Abstract
Central nervous system (CNS) vasculopathy associated with Varicella Zoster Virus (VZV) infection, usually manifesting as stroke due to ischemic lesions by involvement of small arteries, is frequently misdiagnosed. Immunocompromised patients have a particularly higher risk of severe disease and also CNS involvement during or following VZV presentations. We report a case of an 84-year-old man, with myelodysplastic syndrome, who presented with herpes zoster ophthalmicus complicated with left periocular cellulitis and an abnormal neurological exam. Intravenous treatment with acyclovir and amoxicillin/clavulanic acid was began. VZV DNA was detected in the cerebrospinal fluid (CSF) and brain magnetic resonance imaging revealed three acute ischemic lesions in the left frontal and both cerebellar lobes. A VZV CNS multifocal vasculopathy was diagnosed and treatment with intravenous acyclovir continued for 21 days.
Immunocompromised patients with VZV infection can have a more severe course of disease with disseminated involvement and multifocal vasculopathy. In these patients the CSF detection of anti-VZV IgG as well as VZV DNA can be helpful in the diagnosis of CNS VZV vasculopathy. The antiviral treatment can improve the outcome and should be adjusted taking in consideration the degree of immunosuppression. This clinical case and review of the literature highlights the challenges in the diagnosis and management of VZV CNS vasculopathy in immunocompromised patients.
Keywords: Varicella Zoster Virus, Vasculopathy, Stroke, Immunocompromised, Acyclovir
Introduction
Varicella Zoster Virus (VZV) is a neurotropic herpesvirus and the only human virus that replicates in arteries [1]. The primary infection, usually in childhood, causes varicella, after which the virus becomes latent in sensory nerve ganglia; when cell-mediated immunity decreases (as can occur with advanced age or immunosuppression), VZV can reactivate and manifests as zoster [2]. VZV vasculopathy is described in varicella as well as in zoster [3,4]. Immunocompromised individuals usually have more frequent disseminated disease with central nervous system (CNS) involvement [[5], [6], [7]]. The diagnosis is based on clinical manifestations, neurological symptoms, cerebrospinal fluid (CSF) analysis and imaging abnormalities indicating CNS arterial disease [8]. The treatment with antiviral agents can lead to complete recovery [[7], [8], [9]]. Additional therapy with steroids is controversial [7,8].
Case description
An 84-year-old man with myelodysplastic syndrome diagnosed 4 years prior and treated symptomatically with only one short course of corticosteroids in the past. He also suffered from type 2 diabetes, chronic kidney disease, benign prostatic hypertrophy and had a prior intracerebral hemorrhage. Home medications included gliclazide, sitagliptin, furosemide, tamsulosin and pantoprazole. He presented to the emergency room with a painful vesicular exanthema on his left forehead starting 5 days earlier and gait ataxia. There was no report of fever or other lesions on the rest of the body.
The vital signs on hospital admission were: blood pressure 121/74 mm Hg, pulse 91 beats per minute, periphery oxygen saturation 98% on room air, respiratory rate 16 breaths per minute and body temperature 38 °C. On physical examination the patient had vesicular and pustular cutaneous lesions in the left ophthalmic nerve dermatome and ipsilateral periocular inflammatory signs compatible with cellulitis. The pulmonary, cardiac and abdominal examination was unremarkable. There were no other cutaneous lesions. The neurological examination revealed somnolence, temporal and spatial disorientation, left palpebral ptosis with normal ocular movements and isochoric pupils; decreased muscle strength in the right body (arm and leg) with 4/5 in the Medical Research Council Scale and appendicular and gait ataxia; during observation a motor focal seizure was observed.
His hemoglobin level was 12 g/dL (reference range, 12–16), platelet count was 52.000 per cubic mm (reference range, 150.000–400.000), renal-function was altered (creatinine 1.61 mg/dL; urea 50 mg/dL) with a MDRD glomerular filtration rate of 43.7 mL/min/1.73 m2, and the C-reactive protein was 76.5 mg/L (reference range, <3.0). Total white-cell count, coagulation assays and liver function tests were normal, and the antibody/antigen HIV ELISA test was negative. The thoracic x-ray and the brain computer tomography (CT) were unremarkable.
The patient was admitted to the infectious diseases ward. Intravenous treatment with acyclovir 10 mg/kg three times daily plus amoxicillin/clavulanic acid 1.2 g each 8-hours, were started. Taking into consideration the neurological findings, a central nervous system VZV vasculitis was suspected. Levetiracetam 500 mg twice daily was begun and lumbar puncture, brain magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) were performed.
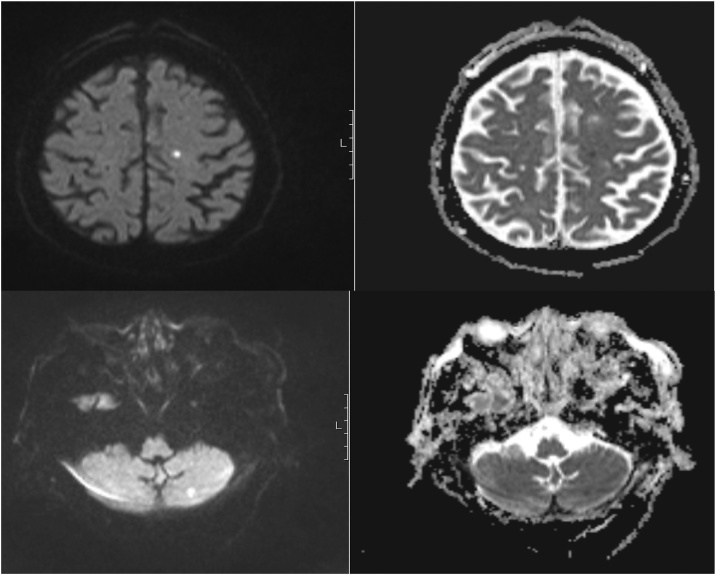
The CSF analyses revealed 4 cells/mm3, protein 0.76 mg/dL (reference range, <0.5), glucose 107 mg/dL (glucose CSF/serum ratio > 0.5 mg/dL) and positivity for VZV by DNA polymerase-chain-reaction (PCR) analysis. VZV serology in CSF was not performed. Brain MRI confirmed the presence of one acute ischemic lesion in the left frontal and two acute ischemic lesions in both cerebellar lobes on T2/T2 FLAIR with MRA showing no irregularities (Fig. 1). A VZV CNS multifocal vasculopathy was confirmed.
Fig. 1.
Brain magnetic resonance imaging, axial DWI (left) and ADC map (right): two small acute ischemic lesions in different arterial territories: left frontal and left cerebellar lobes.
Although promptly beginning of treatment with intravenous acyclovir, the exanthema progressed with bilateral involvement of forehead and new vesicular lesions in front and back thorax as well as abdomen. Taking in consideration the progression of the disease with disseminated zoster we decided to continue treatment with intravenous acyclovir, not add corticosteroids as adjunctive therapy and promptly star physical rehabilitation. Twenty-one days of intravenous acyclovir treatment was completed and the patient was discharged with improvement of neurological symptoms related with VZV infection.
Discussion
VZV vasculopathy can lead to neurological disease such as ischemic or hemorrhagic stroke, aneurysm, dolichoectasia (arterial dilated and elongated), arterial dissection and venous sinus thrombosis [1]. In an adult population the association between VZV vasculopathy and stroke is described in various epidemiological studies. After VZV infection there is an increased risk of stroke of 2–9% in the first year after infection, with a higher risk in people under 40 years old, regardless of the immunological status [4,10]. In children there is also an increased risk of stroke after varicella [11], with VZV vasculopathy being responsible for 31% of all arterial ischemic strokes and 44% of transient cerebral arteriopathy [3,12].
VZV vasculopathy develops due to vascular invasion when the virus spreads transaxonally from the ganglia to the vessel wall [2,6,7,13]. After reaching the adventitia, the virus spreads transmurally infecting all the layers of the vessel, with disruption of the internal elastic lamina, intimal thickening and decreased smooth muscle cells in media, resulting in a granulomatous arteritis [13]. The histological analysis of the vessel wall shows arteries with Cowdry A inclusion bodies, multinucleated giant cells, herpes virions, VZV DNA and VZV antigen [6,7].
Previous studies showed that 50% of the patients have mixed large and small artery involvement, 37% pure small artery involvement and 13% pure large artery disease [8]. Large arteries involvement is more frequent in the anterior and middle cerebral arteries and in the internal and external carotid arteries [8]. Multifocal vasculopathy is more frequent in immunocompromised patients [6,8,14].
The zoster exanthema does not always precede the VZV vasculopathy manifestations [14]. Between 30–40% of patients have CNS manifestations without skin involvement [8,15]. When present, the average time interval until the onset of neurologic symptoms is 4.1 months, ranging from the same day up to 2.5 years [8]. The explanation of the variability in clinical manifestations is related to the pattern of virus spread in the peripheral nerves. When the virus spreads exclusively in a centripetal pattern (toward the spinal cord or brain) CNS complications may develop without concomitant zoster exanthema. When the virus also spreads in centrifugal pattern (toward the skin) the patient will have cutaneous symptoms [6]. In immunocompromised patients herpes zoster can have a more severe course [5]. Although there is no difference in the exanthema occurrence [8], they have more frequently multiple dermatomes or disseminated involvement, internal organs and CNS complications [2]. In AIDS patients disseminated disease is related to VZV reactivation from multiple dorsal root ganglia levels [5,6].
In VZV vasculopathy the CSF analysis revealed mononuclear pleocytosis in 2/3 of the patients [8], usually under 100 cells/mm3 [15]. Considering that 30% of patients do not have pleocytosis, a normal CSF WBC count does not rule out this diagnosis [7,8]. There are no differences in the incidence of CSF pleocytosis in immunocompromised vs. immunocompetent patients [8]. Some patients with VZV infection without CNS involvement can have CSF pleocytosis and moderately elevated levels of CSF proteins [16].
Detection of VZV DNA and presence of anti-VZV IgG can help establishing the diagnosis. The sensitivity of CSF VZV IgG antibody seems to be higher than VZV DNA detection by PCR (93% vs 30%) [8]. The absence of VZV DNA in the CSF does not exclude VZV vasculopathy. VZV DNA is usually detected in the CSF in the first 7 days of symptoms but can persist until 50 days [8,17] while the CSF VZV IgG antibody is usually detected 7 days after symptoms onset [17]. In immunocompromised patients VZV DNA is more frequently identified than in immunocompetent patients (54% vs 16%) [8].
Considering that sensitivity of these assays depend on the time of first symptoms and immunological status, both tests should be performed in order to exclude the diagnosis. The VZV IgG serum/CSF ratio is usually decreased in these patients evidencing a CNS synthesis of IgG [8,14].
Brain MRI or CT reveals abnormalities in 97% of VZV vasculopathy cases [8]. The usual findings are ischemic lesions, although some patients can also present with hemorrhagic lesions [1], more frequently seen in grey-white matter junctions [1,[6], [7], [8]]. The conventional angiography or MRA usually shows vessel stenosis and post-stenotic dilatation in 70% of patients [1,7,8,14], as those with only small-vessel disease the abnormalities are below the resolution limit of the angiography. For that reason, a normal angiography does not rule out VZV vasculopathy [8].
In general population the case-fatality rate of VZV vasculopathy without treatment is 25% [8]. Since there is productive virus infection in the vessel the antiviral treatment can help reducing vessel viral load and inflammation [7]. Although there is no evidence to proof that early treatment prevents VZV vasculopathy, it has been shown that antiviral treatment improves or stabilizes the neurological deficits, the vessel wall enhancement on MRI and the arterial stenosis [9,14].
The usual treatment is acyclovir 10–15 mg/kg each 8-hours for 7–14 days. [7] In VZV CNS infections the intravenous formulation should be used since the oral bioavailability of this drug is very poor (15%–30%) [18]. The CSF levels of intravenous acyclovir are 50% of the levels in the plasma, while the CSF concentration of acyclovir after oral valacyclovir is 20% of plasma levels in patients without damaged blood-brain barrier [19,20]. The intravenous acyclovir has higher mean peak serum concentration (Cmax) and shorter time to reach the Cmax (Tmax) than oral valacyclovir. Taking in consideration the pharmacokinetic and pharmacodynamics profiles of these drugs, intravenous acyclovir is probably a better option to use when there is CNS VZV involvement.
The use of steroids (a short course of oral prednisone 1 mg/kg during 5–7 days) as adjunctive treatment is controversial. The VZV vasculopathy pathological finding of granulomatous arteritis favours the use of steroids in this situation [7]. A previous study showed that 66% of patients treated with acyclovir alone improved or stabilized the symptoms compared with 75% of patients with adjunctive steroids. In the group of patients receiving acyclovir without steroids 13% of the patients died and 20% worsened showing clinical improvement after starting steroids. In the acyclovir plus steroids group, 25% of the patients worsened and 8% had recurrence of the symptoms after treatment was stopped, with clinical improvement after a subsequent treatment with prednisolone. However, this study was performed in an uncontrolled setting with different doses and duration of treatment which may not reflect the real-life data [8]. On the other hand, many patients misdiagnosed with "temporal arteritis" and "CNS vasculitis" worsened with long-term steroids [7].
There is no studies concerning the treatment options in immunocompromised patients, however since these patients usually have more frequent recurrence of symptoms after stopping antiviral treatment, some authors recommend longer courses of antiviral treatment as well as stopping the immunosuppressive treatment if possible and continued oral antiviral treatment for 6–8 weeks after the immunosuppression has been stopped [7].
Conclusion
This case report highlights the clinical presentation, diagnosis and management of an immunocompromised patient with the uncommon association of VZV reactivation and CNS vasculopathy.
VZV vasculopathy should be considered as a possible etiology of stroke, even when there is no simultaneous zoster exanthema. The incidence of skin involvement is not significantly different in immunocompromised patients, nevertheless these patients usually have a more severe course of disease with disseminated and CNS involvement, namely with multifocal vasculopathy.
The concomitant use of CSF VZV DNA and CSF VZV IgG antibody in immunocompromised patients can be helpful in the diagnosis. Even though the overall sensitivity of CSF serology is higher, the antibodies production may be usually delayed and in immunocompromised patients the presence of VZV DNA in CSF is more frequently detected.
Although there are few studies concerning the treatment of VZV vasculopathy in immunocompromised patients, a longer course of antiviral treatment may be needed to avoid relapse. The use of steroids as adjunctive therapy, increasing the immunosuppressive pressure, could compromise the outcome.
References
- 1.Nagel M.A., Gilden D. Developments in varicella zoster virus vasculopathy. Curr Neurol Neurosci Rep. 2016;16(2):12. doi: 10.1007/s11910-015-0614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleinschmidt-DeMasters B.K., Gilden D.H. Varicella-zoster virus infections of the nervous system clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770–780. doi: 10.5858/2001-125-0770-VZVIOT. [DOI] [PubMed] [Google Scholar]
- 3.Askalan R., Laughlin S., Mayank S., Chan A., MacGregor D., Andrew M. Chickenpox and stroke in childhood: a study of frequency and causation. Stroke. 2000;32(6):1257–1262. doi: 10.1161/01.str.32.6.1257. [DOI] [PubMed] [Google Scholar]
- 4.Sreenivasan N., Basit S., Wohlfahrt J., Pasternak B., Munch T.N., Nielsen L.P. The short and long-term risk of stroke after herpes zoster - a nationwide population-based cohort study. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray F., Bélec L., Lescs M.C., Chrétien F., Ciardi A., Hassine D. Varicella-zoster virus infection of the central nervous system in the acquired immune deficiency syndrome. Brain. 1994;117:987–999. doi: 10.1093/brain/117.5.987. [DOI] [PubMed] [Google Scholar]
- 6.Kleinschmidt-DeMasters B.K., Amlie-Lefond C., Gilden D.H. The patterns of varicella zoster virus encephalitis. Hum Pathol. 1996;27:927–938. doi: 10.1016/s0046-8177(96)90220-8. [DOI] [PubMed] [Google Scholar]
- 7.Amlie-Lefond C., Gilden D. Varicella zoster virus: a not uncommon cause of stroke in children and adults. J Stroke Cerebrovasc Dis. 2016;25(7):1561–1569. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagel M.A., Cohrs R.J., Mahalingam R., Wellish M.C., Forghani B., Schiller A. The varicella zoster virus vasculopathies: clinical, CSF, imaging and virological features. Neurology. 2008;70(11):853–860. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng-Ching E., Jones S., Hui F.K., Man S., Gilden D., Bhimraj A. High-resolution MRI vessel wall imaging in varicella zoster virus vasculopathy. J Neurol Sci. 2015;351:168–173. doi: 10.1016/j.jns.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breuer J., Pacou M., Gauthier A., Brown M.M. Brown Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology. 2014;82(3):206–212. doi: 10.1212/WNL.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas S.L., Minassian C., Ganesan V., Langan S.M., Smeeth L. Chickenpox and risk of stroke: a self-controlled case series analysis. Clin Infect Dis. 2014;58(1):61–68. doi: 10.1093/cid/cit659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun K.P., Bulder M.M., Chabrier S., Kirkham F.J., Uiterwaal C.S.P., Tardieu M. The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain. 2009;132:544–557. doi: 10.1093/brain/awn313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagel M.A., Traktinskiy I., Azarkh Y., Kleinschmidt-DeMasters B., Hedley-Whyte T., Russman A. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011;77:364–370. doi: 10.1212/WNL.0b013e3182267bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russman A.N., Lederman R.J., Calabrese L.H., Embi P.J., Forghani B., Gilden D.H. Multifocal varicella-zoster virus vasculopathy without rash. Arch Neurol. 2003;60(11):1607–1609. doi: 10.1001/archneur.60.11.1607. [DOI] [PubMed] [Google Scholar]
- 15.Persson A., Bergstrom T., Lindh M., Namvar L., Studahl M. Varicella-zoster virus CNS disease - viral load, clinical manifestations and sequels. J Clin Virol. 2009;46(3):249–253. doi: 10.1016/j.jcv.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Kasper D.L., Fauci A.S. 3rd edition. Vol. 89. McGraw-Hill Education; 2017. pp. 747–751. (Harrison’s infectious diseases). [Google Scholar]
- 17.Gregoire S.M., VanPesch V., Goffette S., Peeters A., Sindic C.J. Polymerase chain reaction analysis and oligoclonal antibody in the cerebrospinal fluid from 34 patients with varicella-zoster virus infection of the nervous system. J Neurol Neurosurg Psychiatr. 2006;77(8):938–942. doi: 10.1136/jnnp.2006.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagstaff A.J., Faulds D., Goa K.L. Aciclovir: a reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47:153–205. doi: 10.2165/00003495-199447010-00009. [DOI] [PubMed] [Google Scholar]
- 19.Whitley R.J., Blum M.R., Barton N., de Miranda P. Pharmacokinetics of acyclovir in humans following intravenous administration. A model for the development of parenteral antivirals. Am J Med. 1982;73(1A):165–171. doi: 10.1016/0002-9343(82)90084-5. [DOI] [PubMed] [Google Scholar]
- 20.Lycke J., Malmestrom C., Stahle L. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob Agents Chemother. 2003;47:2438–2441. doi: 10.1128/AAC.47.8.2438-2441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]