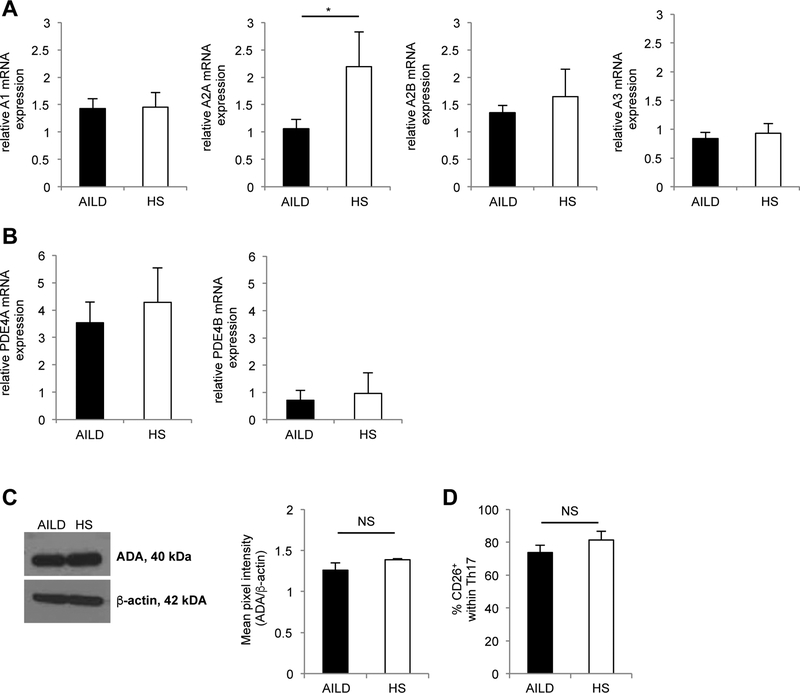
We then explored the mechanisms possibly accounting for low CD39 expression in Th17 cells from AILD patients. As this could result from low adenosinergic responsiveness due to altered adenosine receptor expression, we tested the expression of A1, A2A, A2B and A3 adenosine receptors at mRNA level. Th17 cells from AILD patients displayed markedly lower levels of A2A adenosine receptor compared to HS (Fig. 6A). We also explored whether low levels of CD39 expression were linked directly with high levels of PDE - the enzymes degrading the phosphodiester cAMP bond - or high levels of adenosine catalysis, because of heightened ADA expression.
Figure 6. Low level CD39 expression by Th17 cells is associated with decreased levels of A2A adenosine receptor.
Mean+SEM relative mRNA expression of (A) A1, A2A, A2B and A3 adenosine receptors and of (B) PDE4A and PDE4B in Th17 cells from AILD (n=6) and HS (n=4). (C) Expression of ADA by Th17 cells was determined by immunoblot analysis. One representative from 4 independent experiments is shown. Mean+SEM ADA density is also shown (AILD n=8; HS n=4). (D) Frequency of CD26+ cells within Th17 cells in AILD (n=16) and HS (n=6).
However, no substantive differences in the expression of PDE4A, PDE4B and ADA were noted between Th17 cells from AILD patients or from HS (Fig. 6B,C). As ADA is functional at the cell surface, when directly interacting with CD26, the expression of which is increased on human Th17 cells [23], the frequency of CD26+ cells was sought. As shown in Fig. 6D, no significant differences were again observed in the proportion of CD26+ cells within the Th17CD39+ subset between AILD and HS.
4. DISCUSSION
The present study shows that expression of the CD39 ectonucleotidase, which confers immunoregulatory properties upon Th17 cells, is diminished in patients with juvenile AILD. This decrease is associated with impaired generation of immunosuppressive adenosine and defective regulatory properties. The resulting persistence of Th17 cells in such an inflammatory state might play a role in perpetuation of liver injury, either inflicting or being permissive of tissue damage. Our data also indicate that deficient A2A adenosine receptor expression is responsible for impaired CD39 expression by Th17 cells in AILD.
The phenotypic analysis of Th17CD39+ cells in our patients reveals a complex profile. While maintaining conventional Th17 cell markers, such as CCR6, IL-23R and RORC, and overexpressing activation markers and CD161 - a molecule involved in Th17 cell generation [22] - they also overexpress regulatory molecules, such as FOXP3 and IL-10. That Th17 cells can undergo regulation and acquire immunosuppressive properties has been elegantly documented by Esplugues et al in a mouse model of colitis, where pathogenic Th17 cells could be redirected and controlled in the small intestine, becoming capable of producing the immunoregulatory cytokine IL-10 [24]. Recently, a study from the same group has shown that effector Th17 cells can ‘transdifferentiate’ into regulatory cells with potent suppressive capacity in in vivo anti-CD3 and bacterial induced models of inflammation [25]. These authors propose that acquisition of immunoregulatory properties by Th17 cells might provide a mechanism to limit their pathogenic potential [24]. Whether Th17 cells that acquire immunosuppressive phenotypes and function also co-express the CD39 ectonucleotidase was, however, untested.
A comparable clinical study from our group has shown that acquisition of regulatory properties by effector Th17 cells is characterized by up-regulation of CD39, with Th17CD39+ cells exhibiting immunosuppressive properties comparable to those exerted by T-regs, despite retaining some effector Th17 cell phenotypic features [16].
We report herein that patients with juvenile AILD have decreased proportions of circulating Th17CD39+ cells, the lowest frequencies being present in patients with ASC. Interestingly, echoing what was reported in Crohn disease [16], we observed particularly low frequencies of these cells in AILD patients with associated IBD. The reasons as to why the intestinal inflammatory milieu might influence number and function of Th17CD39+ cells remains to be investigated.
Whether lower proportions of Th17CD39+ cells are the result of the immunosuppressive treatment rather than being an intrinsic defect of Th17 cells in acquiring immunoregulatory properties cannot be conclusively answered by our findings. However, we observed no significant differences in the proportion of Th17CD39+ cells between patients tested at remission or during relapse, when higher doses of immunosuppressive drugs were used.
Moreover, patients on prednisolone monotherapy had proportions of Th17CD39+ cells similar to those on prednisolone and azathioprine or MMF, suggesting that these drug regimens do not impact differentially on CD39 expression by Th17 cells.
The decreased Th17CD39+ cell frequency observed in the circulation is unlikely to result from homing of these cells to the liver, as indicated by the immunohistochemistry and immunofluorescence data (Supplementary Fig. 2A,B).
When compared to HS, Th17CD39+ cells of patients with AILD exhibit a less activated phenotype, but more pro-inflammatory properties, as indicated by lower proportions of FOXP3+ and IL-10+ cells and higher frequencies of TNF-α producing lymphocytes. These findings suggest that these cells retain their effector potential, as confirmed by a decreased ability to generate adenosine, likely to result from concurrent impaired expression of the CD73 ectoenzyme.
Interestingly, Th17CD39+ cells do retain the ability to control liver damaging immune reactions, as indicated by the strong negative correlation between the proportion of TGF-β producing Th17CD39+ cells and biochemical indices of disease activity. This immunoregulatory potential, however, may be hindered by their deficient number.
The co-culture experiments performed in the present paper indicate that CD39 is pivotal in conferring immunomodulatory functions to Th17 cells in health, where the effector function (proliferation and cytokine production) of responder cells is effectively dampened upon addition of these cells. Conversely, these immunomodulatory properties are impaired in AILD suggesting that Th17CD39+ cells can persist at an inflammatory state and be instrumental to liver damage. Decreased CD39 expression on Th17 cells is likely to derive, at least in part, from low levels of the A2A adenosine receptor, with consequent decline in the anti-inflammatory activity of adenosine.
Altered immunoregulation could also derive from low generation of adenosine by the Th17 cells themselves or by CD39+ T-regs, as previously shown in AIH [17], or by resistance of Th17 cells to adenosine [16]. The comparable levels of ADA, PDE4A and PDE4B expression between patients and healthy individuals exclude the possibility that the inability to up-regulate CD39 by effector Th17 cells is linked to increased adenosine catalysis or to augmented degradation of cAMP.
Another possibility for impaired up-regulation of CD39 by Th17 cells - not tested in the current manuscript - would involve defective cAMP signaling, which induces CD39 mRNA transcription upon activation of pathways involving the cAMP dependent protein kinase (PKA). These pathways also involve the phosphoinositide 3-kinase (PI3K) and extracellular signal-regulated kinases (ERK), and culminate with the phosphorylation of the cAMP-response element-binding protein (CREB) [27].
In conclusion, it is likely that Th17 cells are involved in the pathogenesis of autoimmune liver disease both as effectors of damage and as immune regulatory cells. Monitoring the number and CD39 expression of such Th17 populations, as well as ectoenzymatic and functional properties in larger prospective studies will help determine how their effector and regulatory potentials vary over time, providing useful information for the clinical implementation of immunomodulatory interventions.
Supplementary Material
Supplementary Figure 1. Phenotype and cytokine profile of CD39+ and CD39− Th17 cell subsets. Representative flow cytometry plots of: (A) CD39 (X axis) and CCR6, IL-23R, RORC, CD69, CD44, CD25, CD73, CD161 and FOXP3 (Y axis) fluorescence; and of (B) CD39 (X axis) and IFNγ, TNF-α, IL-10 and TGF-β (Y axis) fluorescence from one HS. Cells were gated based on their FSC and SSC patterns, then on CD4+ and IL-17+ lymphocytes. CD39+ and CD39− cell populations within the CD4+IL-17+ subset were subsequently analyzed for their phenotype and cytokine profile. Compared to the Th17CD39- subset, Th17CD39+ cells contain higher proportions of IL-10+ and TGF-β+ lymphocytes, lower frequencies of TNF-α+ cells and tend to have less IFNγ producing lymphocytes.
Supplementary Figure 2. Low frequencies of intra-hepatic CD4+CD39+ cells. (A) Immunohistochemistry staining of liver sections showing scarcity of CD4+ and CD39+ single stained cells in liver biopsies obtained from 2 AIH patients studied during an episode of relapse while on immunosuppression (magnification 20×). (B) Immunofluorescence staining of the same biopsies. Alexa 488 (green): CD4; Alexa 594 (red): ENTPD1; Hoechst (blue): nuclei; merge (yellow): CD4+CD39+ cells (magnification 40×). Arrows indicate CD4+CD39+ cells.
Acknowledgments
Financial support: R Liberal: King’s Health Partners Research and Development Challenge Fund, King’s College London, UK. CR Grant: Alex P Mowat PhD Studentship, King’s College Hospital Charity, UK. MS Longhi: the Roger Dobson Fund, King’s College Hospital Charity, UK and Clinician Scientist Fellowship from the MRC, UK. Kenneth Falchuk IBD Research and Education Fund at BIDMC, Boston MA.
Abbreviations:
- AIH
autoimmune hepatitis
- AILD
autoimmune liver disease
- ASC
autoimmune sclerosing cholangitis
- ANA
anti-nuclear antibody
- AST
aspartate aminotransferase
- SMA
smooth muscle antibody
- T-regs
regulatory T-cells
References
- [1].Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. The New England journal of medicine, 2009;361:888–98. [DOI] [PubMed] [Google Scholar]
- [2].Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology, 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- [3].Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity, 2008;28:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. The Journal of biological chemistry, 2007;282:9358–63. [DOI] [PubMed] [Google Scholar]
- [5].Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nature immunology, 2007;8:958–66. [DOI] [PubMed] [Google Scholar]
- [6].Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nature immunology, 2008;9:1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort). Arthritis and rheumatism, 2006;54:1122–31. [DOI] [PubMed] [Google Scholar]
- [8].Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. The New England journal of medicine, 2007;356:580–92. [DOI] [PubMed] [Google Scholar]
- [9].Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Multiple sclerosis, 1999;5:101–4. [DOI] [PubMed] [Google Scholar]
- [10].Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science, 2006;314:1461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao L, Tang Y, You Z, Wang Q, Liang S, Han X et al. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PloS one, 2011;6:e18909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gregorio GV, Portmann B, Karani J, Harrison P, Donaldson PT, Vergani D et al. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology, 2001;33:544–53. [DOI] [PubMed] [Google Scholar]
- [13].Longhi MS, Mitry RR, Samyn M, Scalori A, Hussain MJ, Quaglia A et al. Vigorous activation of monocytes in juvenile autoimmune liver disease escapes the control of regulatory T-cells. Hepatology, 2009;50:130–42. [DOI] [PubMed] [Google Scholar]
- [14].Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. The Journal of experimental medicine, 2007;204:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Advances in pharmacology, 2011;61:301–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Longhi MS, Moss A, Bai A, Wu Y, Huang H, Cheifetz A et al. Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PloS one, 2014;9:e87956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grant CR, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC et al. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology, 2014;59:1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Longhi MS, Hussain MJ, Mitry RR, Arora SK, Mieli-Vergani G, Vergani D et al. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. Journal of immunology, 2006;176:4484–91. [DOI] [PubMed] [Google Scholar]
- [19].Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. Journal of hepatology, 2004;41:31–7. [DOI] [PubMed] [Google Scholar]
- [20].Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science, 2006;314:1792–5. [DOI] [PubMed] [Google Scholar]
- [21].Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology, 2008;48:841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bai A, Moss A, Kokkotou E, Usheva A, Sun X, Cheifetz A et al. CD39 and CD161 modulate Th17 responses in Crohn’s disease. Journal of immunology, 2014;193:3366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bengsch B, Seigel B, Flecken T, Wolanski J, Blum HE, Thimme R. Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26). Journal of immunology, 2012;188:5438–47. [DOI] [PubMed] [Google Scholar]
- [24].Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY et al. Control of TH17 cells occurs in the small intestine. Nature, 2011;475:514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature, 2015;523:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gagliani N, Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liao H, Hyman MC, Baek AE, Fukase K, Pinsky DJ. cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expression. The Journal of biological chemistry, 2010;285:14791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Phenotype and cytokine profile of CD39+ and CD39− Th17 cell subsets. Representative flow cytometry plots of: (A) CD39 (X axis) and CCR6, IL-23R, RORC, CD69, CD44, CD25, CD73, CD161 and FOXP3 (Y axis) fluorescence; and of (B) CD39 (X axis) and IFNγ, TNF-α, IL-10 and TGF-β (Y axis) fluorescence from one HS. Cells were gated based on their FSC and SSC patterns, then on CD4+ and IL-17+ lymphocytes. CD39+ and CD39− cell populations within the CD4+IL-17+ subset were subsequently analyzed for their phenotype and cytokine profile. Compared to the Th17CD39- subset, Th17CD39+ cells contain higher proportions of IL-10+ and TGF-β+ lymphocytes, lower frequencies of TNF-α+ cells and tend to have less IFNγ producing lymphocytes.
Supplementary Figure 2. Low frequencies of intra-hepatic CD4+CD39+ cells. (A) Immunohistochemistry staining of liver sections showing scarcity of CD4+ and CD39+ single stained cells in liver biopsies obtained from 2 AIH patients studied during an episode of relapse while on immunosuppression (magnification 20×). (B) Immunofluorescence staining of the same biopsies. Alexa 488 (green): CD4; Alexa 594 (red): ENTPD1; Hoechst (blue): nuclei; merge (yellow): CD4+CD39+ cells (magnification 40×). Arrows indicate CD4+CD39+ cells.