Abstract
[Purpose] The present study aimed to determine the effects of a task-oriented training on paretic upper extremity functional performance in patients with subacute stroke. [Participants and Methods] Twenty-eight subacute stroke sufferers (mean age: 50.07, standard deviation 9.31 years; mean time since stroke 11.11, standard deviation 6.73 weeks) were randomly allocated to task-oriented training (n=14) or conventional exercise program (n=14) group. They were trained as a hospital-based, individualized training 1 hour a session, 5 sessions a week for 4 weeks. Wolf Motor Function Test (primary outcome), motor portion of Fugl-Meyer assessment upper extremity, and hand function domain of Stroke Impact Scale were assessed at baseline, after 2 and 4 weeks of training. [Results] All participants completed their training programs. At all post-training assessments, the task-oriented training group showed significantly more improvements in all outcomes than the conventional exercise program group. No serious adverse effects were observed during or after the training. [Conclusion] Task-oriented training produced statistically significant and clinically meaningful improvements of paretic upper extremity functional performance in patients with subacute stroke. These beneficial effects were observed after 2 weeks (10 hours) of training. Future investigation is warranted to confirm and expand these findings.
Keywords: Task-oriented training, Upper limb, Sub-acute stroke
INTRODUCTION
The number of stroke sufferers is gradually increasing all over the world1). A stroke leaves sufferers with long-term disabilities1). Impaired upper extremity (UE) function is one of the major causes of functional difficulties after a stroke, and only 5% of stroke sufferers regain the full functional use of the paretic arm2). This can affect the performance of everyday activities and reduce the patient’s health-related quality of life (QoL)3).
Functional recovery after a stroke occurs mainly on the basis of neuroplasticity that is the capactity of the injured brain for recovery and repair4). There is currently a lack of high-quality, evidence-based interventions for the recovery of UE function after a stroke. There is also a lack of evidence about the ideal amount and content of motor training to recover function after a stroke. The task-oriented training (TOT) is one of the moderate-quality evidenced based interventions for promoting beneficial neuroplasticity associated with paretic UE functional performance3). Although a subacute stage of stroke is most likely to benefit from interventions aimed at maximizing neuroplasticity, many previous studies have focused on the effects of TOT for stroke sufferers in the chronic stage5). There are insufficient studies conducted in a subacute stroke and the heterogeneous conditions, specifically the duration of training and outcomes, across the previous studies6). Therefore, it is difficult to draw any conclusions about the effectiveness of TOT to restore UE functional performance in subacute stroke sufferers.
It was hypothesized that TOT applying during the subacute stage (to prevent the development of learned-non use7) and long-term compensatory8) strategies), where comparing with the conventional exercise program (CEP) could provide the most accurate estimate of the effects and might allow for quantification of the practiced duration for TOT on UE functional recovery in the subacute phase. Therefore, the present study aimed to determine the effects of TOT compared with a dose-matched CEP on UE functional performance in patients with subacute stroke using Wolf Motor Function Test (WMFT) that is the commonly used outcome measure. Effects were measured every 2 weeks during the study in order to determine the optimal duration for TOT.
PARTICIPANTS AND METHODS
This study was an assessor-blinded, randomized controlled trial conducted in the Physical Medicine and Rehabilitation Department of Yangon General Hospital in Myanmar. The research protocol was approved by the Ethics Committee of Khon Kaen University (HE 602116), and has been registered at the Thai Clinical Trials Registry (TCTR 20170615002).
The inclusion criteria were participants with ages between 40 and 70 years, unilateral stroke between 1 month and 6 months post stroke, Fugl-Meyer assessment upper extremity (FMA-UE) motor scores between 19 and 58, initiation of active extension of the wrist and fingers, pre-stroke right-handed, able to understand and follow instructions, and stable medical conditions. The participants were excluded if they had other neurological diseases (e.g. Parkinson’s disease or Alzheimer’s disease), musculoskeletal problems (e.g. deformities or a recent fracture) or pain (FMA-UE pain score of 1 for at least 2 joints) in the affected UE, all types of aphasia, or visual problems that could not be corrected.
The sample size was calculated based on the difference in the mean changes of WMFT (quality of movement)9), and assuming 80% power with 5% significance. A total of 28 participants were recruited (with a 15% dropout rate).
The baseline assessments of the outcome measures were performed after written informed consent was obtained from the participants. Participants were then randomly allocated to either TOT (n=14) or CEP (n=14) using a computer-generated list of random numbers with block sizes of 6 and 8 (allocation ratio 1:1). The study groups were enclosed in sequentially numbered, opaque and sealed envelopes. One physical therapist prepared a random allocation sequence before the beginning of the study. The other one assigned the randomized participants into groups.
Participants in both groups were trained as a hospital-based, individualized training for 1 hour a session, 5 sessions a week for 4 weeks (20 hours). All participants were trained under closed supervision of two experienced physical therapists (one for each group). Classic physiologic overload parameters were used for progression of exercise.
In the TOT program, each participant practiced 3 out of 6 selected functional tasks according to his/her preference. Selected tasks were drinking water from a glass, lifting a glass of water to a level of 90° shoulder flexion with an extended elbow, moving 5 crystals from the table to a box, wiping the table with a towel with the elbow extended, grasping and releasing a 6 cm in diameter tennis ball, and combing their hairs. During each 1-hour session, all participants performed warm-up exercise for 10 minutes; they then practiced the selected functional tasks for 50 minutes. During these 50 minutes TOT, a 2.5-minute rest period followed every 15 minutes of continuous practice. Before the training, the tasks were demonstrated for the participants with reference to their unaffected UE. Variables such as speed, distance, or/and resistance progressively increased in difficulty according to the individual’s ability. The physical therapist provided verbal, visual, or proprioceptive feedback and manually assisted the participants to ensure they performed the tasks completely and precisely. The training program was based on the principles of ‘use it and improve it,’ ‘specificity,’ ‘repetition,’ ‘salience,’ and ‘intensity’10).
The CEP included a dose-matched practice of active, active-assisted, and passive movements, stretching, strengthening, and coordination exercises to improve the range of motion, muscle strength, and coordination of the affected UE. In the CEP, the participants focused their rehabilitation efforts on the paretic arm and were trained 20 one-hour training sessions (5 times a week for 4 weeks). Intermittent several brief rest period of total 1 minute was included in a 1-hour session.
This study used WMFT as the primary measure, and FMA-UE and the hand function portion of the Stroke Impact Scale (SIS) version 3.0 were used as secondary measures. The WMFT, comprises 17 items, was used to evaluate the functional performance of paretic UE11). Two test items focus on strength; the other 15 test items consist of simulated functional tasks. Each of the 15 tasks is timed; participants have up to 120 seconds (WMFT-time) for each task. Each task is also graded on a 6-point scale (WMFT-FAS). It exhibits high reliability in stroke survivors (r=0.90 for WMFT-time, and r=0.95 for WMFT-FAS)12). The FMA-UE (maximum score=66) and SIS-hand function (maximum score=25) were used to measure motor recovery and the quality of the hand used, respectively13, 14). These measures have demonstrated high reliability in stroke sufferers (r=0.99 for FMA-UE and intra-class correlation coefficient [ICC]=0.82 for SIS-hand function)14, 15). For all measures except WMFT-time, higher scores indicate better functional ability. All of these measures have been demonstrated to have excellent clinimetric properties for stroke sufferers12, 14, 15).
All measures were assessed at baseline, after 2 weeks, and 4 weeks of training by the blinded assessor who did not know the participants’ group assignments or interventions.
The data were analyzed using a STATA version 10.1 (StataCorp, 4905 Lakeway Drive College Station, Texas 77845, USA). Descriptive statistics, an independent sample t-test, and χ2 tests were used to analyze the data. A paired-t test was used to compare the baseline scores with the post-test scores in each group. An analysis of covariance was used to compare the differences in the outcome measures between the 2 groups, separately at each assessment time point. The baseline outcome measures were used as covariates. A 2-sided p<0.05 was used to indicate the difference between 2 groups. To observe the average effect of WMFT between 2 groups, Cohen d (the effect size) was calculated16).
RESULTS
A total of 142 referred participants were screened for eligibility between August 2017 and January 2018. Of these, 28 were eligible to participate in the study; the data collection was completed by February 2018. All participants completed the study period (Fig. 1). The mean age of the participants was 50.07 ± 9.3 years and 50% were females. The mean post-stroke duration was 11.11 ± 6.7 weeks. The stroke affected the right side in 50% of the participants. Ninety-six percent of the study participants suffered from an ischemic stroke. The demographic and baseline clinical characteristics of the study participants did not differ significantly between the 2 groups (p>0.05) (Table 1).
Fig. 1.
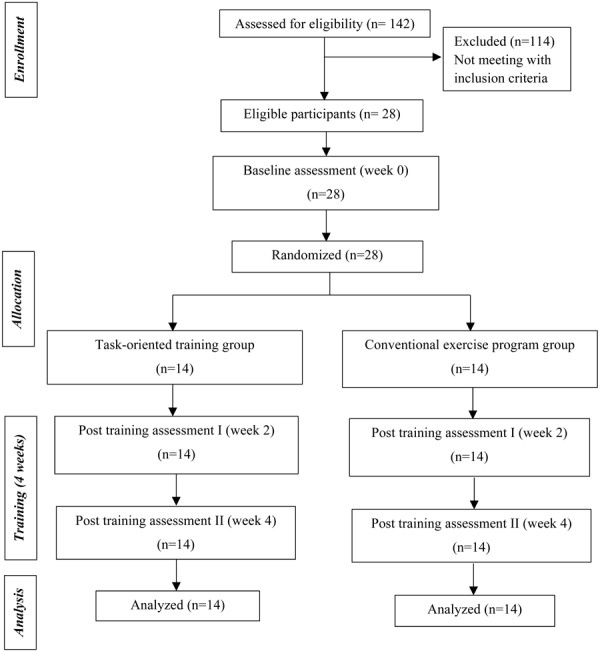
Flow diagram of the participants through each stage of study.
Table 1. Demographic and baseline clinical characteristics of the study participants.
| Characteristics | TOT group (n=14) | CEP group (n=14) | |
|---|---|---|---|
| Age (years) | 55 ± 8.43 | 55.14 ± 10.44 | |
| Post stroke duration (weeks) | 10.79 ± 7.29 | 11.43 ± 6.38 | |
| Male | 8 (57.14%) | 6 (42.86%) | |
| Female | 6 (42.86%) | 8 (57.14%) | |
| Side of hemiplegia | |||
| Right | 7 (50%) | 7 (50%) | |
| Left | 7 (50%) | 7 (50%) | |
| Type of hemiplegia | |||
| Ischemic | 13 (92.86%) | 14 (100.00%) | |
| Hemorrhagic | 1 (7.14%) | 0 (0.00%) | |
| Somatosensory loss | 0 (0.00%) | 0 (0.00%) | |
| WMFT-time (s) | |||
| Maximum scores-120 s | 54.86 ± 22.70 | 52.45 ± 14.47 | |
| WMFT-FAS | |||
| Maximum scores-05 | 1.78 ± 0.41 | 1.76 ± 0.25 | |
| WMFT-weight lifted (lb.) | 0.36 ± 0.63 | 0.43 ± 0.65 | |
| WMFT-grip strength (kg) | 1.86 ± 1.41 | 1.64 ± 1.50 | |
| FMA-UE | |||
| Maximum scores-66 | 30.14 ± 8.25 | 30.86 ± 5.50 | |
| SIS-hand function | |||
| Maximum scores-25 | 5.71 ± 1.54 | 6.07 ± 1.86 | |
Values are expressed as mean ± standard deviation, and numbers (%). CEP: Conventional exercise program; CI: confidence interval; FAS: Functional ability scale; FMA: Fugl-Meyer assessment; s: seconds; SIS: Stroke Impact Scale; TOT: Task-oriented training; UE: Upper extremity.
The results of the present study revealed that the TOT group had significantly greater improvements than the CEP group in WMFT (time, FAS, and grip strength), FMA-UE, and SIS-hand function after 2 weeks (10 hours) and after 4weeks (20 hours) of training (p<0.05) (Table 2). In the WMFT-weight lifted, there were no significant differences between the 2 groups at any post-training assessment.
Table 2. Comparison of adjusted means of outcome measures between task-oriented training and conventional exercise program groups at each assessment time point.
| Outcome | 2 weeks after training | 4 weeks after training | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TOT (n=14) |
CEP (n=14) |
Differences | 95% CI | TOT (n=14) |
CEP (n=14) |
Differences | 95% CI | ||
| WMFT-time (s)† | |||||||||
| Maximum scores-120 s | 31.91 | 46.07 | −14.16** | −21.44 to −6.88 | 23.10 | 41.53 | −18.43** | −27.35 to −9.51 | |
| WMFT-FAS | |||||||||
| Maximum scores-05 | 2.39 | 1.93 | 0.46** | 0.28 to 0.64 | 2.76 | 2.08 | 0.67** | 0.45 to 0.91 | |
| WMFT-weight lifted (lb.) | 1.46 | 1.04 | 0.41 | −0.20 to 1.03 | 1.95 | 1.48 | 0.48 | −0.09 to 1.05 | |
| WMFT-grip strength (kg) | 4.19 | 2.59 | 1.60* | 0.43 to 2.78 | 4.84 | 2.80 | 2.04* | 0.30 to 3.78 | |
| FMA-UE | |||||||||
| Maximum scores-66 | 42.79 | 37.43 | 5.36* | 2.13 to 8.59 | 48.40 | 41.95 | 6.45* | 2.39 to 10.51 | |
| SIS-hand function | |||||||||
| Maximum scores-25 | 10.69 | 7.81 | 2.88** | 1.66 to 4.11 | 12.65 | 9.00 | 3.65* | 1.67 to 5.62 | |
†Negative score means better improvement in WMFT-time(s). CEP: Conventional exercise program; CI: confidence interval; FAS: Functional ability scale; FMA: Fugl-Meyer assessment; s: seconds; SIS: Stroke Impact Scale; TOT: Task-oriented training; UE: Upper extremity. All p-values were calculated through ANCOVA. Statistically significant difference between 2 groups was defined as p<0.05. *p<0.050, **p<0.001.
The TOT group also had greater effect sizes than the CEP group. The values of WMFT-time scores were −0.61 and −1.00; WMFT-FAS scores were 1.22 and 1.56; and WMFT-grip strength scores were 0.75 and 0.80 after 2 weeks and 4 weeks of training, respectively. These values signify medium to large effects.
In addition to the statistically significant improvement, the TOT group had greater clinically meaningful improvements in outcomes than the CEP group. A change of 16% on WMFT-time scores and 17% on WMFT-FAS scores indicate clinically meaningful improvements of the paretic UE in stroke survivors17). In the present study, changes of WMFT-time were 40%, and 56%, after 2 weeks, and 4 weeks of training, respectively in the TOT group and 14%, and 22%, in the CEP group. The change scores of WMFT- FAS were 35%, and 56% at week 2, and at week 4, respectively in the TOT group and 9%, and 18% in the CEP group.
DISCUSSION
This study found that 4 weeks (20 hours) of TOT improved paretic UE functional performance, motor recovery, and quality of hand function in patients with subacute stroke more than 20 hours of CEP. The beneficial effects commenced after 2 weeks (10 hours) of training and continued at a 4-week post-training assessment. The improved functional performance of the paretic UE was indicated by the reduction in WMFT-time scores as well as improvements in WMFT functional ability and grip strength scores. However, scores on the strength item of the WMFT-weight lifted did not differ significantly between the 2 groups. TOT improves an individual’s functional abilities by focusing on skillful, repeated performances of a task rather than seeking to remediate the impairment level18). In this study, TOT led to faster movement and improved quality of movement, both associated with better functional performance, in the paretic UE19). This study also found that TOT led to better task-specific results in functional movement20).
Task-oriented training induces cortical reorganization and is based on motor control, motor learning, and rehabilitation science; active participation and skill acquisition are major components of the patient’s recovery21). It emphasizes the practice of meaningful functional activities, rather than the specific remediation of impairments. Because of the practiced tasks that are meaningful as well as familiar everyday tasks, TOT can induce greater neural plastic changes and transfer to real-life activities22, 23). Task-specific training may restore function by using spared parts of the brain adjacent to the injured and/or recruiting supplementary areas of the brain24). An effective TOT program includes three elements (challenges, progressive and optimal adaptation, and interest) that are critical incorporating the patient’s brain and cognitive and social sciences25).
The positive findings of current study are in line with previous studies comparing TOT with standard care in patients less than six months post-stroke9, 26, 27). However, there were heterogeneity of participants’ baseline UE motor severity and characteristics, outcome measures, practice duration, and the definition of task practice (reaching activities, dressing) across these studies. It is therefore difficult to draw any conclusions about the effectiveness of TOT on UE functional recovery in subacute stroke sufferers. The findings of present study add to existing knowledge about the effectiveness of 4 weeks (20 hours) of TOT on the functional performance of paretic UE in patients with subacute stroke (an average of 11 weeks post-stroke) with mild and moderate UE impairment. In addition, the primary outcome measure used in the present study—which scores on performance time and quality of movement—is the most widely used UE measure.
The underlying mechanism of functional improvement in the paretic UE after TOT may be adaptation through learning to optimize the use of intact end effectors in patients with some voluntary motor control of the wrist and finger extensors after a stroke28). After TOT, increased activity in the sensorimotor and primary motor areas of the lesioned hemisphere plays a critical role in the improvement of functional activities; this has been demonstrated in functional neuroimaging studies29, 30). Richards et al.31) also stated that experience-dependent reorganization of the primary motor cortex in both intact and injured areas of the brain, and functional recovery of paretic arm after task-specific motor trainings. Based on these findings, the meaningful functional improvements in the paretic UE after TOT observed in the present study can be associated with exercise-dependent neural plasticity.
At present, the primary intervention for UE rehabilitation after a stroke is constraint-induced movement therapy (CIMT). Its efficacy has been found to be limited to chronic stroke survivors with 20° wrist extension and 10° finger extension. However, more than 75% of stroke sufferers do not have this level of hand recovery and, therefore, may not benefit from CIMT32). In contrast, the TOT program used in the present study may be suitable for training the paretic UE of subacute stroke sufferers with poor hand recovery.
According to the results of this study, TOT, a top-down training approach targeting functional activities, has a measurable effect not only on paretic UE functional performance but also on the motor recovery and participants’ rated quality of hand function of stroke survivors. In addition, the TOT program used in this study could have favourable effects after only 2 weeks (10 hours) of training for patients with a subacute stroke. Thus, physical therapists and other health care personnel can use this program as an effective, routine therapeutic intervention in a clinical setting.
The limitation of the present study is that functional improvement of the paretic arm cannot be explained from cortical activation patterns. Therefore, further studies using non-invasive brain imaging technology should be conducted to observe the cortical reorganization corresponding to improved paretic UE function after TOT in subacute stroke sufferers.
In conclusion, this study provides support for the use of TOT rather than CEP for improving the functional performance of the paretic UE in subacute stroke sufferers, despite the limitations on the generalizability of the study’s results. Future investigation is warranted to confirm and expand these findings.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
The Back, Neck and Other Joint Pain Research Group, Khon Kaen University, Thailand, provided the research funding.
Acknowledgments
We would like to express our deepest appreciation to Associate Professor Dr. Jarugool Tretriluxana for her valuable advice and suggestions.
All physiotherapists, medical doctors, and staff at the Department of Physical Medicine and Rehabilitation, Yangon General Hospital, Myanmar are gratefully thanked for their helps and supports. Sincere gratitude and appreciation is extended to the patients for their generosity of spirit and willingness to participate in this study.
REFERENCES
- 1.Feigin VL, Krishnamurthi RV, Parmar P, et al. GBD 2013 Writing Group,GBD 2013 Stroke Panel Experts Group: Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology, 2015, 45: 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonifer NM, Anderson KM, Arciniegas DB: Constraint-induced movement therapy after stroke: efficacy for patients with minimal upper-extremity motor ability. Arch Phys Med Rehabil, 2005, 86: 1867–1873. [DOI] [PubMed] [Google Scholar]
- 3.Pollock A, Farmer SE, Brady MC, et al. : Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev, 2014, (11): CD010820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward NS, Cohen LG: Mechanisms underlying recovery of motor function after stroke. Arch Neurol, 2004, 61: 1844–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowden MG, Woodbury ML, Duncan PW: Promoting neuroplasticity and recovery after stroke: future directions for rehabilitation clinical trials. Curr Opin Neurol, 2013, 26: 37–42. [DOI] [PubMed] [Google Scholar]
- 6.Bosch J, O’Donnell MJ, Barreca S, et al. : Does task-oriented practice improve upper extremity motor recovery after stroke?: a systematic review. ISRN Stroke, 2014. [Google Scholar]
- 7.Winstein CJ, Rose DK, Tan SM, et al. : A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: a pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil, 2004, 85: 620–628. [DOI] [PubMed] [Google Scholar]
- 8.Kwakkel G, Kollen BJ, Krebs HI: Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair, 2008, 22: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arya KN, Verma R, Garg RK, et al. : Meaningful task-specific training (MTST) for stroke rehabilitation: a randomized controlled trial. Top Stroke Rehabil, 2012, 19: 193–211. [DOI] [PubMed] [Google Scholar]
- 10.Kleim JA, Jones TA: Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res, 2008, 51: S225–S239. [DOI] [PubMed] [Google Scholar]
- 11.Wolf SL, Catlin PA, Ellis M, et al. : Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke, 2001, 32: 1635–1639. [DOI] [PubMed] [Google Scholar]
- 12.Edwards DF, Lang CE, Wagner JM, et al. : An evaluation of the Wolf Motor Function Test in motor trials early after stroke. Arch Phys Med Rehabil, 2012, 93: 660–668. [DOI] [PubMed] [Google Scholar]
- 13.Fugl-Meyer AR, Jääskö L, Leyman I, et al. : The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med, 1975, 7: 13–31. [PubMed] [Google Scholar]
- 14.Duncan PW, Wallace D, Lai SM, et al. : The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke, 1999, 30: 2131–2140. [DOI] [PubMed] [Google Scholar]
- 15.Gladstone DJ, Danells CJ, Black SE: The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair, 2002, 16: 232–240. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 17.Lang CE, Edwards DF, Birkenmeier RL, et al. : Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil, 2008, 89: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayona NA, Bitensky J, Salter K, et al. : The role of task-specific training in rehabilitation therapies. Top Stroke Rehabil, 2005, 12: 58–65. [DOI] [PubMed] [Google Scholar]
- 19.Winstein CJ, Wolf SL, Dromerick AW, et al. ICARE Investigative Team: Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE): a randomized controlled trial protocol. BMC Neurol, 2013, 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adhikari SP, Tretriluxana J, Chaiyawat P, et al. : Enhanced upper extremity functions with a single session of action-observation-execution and accelerated skill acquisition program in subacute stroke. Stroke Res Treat, 2018, 2018: 1490692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shumway-Cook A, Woollacott M: Motor control: translating research into clinical practice, 4th ed. Philadelphia: Lippincott Williams and Wilkins, 2012. [Google Scholar]
- 22.Carr JH, Shepherd RB: Stroke rehabilitation: guidelines for exercise and training to optimize motor skill. London: Butterworth and Heinemann, 2003. [Google Scholar]
- 23.Winstein CJ, Miller JP, Blanton S, et al. : Methods for a multisite randomized trial to investigate the effect of constraint-induced movement therapy in improving upper extremity function among adults recovering from a cerebrovascular stroke. Neurorehabil Neural Repair, 2003, 17: 137–152. [DOI] [PubMed] [Google Scholar]
- 24.Nudo RJ, Plautz EJ, Frost SB: Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve, 2001, 24: 1000–1019. [DOI] [PubMed] [Google Scholar]
- 25.Stein J, Harvey RL, Macko RF, et al. : Stroke recovery and rehabilitation. New York: Demos Medical Publishing, 2009. [Google Scholar]
- 26.Harris JE, Eng JJ, Miller WC, et al. : A self-administered Graded Repetitive Arm Supplementary Program (GRASP) improves arm function during inpatient stroke rehabilitation: a multi-site randomized controlled trial. Stroke, 2009, 40: 2123–2128. [DOI] [PubMed] [Google Scholar]
- 27.Kwakkel G, Wagenaar RC, Twisk JW, et al. : Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet, 1999, 354: 191–196. [DOI] [PubMed] [Google Scholar]
- 28.Kwakkel G, Veerbeek JM, van Wegen EE, et al. : Constraint-induced movement therapy after stroke. Lancet Neurol, 2015, 14: 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittenberg GF, Chen R, Ishii K, et al. : Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair, 2003, 17: 48–57. [DOI] [PubMed] [Google Scholar]
- 30.Schaechter JD: Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog Neurobiol, 2004, 73: 61–72. [DOI] [PubMed] [Google Scholar]
- 31.Richards LG, Stewart KC, Woodbury ML, et al. : Movement-dependent stroke recovery: a systematic review and meta-analysis of TMS and fMRI evidence. Neuropsychologia, 2008, 46: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf SL, Winstein CJ, Miller JP, et al. EXCITE Investigators: Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA, 2006, 296: 2095–2104. [DOI] [PubMed] [Google Scholar]


