Abstract
[Purpose] The aim of this study was to determine whether the consumption of a leucine-enriched essential amino acid mixture (LEAA), which is known to increase protein synthesis in muscles, alleviates muscle damage and accelerates recovery by ameliorating muscle damage. [Participants and Methods] A double-blind, randomized crossover trial was conducted over a 5-week period. Ten untrained males (age, 23.0 ± 1.6 years) were asked to repeatedly flex and extend their elbows for 10 counts/set × 5 sets at full power while using a dynamometer. The participants took 3.6-g supplements (LEAA mixture or placebo) 3 times daily on day 0 and for the next 7 days. Changes in serum creatine phosphokinase (CPK) activity and myoglobin concentration as markers of muscle tissue damage were evaluated prior to and after exercise and on days 1, 2, 3, 5, and 7. [Results] The relative ratio of the changes in peak serum CPK activity measured on day 5 was significantly lower after taking LEAA than after taking the placebo. [Conclusion] LEAA consumption suppressed exercise-induced elevation of muscle damage markers in blood, which suggests that LEAA could attenuate muscle damage and aid muscle recovery.
Keywords: Amino acids, Leucine, Muscle tissue damage
INTRODUCTION
Repeated performance of high-force, eccentric muscle contractions or unaccustomed exercise can cause tissue damage in the affected muscles1). Muscle tissue damage is accompanied by the leakage of proteins such as creatine phosphokinase (CPK) and myoglobin, from the muscle tissue into the bloodstream2,3,4). Since muscle tissue damage deceases muscle strength and range of motion, it can have a profound effect on the ability to perform subsequent bouts of exercise and therefore adhere to an exercise training program5). Thus, alleviating muscle damage and aiding recovery from muscle damage is necessary for athletes to maximize their performance.
Muscle tissue damage is associated with inflammation and the degeneration of damaged tissue. Structural damage to the sarcolemma caused by the high mechanical forces produced during high-force exercise is accompanied by a net influx of Ca2+ from the interstitium. This abnormal influx has several deleterious effects, including impairment of oxidative phosphorylation and/or activation of a calcium-dependent proteolytic enzyme on the muscle fiber6). The progressive deterioration of the sarcolemma would be accompanied by diffusion of intracellular components, such as CPK and myoglobin, into the interstitium and blood. The presence of these components in the extracellular space, induces active phagocytosis and cellular necrosis. Subsequently, undifferentiated precursors of skeletal muscle cells, known as satellite cells are activated: they proliferate, differentiate, and fuse to form myofibrils, thus repairing muscle tissue7). This process is regulated by intracellular signaling pathways that balance the synthesis and degradation of muscle proteins, such as the mammalian target of rapamycin (mTOR) pathway8). Namely, mTOR promotes muscle regeneration through kinase-independent and kinase-dependent mechanisms at the stages of nascent myofiber formation and myofiber growth, respectively8), whereas rapamycin, an inhibitor of mTOR, impairs both the formation and growth of myofibers during muscle tissue regeneration.
In recent years, researchers found that branched chain amino acids (BCAAs) increases the anabolism and decreases the catabolism of muscle proteins9,10,11). Altered protein turnover during exercise might reduce damage to myofibrillar and/or membrane-associated proteins and reduce muscle fiber disruption, resulting in lower peak values of serum CPK and myoglobin levels after exercise loading. Urinary 3-methylhistidine excretion, an index of myofibrillar protein degradation, was weakened after resistance exercise loading when the nine amino acids known as essential amino acids (EAAs) were ingested with carbohydrates, and this attenuation was associated with elevated cortisol levels12). Oral consumption of amino acids is followed by an increase in their serum concentrations, which immediately increases the rate of muscle protein synthesis13, 14), partly through activation of mTOR signaling4). EAAs are believed to have a particularly important role in the muscle protein synthesis following amino acid intake15,16,17).
Leucine, an EAA, activates mTOR signaling pathway18) and has a key role in the initiation of muscle protein synthesis19,20,21,22,23,24,25,26). In a study of elderly patients, intake of a mixture of essential amino acids including 40% leucine (leucine-enriched essential amino acids, LEAA) activated the mTOR signaling pathway in muscle tissue27). Furthermore, LEAA promoted muscle protein synthesis more strongly than a similar mixture containing 26% leucine in elderly individuals28) and young individuals29) during moderate steady state exercise, which indicates a dose-dependent effect of leucine on muscle protein synthesis.
Because of its effect on protein synthesis in muscle tissue, LEAA has been posited to facilitate recovery from muscle damage, LEAA might strongly affect recovery from muscle damage. Recently, experiments in a rat model demonstrated that LEAA increased muscle protein synthesis and attenuated muscle soreness after eccentric contractions30). However, it remains unclear whether LEAA can alleviate and stimulate recovery from muscle tissue damage after exercise loading in humans.
The aim of the present study was to investigate the effect of LEAA ingestion for 8 days on indirect markers of muscle damage by an isokinetic muscle load in untrained men. We hypothesized that the extent of muscle damage would be attenuated and/or the extent of recovery would be accelerated by LEAA. We measured serum CPK activity, myoglobin concentration, maximal muscle strength, and VAS scores for muscle pain before and after isokinetic muscle loading for 8 days in a randomized double-blind cross-over design.
PARTICIPANTS AND METHODS
Ten healthy males (age, 23.0 ± 1.6 years; height, 174.1 ± 5.8 cm; body weight, 69.0 ± 8.9 kg) who did not routinely perform exercise or habitually consume tobacco, alcohol, or dietary supplements were recruited for this study using by self-report questionnaire. The purpose, procedures, and risks associated with the study were fully explained to the participants, and written informed consent was obtained. This study was performed after receiving approval from the Research Ethics Committee of the University of Tsukuba Graduate School of Physical Education. The ethics approval number is 23-24.
We estimated that nine participants are required to have 80% power to detect a difference in CPK activity threshold of 60% between LEAA and placebo ingestions31), with a 5% alpha level. Taken a drop out ratio of 10% into account, ten participants were estimated to be included into the study.
We used a randomized, double-blind cross-over study design. The participants were randomly divided into two groups, and both groups underwent two 8-day treatment periods separated by a 3-week washout period. On day 0, the elbow flexors of one arm were loaded with exercise, and the recovery of the muscles was evaluated by measurements of maximal muscle strength, blood test and muscle soreness over the following 7 days, during which time the participants were instructed to ingest supplements of LEAA or a placebo three times daily. After the 3-week wash-out, participants went through a second treatment period that was identical to the first but with the other arm and the other supplement. One group ingested LEAA during the first treatment period and placebo on the second, whereas the other group started with the placebo and ended with LEAA.
On the day of exercise loading (day 0), participants were examined early in the morning while in a fasting state and subsequently received a light meal of jelly (200 kcal, protein:fat:carbohydrate=15:20:65). Baseline measurements (maximal muscle strength, blood tests, and muscle soreness) were obtained, and then each participant took 3.6 g supplement. Thirty minutes later, they performed the exercise, which was immediately followed by another 3.6 g supplement. Measurements were repeated after exercise loading in the same manner as described above. On the same day, the participants were also instructed to take a third dose of supplement prior to bedtime.
On days 1, 2, 3, 5, and 7, maximal muscle strength, blood and muscle soreness measurements were repeated 30 minutes after ingesting the supplement while fasting in the morning. In addition, the participants were instructed to ingest supplements at approximately 3:00 PM, and prior to bedtime. On days 4 and 6, when no measurements were performed, the supplements were ingested at approximately 10:00 AM, 3:00 PM, and prior to bedtime. During the experimental period, the participants could lead a normal life, but were also asked to refrain from strenuous exercise and sports. The participants were also asked to monitor meals during the first week after the exercise load and to refrain from consuming alcohol as much as possible.
During the washout period, the participants were again permitted to lead a normal life but to avoid strenuous exercise and sports, and they were asked to ingest meals that were as similar to the initial meals as possible. After a second set of measurements at the end of the washout period, both groups started a new treatment period, changing both the supplement and the arm that was loaded with exercise.
The composition of LEAA supplement was as reported previously28, 29). In particular, it contained 3.6 g of a mixture of all nine essential amino acids (leucine, 1.44 g; lysine, 0.6 g; valine, 0.4 g; isoleucine, 0.39 g; threonine, 0.34 g; phenylalanine, 0.24 g; methionine, 0.12 g; histidine, 0.06 g; and tryptophan, 0.03 g) per pack, whereas the placebo contained 3.6 g of maltitol per pack. Both supplements were processed by Ajinomoto Co., Inc., which were indistinguishable based on their external appearance and taste. The applied dose of LEAA (10.8 g/day) and the timing and duration of the ingestion were also based on a previous study31). The supplements were taken with 200 ml of water.
The participants performed 5 sets of 10 arm curls at each maximum effort using the Biodex System 4 (Biodex Medical Systems, New York, USA), in which the elbow flexor group of one arm was loaded with centripetal and centrifugal movements. This exercise loading, which was based on a previous report32), was as follows: starting with the elbow flexed to 5° and ending at an angle of 125°, each participant performed centripetal movements of the elbow flexors at 60°/s and centrifugal movements at 120°/s.
The maximal isometric strength of elbow flexion (expressed in W) was measured using BIODEX System 4 based on a previous report33). The participants were placed in a sitting posture and approximately 90° of elbow flexion. Prior to a test participants became familiar with the procedures by performing 2–3 submaximal contractions as warm-up. Participants were stabilized in the chair with shoulder and abdominal straps. The anatomical axis of rotation was aligned to the dynamometer axis using visual inspection and manual palpation. The isometric test included a maximal muscle contractions for 5 seconds.
Blood samples were collected from an antecubital vein using winged blood collection needles. Serum was isolated from the collected blood by centrifugation at 3,000 rpm and 4 °C for 15 minutes. To evaluate changes in the serum concentrations of amino acids with time, the concentrations of eight EAAs were measured (with the exception of tryptophan) using an automated JLC-500/V2 amino acid analyzer (JEOL, Tokyo). Serum CPK activity and myoglobin concentrations were measured as markers of muscle damage. Serum CPK activity was assayed using a commercial kit (L-Type CK; Wako Pure Chemical Industries, Ltd., Osaka, Japan). Serum myoglobin concentrations were analyzed using a chemiluminescence-based immunoassay (Chemilumi ACS-Myoglobin, Siemens Healthcare Diagnostics K.K., Tokyo, Japan). The coefficient of variations (CV) for the assays of CPK and myoglobin were 5% and 15%, respectively.
Muscle soreness was evaluated using a visual analysis scale (VAS) as previously reported34). The participants had to flex the elbow joint from an extended position to a fully flexed position and extend the elbow joint from a flexed to a fully extended position in approximately 2 s at complete rest under the guidance of the investigators. The VAS incorporated a 100-mm line marked with a 0 at one end, indicating no discomfort, and a 10 at the other end represented substantial pain. The participant placed the mark with a pen using their free hand.
Differences in changes in blood amino acid concentrations between LEAA and placebo ingestion were evaluated using a paired t-test. Serum CPK activities and myoglobin concentrations were log-transformed and presented as back transformation values. Differences in maximal muscle strength, log-transformed CPK activity, log-transformed myoglobin concentration and VAS scores for muscle soreness at each time point for the two supplement ingestions were evaluated using a mixed-effects model. Differences in the mean changes relative to the pre-exercise load value in log-transformed CPK activity and log-transformed myoglobin concentration at peak (day 5 and 3, respectively) were also evaluated using a mixed-effected model. These values were treated as response variables; treatment, time point and treatment by time point interaction were treated as fixed effects; and the subject was treated as a random effect. The analyses were performed using SAS version 9.2, and a p-value of 0.05 was considered statistically significant.
RESULTS
Blood leucine and total EAA levels were increased in LEAA ingestion compared with placebo ingestion (Leu: 215 ± 15.6 μM vs. 148 ± 16.1 μM [p=0.016], total EAAs: 1,084 ± 65 μM vs. 894 ± 90 μM [p=0.099], respectively) after supplementation on the first day. On the following day, leucine and total EAAs levels also were significantly higher in LEAA ingestion than in placebo ingestion (Leu: 264 ± 24.4 μM vs. 109 ± 9.5 μM [p=0.0002], EAAs: 1,085 ± 91 μM vs. 681 ± 52 μM [p=0.007], respectively) 30 minutes after supplementation, and they were significantly higher throughout the whole experimental period.
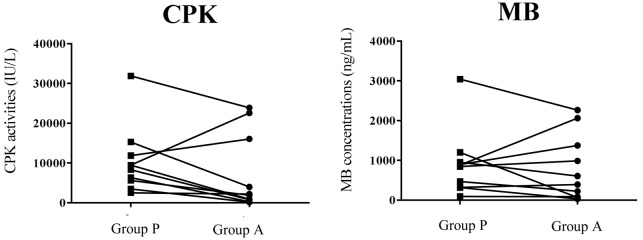
Exercise load caused a marked increase in blood muscle damage markers (CPK activity and myoglobin concentration), as shown in Table 1. Maximum CPK values were observed on day 5 after exercise loading and subsequently decreased. The average of the peak CPK values were 7,811 IU/l in placebo ingestion and 2,380 IU/l in LEAA ingestion. The individual plots (n=10) of serum CPK activities on the peak day for placebo ingestion and LEAA ingestion as were shown in Fig. 1. As shown on Fig. 2, the increase in serum CPK activity relative to the pre-exercise load value was significantly lower in LEAA ingestion than in placebo ingestion (12.7-fold vs. 55-fold; p=0.021). Serum myoglobin concentrations was also higher after the exercise loading, reaching maximum values on day 3 and then gradually decreasing. The relative ration increase in blood myoglobin value at the peak (day 3) in LEAA ingestion was lower than that in placebo ingestion (9.5-fold vs. 18.5-fold), however the difference was not significant (p=0.19). The individual plots of myoglobin levels and the ratios of myoglobin changes relative to the pre-exercise load values are shown in Figs. 1 and 2.
Table 1. Changes in maximal muscle strength, serum myoglobin concentrations, creatin phosphokinase (CPK) activities, and VAS scores for muscle pain. There was no significantly difference between Placebo and LEAA ingestion. CI means confidence interval. Group P and Group A mean placebo group and LEAA group.
| Mesurement item | Group | Pre | Post | Day 1 | Day 2 | Day 3 | Day 5 | Day 7 | |
|---|---|---|---|---|---|---|---|---|---|
| Muscle power (W) | Group P | Mean | 49.7 | 31.1 | 36.1 | 37 | 36.5 | 40.5 | 39.6 |
| 95%CI (Lower) | 41.9 | 23.4 | 28.3 | 29.3 | 28.7 | 32.7 | 31.8 | ||
| 95%CI (Upper) | 57.5 | 38.9 | 43.9 | 44.8 | 44.3 | 48.2 | 47.4 | ||
| Group A | Mean | 48 | 30 | 37.2 | 37.2 | 35.9 | 41.4 | 43.7 | |
| 95%CI (Lower) | 40.2 | 22.2 | 29.4 | 29.4 | 28.1 | 33.6 | 35.9 | ||
| 95%CI (Upper) | 55.7 | 37.7 | 45 | 45 | 43.6 | 49.1 | 51.5 | ||
| MB (ng/ml) | Group P | Mean | 33.2 | 36.1 | 59.4 | 440 | 615 | 200 | 67 |
| 95%CI (Lower) | 18.2 | 19.9 | 32.7 | 242 | 338 | 110 | 36.8 | ||
| 95%CI (Upper) | 60.3 | 65.7 | 108.2 | 801 | 1,120 | 363 | 122 | ||
| Group A | Mean | 40.6 | 42 | 64.8 | 231 | 386 | 113 | 68 | |
| 95%CI (Lower) | 22.3 | 23.1 | 35.6 | 127 | 212 | 62.1 | 37.4 | ||
| 95%CI (Upper) | 73.8 | 76.4 | 117.9 | 420 | 703 | 205 | 124 | ||
| CPK (IU/L) | Group P | Mean | 142 | 144 | 247 | 1,412 | 5,195 | 7,811 | 1,660 |
| 95%CI (Lower) | 65.8 | 66.8 | 115 | 657 | 2,416 | 3,633 | 772 | ||
| 95%CI (Upper) | 304 | 309 | 531 | 3,036 | 11,169 | 16,792 | 3,568 | ||
| Group A | Mean | 187 | 187 | 286 | 810 | 3,006 | 2,380 | 1,112 | |
| 95%CI (Lower) | 86.9 | 87.2 | 133 | 377 | 1,398 | 1,107 | 517 | ||
| 95%CI (Upper) | 402 | 403 | 615 | 1,741 | 6,462 | 5,116 | 2,390 | ||
| VAS scores for muscle pain | Group P | Mean | 0 | 3.1 | 5.3 | 6 | 5.3 | 2.4 | 1 |
| 95%CI (Lower) | −1.4 | 1.7 | 3.9 | 4.7 | 3.9 | 1.1 | −0.4 | ||
| 95%CI (Upper) | 1.3 | 4.4 | 6.6 | 7.4 | 6.6 | 3.7 | 2.3 | ||
| Group A | Mean | 0.1 | 3 | 4.3 | 5.5 | 5.2 | 1.7 | 1 | |
| 95%CI (Lower) | −1.2 | 1.6 | 3 | 4.2 | 3.9 | 0.4 | −0.3 | ||
| 95%CI (Upper) | 1.5 | 4.3 | 5.7 | 6.8 | 6.6 | 3.1 | 2.4 | ||
Fig. 1.
Individual plot (n=10) of serum creatin phosphokinase (CPK) activities (left) and myoglobin concentrations (right) on the days at which maximum levels were observed (days 5 and 3, respectively) for placebo and LEAA ingestion. Group P and Group A mean placebo group and LEAA group.
Fig. 2.
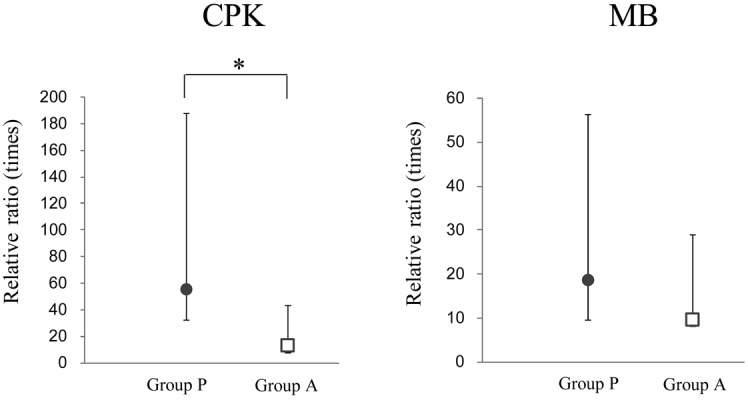
Mean ± 95% confidence interval (n=10) of the ratio of changes relative to pre-exercise load values (ratio=values at each point/values at Pre) for serum creatin phosphokinase (CPK) activity (left) and myoglobin concentration (MB) (right) on the days at which maximum concentrations were observed (days 5 and 3, respectively). Group P and Group A mean placebo group and LEAA group. *Significant difference between placebo and LEAA ingestion (p<0.05).
Maximal muscle strength was reduced by approximately 40% immediately after the exercise load and later recovered in gradual increments (Table 1). No significant differences in maximal muscle strength were observed between supplement ingestions (p=0.64 at day 2).
The VAS scores for muscle pain increased gradually after the exercise loading, reaching maximum values at day 2 and subsequently decreasing. The VAS scores for muscle pain in LEAA ingestion were lower than those in placebo ingestion, however no significant difference was observed (p=0.58 at day2).
DISCUSSION
In this randomized, double-blind, placebo-controlled crossover study, we demonstrated that ingesting LEAA suppressed the peak serum CPK activity at an isokinetic exercise loading in untrained men.
Our results showed that the 3.6 g dose (21 mg leucine per body weight) of orally administered LEAA supplement was efficiently absorbed and was sufficient to elevate serum levels of EAA and leucine for several hours after ingestion. The increases of serum levels of leucine and EAAs in this study were similar to those observed in a study by Bukhari et al. in which 3 g of LEAA were ingested by older people (age; 66 ± 1 years old)35), although the timing of measurements were different. Whereas serum levels of EAAs were measured 30 minutes after supplementation in our study, Bukhari et al. performed measurements in a time-course manner, and reported that serum concentration of amino acids (including leucine) peaked at up to 40–60 minutes, and lasted LEAA ingestion stimulated muscle protein synthesis and albumin protein synthesis in older women. These findings suggested that leucine levels after LEAA ingestion were sufficiently increased to stimulate muscle protein synthesis.
In this study, peak serum CPK activity was measured 5 days after the exercise loading. In a previous study of endurance runners, total serum CPK activity was markedly elevated 48 hours when they trained during the first week post-exercise36). The increase of CPK following eccentric exercise reached a maximum at 96 hours after exercise, and additional exercise produced only small increases, probably due to accelerated enzymatic clearance37). We found that the relative increase of the peak serum CPK activity was significantly lower in LEAA ingestion than in placebo ingestion (Fig. 2). Previous studies showed that changes in serum CPK activity correlate with the magnitude of the muscle damage induced by exercise19, 38, 39). Thus, our findings indicate that LEAA intake decreases the level of muscle damage and promotes recovery after exercise in humans, although the mechanism by which LEAA mediates these effects was not investigated.
Despite the beneficial effect of LEAA on the recovery of muscle damage, no such effect was found on the maximal isometric strength of elbow flexion and DOMS. Previous findings related to the effect of BCAA on maximal muscle contraction have also been controversial. In a study with untrained women, BCAA supplementation suppressed the decrease in leg muscle force that occurs during maximal voluntary isometric contraction, and this effect was associated with decreased soreness40). In another study with untrained college-age men, participants ingesting BCAA supplements produced higher torque levels during knee flexion 48 hours after endurance exercise compared with controls41). However, Nosaka et al. reported that BCAA supplementation in non-athletes did not affect the recovery of maximal voluntary contraction after 30 minutes of an arm curl exercise with a wristband weight set to 9% of their maximal isometric strength; whereas reduced serum CPK and myoglobin levels and attenuated muscle soreness were observed after BCAA ingestion31). No differences in serum myofiber concentrations or muscle function were detected after BCAA supplementation, despite the observation of reduced overall soreness in the quadriceps muscle and knee42). Thus, further studies are needed to fully explain how muscle function can be improved via amino acid supplementation after exercise.
A limitation of our investigation might be sample size. The extent of muscle damage after exercise loading was very wide in variation range in this study. More participants would be needed to obtain more reliable data.
In conclusion, LEAA consumption suppressed exercise-induced elevation of muscle damage markers in blood, suggesting LEAA could attenuate muscle damage and aid muscle recovery.
Conflict of interest
None.
REFERENCES
- 1.Clarkson PM, Hubal MJ: Exercise-induced muscle damage in humans. Am J Phys Med Rehabil, 2002, 81: S52–S69. [DOI] [PubMed] [Google Scholar]
- 2.Clarkson PM, Byrnes WC, McCormick KM, et al. : Muscle soreness and serum creatine kinase activity following isometric, eccentric, and concentric exercise. Int J Sports Med, 1986, 7: 152–155. [DOI] [PubMed] [Google Scholar]
- 3.Clarkson PM, Nosaka K, Braun B: Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc, 1992, 24: 512–520. [PubMed] [Google Scholar]
- 4.Munjal DD, McFadden JA, Matix PA, et al. : Changes in serum myoglobin, total creatine kinase, lactate dehydrogenase and creatine kinase MB levels in runners. Clin Biochem, 1983, 16: 195–199. [DOI] [PubMed] [Google Scholar]
- 5.Howatson G, van Someren KA: The prevention and treatment of exercise-induced muscle damage. Sports Med, 2008, 38: 483–503. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong RB: Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc, 1984, 16: 529–538. [PubMed] [Google Scholar]
- 7.Chargé SB, Rudnicki MA: Cellular and molecular regulation of muscle regeneration. Physiol Rev, 2004, 84: 209–238. [DOI] [PubMed] [Google Scholar]
- 8.Ge Y, Wu AL, Warnes C, et al. : mTOR regulates skeletal muscle regeneration in vivo through kinase-dependent and kinase-independent mechanisms. Am J Physiol Cell Physiol, 2009, 297: C1434–C1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. : Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab, 2004, 286: E321–E328. [DOI] [PubMed] [Google Scholar]
- 10.Tipton KD, Ferrando AA, Phillips SM, et al. : Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol, 1999, 276: E628–E634. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe RR: Effects of amino acid intake on anabolic processes. Can J Appl Physiol, 2001, 26: S220–S227. [DOI] [PubMed] [Google Scholar]
- 12.Bird SP, Tarpenning KM, Marino FE: Independent and combined effects of liquid carbohydrate/essential amino acid ingestion on hormonal and muscular adaptations following resistance training in untrained men. Eur J Appl Physiol, 2006, 97: 225–238. [DOI] [PubMed] [Google Scholar]
- 13.Bohé J, Low A, Wolfe RR, et al. : Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol, 2003, 552: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tipton KD, Borsheim E, Wolf SE, et al. : Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab, 2003, 284: E76–E89. [DOI] [PubMed] [Google Scholar]
- 15.Børsheim E, Tipton KD, Wolf SE, et al. : Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab, 2002, 283: E648–E657. [DOI] [PubMed] [Google Scholar]
- 16.Tipton KD, Gurkin BE, Matin S, et al. : Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem, 1999, 10: 89–95. [DOI] [PubMed] [Google Scholar]
- 17.Volpi E, Kobayashi H, Sheffield-Moore M, et al. : Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr, 2003, 78: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshizawa F, Sekizawa H, Hirayama S, et al. : Time course of leucine-induced 4E-BP1 and S6K1 phosphorylation in the liver and skeletal muscle of rats. J Nutr Sci Vitaminol (Tokyo), 2001, 47: 311–315. [DOI] [PubMed] [Google Scholar]
- 19.Anthony JC, Yoshizawa F, Anthony TG, et al. : Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr, 2000, 130: 2413–2419. [DOI] [PubMed] [Google Scholar]
- 20.Buse MG, Reid SS: Leucine. A possible regulator of protein turnover in muscle. J Clin Invest, 1975, 56: 1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buse MG, Atwell R, Mancusi V: In vitro effect of branched chain amino acids on the ribosomal cycle in muscles of fasted rats. Horm Metab Res, 1979, 11: 289–292. [PubMed] [Google Scholar]
- 22.Drummond MJ, Rasmussen BB: Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care, 2008, 11: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gran P, Cameron-Smith D: The actions of exogenous leucine on mTOR signalling and amino acid transporters in human myotubes. BMC Physiol, 2011, 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JB, Jefferson LS: Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim Biophys Acta, 1978, 544: 351–359. [DOI] [PubMed] [Google Scholar]
- 25.Norton LE, Layman DK: Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J Nutr, 2006, 136: 533S–537S. [DOI] [PubMed] [Google Scholar]
- 26.Stipanuk MH: Leucine and protein synthesis: mTOR and beyond. Nutr Rev, 2007, 65: 122–129. [DOI] [PubMed] [Google Scholar]
- 27.Dickinson JM, Gundermann DM, Walker DK, et al. : Leucine-enriched amino acid ingestion after resistance exercise prolongs myofibrillar protein synthesis and amino acid transporter expression in older men. J Nutr, 2014, 144: 1694–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. : A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab, 2006, 291: E381–E387. [DOI] [PubMed] [Google Scholar]
- 29.Pasiakos SM, McClung HL, McClung JP, et al. : Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr, 2011, 94: 809–818. [DOI] [PubMed] [Google Scholar]
- 30.Kato H, Suzuki H, Mimura M, et al. : Leucine-enriched essential amino acids attenuate muscle soreness and improve muscle protein synthesis after eccentric contractions in rats. Amino Acids, 2015, 47: 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nosaka K, Sacco P, Mawatari K: Effects of amino acid supplementation on muscle soreness and damage. Int J Sport Nutr Exerc Metab, 2006, 16: 620–635. [DOI] [PubMed] [Google Scholar]
- 32.Evans RK, Knight KL, Draper DO, et al. : Effects of warm-up before eccentric exercise on indirect markers of muscle damage. Med Sci Sports Exerc, 2002, 34: 1892–1899. [DOI] [PubMed] [Google Scholar]
- 33.Bassan NM, Simões LB, Cesar TE, et al. : Reliability of isometric and isokinetic peak torque of elbow flexors and elbow extensors muscles in trained swimmers. Rev Bras Cineantropom Desempenho Hum, 2015, 17: 507–516. [Google Scholar]
- 34.Newton MJ, Sacco P, Chapman D, et al. : Do dominant and non-dominant arms respond similarly to maximal eccentric exercise of the elbow flexors? J Sci Med Sport, 2013, 16: 166–171. [DOI] [PubMed] [Google Scholar]
- 35.Bukhari SS, Phillips BE, Wilkinson DJ, et al. : Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metab, 2015, 308: E1056–E1065. [DOI] [PubMed] [Google Scholar]
- 36.Stäubli M, Roessler B, Köchli HP, et al. : Creatine kinase and creatine kinase MB in endurance runners and in patients with myocardial infarction. Eur J Appl Physiol Occup Physiol, 1985, 54: 40–45. [DOI] [PubMed] [Google Scholar]
- 37.Hyatt JP, Clarkson PM: Creatine kinase release and clearance using MM variants following repeated bouts of eccentric exercise. Med Sci Sports Exerc, 1998, 30: 1059–1065. [DOI] [PubMed] [Google Scholar]
- 38.Brancaccio P, Maffulli N, Limongelli FM: Creatine kinase monitoring in sport medicine. Br Med Bull, 2007, 81-82: 209–230. [DOI] [PubMed] [Google Scholar]
- 39.Brancaccio P, Maffulli N, Buonauro R, et al. : Serum enzyme monitoring in sports medicine. Clin Sports Med, 2008, 27: 1–18, viivii. [DOI] [PubMed] [Google Scholar]
- 40.Shimomura Y, Inaguma A, Watanabe S, et al. : Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int J Sport Nutr Exerc Metab, 2010, 20: 236–244. [DOI] [PubMed] [Google Scholar]
- 41.Greer BK, Woodard JL, White JP, et al. : Branched-chain amino acid supplementation and indicators of muscle damage after endurance exercise. Int J Sport Nutr Exerc Metab, 2007, 17: 595–607. [DOI] [PubMed] [Google Scholar]
- 42.Jackman SR, Witard OC, Jeukendrup AE, et al. : Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Med Sci Sports Exerc, 2010, 42: 962–970. [DOI] [PubMed] [Google Scholar]