Abstract
Background
The time of day that people exercise could have an influence on the efficacy of exercise for weight loss, via differences in adherence and/or physiological adaptations. However, there is currently no evidence to support an optimal time of day for exercise to maximise efficacy.
Purpose
To examine the feasibility and acceptability of prescribed morning and evening exercise.
Methods
Twenty inactive, overweight adults aged 18–60 years were recruited for a 12-week intervention and randomized to one of three groups using a 2:2:1 random allocation ratio: i) morning exercise (AM; n = 9); ii) evening exercise (PM; n = 7); or iii) waitlist control (CON; n = 4). Exercise groups were prescribed self-paced walking or running on a treadmill to achieve a weekly total of 250 min. Feasibility and acceptability data were collected, and physiological and behavioural outcomes associated with energy balance were measured at baseline, mid- and post-intervention.
Results
Attrition was low (n = 2 dropped out), with high measurement completion rates (>80%). The intervention groups had high adherence rates to exercise sessions (94% and 87% for the AM and PM groups, respectively). No adverse events resulting from the intervention were reported. Both intervention groups displayed improvements to their cardiometabolic risk profile; cardiorespiratory fitness improved by 5.2 ± 4.7, and 4.6 ± 4.5 mL kg−1.min−1 and body fat percentage reduced by 1.2 ± 1.4, and −0.6 ± 1.2% for AM and PM groups, respectively.
Conclusion
This feasibility study provides evidence that morning and evening exercise interventions are feasible, and also provides justification for a large-scale randomized controlled trial.
Trial registration
This trial is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12616000457448p, 7/4/2016).
Keywords: Exercise, Time of day, Feasibility, Randomized controlled trial, Energy balance
Abbreviations: 3-FU, 3-month follow-up; 6-FU, 6-month follow-up; AM, morning exercise; BL, baseline; BMI, body mass index; CON, control; DXA, dual x-ray absorptiometry; LFPQ, Leeds food preference questionnaire; MARCA, Multimedia activity recall for children and adults; MEQ, morningness-eveningness questionnaire; MVPA, moderate-vigorous physical activity; PAL, physical activity level; PM, evening exercise; PSQI, Pittsburgh sleep quality index; RPE, ratings of perceived exertion; RMR, resting metabolic rate; TFEQ, three-factor eating questionnaire; VAS, visual analogue scale; VO2peak, peak oxygen uptake
1. Introduction
Systems and functions of the body operate around an ∼24 h cycle known as the circadian clock. Circadian rhythms regulate several physiological processes that influence athletic performance, such as heart rate and oxygen uptake (VO2) [1,2]. Perhaps, in part, because of these rhythms, there is a plethora of research which has compared the effect of morning and evening exercise on exercise performance [[3], [4], [5]]. However, interventions investigating the effects of exercise training on health outcomes tend not to compare, nor control, the time of day at which exercise is prescribed. Participation in regular physical activity has well-documented health benefits including reducing the risk of cardiovascular disease, type II diabetes, obesity and some cancers [6]. The time of day that people choose to exercise may influence adherence, and hence the effectiveness to achieve the desired outcomes. As such, in order to optimise exercise prescription, the time of day of training should be considered. Given the popular view that exercising in the morning in a fasted state may be more effective in eliciting weight loss [7], research in this area may have important practical implications.
The single study (to our knowledge) that has compared the effectiveness of morning versus evening exercise on health outcomes has identified inherent limitations including, small sample size (n = 48), lack of generalised results (overweight women aged 20-45y only), and short-term duration (6-weeks) [8]. In addition, the time periods in which participants trained (morning, 0800–1000; and evening, 1400–1600) are not conducive to regular work-hours, and do not coincide with circadian peaks. In light of these limitations, and due to the complexities in understanding the impact of time of exercise on health outcomes, we propose a more rigorous protocol be considered. This will provide the opportunity to explain any differences observed between morning and evening exercise, and consider some additional health outcomes and components of energy balance, which will provide further insight than previous studies [8].
The current study was intentionally designed to assess the feasibility, safety and acceptability of morning versus evening exercise in a rigorously designed randomized controlled trial. The Medical Research Council and National Institute for Health Research both advocate for conducting feasibility studies to assess whether it is appropriate to upscale to a larger trial, and inform large-scale, complex interventions [9]. The assessment of feasibility and acceptability were defined by: recruitment and consent rates; measurement completion rate; adherence to, and enjoyment of, exercise sessions; retention rate; loss-to-follow-up; and adverse events [10]. Based on documented drop-out rates of lifestyle interventions [11], the trial will be considered feasible if adherence to the intervention, rate of completion of measurements, and retention rate are all ≥80%. Bowen et al. [12], proposed eight key areas of focus for feasibility studies; (i) acceptability; (ii) demand; (iii) implementation; (iv) practicality, (v) adaptation; (vi) integration; (vii) expansion; and (viii) limited-efficacy testing, which will provide the framework for discussion.
2. Materials and methods
2.1. Study design and ethics approval
The study design is a three-armed, randomized controlled trial design. Twenty participants were randomly assigned to one of three groups, a waitlist control group (CON; n = 4), or one of two intervention groups: a morning exercise group (AM; n = 9), or an evening exercise group (PM; n = 7). This study is registered with the Australian New Zealand Clinical Trials Registry (Trial Registration: ACTRN12616000457448p, 7/4/2016) and been approved by the Bellberry Human Research Ethics Committee (HREC2016-02-130). All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all individual participants included in this study.
2.2. Participants
Males and females aged 18–60 years who met the eligibility criteria were included in the study. The inclusion criteria were [1]: being insufficiently active (<150 min of moderate-vigorous physical activity per week, by self-report) [2]; being overweight or obese (body mass index (BMI) ≥25 kg/m2); and [3] weight stable in the previous three months (±3 kg by self-report). The exclusion criteria were [1] pregnancy, or plans to become pregnant over the course of the study [2]; participation in shift work [3]; current participation in a weight loss program [4]; any reported use of medication or supplements that would affect food intake, appetite or physical activity levels, weight loss, or metabolism [4]; having a body weight >159 kg (due to limitations of the dual-energy x-ray absorptiometry (DXA) machine); and [5] inability to complete the study within the designated time period [13].
Participants were recruited from the local community and metropolitan universities via electronic media and print advertising. Interested individuals were provided with a study description and details regarding the participant expectations and time commitments required to complete the study. Potential participants were screened for eligibility by web-based or telephone survey, which included stage one of the Adult Pre-exercise Screening System, developed by Exercise and Sports Science Australia [14]. Individuals deemed eligible and who obtained medical clearance, or did not require it, were scheduled for their baseline assessment. Ineligible individuals, and those who failed to obtain medical clearance were excluded from the study. During screening, demographic information (i.e. age, sex, employment status and work hours, educational attainment, marital status and details of any dependents) were collected. Participants did not receive any compensation for their participation in the study.
2.3. Randomization and blinding
After completing baseline assessments (2 x face-to-face appointments and 2 x telephone interviews), participants were randomly allocated to one of three groups; AM, PM or CON, at a 2:2:1 ratio using permuted block randomization with multiple, randomized block sizes. This process used a list of identification numbers that is sent to a researcher independent to the study who was blinded to the identity of the participants. Unequal allocation offers several advantages over equal allocation such as: improved acceptability of the trial, increased exposure to the intervention, increased power for secondary analyses, a way to offset a higher attrition in one of the groups, and trial efficacy [15] and has been used in previous exercise trials [16,17]. Due to the nature of the exercise intervention, it was not possible to blind the participants to group allocation. However, participants were not informed of the hypothesis of the study.
2.4. Intervention groups
The two intervention conditions comprised of a 12-week exercise program in which participants were prescribed a minimum of 250 min of moderate-vigorous exercise per week; the dose of exercise recommended by the American College of Sports Medicine to elicit clinically significant weight loss [18]. There were no caveats to restrict additional exercise outside of the intervention, however, participants were asked to complete the minimum dose of exercise during their respective time frame, conducive to their group allocation. That is, the AM group were required to complete at least 250 min/week of exercise between 06:00–09:00, and the PM group between 16:00–19:00. These time periods were chosen to coincide with diurnal hormone patterns, and also for convenience based on when most people could accommodate exercise (i.e. before or after work) to enhance any physiological adaptations which may occur as a result of exercising at either time of day, and maximise adherence to the sessions [19].
The exercise program included both supervised and unsupervised exercise sessions. Participants completed an initial four-week supervised exercise training phase, of five 50 min sessions per week. Over the remaining eight weeks, exercise sessions were tapered by one session per fortnight until two sessions per week was reached, which was sustained for the remainder of the intervention. Supervised sessions consisted of self-paced brisk-walking or running on a motor-driven treadmill. Participants were free to adjust the speed and/or incline to achieve the desired exercise load. All sessions include a 3-min warm-up at a standard intensity level, and a 3-min cool-down/stretching period. All supervised exercise sessions were conducted at the School of Human Movement and Nutrition Sciences at The University of Queensland, St. Lucia, Australia. Both intervention groups received the same instructions during the exercise sessions, including verbal encouragement and equal motivation. Participants were encouraged to continue to exercise for 250 min/week, but the unsupervised sessions were not prescribed. Participants were asked to keep an exercise diary to record information about each unsupervised session, including the type, mode, time of day, and duration of the activity. Exercise diaries were collected weekly during the participants last supervised exercise session for the week.
The secondary component of the intervention involved several constituents of theoretical approaches to motivate behaviour change and promote weight loss, which is especially important for long term adherence [20]. Informational and behavioural approaches were the focus of the intervention. Strategies used to enhance behaviour change are outlined in Table 1, and are similar to methods employed by Garaulet et al. [21] and Norton et al. [22] and recommended by the European Food Information Council [23]. Both intervention groups will receive equal instructions and support.
Table 1.
Behaviour change techniques.
| Theoretical component | Description |
|---|---|
| Self-efficacy (self-monitoring strategies) | Participants will record their behaviour using exercise diaries. The act of keeping daily records requires conscious thought about activity levels and serves as a reminder to exercise [24]. Additionally, participants will weigh themselves weekly to promote weight loss and reduce the risk of weight regain [17,25]. |
| Goal setting | During the first week of the intervention, participants will receive their results from baseline testing (excluding questionnaire data) to assist in setting relevant goals. Participants will then be taught goal-setting techniques [26] and instructed to set at least one goal specific to exercise. Goals and progress will be reviewed by the participant and PI every three weeks. Participants will be introduced to the ‘body weight planner’ (https://www.niddk.nih.gov/health-information/health-topics/weight-control/body-weight-planner/Pages/bwp.aspx); a web-based energy balance prediction model for setting realistic goals for weight loss and weight-maintenance [27]. |
| Relapse prevention | Problem solving and coping strategies will be addressed by discussion [28,29]. |
| Outcome expectancy | Participants will be aware of, and given realistic expectations of the potential benefits of exercise, such as improved fitness and weight loss, to reduce rate of attrition [21,30]. |
| Education and skill development | Participants will be given instructions, feedback and guidance about use of exercise equipment, exercise technique, and recognising cues for injury avoidance. Participants will also be familiarised with basic concepts in nutrition such as the Australian Dietary Guidelines and portion size control [31] and encouraged to visit the UQ St Lucia Dietetics Practice. |
| Prompting | Participants will be sent email/SMS reminders to attend their supervised exercise sessions. After their session, participants will be prompted to incorporate some active transport or active leisure time each week. During phase 2, participants will be reminded to complete the required dose of unsupervised exercise. |
| Encouragement and support | Participants will be regularly asked about their progress and any potential barriers to exercise will be identified and methods to help overcome them will be discussed. |
| Environment | During the supervised exercise sessions, music will be played to increase motivation and exercise performance [32] |
2.5. Control group
Participants assigned to the control group undertook the same outcome testing measures as the intervention groups, but were given no specific instructions other than to continue with their typical everyday activities. For consistency, participants in the control group also received baseline results, but were not provided with any additional advice. They were assigned to a wait-list group to receive the 12-week exercise program upon completion of all study assessments and were allowed to choose whether they would like to exercise in either the morning, or in the evening.
2.6. Outcome measures
2.6.1. Feasibility measures
2.6.1.1. Recruitment and consent rates
Recruitment rate was defined as the number of individuals recruited from those interested. Interested individuals were considered (i) individuals who met the inclusion criteria but declined further participation; (ii) high-risk individuals who failed to obtain medical clearance; and (iii) individuals who did not meet the eligibility criteria. Consent rate was calculated as a percentage of individuals who consented to further involvement in the study, of those deemed eligible.
2.6.1.2. Measurement completion rate
Measurement completion rate was defined as the number of participants who were able to complete each outcome measure. Completion rates were calculated at all three time points.
2.6.1.3. Adherence and perceived enjoyment of exercise sessions
Adherence to, and perceived enjoyment of, exercise sessions gives an indication of intervention acceptability. Adherence to exercise sessions was measured by the total number of supervised sessions attended out of the maximum 42 sessions. The shortened version of the physical activity enjoyment scale (PACES), PACES-8, was used to assess and compare participant enjoyment between the exercise intervention groups, measured twice during the intervention; immediately after a training session in weeks 1 and 12. PACES-8 uses a 7-point bipolar rating scale; participants are asked to provide a rating to reflect their agreement with one of two bi-polar statements at each end of the continuum, related to an aspect of enjoyment (e.g. ‘I enjoy it’ – ‘I hate it’) and has shown good test-retest reliability [33].
2.6.1.4. Retention rate
The retention rate was defined as the number of participants who remained in the study, that is, the number of participants who did not formally drop out of the study.
2.6.1.5. Loss-to-follow-up
Loss-to-follow-up was defined as participants who withdrew or dropped out and did not consent to a follow-up assessment. Reasons for withdrawing were recorded, where voluntarily provided by participants. Participants who withdrew from the study after randomization (i.e. before beginning the intervention) were also recorded, separately.
2.6.1.6. Adverse events
Adverse events were recorded and classified as ‘related’ or ‘unrelated’ to the study.
2.6.2. Outcome variables
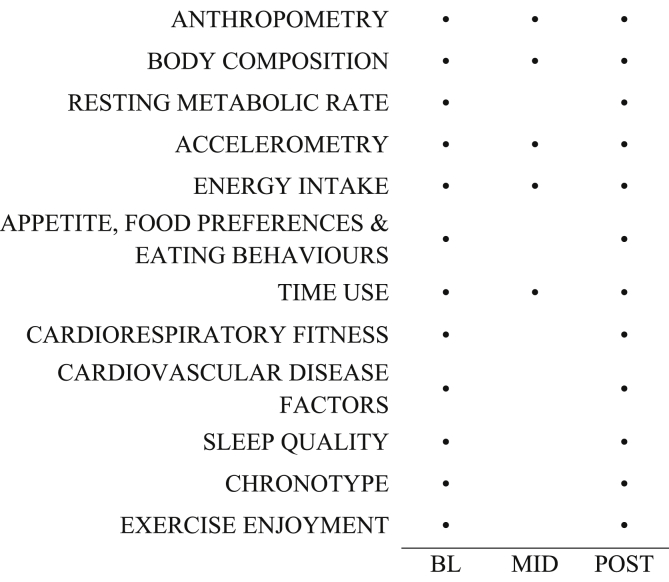
Each participant underwent a number of measurements at: baseline (prior to randomization), mid-intervention (weeks 5–6), and post-intervention (weeks 12–13). Fig. 1 provides an overview of the outcome measures assessed at each time point.
Fig. 1.
Overview of protocol by outcome measure.
BL = baseline, MID = mid-intervention, POST = post-intervention.
2.6.2.1. Anthropometry
Standing height (SECA 217-172-1009, Hamburg, Germany) was measured to the nearest 0.1 cm at baseline; body mass (A&D Mercury Load Cell Digitizer; A&D Weighting, Melbourne, Australia) and waist circumference (Lufkin W606M retractable steel tape; Cooper Tools, Chicago, USA) were measured to the nearest 0.05 kg and 0.1 cm, respectively. All anthropometric measures were taken in accordance with the International Society for the Advancement of Kinathropometry (ISAK) data collection procedures [34].
2.6.2.2. Body composition
Fat mass (kg), fat-free mass (kg), percent fat mass (%), and visceral adipose tissue (cm3) were assessed by dual-energy x-ray absorptiometry (DXA; Hologic Discovery W, Waltham, USA). Participants were instructed to remove shoes and any potential artefacts (e.g. jewellery) and asked to lay supine on the centre of the scanning table and re-positioned by the DXA operator as required. In accordance with Best Practice DXA scanning protocol, participants were asked to present in a fasted, rested, and euhydrated state, and repeat scans were scheduled at a similar time of the day to minimise measurement error [35]. Scans were analysed using the APEX system software version.
2.6.2.3. Resting metabolic rate
Indirect calorimetry was used to measure resting metabolic rate (RMR) via a ventilated hood and metabolic cart (TrueOne 2400 Metabolic Measurement System, ParvoMedics, Sandy, Utah, USA) at baseline and post-intervention. Participants arrived for a morning visit following an overnight fast (12 h) and having abstained from exercise (including active transport) for at least 14 h [36]. Participants were asked to lay supine in a comfortable position with their head on a pillow, and to remain motionless and awake. Following a 15 min equilibration period, the participant's head was covered by a transparent ventilated plastic hood, with a drape wrapped around the upper body to avoid leakage of air, for 30 min. During the first 10 min of measurement, the dilution pump flow rate was adjusted (approximately bodyweight (kg) divided by 3) until steady state was reached (≤10% coefficient of variation for VO2 and VCO2). The measurement protocol was developed according to best practice based on a methodological review by Compher et al. [36]. Resting energy expenditure (kcal/day) is calculated with the system software (TrueOne 32 RMR, version 4.3.4), using the modified Weir equation (5.616 x VO2 + 1.584 x VCO2). The first 10 min of data was discarded, and RMR (kcal/day) was defined as the average of the final 20 min of measurement.
2.6.2.4. Accelerometry
To objectively measure physical activity (light, moderate and vigorous) and sedentary time, the GENEActiv (Activinsights Ltd., Cambridgeshire, UK), participants were asked to wear a small, lightweight, waterproof tri-axial accelerometer on their non-dominant wrist, continuously for seven consecutive days. Participants were provided with written instructions about how to use the accelerometer, and were asked to complete a brief log during the monitoring period to record non-wear time (such as removal for bathing, showering or swimming), cycling and sleep/wake times [37]. GENEActivs were sampled at 30 Hz and the downloaded.bin files were converted to 15 s epoch.csv files. Accelerometry profiles were cleaned and checked for valid days prior to any data analysis. As in other studies, a valid day will be defined as minimum wear time of ≥16 h, from at least four of the seven days of monitoring, and must include both weekend days [38]. Compared to indirect calorimetry, GENEActivs have demonstrated strong correlations for criterion validity (Pearson's r = 0.79–0.98) for both physical activity and sedentary behaviour [39,40].
2.6.2.5. Energy intake
Energy intake was measured using a five-step multiple-pass 24 h dietary interview. Participants received cues using standardised probing questions [41] to help remember and report all foods. The multiple-pass method has been established as a reliable and valid tool compared to doubly labelled water [42]. At each time point, food recalls were administered on two occasions approximately one week apart, recalling the previous day's food and beverage intake. Therefore, each measurement consists of two recalled days. Where possible, participants were asked to recall the same two days of the week to account for any particular dietary habits, and recall days will include both a weekend, and weekday. Data obtained from the food recalls were then entered into a computerised nutrition database (FoodWorks® Premium Version 8, Xyris software, Brisbane, Australia) for analysis.
2.6.2.6. Measures of appetite, food preferences and eating behaviours
2.6.2.6.1. Visual analogue scales
Visual analogue scales (VAS) were administered to measure subjective appetite sensations. The VAS uses a 100 mm horizontal line representing a continuum and participants place a mark on the scale to reflect the intensity of a particular sensation, in this case, ‘hunger’, ‘fullness’ and ‘motivation to eat’. VAS data were collected at baseline and post-intervention under standardised conditions; at the same time of day and fasted, for all groups. VAS have shown good test-retest results [43].
2.6.2.6.2. Leeds food preference questionnaire
The Leeds Food Preference Questionnaire (LFPQ) is a computer-based tool designed to measure the reward value of food, based on processes of ‘liking’ and ‘wanting’ [44]. Participants are presented with visual food stimuli, varying in fat content and taste (high-fat, low-fat, sweet and savoury items). To measure ‘implicit wanting’, participants are presented with two foods from different categories and asked to choose which they would ‘prefer to eat right now’. A standardised implicit wanting score for each food category is then calculated as a function of the reaction time in selecting a certain food, adjusted for the frequency of choice for each category [45]. To measure ‘explicit liking’, individual foods are presented at random, and participants are asked to rate ‘how pleasant it would be to taste some of this food right now’ on a 100 mm VAS anchored at each end with statements ‘not at all’, and ‘extremely’, and mean ratings for each food category can be calculated using experiment generator software (E-prime v. 2.0, Psychology Software Tools, Sharpsburg, Pennsylvania, USA). The LFPQ has shown good test-retest reliability, both on immediate repetition and after one week (r = 0.61–0.95), and has shown sensitivity to acute dietary manipulations and satisfactory concurrent validity with other behavioural paradigms of food reward [46].
2.6.2.6.3. Three-factor eating questionnaire
The three-factor eating questionnaire (TFEQ) is a 51-item self-report questionnaire that measures three eating behaviour traits: restraint, disinhibition, and hunger. The questionnaire is composed of two sections; the first 36-items use a dichotomous format (true/false), and the latter 15-items use a four-point Likert scale format [47]. The TFEQ was administered under standardised conditions; at the same time of day and fasted. The TFEQ has demonstrated good test-retest reliability (r = 0.91), moderate internal consistency (Cronbach's α ranging from 0.79 to 0.92), and good convergent and discriminant validity [48]. Furthermore, the reliability and construct validity of each of the three factors in the TFEQ have been individually tested and established in several studies among different weight groups (healthy, overweight and obese) [48,49].
2.6.2.7. Time use
Self-reported use of time was measured using the adult version of the Multimedia Activity Recall for Children and Adults (MARCA) [50]; a computerised 24 h recall tool which asks participants to recall all activities from their previous day (midnight to midnight), in increments as small as 5 min. The MARCA was administered using a computer-assisted telephone interview in an open-ended format, using meal times as reference points in a segmented day format, on two occasions approximately one week apart. During the recall, the interviewer chose from a list of over 500 activities, organised by categories: ‘inactivity’, ‘transport’, ‘sport or recreation’, ‘occupation’, ‘self-care’, ‘home activities’, and ‘other’. At each time point, two consecutive days were recalled; therefore, each measurement consists of four recalled days. Where possible, participants recalled the same day of the week to account for any particular habits, although a minimum of one weekend day and three weekdays were required.
The adult version of the MARCA has excellent test-retest reliability (0.990–0.997) for moderate-vigorous physical activity (MVPA) and major activity sets (physical activity level (PAL), sleep and screen time) [51].
2.6.2.8. Cardiorespiratory fitness
Cardiorespiratory fitness was assessed via indirect calorimetry. Participants were required to walk/run on a treadmill until volitional fatigue and peak oxygen uptake (VO2peak) was measured (TrueOne 2400 Metabolic Measurement System, ParvoMedics, Sandy, Utah, USA). Participants were asked to avoid exercise and any stimulants (such as caffeine, and tobacco) and alcohol in the 24 h prior, and to avoid eating in the 2 h prior to the test. The Modified Bruce protocol was used to measure peak oxygen uptake due to its lower initial intensity, which was more suitable for the previously insufficiently active participants included in this study. VO2peak was recorded as the highest mean value attained during two 30-s periods, before volitional exhaustion [52].
2.6.2.9. Cardiovascular disease factors
Blood pressure, lipid profiles and blood glucose were sampled at baseline and post-intervention. Participants were instructed to be fasted and rested for at least 12 h prior to measurement. Samples of capillary blood were extracted via a contact-activated lancet (BD, Franklin Lakes, New Jersey) and processed using the CardioChek PA (Polymer Technology Services, Inc., Indianapolis, IN, USA) and AccuChek Performa (Model NC, Roche, Mannheim, Germany) analysers; handheld, battery-operated reflectance spectrophotometers to measure blood lipids (triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol), and blood glucose, respectively. Resting blood pressure was taken immediately after RMR measurement using an automated sphygmomanometer (Model HEM-7322, Omron Healthcare Co., Kyoto, Japan). Measurement was repeated after a 5 min interval, and the mean recorded.
2.6.2.10. Sleep quality
The Pittsburgh Sleep Quality Index (PSQI) was used to assess subjective sleep quality and disturbances over a 1-month time interval at baseline and post-intervention. The PSQI is a self-rated questionnaire routinely used in sleep research which measures factors assessed in clinical diagnosis of sleep disorders: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction, and summed to generate a global PSQI score ranging from 0 to 21; higher scores indicating a worse sleep quality [53]. The PSQI has good test-retest reliability, internal homogeneity and validity, and has been used frequently in exercise and weight loss research [[54], [55], [56]].
2.6.2.11. Chronotype
The Morningness-Eveningness Questionnaire (MEQ) is a reliable 19-item self-rated questionnaire which uses Likert-type responses to evaluate whether a person is a morning- or evening-type based on factors of sleep/wake patterns, and preferred physical and mental activity times [57]. The MEQ is the most frequently used self-evaluation technique for identifying morning- or evening-types and is routinely used in chronobiological and chronopsychological research and weight loss intervention studies [58], and is also recommended for use when the goal is to assess characteristics that change under specific situations [59]. The MEQ has been correlated with changes in behavioural and physiological rhythms such as body temperature (57), and melatonin and cortisol [60]. The original cut-offs prescribed by Horne & Ostberg indicate that a score of 70–86 determines ‘definitely morning types’, 50–69 classifies ‘moderately morning types’, a score between 42 and 58 qualifies as ‘neither type’, and evening types are scored as ≤41; ‘moderately’ and ‘definitely’ evening types are 31–41 and 16–30, respectively. Taillard et al. [61] adapted the scoring system for a middle-aged population, which has since been adopted by other researchers [62]. For participants aged <40 y, the original cut-offs were used, for adults aged ≥40 y, the cut-offs proposed by Taillard et al. (2004) which suggest that a score <53 indicates an evening-type and scores >64 indicate morning-types, were adopted.
2.6.2.12. Exercise intensity
To measure exercise intensity, participants wore a heart rate monitor (Polar RCX3, Polar, Kempele, Finland) during each supervised training session. Heart rate was recorded at 5-s intervals throughout the training sessions. Exercise intensity was measured as a percentage of peak heart rate, established during their prior VO2peak test.
Rating of perceived exertion (RPE) were also recorded using Borg's original 6- to 20-point scale [63] at the end of the warm-up (at 3-min), half-way through the session (at 22-min), and at the end of the session, immediately prior to cool-down (at 47-min). Participants were familiarised with Borg's scale prior to their VO2peak test at their baseline visit. Treadmill speed and incline were also recorded at 5-min intervals.
2.7. Statistical analysis
Descriptive statistics were used to describe the baseline characteristics and summarise the feasibility data, with all continuous variables presented as mean and standard deviation (±SD), and for categorical variables as the total number and percentage of responses. All data were calculated using Microsoft Excel 2016. As this was a feasibility study, effectiveness data are reported, but not analysed for statistical differences [64]. Group characteristics at baseline were not tested for differences, as per the 2010 CONSORT statement [65].
3. Results
3.1. Feasibility outcomes
Forty-nine individuals expressed interest in the study; of which, 30 met the eligibility criteria, nine were conditionally eligible, and 10 were ineligible. Four of the nine participants considered ‘conditionally eligible’ obtained the required medical clearance, two of which subsequently enrolled in the study. In total, 20 individuals consented to involvement resulting in a recruitment rate of 20/49 (41%), and a consent rate of 20/34 (59%). Most participants were in full-time employment in mainly professional or clerical positions, 70% were female, and most were university-educated. Table 2 shows the baseline characteristics of participants.
Table 2.
Baseline characteristics of participants.
| AM | PM | CON | ||
|---|---|---|---|---|
| n (n female) | 9 [7] | 7 [3] | 4 [4] | |
| Age (years) | 39 ± 13 | 37 ± 9 | 44 ± 11 | |
| Height (m) | 1.68 ± 0.1 | 1.73 ± 0.1 | 1.63 ± 0.1 | |
| Weight (kg) | 90.64 ± 13.5 | 91.74 ± 13.9 | 73.51 ± 2.4 | |
| Chronotype | Morning-type | 2 (22%) | 3 (43%) | 3 (75%) |
| Evening-type | 1 (11%) | 1 (14%) | 1 (25%) | |
| Neither-type | 6 (67%) | 3 (43%) | 0 | |
Data presented as number or M±SD.
AM = morning exercise group, CON = control group, PM = evening exercise group.
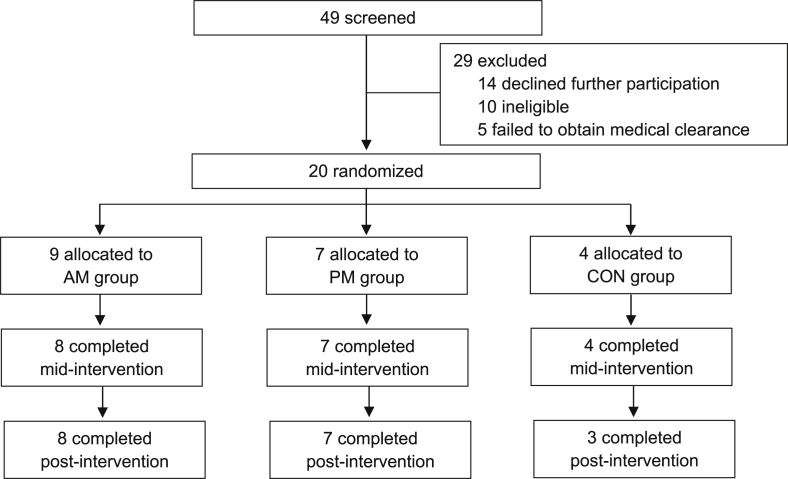
All enrolled participants (n = 20) progressed in the study after randomization (i.e. no participants withdrew after being randomized, and before the intervention), Fig. 2. Sixteen participants were randomized to an intervention group (n = 9 AM; n = 7 PM) and four were randomized to the control group. Ninety-percent (n = 18) of participants remained in the trial from baseline to end of intervention, and lack of time was cited as the reason for drop out for both participants who withdrew (n = 2; AM = 1, CON = 1). Fifteen out of the 16 participants assigned to the intervention groups completed the 12-week exercise intervention, with exercise session adherence rates of 94% and 87% for AM and PM, respectively. One adverse event was recorded, which was unrelated to the study and did not lead to withdrawal (lower limb injury). Measurement completion rates were ≥80% across the whole sample at each time point (baseline = 97%, mid-intervention = 87%, and post-intervention = 80%).
Fig. 2.
CONSORT flow diagram of participant progression through the study.
AM = morning exercise group, CON = control group, PM = evening exercise group.
Rates of adherence to the supervised exercise sessions were favourable. Of a total 42 sessions prescribed over the 12-week program, the AM group completed 39.5 ± 2.3 (94%) sessions, and PM completed 35.5 ± 6.1 (87%). Perceived enjoyment to the exercise sessions were similar between groups; AM = 67 ± 21%, and PM = 64 ± 28%. Self-selected exercise intensity was similar between groups; most of the session was spent at a vigorous intensity (64% and 59% for AM and PM respectively), and similar time was spent in moderate and high intensity zones (AM = 19% and 16%, PM = 22% and 17%, respectively).
3.2. Outcome variables
There was encouraging evidence of improvements in physiological variables between baseline and post-intervention in the intervention groups compared with control (Table 3). Due to the small amount of effect data available in feasibility studies, and the subsequent degree of uncertainty in drawing any conclusions, outcome data are considered to be preliminary, as participant numbers are not powered for statistical significance.
Table 3.
Physiological outcomes at baseline and post-intervention in the intervention and control groups.
| AM |
PM |
CON |
|||||
|---|---|---|---|---|---|---|---|
| BL | POST | BL | POST | BL | POST | ||
| BMI (kg/m2) | 31.9 ± 4.1 | 30.4 ± 4.5 | 30.6 ± 3.4 | 29.5 ± 3.6 | 27.0 ± 1.2 | 27.7 ± 0.7 | |
| Waist circumference (cm) | 95.2 ± 10.2 | 89.5 ± 7.3 | 105.5 ± 6.2 | 97.7 ± 13.1 | 86.4 ± 1.8 | 83.0 ± 1.2 | |
| Body fat (%) | 41.7 ± 8.1 | 40.5 ± 9.8 | 40.6 ± 8.9 | 40.0 ± 8.3 | 39.7 ± 8.1 | 43.3 ± 7.0 | |
| Fat mass (kg) | 36.1 ± 9.1 | 34.6 ± 9.5 | 38.8 ± 13.0 | 36.9 ± 12.9 | 35.0 ± 8.9 | 34.4 ± 8.7 | |
| Lean mass (kg) | 48.4 ± 8.0 | 47.6 ± 7.9 | 48.3 ± 8.9 | 48.2 ± 9.8 | 46.1 ± 6.1 | 46.4 ± 7.6 | |
| RMR (kcal) | 1543 ± 183 | 1520 ± 102 | 1607 ± 148 | 1550 ± 176 | 1350 ± 160 | 1305 ± 84 | |
| VO2peak (mL.kg−1.min−1) | 30.7 ± 5.1 | 35.9 ± 8.3 | 28.5 ± 7.0 | 33.1 ± 8.7 | 27.5 ± 3.8 | 26.8 ± 4.8 | |
| Cardiovascular disease risk factors | Blood glucose (mmol/L) | 5.6 ± 0.7 | 5.5 ± 0.5 | 6.0 ± 1.0 | 5.3 ± 0.4 | 5.1 ± 0.3 | 5.5 ± 0.7 |
| Total cholesterol (mmol/L) | 5.4 ± 0.6 | 4.7 ± 0.6 | 4.7 ± 0.6 | 4.2 ± 0.5 | 5.2 ± 1.4 | 5.0 ± 0.9 | |
| HDL (mmol/L) | 1.7 ± 0.4 | 1.5 ± 0.4 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.6 ± 0.3 | 1.5 ± 0.3 | |
| LDL (mmol/L) | 3.1 ± 0.7 | 2.7 ± 0.6 | 2.6 ± 0.6 | 2.4 ± 0.4 | 2.7 ± 0.9 | 2.8 ± 0.8 | |
| TC:HDL (mmol/L) | 3.3 ± 0.8 | 3.4 ± 0.9 | 4.3 ± 0.3 | 4.4 ± 0.6 | 3.3 ± 0.7 | 3.3 ± 0.6 | |
| Triglycerides (mmol/L) | 1.3 ± 0.8 | 1.0 ± 0.4 | 1.4 ± 1.1 | 1.2 ± 0.5 | 2.0 ± 1.0 | 1.4 ± 0.2 | |
| Resting SBP (mmHg) | 119 ± 10 | 114 ± 7 | 135 ± 6 | 121 ± 8 | 123 ± 28 | 126 ± 22 | |
| Resting DBP (mmHg) | 84 ± 8 | 80 ± 6 | 86 ± 9 | 84 ± 10 | 87 ± 16 | 89 ± 16 | |
Data presented as number or M±SD.
AM = morning exercise group, BMI = body mass index, CON = control group, DBP = diastolic blood pressure, HDL = high-density lipoprotein, LDL = low density lipoprotein, mmHg = millimetres of mercury, mmol/L = millimoles per litre, PM = evening exercise, RMR = resting metabolic rate, SBP = systolic blood pressure, TC = total cholesterol, VO2peak = peak oxygen uptake.
4. Discussion
The primary aim of this study is to investigate the feasibility and acceptability of prescribing morning or evening exercise to inactive, overweight and obese individuals, and to assess whether it is appropriate to upscale to a larger trial. A feasibility study asks whether something can be done, should we proceed with it, and if so, how? [66] To accurately report and assess the acceptability of our randomized feasibility study, we have considered the eight general areas of focus addressed by feasibility studies, proposed by Bowen et al. [12]. Addressing each area will assist in optimising the design and conduct of any subsequent large-scale trials.
4.1. Acceptability
Acceptability refers to how well the program is received by participants. Clearly for an exercise intervention to be feasible, participation in both the exercise intervention, and outcome measurements, is crucial. Thirty-four individuals were deemed eligible for the study after screening, 20 of which enrolled in the study after understanding and consenting to the rigorous protocol. Due to the number of outcome measures included in our protocol, there were concerns about the burden on participants and the potential consequences to measurement completion rates. For each testing time point, measurement completion rate was ≥80%, which is in accordance with the acceptable feasibility limits [10]. Further evidence to support the acceptability of the intervention is the level of perceived enjoyment of the exercise sessions. Participants in both morning and evening groups reported similar enjoyment ratings (>60%, Table 1).
4.2. Demand
Demand for the intervention can be assessed by the number of expressions of interest for the study. Within a two-week period of study advertisement, 49 participants expressed interest. If the consent rate is a proxy for level of demand for this program, then based on this feasibility study, 59% of individuals would likely participate in the program, if offered to them.
4.3. Implementation
Implementation refers to the extent to which the intervention can be successfully delivered as planned. In accordance with the study protocol, all participants allocated to the intervention groups began the exercise program within 2-weeks of completing baseline assessments. In contrast to other exercise interventions, with rates of attrition between 25 and 50%, and mean adherence rates reported as 66% [67], participant retention rate to our 12-week intervention was 90% and adherence to exercise sessions was high (91%). In addition, there were no adverse events reported during any of the exercise sessions.
4.4. Practicality
The extent to which the intervention can be carried out using existing means and resources is termed the ‘practicality’. The testing and training protocol we have proposed is onerous on both the participant and researcher/s. Calculating the time required for study participation provides an estimate of the expected study duration on a large scale. Including recruitment, it took <19 weeks for 18 participants to complete the study. Completion of the 12-week exercise program from commencement of the first participant to completion of the last participant, was <16 weeks. This relatively short duration to complete the study is a positive outcome, and is promising for a larger scale study. However, practicality also refers to the extent to which the intervention can be delivered, within the available resources. Due to the nature of the intervention, comparing morning and evening exercise, training times are restrictive. Therefore, if this feasibility study lead to a large-scale trial, adoption of a block-enrolment strategy may be a viable option to ensure availability of equipment.
4.5. Adaptation
Adaptation focuses on how well the program is performed, when program contents or procedures are amended. There are a number of measures included in the study protocol which may cause discomfort to participants For example, there have been reports of intolerance to measurement of RMR via a ventilated hood and metabolic cart. Specifically, user complaints of odour, and feelings of claustrophobia when using the hood have been reported [68]. There were no individuals who expressed concern or discomfort during this procedure, or other procedures which may be considered invasive or uncomfortable (e.g. blood sampling or VO2peak testing). Therefore, our data indicate we do not need to adapt the protocol.
4.6. Integration
Integration focuses on the change that occurs within the social/physical environment, as a result of the intervention. As a consequence of the random allocation, some participants were in a group which did not match their chronotype, and/or their preferred time to exercise. Despite this, no individuals reported ‘unfavourable group allocation’ as their reason for study withdrawal. This may add to the literature supporting temporal consistency, i.e. performing a behaviour at a specific time, such as regularly exercising at 6am, is important for long-term adherence and helps create a ‘protected time’ for new habits [69], irrespective of the time an individual may like to perform the activity.
4.7. Expansion
Expansion examines the potential success of an already-successful intervention, in a different setting or population. Alizadeh et al. [8] compared 6-weeks of morning and evening aerobic exercise on anthropometric indices, energy intake, and subjective appetite sensations. Both exercise groups were effective in reducing body mass over their 6-week trial period, suggesting that manipulating the time of day that individuals train is efficacious for weight loss. From their sample of 48 participants, they reported a drop out rate of 23% (n = 11). The small sample size of women-only and limited diversity of outcome measures was a limitation of this study. Therefore, our feasibility study expands on the work of Alizadeh and colleagues to include a more diverse population of men and women, with a number of additional outcome measures.
4.8. Limited efficacy testing
Feasibility studies are designed to test the practicality of an intervention, rather than the effectiveness of interventions, and are often conducted with small samples with limited statistical power and shorter follow-up periods [10]. The aim of this study was to investigate individual's tolerance and response to the rigorous testing and training protocol, prior to progressing to a larger study. Therefore, a small convenience sample of 20 adults were recruited from students and staff members at The University of Queensland, Australia. Due to available resources (exercise training equipment), 20 participants was considered the reasonable cohort size that could be started. Obtaining follow-up data following the cessation of an intervention is important to capture the residual effects of the intervention. However, as this study aimed to test only the feasibility of the intervention, we have not reported these data. If this study were to lead to a large-scale randomized controlled trial in the future, we recommend collecting follow-up data.
4.9. Conclusions
While there is agreement that regular exercise plays an important role in improving general health and maintaining energy balance, there remains a distinct lack of evidence regarding an optimal time of day for exercise to maximise efficacy. Therefore, a multifactorial study in a free-living context would better help understand the relationship between exercise time of day, and efficacy for health outcomes. However, in the absence of much previous work, feasibility of this exercise prescription first needed to be assessed.
Findings from our randomized feasibility study have addressed several previously unknown questions regarding the safety, feasibility and acceptability of morning and evening exercise sessions. Based on our threshold definition of 80% adherence to the intervention and rate of completion of measurements, we consider this intervention to be feasible. Based on these data, and using these procedures, it is possible to design and undertake a large scale randomized controlled trial with a sample size that is adequately powered to detect change. Due to the magnitude of poor health outcomes associated with overweight and obesity [70], we recommend the primary efficacy outcome of a future randomized controlled trial focus on changes in body composition. If, by manipulating the time of day at which exercise is prescribed, we can identify favourable changes in the way people restructure their time, adhere better to the program, and improve their diet and associated eating behaviours, recommendations could be developed to promote exercise at a certain time of day.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
PB is supported by an Australian Government Research Training Program (RTP) Scholarship.
Contributor Information
Paige G. Brooker, Email: p.brooker@uq.edu.au.
Sjaan R. Gomersall, Email: s.gomersall1@uq.edu.au.
Neil A. King, Email: n.king@qut.edu.au.
Michael D. Leveritt, Email: michael.leveritt@uq.edu.au.
References
- 1.Drust B., Waterhouse J., Atkinson G., Edwards B., Reilly T. Circadian rhythms in sports performance-an update. Chronobiol. Int. 2005;22(1):21–44. doi: 10.1081/cbi-200041039. [DOI] [PubMed] [Google Scholar]
- 2.Trine M.R., Morgan W.P. Influence of time of day on psychological responses to exercise. A review. Sports Med. 1995;20(5):328–337. doi: 10.2165/00007256-199520050-00004. [DOI] [PubMed] [Google Scholar]
- 3.Seo D.Y., Lee S., Kim N., Ko K.S., Rhee B.D., Park B.J. Morning and evening exercise. Integrat. Med. Res. 2013;2(4):139–144. doi: 10.1016/j.imr.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson G., Reilly T. Circadian variation in sports performance. Sports Med. 1996;21(4):292–312. doi: 10.2165/00007256-199621040-00005. [DOI] [PubMed] [Google Scholar]
- 5.Chtourou H., Souissi N. The effect of training at a specific time of day: a review. J. Strength Condit Res. 2012;26(7):1984–2005. doi: 10.1519/JSC.0b013e31825770a7. [DOI] [PubMed] [Google Scholar]
- 6.Australian Bureau of Statistics . 2013. Australian Health Survey: Physical Activity. 2011-12 cat. No. 4364.0.55.004. [Google Scholar]
- 7.McCarty M.F. Optimizing exercise for fat loss. Med. Hypotheses. 1995;44(5):325–330. doi: 10.1016/0306-9877(95)90258-9. [DOI] [PubMed] [Google Scholar]
- 8.Alizadeh Z., Younespour S., Tabesh M.R., Haghravan S. Comparison between the effect of 6 weeks of morning or evening aerobic exercise on appetite and anthropometric indices: a randomized controlled trial. Clin. Obes. 2017;7(3):157–165. doi: 10.1111/cob.12187. [DOI] [PubMed] [Google Scholar]
- 9.Craig P., Dieppe P., Macintyre S., Mitchie S., Nazareth I., Petticrew M. Developing and evaluating complex interventions: the new medical research council guidance. BMJ. 2008;337 doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore C.G., Carter R.E., Nietert P.J., Stewart P.W. Recommendations for planning pilot studies in clinical and translational research. Clin. Trans. Sci. 2011;4(5):332–337. doi: 10.1111/j.1752-8062.2011.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groeneveld I.F., Proper K.I., van der Beek A.J., Hildebrandt V.H., van Mechelen W. Factors associated with non-participation and drop-out in a lifestyle intervention for workers with an elevated risk of cardiovascular disease. Int. J. Behav. Nutr. Phys. Activ. 2009;6(1):1–9. doi: 10.1186/1479-5868-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowen D.J., Kreuter M., Spring B., Cofta-Woerpel L., Linnan L., Weiner D. How we design feasibility studies. Am. J. Prev. Med. 2009;36(5):452–457. doi: 10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troiano R.P., Berrigan D., Dodd K.W., Mâsse L.C., Tilert T., McDowell M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008;40(1):181. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 14.Exercise and Sports Science Australia Adult Pre-exercise Screening System. https://www.essa.org.au/for-gps/adult-pre-exercise-screening-system/ (APSS) 2012 [cited 2015 15 October]. Available from:
- 15.Dumville J.C., Hahn S., Miles J.N.V., Torgerson D.J. The use of unequal randomisation ratios in clinical trials: a review. Contemp. Clin. Trials. 2006;27(1):1–12. doi: 10.1016/j.cct.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly J.E., Kirk E.P., Jacobsen D.J., Hill J.O., Sullivan D.K., Johnson S.L. Effects of 16 mo of verified, supervised aerobic exercise on macronutrient intake in overweight men and women: the Midwest Exercise Trial. Am. J. Clin. Nutr. 2003;78(5):950. doi: 10.1093/ajcn/78.5.950. [DOI] [PubMed] [Google Scholar]
- 17.Racette S.B., Weiss E.P., Villareal D.T., Arif H., Steger-May K., Schechtman K.B. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J. Gerontol. 2006;61(9):943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly J.E., Blair S.N., Jakicic J.M., Manore M.M., Rankin J.W., Smith B.K. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009;41(2):459. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 19.Maraki M., Tsofliou F., Pitsiladis Y.P., Malkova D., Mutrie N., Higgins S. Acute effects of a single exercise class on appetite, energy intake and mood. Is there a time of day effect? Appetite. 2005;45(3):272–278. doi: 10.1016/j.appet.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Davis R., Campbell R., Hildon Z., Hobbs L., Michie S. Theories of behaviour and behaviour change across the social and behavioural sciences: a scoping review. Health Psychol. Rev. 2015;9(3):323–344. doi: 10.1080/17437199.2014.941722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garaulet M., Pérez‐Llamas F., Zamora S., Tebar F.J. Weight loss and possible reasons for dropping out of a dietary/behavioural programme in the treatment of overweight patients. J. Hum. Nutr. Diet. 1999;12(3):219–227. [Google Scholar]
- 22.Norton L.H., Norton K.I., Lewis N., Dollman J. A comparison of two short-term intensive physical activity interventions: methodological considerations. Int. J. Behav. Nutr. Phys. Activ. 2011;8(1):133. doi: 10.1186/1479-5868-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Council T.E.F.I. Motivating behaviour change. 2014. http://www.eufic.org/article/en/expid/Motivating-behaviour-change/ [cited 2015 15 October]. Available from:
- 24.Gleeson-Kreig J.M. Self-monitoring of physical activity: effects on self-efficacy and behavior in people with type 2 diabetes. Diabetes Educat. 2006;32(1):69–77. doi: 10.1177/0145721705284285. [DOI] [PubMed] [Google Scholar]
- 25.Burke L.E. Self-monitoring in weight loss: a systematic review of the literature. J. Am. Diet Assoc. 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts S.S. Set goals the SMART way. (THE DIABETES ADVISOR) (specific, measurable, achievable, realistic, and time-bound) Diabetes Forecast. 2007;60(5):43. [Google Scholar]
- 27.Hall K.D., Sacks G., Chandramohan D., Chow C.C., Wang Y.C., Gortmaker S.L. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutsaerts M.A.Q., Kuchenbecker W.K.H., Mol B.W., Land J.A., Hoek A. Dropout is a problem in lifestyle intervention programs for overweight and obese infertile women: a systematic review. Hum. Reprod. 2013 Apr;28(4):979–986. doi: 10.1093/humrep/det026. [DOI] [PubMed] [Google Scholar]
- 29.Messier V., Hayek J., Karelis A.D., Messier L., Doucet E., Prud'homme D. Anthropometric, metabolic, psychosocial and dietary factors associated with dropout in overweight and obese postmenopausal women engaged in a 6-month weight loss programme: a MONET study. Br. J. Nutr. 2010;103(8):1230–1235. doi: 10.1017/S0007114509993023. [DOI] [PubMed] [Google Scholar]
- 30.Dalle Grave R., Calugi S., Molinari E., Petroni M.L., Bondi M., Compare A. Weight loss expectations in obese patients and treatment attrition: an observational multicenter study. Obes. Res. 2005;13(11):1961–1969. doi: 10.1038/oby.2005.241. [DOI] [PubMed] [Google Scholar]
- 31.National Health and Medical Research Council . 2013. Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults, Adolescents and Children in Australia. Canberra, Australia. [Google Scholar]
- 32.Hayakawa Y., Miki H., Takada K., Tanaka K. Effects of music on mood during bench stepping exercise. Percept. Mot. Skills. 2000;90(1):307–314. doi: 10.2466/pms.2000.90.1.307. [DOI] [PubMed] [Google Scholar]
- 33.Kendzierski D., DeCarlo K.J. Physical activity enjoyment scale: two validation studies. J. Sport Exerc. Psychol. 1991;13(1):50–64. [Google Scholar]
- 34.Stewart A.D., Marfell-Jones M., Olds T., de Ridder H. 2011. International Standards for Anthropometric Assessment. null p. [Google Scholar]
- 35.Nana A., Slater G.J., Hopkins W.G., Halson S.L. Importance of standardized DXA protocol for assessing physique changes in athletes. Int. J. Sport Nutr. Exerc. Metabol. 2013 Jun;26(3):259–267. doi: 10.1123/ijsnem.2013-0111. [DOI] [PubMed] [Google Scholar]
- 36.Compher C., Frankenfield D., Keim N., Roth-Yousey L. Evidence anal working G, evidence analysis working G. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J. Am. Diet Assoc. 2006;106(6):881–903. doi: 10.1016/j.jada.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Sabia S., van Hees V.T., Shipley M.J., Trenell M.I., Hagger-Johnson G., Elbaz A. Association between questionnaire- and accelerometer-assessed physical activity: the role of sociodemographic factors. Am. J. Epidemiol. 2014;179(6):781–790. doi: 10.1093/aje/kwt330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Hees V.T., Renström F., Wright A., Gradmark A., Catt M., Chen K.Y. Estimation of daily energy expenditure in pregnant and Non-Pregnant women using a Wrist-Worn Tri-Axial accelerometer. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esliger D.W., Rowlands A.V., Hurst T.L., Catt M., Murray P., Eston R.G. Validation of the GENEA accelerometer. Med. Sci. Sports Exerc. 2011;43(6):1085–1093. doi: 10.1249/MSS.0b013e31820513be. [DOI] [PubMed] [Google Scholar]
- 40.Phillips L.R.S., Parfitt G., Rowlands A.V. Calibration of the GENEA accelerometer for assessment of physical activity intensity in children. J. Sci. Med. Sport. 2013;16(2):124–128. doi: 10.1016/j.jsams.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Gibson R.S. Oxford University Press; New York: 2005. Principles of Nutritional Assessment. [Google Scholar]
- 42.Melanson E.L., Keadle S.K., Donnelly J.E., Braun B., King N.A. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Med. Sci. Sports Exerc. 2013;45(8):1600–1609. doi: 10.1249/MSS.0b013e31828ba942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flint A., Raben A., Blundell J.E., Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000;24(1):38. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 44.Finlayson G., King N., Blundell J.E. Is it possible to dissociate ‘liking’ and ‘wanting’ for foods in humans? A novel experimental procedure. Physiol. Behav. 2007;90(1):36–42. doi: 10.1016/j.physbeh.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 45.Finlayson G., King N., Blundell J. The role of implicit wanting in relation to explicit liking and wanting for food: implications for appetite control. Appetite. 2008;50(1):120–127. doi: 10.1016/j.appet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Finlayson G., Caudwell P., Gibbons C., Hopkins M., King N., Blundell J. Low fat loss response after medium-term supervised exercise in obese is associated with exercise-induced increase in food reward. J. Obes. 2011;2011:1–8. doi: 10.1155/2011/615624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stunkard A.J., Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 48.Allison D.B., Kalinsky L.B., Gorman B.S. A comparison of the psychometric properties of three measures of dietary restraint. Psychol. Assess. 1992;4(3):391. [Google Scholar]
- 49.Bryant E.J., Caudwell P., Hopkins M.E., King N.A., Blundell J.E. Psycho-markers of weight loss. The roles of TFEQ Disinhibition and Restraint in exercise-induced weight management. Appetite. 2012;58(1):234–241. doi: 10.1016/j.appet.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Gomersall Olds TS., Ridley K. Development and evaluation of an adult use-of-time instrument with an energy expenditure focus. J. Sci. Med. Sport. 2011;14(2):143–148. doi: 10.1016/j.jsams.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Hills A.P., Mokhtar N., Byrne N.M. Assessment of physical activity and energy expenditure: an overview of objective measures. Frontiers Nutr. 2014;1:5. doi: 10.3389/fnut.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robergs R.A., Dwyer D., Astorino T. Recommendations for improved data processing from expired gas analysis indirect calorimetry. Sports Med. 2010;40(2):95–111. doi: 10.2165/11319670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 53.Buysse D.J., Reynolds C.F., Iii, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatr. Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 54.Chaput J.-P., Drapeau V., Hetherington M., Lemieux S., Provencher V., Tremblay A. Psychobiological impact of a progressive weight loss program in obese men. Physiol. Behav. 2005;86(1–2):224–232. doi: 10.1016/j.physbeh.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 55.Curcio G., Tempesta D., Scarlata S., Marzano C., Moroni F., Rossini P.M. Validity of the Italian version of the Pittsburgh sleep quality index (PSQI) Neurol. Sci. 2013;34(4):511–519. doi: 10.1007/s10072-012-1085-y. [DOI] [PubMed] [Google Scholar]
- 56.Buman M.P., King A.C. Exercise as a treatment to enhance sleep. Am. J. Lifestyle Med. 2010;4(6):500–514. [Google Scholar]
- 57.Horne J.A., Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4(2):97. [PubMed] [Google Scholar]
- 58.Garaulet M., Gómez-Abellán P., Alburquerque-Béjar J.J., Lee Y.C., Ordovás J.M., Scheer F.A.J.L. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2005;37(4):604–611. doi: 10.1038/ijo.2012.229. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levandovski R., Sasso E., Hidalgo M.P. Chronotype: a review of the advances, limits and applicability of the main instruments used in the literature to assess human phenotype. Trend. Psychiat. Psychother. 2013;35(1):3. doi: 10.1590/s2237-60892013000100002. [DOI] [PubMed] [Google Scholar]
- 60.Bailey S.L., Heitkemper M.M. Circadian rhythmicity of cortisol and body temperature: morningness-eveningness effects. Chronobiol. Int. 2001;18(2):249–261. doi: 10.1081/cbi-100103189. [DOI] [PubMed] [Google Scholar]
- 61.Taillard J., Philip P., Chastang J.F., Bioulac B. Validation of Horne and Ostberg morningness-eveningness questionnaire in a middle-aged population of French workers. J. Biol. Rhythm. 2004;19(1):76–86. doi: 10.1177/0748730403259849. [DOI] [PubMed] [Google Scholar]
- 62.Paine S.-J., Gander P.H., Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30-49 Years) J. Biol. Rhythm. 2006;21(1):68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
- 63.Borg G. Mouvement Publications; Ithaca, NY: 1985. An Introduction to Borg's RPE-Scale. [Google Scholar]
- 64.Loscalzo J. Pilot trials in clinical research. Circulation. 2009;119(13):1694. doi: 10.1161/CIRCULATIONAHA.109.861625. [DOI] [PubMed] [Google Scholar]
- 65.de Boer M.R., Waterlander W.E., Kuijper L.D.J., Steenhuis I.H.M., Twisk J.W.R. Testing for baseline differences in randomized controlled trials: an unhealthy research behavior that is hard to eradicate. Int. J. Behav. Nutr. Phys. Activ. 2015;12(1):4. doi: 10.1186/s12966-015-0162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eldridge S.M., Lancaster G.A., Campbell M.J., Thabane L., Hopewell S., Coleman C.L. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dishman R.K. Human Kinetics Books; Champaign: 1988. Exercise Adherence: its Impact on Public Health. [Google Scholar]
- 68.Cooper J.A. Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J. Am. Diet Assoc. 2009;109(1):128–132. doi: 10.1016/j.jada.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaushal N., Rhodes R.E. Exercise habit formation in new gym members: a longitudinal study. J. Behav. Med. 2015;38(4):652–663. doi: 10.1007/s10865-015-9640-7. [DOI] [PubMed] [Google Scholar]
- 70.Stein C.J., Colditz G.A. The epidemic of obesity. J. Clin. Endocrinol. Metab. 2004;89(6):2522–2525. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]