Abstract
[Purpose] This study investigated the effect of forward head posture on upper and lower thoracic shape in adults to better understand the relationship between a forward head posture and respiratory function. [Participants and Methods] Fifteen healthy males were recruited after obtaining informed consent from all participants. All participants were instructed to respire in both the forward and neutral head postures while seated. Respiratory function was assessed using spirometry. Thoracic shape during respiration was assessed using 23 markers on both the upper and the lower thorax and compared between the 2 postures. [Results] Forced vital capacity, expiratory and inspiratory reserve volumes, forced expiratory volume at 1 second, and the peak flow rate observed with the forward head posture were significantly lower than that with the neutral head posture. The upper thorax showed a greater forward shift and the lower thorax showed a greater forward and inward shift with the forward head posture than with the neutral head posture. No significant difference in upper thoracic mobility was observed during respiration between the forward head posture and the neutral head posture. However, mobility of the lower thorax during respiration was significantly reduced with the forward head posture. [Conclusion] The forward head posture causes expansion of the upper thorax and contraction of the lower thorax, and these morphological changes cause decreased respiratory function.
Key words: Forward head posture, Thoracic shape and movement, Spirometry
INTRODUCTION
Because of the rising popularity of media devices such as smartphones and computers, frequent users often exhibit incorrect posture1). Forward head posture (FHP) is a poor habitual neck posture that is defined by hyperextension of the upper cervical vertebrae and forward translation of the cervical vertebrae2) (Fig. 1).
Fig. 1.
Two task postures used in the experiments.
FHP evaluation is clinically important for diagnosis and rehabilitation treatment3). FHP increases compressive loading on tissues in the cervical spine, particularly the facet joints and ligaments2, 4). Studies have reported that symptoms including neck pain, headache, temporomandibular pain, and musculoskeletal disorders are related to FHP3, 5,6,7). In addition, FHP greatly influences respiratory function by weakening the respiratory muscles8, 9). In a prior study, Han et al.10) showed that forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1.0), and accessory respiratory muscle activity were significantly lower in the FHP group, when compared with the normal group. Additionally, work by Silveira et al.11) showed that respiratory function is affected by FHP; another study suggested that inappropriate posture may impair respiratory function, particularly among patients with respiratory diseases12). Finally, it was reported that FHP resulted in weakened respiratory function1, 13); this was improved by manual therapy14) and therapeutic exercise1, 13, 14).
Given the reasons outlined above, it is reasonable to suspect that morphological changes to thoracic shape, as a result of FHP, affect respiratory function. In fact, Szczygieł et al.15) reported that FHP caused limited movement of lower ribs during inspiration. However, to the best of our knowledge, no morphological analysis of the thoracic shape or respiratory movement associated with FHP has been reported. Therefore, the objective of the present study was to evaluate how much FHP affects upper and lower thoracic shape during respiration to better understand the influence of FHP on respiratory function.
PARTICIPANTS AND METHODS
The present study was conducted at Bunkyo Gakuin University between 2015 and 2017. The study was conducted in accordance with all dictates contained in the Declaration of Helsinki. The study protocol was reviewed and approved by the Bunkyo Gakuin University ethics committee (approval No. 2014-MSJ17).
Fifteen male participants, comprising university students and those from a previous pool of study participants, volunteered to participate in the study (Table 1). All participants gave their written, informed consent. Participants had no history of smoking, neck or respiratory diseases or traumas, anamneses of thoracotomy and laparotomy, or obvious spinal and thoracic deformations.
Table 1. Participant characteristics.
| Participants Male (%) |
N=15 100 |
|
|---|---|---|
| Variable | Mean ± SD | Range |
| Age (years) | 26.8 ± 4.5 | 20–36 |
| Height (cm) | 170.0 ± 7.8 | 160.0–194.0 |
| Seated height (cm) | 91.9 ± 3.7 | 86.0–103.5 |
| Weight (kg) | 65.3 ± 8.6 | 50.0–85.0 |
| BMI (kg/m2) | 22.6 ± 2.7 | 17.7–27.4 |
BMI: body mass index; SD: standard deviation.
Participants were tested using Record software (Record AS, Staubø, Norway). They were placed in an upright, seated position with 90° flexion of the shoulder in the scapular plane, feet flat on the floor, and 90° flexion of the hip and knee joints. For all experiments, two postures were attained: neutral head posture (NHP) and FHP. NHP was defined as a relaxed head position with the head maintained in the horizontal position. FHP was defined as a position where the head is maximally forward and maintained in the horizontal position (Fig. 1).
Kinematic data on the thoracic shape in the horizontal plane during deep breathing were collected using a three-dimensional motion analyser Vicon MX (Vicon, Inc., CA, USA) at a sampling rate of 100 Hz. A total of 23 infrared, reflective markers on the skin, each with a diameter of 9.5 mm, were placed at specific points across the front of the upper thorax and the back of the lower thorax (Fig. 2), which can measure the shape of upper and lower ribs. The back of the upper thorax was excluded due to reflections off of the scapula. The front of the lower thorax was also excluded because of reflections off of the fifth and sixth ribs, which are upper thoracic structures. Markers were placed in the following locations: the centre of the sternum at the third sternocostal joint (Centre A), the right side of the upper thorax (Fig. 1: A1, A2, A3, A4 and A5 were placed horizontally at the Centre A), the left side of the upper thorax (A6, A7, A8, A9 and A10 were placed horizontally at the Centre A), spinous process point with the same height as the Centre A (Back Centre A), the spinous process point with the same height as the xiphoid (Centre B), the right side of the lower thorax (B1, B2, B3, B4 and B5 were placed horizontally at the Centre B), and the left side of the lower thorax (B6, B7, B8, B9 and B10 were placed horizontally at the Centre B). The distance between each marker was based on the participant’s physical size, which was set to 5% of the thorax circumference.
Fig. 2.
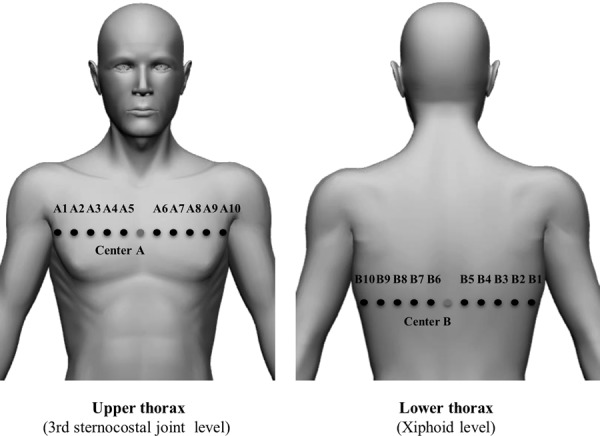
Marker placements. Note that Back Centre A is set at the opposite side of Centre A.
Using a value conversion program, each marker’s position was converted to a numerical coordinate with X and Y axes. On the upper thorax, the X axis was defined as a line connecting A1 and A10. The Y axis was defined as a perpendicular line extending from Back Centre A to the X axis. On the lower thorax, the X axis was defined as a line connecting B1 and B10. The Y axis was defined as a perpendicular line extending from Centre B to the X axis. After conversion of the markers’ values, a Y-value (anteroposterior diameter) and X-value (lateral diameter) were calculated for each marker. The value of each marker was based on the participant’s physical size, which was set to divided by the lateral diameter value of the thorax.
Each marker’s movement was used to record changes in thoracic anteroposterior diameter and lateral diameter in the resting and testing positions during deep breathing (maximal inspiratory position, maximal expiratory position)—a method demonstrated by Nakabo et al16). The oral instructions provided to participants were to ‘Just relax and breathe as normal as possible’ during quiet breathing and to ‘Take a deep, natural breaths’ during deep, volitional breathing, in accordance with the method outlined by Shobo et al17). In addition, participant head and trunk position were monitored throughout testing so as not to shift during measurement.
Change in each thoracic marker position and thoracic mobility with NHP or FHP were calculated from the amount of change in the movement of the infrared reflective markers attached to the body surface. A horizontal, cross-sectional view of the upper thorax was visualised using 12 markers on the anterior aspect of the third sternocostal joint. A horizontal, cross-sectional view of the lower thorax was visualised using 11 markers on the posterior aspect of the xiphoid process. Upper and lower thoracic mobility was calculated by subtracting the maximum expiratory value from the maximum inspiratory value.
A spirometer (Autospiro AS-507, Minato Medical Science, Osaka, Japan) was used to assess pulmonary function with NHP and FHP for each participant. Resulting measurement items included: FVC, expiratory reserve volume (ERV), inspiratory reserve volume (IRV), FEV1.0, and peak flow rate (PFR). Each test was repeated three times in each position, and the resulting values were averaged for each participant.
For all kinematic variables, the mean of measured values obtained across three consistent breath trials in the NHP and FHP conditions (as described above) was used for data analyses. For statistical analyses, a within-parameter comparison between the NHP and FHP conditions was performed using the Wilcoxon signed-rank test. A p-value <0.05 was considered statistically significant. Evaluation and analysis of all data was performed using IBM SPSS Statistics for Windows, Version 24.0 (Armonk, NY, USA: IBM Corp.).
RESULTS
FVC, ERV, IRV, FEV1.0, and PFR were significantly lower with FHP than with NHP (Table 2).
Table 2. Comparison of respiratory function between NHP and FHP.
| NHP | FHP | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| FVC** (l) | 4.44 ± 0.70 | 4.19 ± 0.67 |
| IRV** (l) | 1.94 ± 0.42 | 1.78 ± 0.40 |
| ERV** (l) | 2.00 ± 0.39 | 1.86 ± 0.41 |
| FEV1.0** (l) | 3.78 ± 0.58 | 3.55 ± 0.59 |
| PFR** (l/sec) | 8.84 ± 2.08 | 8.01 ± 2.37 |
ERV: expiratory reserve volume; FEV1.0: forced expiratory volume in 1 second; FVC: forced vital capacity; IRV: inspiratory reserve volume; l: litre; PFR: peak flow rate; SD: standard deviation. **p<0.01 NHP vs. FHP.
At rest, the upper thorax was found to be shifted significantly more forward with FHP than NHP. The lower thorax was found to be shifted significantly more forward and inward with FHP relative to NHP. In the maximal inspiratory position, the upper thorax was found to be shifted significantly forward with FHP relative to NHP, and the left upper thorax, specifically, was shifted significantly outward with FHP relative to NHP. The lower thorax was found to be shifted significantly backward and outward with NHP relative to FHP. In the maximal expiratory position, the upper thorax was found to be shifted significantly more backward with NHP relative to FHP, while the left upper thorax was shifted significantly inward with NHP relative to FHP. The lower thorax was significantly shifted forward and inward with FHP relative to NHP, and the right lower thorax was shifted significantly inward with FHP relative to NHP (Fig. 3).
Fig. 3.
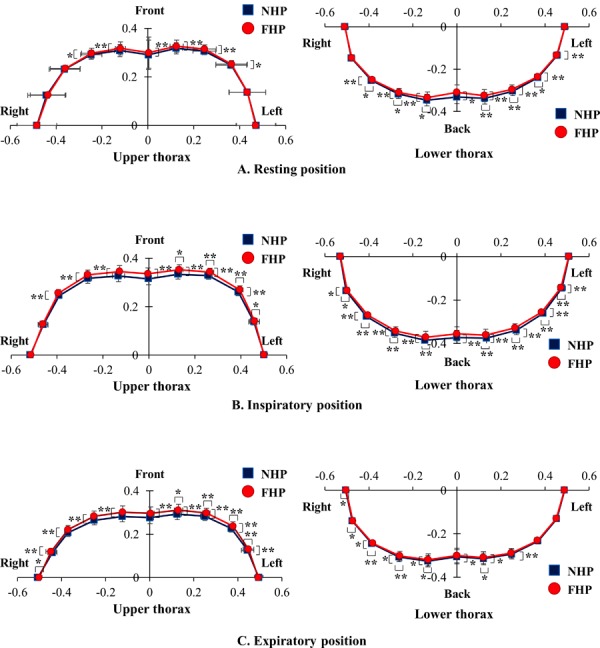
Horizontal location change of the upper and lower thoracic markers between neutral head posture (NHP) and forward head posture (FHP). A) Resting position. B) Inspiratory position. C) Expiratory position. See the section PARTICIPANTS AND METHODS for details concerning the X and Y axes. *p<0.05, **p<0.01 NHP vs. FHP.
There was no significant difference in the participants’ upper thoracic mobility along either the X (the lateral direction) or Y-axis directions (the anteroposterior direction) during respiration with NHP and FHP (Fig. 4). However, mobility along both axes in the lower thorax during respiration was significantly reduced with FHP relative to NHP (Fig. 4).
Fig. 4.
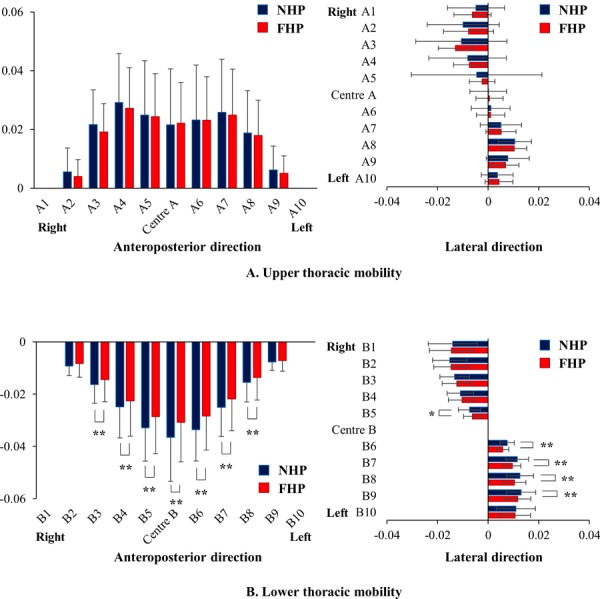
Comparison of upper and lower thoracic mobility between neutral head posture (NHP) and forward head posture (FHP) during respiration. See the section PARTICIPANTS AND METHODS for details concerning the X and Y axes. A) Comparison of upper thoracic mobility in the Y-axis (anteroposterior) and X-axis (lateral) directions between NHP and FHP. B) Comparison of lower thoracic mobility in the Y-axis (anteroposterior) and X-axis (lateral) directions between NHP and FHP. *p<0.05, **p<0.01 NHP vs. FHP.
DISCUSSION
Thoracic shape and respiratory function in the two neck postures were investigated in the present study. Our results revealed that the shape of the thorax changed and that respiratory function decreased with FHP, relative to NHP in the same person. Lee et al.18) reported that, even among participants without neck pain, assuming FHP led to a statistically significant decrease in FVC. Similarly, Han et al.10) and Almeida et al.19) reported FHP was associated with a statistically significant decrease in FVC and FEV1.0 levels compared to controls. The results of this study are consistent with those reports. Therefore, it is assumed that these previous results may have been associated with FHP-induced changes in thoracic shape and reduction of thoracic mobility.
A critical finding in the present study was that the shape of the thorax was significantly changed by FHP. FHP induced expansion of the upper thorax and contraction of the lower thorax. In addition, the characteristic thoracic shape in FHP was maintained during respiration. Therefore, decrements to respiratory function can be explained by the restriction of thoracic motion during respiration caused by this characteristic thoracic shape resulting from FHP. The expansion of the upper thorax with FHP seems to act to restrict the contraction of the upper thorax during expiration and consequently cause ERV, FEV1.0, and PFR to decrease. Conversely, contraction of the lower thorax with FHP may restrict the expansion of the lower thorax during inspiration and cause FVC and IRV decrements.
The second finding presented here was that mobility in the lower thorax was decreased by FHP during respiration. In particular, lower thorax mobility along the Y-axis (the anteroposterior direction) was significantly reduced. One possible explanation for this is that FHP has been reported to contribute to abdominal muscle shortening20). This abdominal muscle shortening, resulting from FHP, can lead to restricted expansion due to a decreased anteroposterior diameter of the lower thorax. Decreased anteroposterior diameter of the lower thorax can result in a reduction of lower thoracic mobility in the anteroposterior direction, which can in turn result in reduced diaphragm excursion, as the diaphragm is located at the level of the lower thorax. It has been previously demonstrated that greater diaphragm excursion increases ventilatory volume, and that there is a positive correlation between diaphragmatic excursion and inspired volumes21,22,23,24). In addition, Zacharkow et al.25) reported that the diaphragm relaxes as the anteroposterior diameter of the thorax decreases. Therefore, the results of the present suggest that the contractile and mobility reductions to the lower thorax may decrease the function of the diaphragm, resulting in decreased respiratory function.
The present study has some limitations, which warrant discussion. The first limitation is that the experiments were conducted only on young males; thus, there was no examination of the effects of FHP on people of different genders and ages. The second limitation of this study was that the markers in this study were placed on the skin; therefore, skin motion artefacts may have impacted the results26, 27). However, a previous study revealed that a profile of body movements represented by skin-mounted markers was similar to the profile represented by bone-pinned markers28). Therefore, the methodology used in the present study, involving skin-mounted markers, may have a minor impact on the assessment of trunk movements29). However, we could not analyse thoracic motion in the vertical plane because of deviations between markers on the skin and the skeleton, which may interfere with this assessment. Finally, diaphragm activity associated with FHP was not evaluated because simultaneous measurement was not possible with the available experimental methods. Therefore, additional research is needed to specifically examine diaphragmatic activity in FHP under measurement conditions similar to those used in this study.
Despite these limitations, the present study makes it clear that the characteristics of thoracic shape and mobility during volitional deep breathing are impacted by head position, and that FHP may adversely influence respiratory health and fitness.
Funding
This work was supported by the JSPS KAKENHI (grant number JP17K01584) and IUHW Research Grants.
Conflict of interest
The authors declare no conflicts.
Acknowledgments
The authors would like to thank Dr. Ning Qu and Dr. Naoyuki Hatayama for their constructive comments and technical support before preparation of the first draft; Dr. Hidenobu Miyaso, Dr. Zhong-Lian Li, Dr. Takuya Omotehara, and Dr. Hirohisa Koseki for their careful reading and useful comments on the first draft of the manuscript; Ms. Tomoko Kawasaki, Mr. Toshihiko Matsuda, and Mr. Tatsuya Endo for their excellent technical assistance; and Ms. Yuka Kobayashi and Ms. Yuki Ogawa for their excellent secretarial assistance.
REFERENCES
- 1.Lee NK, Jung SI, Lee DY, et al. : Effects of exercise on cervical angle and respiratory function in smartphone users. Osong Public Health Res Perspect, 2017, 8: 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kendall F, McCreary E, Provanc P, et al.: Muscles: testing and function, with posture and pain, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2005. [Google Scholar]
- 3.Fernández-de-las-Peñas C, Alonso-Blanco C, Cuadrado ML, et al. : Forward head posture and neck mobility in chronic tension-type headache: a blinded, controlled study. Cephalalgia, 2006, 26: 314–319. [DOI] [PubMed] [Google Scholar]
- 4.Fiebert IM, Roach KE, Yang SS, et al. : Cervical range of motion and strength during resting and neutral head postures in healthy young adults. J Back Musculoskeletal Rehabil, 1999, 12: 165–178. [Google Scholar]
- 5.Szeto GP, Straker L, Raine S: A field comparison of neck and shoulder postures in symptomatic and asymptomatic office workers. Appl Ergon, 2002, 33: 75–84. [DOI] [PubMed] [Google Scholar]
- 6.Lee WY, Okeson JP, Lindroth J: The relationship between forward head posture and temporomandibular disorders. J Orofac Pain, 1995, 9: 161–167. [PubMed] [Google Scholar]
- 7.Ciancaglini R, Testa M, Radaelli G: Association of neck pain with symptoms of temporomandibular dysfunction in the general adult population. Scand J Rehabil Med, 1999, 31: 17–22. [DOI] [PubMed] [Google Scholar]
- 8.Kapreli E, Vourazanis E, Strimpakos N: Neck pain causes respiratory dysfunction. Med Hypotheses, 2008, 70: 1009–1013. [DOI] [PubMed] [Google Scholar]
- 9.Kapreli E, Vourazanis E, Billis E, et al. : Respiratory dysfunction in chronic neck pain patients. A pilot study. Cephalalgia, 2009, 29: 701–710. [DOI] [PubMed] [Google Scholar]
- 10.Han J, Park S, Kim Y, et al. : Effects of forward head posture on forced vital capacity and respiratory muscles activity. J Phys Ther Sci, 2016, 28: 128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silveira W, Mello FC, Guimarães FS, et al. : Postural alterations and pulmonary function of mouth-breathing children. Rev Bras Otorrinolaringol (Engl Ed), 2010, 76: 683–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimitriadis Z, Kapreli E, Strimpakos N, et al. : Respiratory weakness in patients with chronic neck pain. Man Ther, 2013, 18: 248–253. [DOI] [PubMed] [Google Scholar]
- 13.Kim SY, Kim NS, Kim LJ: Effects of cervical sustained natural apophyseal glide on forward head posture and respiratory function. J Phys Ther Sci, 2015, 27: 1851–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-de-Uralde-Villanueva I, Candelas-Fernández P, de-Diego-Cano B, et al. : The effectiveness of combining inspiratory muscle training with manual therapy and a therapeutic exercise program on maximum inspiratory pressure in adults with asthma: a randomized clinical trial. Clin Rehabil, 2018, 32: 752–765. [DOI] [PubMed] [Google Scholar]
- 15.Szczygieł E, Węglarz K, Piotrowski K, et al. : Biomechanical influences on head posture and the respiratory movements of the chest. Acta Bioeng Biomech, 2015, 17: 143–148. [PubMed] [Google Scholar]
- 16.Nakabo T, Yamamoto S: Influence of kyphosis on chest wall motion -Comparison of slump sitting and straight sitting- (in Japanese with English abstract). Rigakuryoho Kagaku, 2009, 24: 697–701. [Google Scholar]
- 17.Shōbo A, Kakizaki F: The relationship between thoracic configuration and changes in volumes of hemithoraces in upright sitting. J Phys Ther Sci, 2016, 28: 3205–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YM, Gong WT, Kim BK: Correlation between cervical lordosis, vital capacity, T-spine ROM and equilibrium. J Phys Ther Sci, 2011, 23: 103–105. [Google Scholar]
- 19.Almeida VP, Guimarães FS, Moço VJ, et al. : [Correlation between pulmonary function, posture, and body composition in patients with asthma]. Rev Port Pneumol, 2013, 19: 204–210 (In Portuguese). [DOI] [PubMed] [Google Scholar]
- 20.Ito Y, Yamada T, Takeda M: Investigation of respiratory function and breathing pattern in elderly people with kyphosis posture (in Japanese with English abstract). Rigakuryoho Kagaku, 2007, 22: 353–358. [Google Scholar]
- 21.Houston JG, Angus RM, Cowan MD, et al. : Ultrasound assessment of normal hemidiaphragmatic movement: relation to inspiratory volume. Thorax, 1994, 49: 500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen E, Mier A, Heywood P, et al. : Excursion-volume relation of the right hemidiaphragm measured by ultrasonography and respiratory airflow measurements. Thorax, 1994, 49: 885–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott S, Fuld JP, Carter R, et al. : Diaphragm ultrasonography as an alternative to whole-body plethysmography in pulmonary function testing. J Ultrasound Med, 2006, 25: 225–232. [DOI] [PubMed] [Google Scholar]
- 24.Boussuges A, Gole Y, Blanc P: Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest, 2009, 135: 391–400. [DOI] [PubMed] [Google Scholar]
- 25.Zacharkow D: Posture: sitting, chair design and exercise. Springfield: Charles C Thomas, 1988. [Google Scholar]
- 26.Andersen MS, Damsgaard M, Rasmussen J, et al. : A linear soft tissue artefact model for human movement analysis: proof of concept using in vivo data. Gait Posture, 2012, 35: 606–611. [DOI] [PubMed] [Google Scholar]
- 27.Nester CJ, Liu AM, Ward E, et al. : Error in the description of foot kinematics due to violation of rigid body assumptions. J Biomech, 2010, 43: 666–672. [DOI] [PubMed] [Google Scholar]
- 28.Gal J, Herzog W, Kawchuk G, et al. : Measurements of vertebral translations using bone pins, surface markers and accelerometers. Clin Biomech (Bristol, Avon), 1997, 12: 337–340. [DOI] [PubMed] [Google Scholar]
- 29.Kudo S, Fujimoto M, Sato T, et al. : Quantitative evaluation of linked rigid-body representations of the trunk. Gait Posture, 2018, 63: 119–123. [DOI] [PubMed] [Google Scholar]


