Abstract
Background
Improving medication adherence is one of the most effective approaches to improving the health outcomes of patients with diabetes. To date, enhancing diabetes medication adherence has occurred by improving diabetes-related knowledge. Unfortunately, behavior change often does not follow knowledge change. Enhancing communication between patients and healthcare professionals through addressing health literacy-related psychosocial attributes is critical.
Objective
Examine whether a patient-centered intervention augmenting usual care with a health literacy-psychosocial support intervention will improve medication adherence for patients with diabetes, compared to usual care.
Methods
This study is a randomized controlled trial with an intervention mixed methods design. Fifty participants being enrolled are English-speaking, 18–80 years old with diagnosed diabetes, take at least one diabetes medication, have low diabetes medication adherence (proportion of days covered less than 80% or based on clinical notes), and have poor diabetes control (hemoglobin A1c of ≥8%). Participants will be allocated to either a control group receiving usual care (n = 25) or an intervention group (n = 25) receiving usual care and a 6-session intervention focusing on the modifiable psychosocial factors that may influence medication adherence. A questionnaire will be administered at baseline and at the end of the intervention to all participants to assess the effectiveness of the intervention. Fifteen participants from the intervention group will be interviewed to explore participants’ experiences and perceptions of the intervention processes and outcomes.
Conclusions
The trial will examine if a patient-centered intervention that addresses patients’ health literacy and focuses on modifiable psychosocial factors will improve medication adherence among patients with diabetes.
1. Introduction
Diabetes was the 7th leading cause of death in the U.S. in 2016, and 1 in 11 Americans have diabetes [1]. The costs of prescription medications to treat diabetes and its complication comprise 45% of the total costs of diabetes care ($327 billion) in 2017. One of the lowest adherence rates of diabetes medications was 31% [2], and the World Health Organization noted that increasing the effectiveness of adherence interventions may have a greater impact on patient health outcomes than any improvement in specific medical treatment [3].
Diabetes management is multifaceted. One of the most effective approaches for improving the health outcomes of patients with diabetes is to improve their medication adherence [4,5]. To date, enhancing medication adherence has occurred by improving diabetes-related knowledge using readable self-care materials or one-on-one teaching by a diabetes educator or the treating clinician [6]. Unfortunately, behavior change often does not follow only from knowledge change [7,8]. Behavior change is more likely to occur with a combination of education, motivation, and behavioral skills [9]. While information is knowledge about the disease and medicines, motivation depends on several factors, mainly, patient's beliefs in medicines and perceptions of their illness. Behavioral skills are related to the patient's perceived self-efficacy in taking the medicines as prescribed. Another critical factor to be considered to achieve behavior change by enhancing communication between patients and healthcare professionals is addressing health literacy-related attributes [10].
Health literacy is “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions” [11,12]. Health literacy includes a wide variety of skills and attributes including social and individual factors, such as cultural and conceptual knowledge, speaking, listening, numeracy, writing, and reading skills [12,13]. Beyond these skills, health literacy encompasses decision making/critical thinking, evaluation, responsibility, self-efficacy, and navigation [[14], [15], [16]]. Enhancing health literacy is associated with improved self-efficacy, defined as an individual's belief that can facilitate one to perform given tasks and attain desired goals [17,18]. Patients with higher health literacy may feel more confident in their ability to perform self-care behaviors, including medication adherence, and may be more likely to do them. Recent studies have revealed a positive association between health literacy and diabetes self-efficacy [19,20].
Our previous study conducted with patients with type 2 diabetes showed that patients’ self-efficacy in medication use and beliefs in medicines/illness are two crucial factors that influence medication adherence [[21], [22], [23], [24]]. Our pilot work and prior literature showed that health literacy indirectly influences medication adherence of patients with diabetes [23,[25], [26], [27]] via other psychosocial factors, such as improved self-efficacy and illness and medication beliefs [21,[28], [29], [30], [31]]. Hence, health literacy interventions that address more understandable text may not work in isolation to improve medication adherence [[21], [22], [23], [24]]. A comprehensive intervention that addresses health literacy as well as psychosocial components, such as self-efficacy and illness and medication beliefs, may be a more effective strategy to improve medication adherence than an approach focusing only on health literacy.
2. Methods
2.1. Study overview
This study (Project ADHERE) proposes that a clinical pharmacist's inclusion of health literacy, psychosocial support, and self-efficacy aspects into tailored diabetes care will be a more effective intervention to improve medication adherence; rather than usual care. Researchers have reported that the strategies for improving health literacy may include building patients' capacity to be self-motivated in their disease self-management [20,[31], [32], [33], [34]]. Project ADHERE will focus on an intervention from a clinical pharmacist because pharmacists are an underused resource for clinical support in patients with uncontrolled diabetes [35]. To design a feasible intervention that fits the existing practice in diabetes care, the investigators completed four days of shadowing and observation at the diabetes clinic led by the clinical pharmacist and observed the current clinic workflow and process at the site. With the proposed intervention, the clinical pharmacist will identify patients' concerns and barriers to medication taking and self-care with diabetes with an emphasis on their self-efficacy, beliefs in medicine, and illness perceptions. Then the pharmacist will provide individualized plans and collaboratively set specific goals with each patient by strengthening their self-efficacy in medication use, and health literacy skills in navigating health information for diabetes self-care. With the awareness of patients' health literacy levels, health information communicated by the pharmacist can be tailored to be more understandable for patients with diabetes.
2.2. Study design
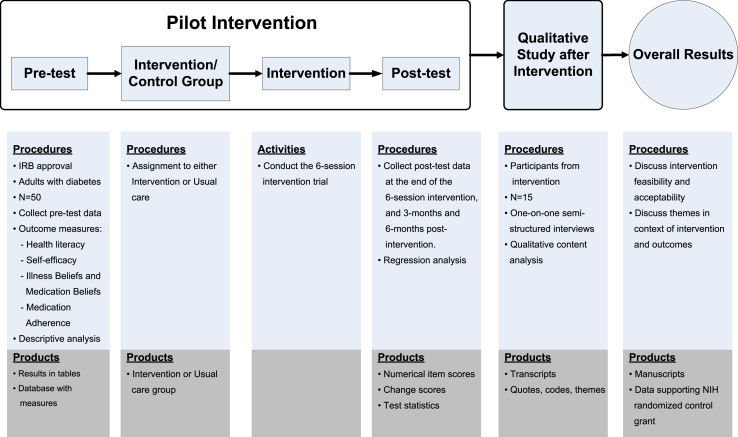
This is a prospective longitudinal randomized controlled trial (RCT) with two arms at a single healthcare facility. This study is being conducted in 2 phases using an intervention mixed methods design. We will pilot test the intervention in phase 1, and then explore the feasibility, acceptability, and outcomes of the trial in phase 2. The rationale for this design is that neither quantitative nor qualitative methods are sufficient in explaining the intervention outcomes. Mixing both methods gives a more complete analysis and augments the intervention trial [32]. This method will involve collecting both quantitative and qualitative data sequentially and then integrating the data to fully assess the feasibility, acceptability, impact, and scalability of the intervention. The qualitative results collected after the intervention will allow us to further explain the outcomes, examine participants’ experiences, and modify the methods in a follow-up trial and/or dissemination study. Also, it will help us understand how the participants view the results of the trial and determine the potential for long-term sustained effects of the intervention after the trial. An overview of how phase 1 and 2 aligns with the mixed-methods approach is shown in Fig. 1. Participants will receive a total incentive of US$150 with US$20 provided upon completion of the first survey after enrollment, US$20 provided at the end of the 6-session baseline intervention, US$50 and US$60 after 3-months and 6-months post-intervention follow-up. Additional interviews of 15 participants receiving the intervention will be conducted in phase 2, and participants will be compensated an additional US$25 upon completing the interview. The Health Sciences Institutional Review Board at the University of Wisconsin-Madison and the VA Research and Development Committee approved the study procedures (UW IRB: 2017-0951). The study is registered on ClinicalTrials.gov [NCT03406923].
Fig. 1.
An intervention mixed methods design to improve medication adherence among adults with diabetes using a health literacy/psychosocial support intervention.
2.3. Study aims and hypotheses
The goal of phase 1 is to compare the effects on medication adherence between participants receiving usual care only and those receiving usual care augmented with a 6-session health literacy-psychosocial support intervention.Project ADHERE, is intended to (1) improve patients' self-efficacy in managing medications, (2) shift negative illness beliefs and medication beliefs to be positive, and (3) improve medication adherence in adults with poorly controlled diabetes who are non-adherent to their diabetes medicines. We will use an interviewer-administered questionnaire to measure the changes in participants' psychosocial factors related to medication adherence over four assessment periods: at baseline, at the end of the 6-session intervention, and 3-months and 6-months post-intervention. We will repeat the questionnaires either in-person or by sending the participant a paper copy and having a researcher follow up by phone to possibly administer the questionnaire. In phase 1, we aim to assess the effectiveness of the intervention to enhance health literacy-related attributes by increasing patients' self-efficacy and addressing patients’ negative beliefs in medicine and illness.
The goal of phase 2 is to assess participants' experiences and perceptions of the intervention processes and outcomes including self-efficacy, beliefs, and medication adherence. Using a phenomenology qualitative approach [33], we will conduct semi-structured 60-minute in-depth interviews with a subsample of the intervention participants. Qualitative methods provide rich and detailed information about how individuals experience and understand events [34]. Phenomenology is an appropriate qualitative method when researchers want to describe an event, activity, or phenomenon [35]. In this type of study, methods such as conducting interviews are used to understand the meaning participants place on whatever is being examined and rely on participants' own perspectives to provide insight. Interviews are particularly useful in this study because it will provide great detail and depth about each participant's intervention experience and perspective [36]. In phase 2, we aim to examine the acceptability and sustainability of the intervention.
Compared to traditional patient education and medication counseling, we hypothesize that a patient-centered intervention that addresses the patient's health literacy and focuses on psychosocial factors, such as self-efficacy, illness beliefs, and beliefs in medicine, will improve medication adherence and hemoglobin A1C values for patients with diabetes.
2.4. Study site
The study will be conducted in the pharmacist-led diabetes clinic at William S. Middleton Memorial Veterans Hospital in Madison, WI. This facility provides a full range of outpatient services for 130,000 veterans living in 15 counties in south-central Wisconsin and 5 counties in northwestern Illinois [37]. The pharmacist-led diabetes clinic takes care of patients who are referred from their primary care provider for poor glycemic control.
2.5. Participants
This two-year study composed of two phases will start participant recruitment in December 2018. We will recruit 50 patients in phase 1 and 15 patients (sampled from completers of phase 1) in phase 2 from the pharmacist-led diabetes clinic. Eligibility criteria for the study enrollment in phase 1 include: (1) English-speaking men and women 18–80 years old with diabetes, (2) currently taking at least one medication prescribed for glucose control (oral or injectable medications), (3) medication adherence measured using the proportion of days covered (PDC) of diabetes medications less than 80%, obtained from pharmacy claims, clinical notes in medical records indicating nonadherence, or previously known non-adherence with injectable medications, and (4) one A1C measure of 8% or greater in the last 18 months. Fifteen individuals who complete the intervention and follow-up data collection in phase 1 will be eligible to participate in phase 2.
2.6. Recruitment
Convenience sampling will be used for patient recruitment. A list of eligible patients will be generated from the electronic medical records at the Veteran Affairs (VA) hospital. Study team members will work with data analyst staff to query the electronic health record database and identify the patients at the diabetes clinic who meet the inclusion criteria. Patients with diabetes will be identified based on International Classification of Diseases, Tenth Revision, Clinical Modification diabetes diagnosis codes (ICD10 code E11). The PDC measure will be calculated by the study team to identify patients with a PDC less than 80% (medication non-adherent). The pharmacist will identify non-adherent users by reviewing diabetes clinic notes; if patients reported missing 2 or more insulin doses in the prior two weeks, they will also be classified as non-adherent.
We will retrieve contact addresses of eligible patients and send by US mail a one-page study information sheet and informed consent at least 1 month ahead of each eligible patient's next appointment to inform them about the study. The clinical pharmacist responsible for the intervention will contact eligible patients via a phone call to answer questions and ask if they want to participate in the study. This phone call will occur 1 or 2 weeks before their next clinic appointment. If patients are interested in participating in the study, they will be asked to arrive 1 h before their next scheduled clinical pharmacist appointment. Trained researchers will meet with potential patients on-site at the scheduled time. The researchers will confirm the patient's interest in participating in the study, provide more information, answer questions, and obtain informed consent.
After the completion of phase 1, a convenient sampling will be used to recruit 15 individuals who received the intervention and completed follow-up data collection for participation in phase 2. Upon completion of the final survey, the researcher will invite the participant to complete the semi-structured interview. If the participant agrees, the researcher will either schedule a time to conduct the interview or get permission to contact the participant at a later date to schedule the interview.
2.7. Randomization
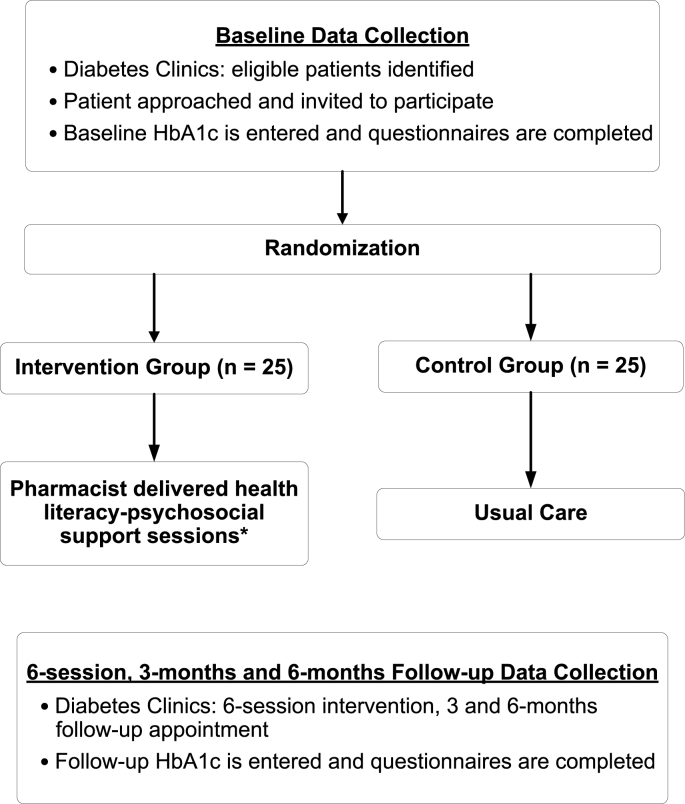
Participants will be randomly assigned, using concealed allocation to a control group (n = 25) or intervention group (n = 25) after enrollment [38]. We will use variable permuted-block randomization to allocate participants [39]. The randomization process in phase 1 is further described in Fig. 2.
Fig. 2.
The randomization process in phase.
*Details are described in Table 1.
2.8. Study arms
Participants will be assigned to either of the two study arms, which are usual care at the diabetes clinic (control group) or usual care at the diabetes clinic augmented with a 6-session ADHERE intervention (intervention group).
Usual care consists of the clinical pharmacist: (1) confirming if the patient understands how to take medications correctly, (2) adjusting diabetes medications with prescriber confirmation, (3) monitoring patient's A1C values periodically to assess diabetes management, (4) screening for and monitoring short and long-term disease complications, (5) assessing blood pressure and lipid management, (6) prescribing medications needed for complications, blood pressure and lipids, (7) sending consults/referrals for additional care as needed, and (8) triaging non-diabetes care needs that patients bring with them to clinic.
Augmented care ADHERE 6-session intervention consists of:
-
1.
The pharmacist identifying patients' concerns and barriers to medication taking and self-care with an emphasis on self-efficacy, negative beliefs in medicine and illness (Table 1).
-
2.
The patient will identify the specific areas they initially would like to focus on for the intervention.
-
3.
Area(s) to work on and specific goals are negotiated between pharmacist and participant.
-
4.
The pharmacist will then provide individualized plans and set specific goals with each patient by strengthening their self-efficacy in medication use and health literacy skills in navigating health information for diabetes self-care, and address their beliefs about medicines and diabetes (depending on the patient's self-identified agenda and goal).
-
5.
Session 1 will consist of an initial 45-minute face-to-face interaction between the participant and the clinical pharmacist.
-
6.
Sessions 2–5 are 10-minute sessions that will take place every 2–3 weeks by telephone when the clinical pharmacist communicates with participants to discuss their agreed upon goals, action plans, and beliefs. Progress towards goals will be assessed at each patient interaction, with adjustments made depending on any changes that occurred. Additional steps towards goals will also be made.
-
7.
Session 6 will be a 45-minute face-to-face session to re-examine participants' goals.
Table 1.
Contents of the 6-session HL-PSY intervention.
| Details of the intervention | |
|---|---|
| Session 1 | Discuss participants' self-management goals, self-efficacy and details of the intervention based on baseline evaluation of their psychosocial factors. |
| Session 2- Session 5 | Reinforcement of participants' psychosocial factors to improve their medication adherence and self-management skills |
| Session 6 | Reexamination of participants' goals of diabetes management and psychosocial factors |
Note: Session 1 and 6 will be conducted via a face-to-face interaction, and session 2 to 5 will be conducted via phone-call follow-up.
The methods described for the intervention align with the current clinic workflow and will not require a substantial change to the current system for counseling patients. The two face-to-face sessions (sessions 1 and 6) will take place during a regularly-scheduled clinic appointment. Upon enrollment in the study, the clinical pharmacist and research staff will inform the participant about the sequence of appointments for sessions 2–6. Phone contacts between face-to-face visits is a standard mode of communication between the pharmacist and patients in between face-to-face appointments. This will ensure that the research appointment to complete the questionnaires with the researchers will not interrupt the normal clinical flow.
2.9. Data collection
2.9.1. Phase 1
A questionnaire will be administered to both groups to collect baseline information including participants' demographics, health literacy levels, psychosocial factors (beliefs in medicines, illness perception, medication self-efficacy), and self-reported medication adherence. Participants’ clinical information will be electronically extracted from electronic medical record data. A 20-minute paper survey will be administered to all participants over four assessment points: at the baseline, at the end of the 6-session intervention, and 3-months and 6-months post-intervention. Survey information will be collected in person at baseline and session 6 of the intervention. Follow up survey information at 3 and 6-months post-intervention will be collected either in-person or over the phone with a mailed reminder letter and survey sent one week before the survey interview. The method of 3 and 6-month follow-up surveys will be determined by participant preferences and availability. In addition, sessions 1 and 6 of the intervention (the face-to-face interaction) will be audio recorded to analyze and identify intervention elements that may need modification for feasibility, dissemination, and sustainability purposes.
2.9.2. Phase 2
Fifteen participants who completed both baseline and all three follow-up questionnaires will be interviewed in a one-on-one meeting. A 60-minute in-depth semi-structured interview will be conducted in a private office at the VA hospital or a private space convenient to the participant. All interviews will be audio recorded and transcribed by a certified professional transcriptionist. Each audio-recording will be stripped of all direct participant identifiers and assigned a study code that is linked to identifiable information such as name or medical record number. Any personal, sensitive or identifiable information that is revealed will be scrubbed from the audio recording and not included in transcripts.
2.10. Outcome measures
All study measurements will be performed by trained research personnel. Measures will be assessed at parallel time points in both study arms in phase 1. Table 2 summarizes the study measures and the timing of outcome assessment. The outcome measures are described in detail below.
Table 2.
Summary and timing of study measures.
| Outcome | Method | Baseline | Phase 1 |
6–month post-intervention | Phase 2 |
|
|---|---|---|---|---|---|---|
| Completion of the 6-session intervention | 3–month post-intervention | After completion of phase 1 | ||||
| Primary outcome | ||||||
| Medication adherence | ARMS-D | X | X | X | ||
| PDC | X | X | X | |||
| Interview | X | |||||
| Secondary outcomes | ||||||
| Health literacy | NVS | X | X | |||
| Belief in medicine | BMQ | X | X | X | X | |
| Illness perception | BIPQ | X | X | X | X | |
| Self-efficacy | SEAMS | X | X | X | X | |
| Additional outcomes | ||||||
| Diabetes control | A1C | X | X | |||
Abbreviations: BIPQ: the Brief Illness Perception Questionnaire, BMQ: the Belief about Medicines Questionnaire, A1C: hemoglobin A1c, ARMS-D: the 11-item Adherence to Refills and Medications Scale for Diabetes Scale, PDC: proportion of days covered, SEAMS: the Self-efficacy for Appropriate Medication Use Scale.
2.10.1. Phase 1: primary outcome
In phase 1, the primary outcome, medication adherence, is measured by the 11-item Adherence to Refills and Medications Scale for Diabetes (ARMS-D) [40] and the PDC [41]. The ARMS-D will be used to assess self-reported medication adherence [40,42], and this measure is currently validated for use in patients with diabetes taking oral diabetes medications and/or insulin [42,43]. In addition, we will work with the VA data analysts to calculate the PDC by looking at patient adherence based on VA pharmacy claims prescription refill data. Studies have found that measures of medication adherence based on pharmacy claims are a reliable source of medication exposure [44]. Thus, pharmacy claims provide a reliable tool to measure adherence to long-term medications including glucose-lowering medications. The PDC is the leading method used to calculate medication adherence at a population level. Patients 18 years and older with a PDC of 80% or more during the measurement period are considered adherent. We will use clinical notes in medical records indicating if the patient was non-adherent during the past 12 months. Both subjective and objective measures will be complementary to capture participants’ medication adherence.
2.10.2. Phase 1: secondary outcomes
Health literacy will be measured using the 6-item Newest Vital Sign (NVS) [45]. Since the NVS has only been validated and tested using a face-to-face administration, the current study will use the same approach to collect baseline health literacy information. The NVS takes 3–5 min to complete. Each question will be scored “0” for incorrect and “1” for correct yielding a total score ranging from 0 to 6, with higher scores indicating better health literacy [45,46]. The health literacy will be measured at baseline and 6 months after the intervention is completed.
The participant's psychosocial factors will be assessed using the Brief Illness Perception Questionnaire (BIPQ) [47], the Belief about Medicines Questionnaire (BMQ) [48], and the Self-efficacy for Appropriate Medication Use Scale (SEAMS) [17]. These factors will be measured at four assessment periods throughout phase 1. The 9-item BIPQ was developed with patient groups, including patients with diabetes, and assesses patient beliefs about diabetes including timeline, consequences, identity (symptoms), personal control over diabetes, treatment control (helpfulness of diabetes medication), emotional responses, concern, and illness coherence, using single items [48]. A 9th question asks individuals to rank the most important factors they believe caused their illness. Each item is assessed on a scale of 0–10 with greater scores representing a more threatening view of diabetes. The 10-item BMQ has the necessity beliefs and concern beliefs sub-scale (five items each) [48]. The scale has five-point Likert-type responses ranging from strongly disagree to strongly agree. Each sub-scale has scores ranging from 5 to 25, with a higher score meaning stronger concern or necessity beliefs about the medicine. Medication self-efficacy will be measured using the 13-item SEAMS [17]. The SEAMS is a self-reported instrument that measures medication self-efficacy in chronic disease management. With the SEAMS, patients are asked to indicate, under a number of different circumstances, their level of self-efficacy regarding taking medications correctly [17]. The total score ranges from 13 to 39 with higher scores indicating more self-efficacy in adhering to medication use. In total, it will take about 20 min to complete these surveys.
2.10.3. Phase 1: additional outcomes
Diabetes control measured with A1C will be electronically abstracted from electronic medical records using the most recent value for each participant within the prior six months. Lower A1C values will represent better glycemic control, with values ≤7.0% recommended for people with diabetes if it can be done safely [49]. For other patients, the current Standards of Medical Care in Diabetes provided by the American Diabetes Association recommend individualized glycemic goals [50,51]. The A1C will be accessed at the baseline for recruitment and at 6-months post-intervention to evaluate the program effectiveness.
Participants’ sociodemographic information will be collected at study enrollment including age, gender, ethnicity, highest education level, and the annual household income level. Clinical characteristics will include self-reported health status, the number of medications used for diabetes, duration of diagnosis of diabetes mellitus, and what comorbidities the patient has.
2.10.4. Phase 2: primary outcome
Semi-structured interviews will be conducted after the completion of phase 1. These one-on-one, face-to-face interviews with 15 intervention participants will take place in a private office or space. The interviews will focus on exploring participants' experiences of the intervention processes and outcomes. We want to understand participants' viewpoints of the intervention and its impact on taking diabetes medicines. In addition, we want to collect participants' feedback regarding the intervention's feasibility, acceptability, delivery, and content. Table 3 shows the sample interview questions.
Table 3.
Sample interview questions.
| Item | Questions |
|---|---|
| 1. | What did you like specifically about the intervention/how it was delivered-why? |
| 2. | What parts of the intervention did you find most useful? |
| 3. | What parts of the content were unclear/not relevant? |
| 4. | How did the intervention influence your self-efficacy in taking medications? |
| 5. | How did the intervention influence how you took your diabetes medicines? |
| 6. | How did the intervention influence your beliefs about diabetes? |
| 7. | How did the intervention influence your beliefs about the diabetes medicines you take? |
| 8. | Do you think adding health literacy-psychosocial support in diabetes care would be beneficial/feasible? How? Where? |
| 9. | What new skills have you learned from this intervention? Was it possible to use the skills you learned at home after the intervention? Why or Why not? |
2.11. Data analysis plan
2.11.1. Phase 1 analysis
The general linear mixed model (GLMM) will be used to model group means (perceived level of illness beliefs, medication beliefs, self-reported adherence and A1C values) as fixed effects while simultaneously modeling for individual subject variables as random effects over time. Baseline outcome measures will be incorporated as adjusting covariates, along with other anticipated covariates. The mixed model has several unique abilities: (1) to characterize group and individual behavior patterns in a formal way, (2) to acknowledge both group and individual differences, and (3) to incorporate additional covariates in the analysis. The program NCSS Version 11 (2016) will be used to construct the GLMM models. Both between group and within time contrasts will be assessed.
The mean PDC of patients in the 12-month pre- and post-index periods will be compared within both the intervention and reference groups using paired t-tests. The pre-index PDC will be compared between the intervention and reference groups using an independent t-test. The post-index PDC will be compared between groups, while controlling for the pre-index PDC and any poorly balanced baseline covariates, using multivariable linear regression. Additionally, PDC will be categorized to classify patients as non-adherent (PDC < 20%), partially adherent (20% ≤ PDC < 80%), or adherent (PDC ≥ 80%) [52]. Chi-square tests will be used to compare the proportion of patients in each category in the pre- and post-index period. The likelihood of being classified as adherent (PDC ≥ 80% vs. PDC <80%) in the post-index period will be compared between groups using a multivariable logistic regression while adjusting for PDC category during the pre-index period and any poorly balanced baseline covariates.
2.11.2. Phase 2 analysis
All face-to-face interviews will be audio recorded and transcribed verbatim by a professional transcriber. A research team member will then verify the transcripts against the audio recordings. A qualitative content analysis will be conducted and NVivo 10 (QSR International-Melbourne) will be used to organize and categorize the themes. For analysis, (1) the transcripts will be initially read to achieve immersion; (2) the data will be read line by line to capture key thoughts; (3) the labels and codes will be created; (4) the themes and categories will be developed and organized; and (5) a conceptual model for how the themes are linked will be developed [53]. Feedback after initial coding of the transcripts will improve the discussion for subsequent interviews and prompt further questioning on emerging themes. A comparison of themes across individual participant responses will help explore the similarities, differences, and interconnections across the codes and participants. We will also document emerging relationships between themes. All analysis will occur until data saturation (i.e., when the researcher cannot find new dimensions within the data) [35,54,55]. A project assistant and the principal investigator will code the transcripts independently. After coding, similarities and divergences will be discussed. The agreement will be reached on all codes before results interpretation. After analysis, data will be discussed in the context of the intervention and outcomes. To check for accurateness and resonance with participant experiences, at the completion of the qualitative data analysis, a summary of the results of the interview will be given to four interview participants to conduct member checking [56]. These individuals will be selected based on their indicated interest in future and related research opportunities.
2.11.3. Sample size and power calculation
Since this is a pilot study and is concerned with the assessment of (1) feasibility, (2) data collection, and (3) measurement adequacy, reliance on a priori statistical power estimates is not the same as with a confirmatory study. Numerous rules-of-thumb and recommendations abound regarding sample size appropriateness to maintain rationale about measurement precision in a feasibility study [[57], [58], [59]]. An audit of sample sizes for exploratory studies in the United Kingdom discovered a median of 36 subjects per arm (minimum of 10 to a maximum of 300) [59]. Although rules-of-thumb provide guidance, we provide estimates of minimum detectable effect sizes. In phase 1, a sampling of 50 total subjects (25 per arm), would provide sufficient power (1-beta = 0.80, two-tailed alpha <0.05) to detect a large effect (Cohen's d = 0.80), which is calculated to indicate a significant difference in the outcomes measured (i.e., medication adherence and A1C level) between intervention and control groups [60]. The results of these estimates in conjunction with the rules-of-thumb provide our sampling justification. There is no rigid rule of how large sample size is for a qualitative interview, and a sample size of 15–30 is sufficient for a content analysis approach [36,61]. A total of 15 individuals recruited in phase 2 is appropriate for qualitative interviews. If saturation is not achieved, more patients will be recruited until saturation occurs.
3. Discussion
To our knowledge, this is the first longitudinal randomized control trial which aims to improve participants’ medication adherence by identifying and addressing various psychosocial factors and health literacy simultaneously. While several factors influence medication adherence including patient, provider and system-based factors, few factors are modifiable in diabetes care [6,10,13]. Bailey et al. proposed a theoretical framework that illustrates the possible mechanism between health literacy, diabetes-related behaviors, and health outcomes. It has been suggested that future studies should integrate health literacy as an important element in intervention design to confirm the causal relationship of health literacy and its attributing factors to health outcomes [62]. A review conducted by Von Wagner et al. provided a framework to illustrate how health literacy influences health outcomes mediated by a range of health actions. These authors also recommended applying health literacy in longitudinal research to investigate how health literacy impacts health outcomes through its related mediators (e.g., self-efficacy, beliefs, and motivation) [10]. The intervention developed in this study focuses on the modifiable psychosocial factors shown in our preliminary data to influence medication adherence [[21], [22], [23], [24]]. Also, a prospective longitudinal RCT with two arms will be conducted over four assessment periods to minimize the possible biases (e.g., selection bias) that jeopardizes causal inferences.
To ensure that the intervention would fit into the existing practice in diabetes care, the investigators completed four days of shadowing and observations at the diabetes clinic led by the clinical pharmacist and observed the current clinic workflow and process at the site. Drawing on these observations, the study team is confident that the intervention is in line with the current clinic workflow and will not require a substantial change to the current system for counseling diabetes patients. As mentioned before, knowledge change often does not lead to behavior change. Hence, the intervention will innovatively focus on moving knowledge towards action as the clinical pharmacist works with patients in assessing health literacy, identifying their barriers to medication use, including lack of self-efficacy, addressing negative beliefs about diabetes and diabetes medications; towards problem solving, and developing goals and action plans that will improve medication adherence and glycemic control.
The tailored intervention in this study will improve patients' medication adherence and glycemic control by using two strategies to: (1) address health literacy through reducing the complexity of diabetes content disseminated to patients during medication counseling and (2) address health literacy by enhancing patient-pharmacist communication [[63], [64], [65]]. The second strategy aims to improve the psychosocial support offered to patients by building self-efficacy and addressing negative beliefs about medicines and diabetes [[66], [67], [68]]. Together, the patient and the pharmacist can work together towards goal setting, problem solving, and negotiation of competing priorities. The proposed intervention may not only improve the pharmacist's awareness of patients' needs, but may also empower patients with more capacity to make informed decisions, which facilitates shared decision making in diabetes care.
The research team anticipates that there will be challenges throughout the study and has strategized on how to address these challenges, when possible. This study will include participants taking oral glucose-lowering agents alone, injectable agents alone, or both of these agents. It is possible that there are different treatment beliefs between patients taking oral diabetes medications and injectable medications. We will account for these differences in our statistical analysis, if it is shown that differences in treatment beliefs exist. In addition, we are working with a VA population, which has a minority of female patients, especially in older age groups where diabetes is more common. We will increase recruiting efforts for females in an effort to balance the genders in both control and intervention groups. The additional use of time for this tailored approach is a limitation to the study's scalability. However, taking into consideration the low success rates of existing interventions, low patient medication adherence with diabetes medications, as well as the literature showing the significance of psychosocial interventions; it is imperative that we conduct this intervention to determine its feasibility and use the qualitative data from phase 2 to modify the protocol for scalability.
Overall, this patient-centered randomized control trial should offer valuable insight on the effectiveness, acceptability, and sustainability of tailored health literacy-psychosocial strategies to improve the medication adherence of patients with diabetes. Findings from this study will expand our understanding of the problems of medication non-adherence that patients encounter and will contribute to potential solutions to address these issues to overcome the barriers to diabetes self-management.
Funding sources
This study was funded by Merck Sharp & Dohme, United States, grant number 57298.
Conflicts of interest and disclosures
None of the authors has any conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100326.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.American Diabetes Association . 2018. The Cost of Diabetes.http://www.diabetes.org/advocacy/news-events/cost-of-diabetes.html [Google Scholar]
- 2.Donnan P.T., MacDonald T.M., Morris A.D. Adherence to prescribed oral hypoglycaemic medication in a population of patients with Type 2 diabetes: a retrospective cohort study. Diabet. Med. 2002;19(4):279–284. doi: 10.1046/j.1464-5491.2002.00689.x. [DOI] [PubMed] [Google Scholar]
- 3.Haynes R.B., McDonald H., Garg A.X., Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst. Rev. 2002;2:CD000011. doi: 10.1002/14651858.CD000011. [DOI] [PubMed] [Google Scholar]
- 4.Campbell R.K. Recommendations for improving adherence to type 2 diabetes mellitus therapy-focus on optimizing insulin-based therapy. Am. J. Manag. Care. 2012;18(3 Suppl):S55–S61. [PubMed] [Google Scholar]
- 5.Nau D.P. Recommendations for improving adherence to type 2 diabetes mellitus therapy--focus on optimizing oral and non-insulin therapies. Am. J. Manag. Care. 2012;18(3 Suppl):S49–S54. [PubMed] [Google Scholar]
- 6.Al Sayah F., Majumdar S.R., Williams B., Robertson S., Johnson J.A. Health literacy and health outcomes in diabetes: a systematic review. J. Gen. Intern. Med. 2013;28(3):444–452. doi: 10.1007/s11606-012-2241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S.H., Yu X. The mediating effect of self-efficacy on the relationship between health literacy and health status in Korean older adults: a short report. Aging Ment. Health. 2010;14(7):870–873. doi: 10.1080/13607861003801011. [DOI] [PubMed] [Google Scholar]
- 8.Bijl J.V., Poelgeest-Eeltink A.V., Shortridge-Baggett L. The psychometric properties of the diabetes management self-efficacy scale for patients with type 2 diabetes mellitus. J. Adv. Nurs. 1999;30(2):352–359. doi: 10.1046/j.1365-2648.1999.01077.x. [DOI] [PubMed] [Google Scholar]
- 9.Fisher J.D., Fisher W.A. Changing AIDS-risk behavior. Psychol. Bull. 1992;111(3):455. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 10.von Wagner C., Steptoe A., Wolf M.S., Wardle J. Health literacy and health actions: a review and a framework from health psychology. Health Educ. Behav. 2009;36(5):860–877. doi: 10.1177/1090198108322819. [DOI] [PubMed] [Google Scholar]
- 11.Ratzan S.C. Health literacy: communication for the public good. Health Promot. Int. 2001;16(2):207–214. doi: 10.1093/heapro/16.2.207. [DOI] [PubMed] [Google Scholar]
- 12.Kindig D.A., Panzer A.M., Nielsen-Bohlman L. National Academies Press; 2004. Health Literacy: a Prescription to End Confusion. [PubMed] [Google Scholar]
- 13.Paasche-Orlow M.K., Wolf M.S. The causal pathways linking health literacy to health outcomes. Am. J. Health Behav. 2007;31(Suppl 1):S19–S26. doi: 10.5555/ajhb.2007.31.supp.S19. [DOI] [PubMed] [Google Scholar]
- 14.Sørensen K., Van den Broucke S., Fullam J., Doyle G., Pelikan J., Slonska Z., Brand H. Health literacy and public health: a systematic review and integration of definitions and models. BMC Public Health. 2012;12:80. doi: 10.1186/1471-2458-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haun J.N., Valerio M.A., McCormack L.A., Sorensen K., Paasche-Orlow M.K. Health literacy measurement: an inventory and descriptive summary of 51 instruments. J. Health Commun. 2014;19(Suppl 2):302–333. doi: 10.1080/10810730.2014.936571. [DOI] [PubMed] [Google Scholar]
- 16.Baker D.W. The meaning and the measure of health literacy. J. Gen. Intern. Med. 2006;21(8):878–883. doi: 10.1111/j.1525-1497.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Risser J., Jacobson T.A., Kripalani S. Development and psychometric evaluation of the Self-efficacy for Appropriate Medication Use Scale (SEAMS) in low-literacy patients with chronic disease. J. Nurs. Meas. 2007;15(3):203–219. doi: 10.1891/106137407783095757. [DOI] [PubMed] [Google Scholar]
- 18.Bodenheimer T., Lorig K., Holman H., Grumbach K. Patient self-management of chronic disease in primary care. J. Am. Med. Assoc. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 19.Osborn C.Y., Cavanaugh K., Wallston K.A., Rothman R.L. Self-efficacy links health literacy and numeracy to glycemic control. J. Health Commun. 2010;15(Suppl 2):146–158. doi: 10.1080/10810730.2010.499980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavanaugh K., Wallston K.A., Gebretsadik T., Shintani A., Huizinga M.M., Davis D., Gregory R.P., Malone R., Pignone M., DeWalt D., Elasy T.A., Rothman R.L. Addressing literacy and numeracy to improve diabetes care: two randomized controlled trials. Diabetes Care. 2009;32(12):2149–2155. doi: 10.2337/dc09-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiyanbola O.O., Unni E., Huang Y.M., Lanier C. The association of health literacy with illness perceptions, medication beliefs, and medication adherence among individuals with type 2 diabetes. Res. Soc. Adm. Pharm. 2018;14(9):824–830. doi: 10.1016/j.sapharm.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y.M., Shiyanbola O.O., Smith P.D. Association of health literacy and medication self-efficacy with medication adherence and diabetes control. Patient Prefer. Adherence. 2018;12:793–802. doi: 10.2147/PPA.S153312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y.M., Shiyanbola O.O., Chan H.Y. A path model linking health literacy, medication self-efficacy, medication adherence, and glycemic control. Patient Educ. Counsel. 2018;101(11):1906–1913. doi: 10.1016/j.pec.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Shiyanbola O.O., Unni E., Huang Y.M., Lanier C. Using the extended self-regulatory model to characterise diabetes medication adherence: a cross-sectional study. BMJ Open. 2018;8(11):e022803. doi: 10.1136/bmjopen-2018-022803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loke Y.K., Hinz I., Wang X., Salter C. Systematic review of consistency between adherence to cardiovascular or diabetes medication and health literacy in older adults. Ann. Pharmacother. 2012;46(6):863–872. doi: 10.1345/aph.1Q718. [DOI] [PubMed] [Google Scholar]
- 26.Bains S.S., Egede L.E. Associations between health literacy, diabetes knowledge, self-care behaviors, and glycemic control in a low income population with type 2 diabetes. Diabetes Technol. Ther. 2011;13(3):335–341. doi: 10.1089/dia.2010.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S., Love F., Quistberg D.A., Shea J.A. Association of health literacy with self-management behavior in patients with diabetes. Diabetes Care. 2004;27(12):2980–2982. doi: 10.2337/diacare.27.12.2980. [DOI] [PubMed] [Google Scholar]
- 28.Kale M.S., Federman A.D., Krauskopf K., Wolf M., O'Conor R., Martynenko M., Leventhal H., Wisnivesky J.P. The Association of Health Literacy with Illness and Medication Beliefs among Patients with Chronic Obstructive Pulmonary Disease. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y.J., Shin S.J., Wang R.H., Lin K.D., Lee Y.L., Wang Y.H. Pathways of empowerment perceptions, health literacy, self-efficacy, and self-care behaviors to glycemic control in patients with type 2 diabetes mellitus. Patient Educ. Counsel. 2016;99(2):287–294. doi: 10.1016/j.pec.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Lee E.H., Lee Y.W., Moon S.H. A structural equation model linking health literacy to self-efficacy, self-care activities, and health-related quality of life in patients with type 2 diabetes. Asian Nurs. Res. (Korean Soc. Nurs. Sci.) 2016;10(1):82–87. doi: 10.1016/j.anr.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Hofer R., Choi H., Mase R., Fagerlin A., Spencer M., Heisler M. Mediators and moderators of improvements in medication adherence. Health Educ. Behav. 2017;44(2):285–296. doi: 10.1177/1090198116656331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creswell J.W., Fetters M.D., Ivankova N.V. Designing a mixed methods study in primary care. Ann. Fam. Med. 2004;2(1):7–12. doi: 10.1370/afm.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis S. Qualitative inquiry and research design: choosing among five approaches. Health Promot. Pract. 2015;16(4):473–475. [Google Scholar]
- 34.Social Science Research Institute, Duke University, Tipsheet-Qualitative Interviews. https://dism.ssri.duke.edu/survey-help/tipsheets/tipsheet-qualitative-interviews. (Accessed July 14 2018).
- 35.Charmaz K., Belgrave L. Vol. 2. 2012. pp. 347–365. (Qualitative Interviewing and Grounded Theory Analysis, The SAGE Handbook of Interview Research: The Complexity of the Craft). [Google Scholar]
- 36.Turner D.W., III Qualitative interview design: a practical guide for novice investigators. Qual. Rep. 2010;15(3):754–760. [Google Scholar]
- 37.U.S. Department of Veterans Affairs, about the William S. Middleton Memorial Veterans Hospital. https://www.madison.va.gov/about/index.asp. (Accessed July 14 2018).
- 38.Viera A.J., Bangdiwala S.I. Eliminating bias in randomized controlled trials: importance of allocation concealment and masking. Fam. Med. 2007;39(2):132. [PubMed] [Google Scholar]
- 39.Matts J.P., Lachin J.M. Properties of permuted-block randomization in clinical trials. Contr. Clin. Trials. 1988;9(4):327–344. doi: 10.1016/0197-2456(88)90047-5. [DOI] [PubMed] [Google Scholar]
- 40.Mayberry L.S., Gonzalez J.S., Wallston K.A., Kripalani S., Osborn C.Y. The ARMS-D out performs the SDSCA, but both are reliable, valid, and predict glycemic control. Diabetes Res. Clin. Pract. 2013;102(2):96–104. doi: 10.1016/j.diabres.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrade S.E., Kahler K.H., Frech F., Chan K.A. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol. Drug Saf. 2006;15(8):565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 42.Osborn C.Y., Gonzalez J.S. Measuring insulin adherence among adults with type 2 diabetes. J. Behav. Med. 2016;39(4):633–641. doi: 10.1007/s10865-016-9741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim C.-J., Schlenk E.A., Ahn J.-A., Kim M., Park E., Park J. Evaluation of the measurement properties of self-reported medication adherence instruments among people at risk for metabolic syndrome: a systematic review. Diabetes Educat. 2016;42(5):618–634. doi: 10.1177/0145721716655400. [DOI] [PubMed] [Google Scholar]
- 44.Nau D.P. Pharmacy Quality Alliance; Springfield, VA: 2012. Proportion of Days Covered (PDC) as a Preferred Method of Measuring Medication Adherence. [Google Scholar]
- 45.Weiss B.D., Mays M.Z., Martz W., Castro K.M., DeWalt D.A., Pignone M.P., Mockbee J., Hale F.A. Quick assessment of literacy in primary care: the newest vital sign. Ann. Fam. Med. 2005;3(6):514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y.M., Shiyanbola O.O., Smith P.D., Chan H.Y. Quick screen of patients' numeracy and document literacy skills: the factor structure of the newest vital sign. Patient Prefer. Adherence. 2018;12:853–859. doi: 10.2147/PPA.S165994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broadbent E., Petrie K.J., Main J., Weinman J. The brief illness perception questionnaire. J. Psychosom. Res. 2006;60(6):631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 48.Horne R., Weinman J., Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol. Health. 1999;14(1):1–24. [Google Scholar]
- 49.Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M., Peters A.L., Tsapas A., Wender R., Matthews D.R. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American diabetes association and the European Association for the study of diabetes. Diabetologia. 2015;58(3):429–442. doi: 10.1007/s00125-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 50.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Supplement 1):S55–S64. doi: 10.2337/dc18-S006. [DOI] [PubMed] [Google Scholar]
- 51.American Diabetes Association 11. Older adults: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Supplement 1):S119–S125. doi: 10.2337/dc18-S011. [DOI] [PubMed] [Google Scholar]
- 52.de Vries F.M., Denig P., Vegter S., Bos H.J., Postma M.J., Hak E. Does a cardiovascular event change adherence to statin treatment in patients with type 2 diabetes? A matched cohort design. Curr. Med. Res. Opin. 2015;31(4):595–602. doi: 10.1185/03007995.2015.1011780. [DOI] [PubMed] [Google Scholar]
- 53.Hsieh H.F., Shannon S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 54.Pope C., Ziebland S., Mays N. Qualitative research in health care. Anal. Qual. Data, BMJ. 2000;320(7227):114–116. doi: 10.1136/bmj.320.7227.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richards L. Sage; 2014. Handling Qualitative Data: A Practical Guide. [Google Scholar]
- 56.Birt L., Scott S., Cavers D., Campbell C., Walter F. Member checking: a tool to enhance trustworthiness or merely a nod to validation? Qual. Health Res. 2016;26(13):1802–1811. doi: 10.1177/1049732316654870. [DOI] [PubMed] [Google Scholar]
- 57.Hertzog M.A. Considerations in determining sample size for pilot studies. Res. Nurs. Health. 2008;31(2):180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 58.Julious S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut. Stat. 2005;4(4):287–291. [Google Scholar]
- 59.Billingham S.A., Whitehead A.L., Julious S.A. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med. Res. Methodol. 2013;13(1):104. doi: 10.1186/1471-2288-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen J. second ed. Erlbaum Associates; Hillsdale: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 61.Creswell J.W., Miller D.L. Determining validity in qualitative inquiry. Theory Into Pract. 2000;39(3):124–130. [Google Scholar]
- 62.Bailey S.C., Brega A.G., Crutchfield T.M., Elasy T., Herr H., Kaphingst K., Karter A.J., Moreland-Russell S., Osborn C.Y., Pignone M., Rothman R., Schillinger D. Update on health literacy and diabetes. Diabetes Educat. 2014;40(5):581–604. doi: 10.1177/0145721714540220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berkman N.D., Sheridan S.L., Donahue K.E., Halpern D.J., Viera A., Crotty K., Holland A., Brasure M., Lohr K.N., Harden E. Health literacy interventions and outcomes: an updated systematic review. Evid. Rep. Technol. Assess. 2011;199(1):941. [PMC free article] [PubMed] [Google Scholar]
- 64.Haynes R.B., Ackloo E., Sahota N., McDonald H.P., Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst. Rev. 2008;2(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 65.Ratanawongsa N., Karter A.J., Parker M.M., Lyles C.R., Heisler M., Moffet H.H., Adler N., Warton E.M., Schillinger D. Communication and medication refill adherence: the diabetes study of northern California. JAMA Intern Med. 2013;173(3):210–218. doi: 10.1001/jamainternmed.2013.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDonald H.P., Garg A.X., Haynes R.B. Interventions to enhance patient adherence to medication prescriptions: scientific review. J. Am. Med. Assoc. 2002;288(22):2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 67.Peyrot M., Rubin R.R. Behavioral and psychosocial interventions in diabetes: a conceptual review. Diabetes Care. 2007;30(10):2433–2440. doi: 10.2337/dc07-1222. [DOI] [PubMed] [Google Scholar]
- 68.Gonzalez J.S., Tanenbaum M.L., Commissariat P.V. Psychosocial factors in medication adherence and diabetes self-management: implications for research and practice. Am. Psychol. 2016;71(7):539. doi: 10.1037/a0040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.