Abstract
Ecosystem Health, Conservation Medicine, EcoHealth, One Health, Planetary Health and GeoHealth are inter-related disciplines that underpin a shared understanding of the functional prerequisites of health, sustainable vitality and wellbeing. All of these are based on recognition that health interconnects species across the planet, and they offer ways to more effectively tackle complex real-world challenges. Herein we present a bibliometric analysis to document usage of a subset of such terms by journals over time. We also provide examples of parasitic and vector-borne diseases, including malaria, toxoplasmosis, baylisascariasis, and Lyme disease. These and many other diseases have persisted, emerged or re-emerged, and caused great harm to human and animal populations in developed and low income, biodiverse nations around the world, largely because of societal drivers that undermined natural processes of disease prevention and control, which had developed through co-evolution over millennia. Shortcomings in addressing drivers has arisen from a lack or coordinated efforts among researchers, health stewards, societies at large, and governments. Fortunately, specialists collaborating under transdisciplinary and socio-ecological health umbrellas are increasingly integrating established and new techniques for disease modeling, prediction, diagnosis, treatment, control, and prevention. Such approaches often emphasize conservation of biodiversity for health protection, and they provide novel opportunities to increase the efficiency and probability of success.
Keywords: Baylisascariasis, Conservation Medicine, EcoHealth, Ecosystem Health, GeoHealth, Integrative research, Lyme disease, Malaria, One Health, Planetary Health, Social-ecological systems, Toxoplasmosis, Transdisciplinarity
1. Introduction
Most of us have been trained to work within the constraints of traditional disciplinary frameworks, and we continue to do so, even when the problems at the heart of our studies span human medical, veterinary medical, environmental and/or ecological sciences. Few of us can bear the time and effort required to learn the jargon, concepts, theories, and models, let alone normative conventions, of other fields. Moreover, until recently, many researchers and academics have not possessed the soft skills needed to relay the co-constructions of knowledge required for sustainable solutions to the lay public and policy makers.
The sequential emergence of Ecosystem Health, Conservation Medicine, EcoHealth, One Health and more recently Planetary Health and GeoHealth (Aguirre et al., 2012, Aguirre et al., 2016; Almada et al., 2017; Wilcox et al., 2012; 2019; Whitmee et al., 2015) attest to a growing interest in interdisciplinary and transdisciplinary research and its application to confront issues that straddle health and ecological sciences and that stretch further to encompass social science and policy frameworks. Now, diverse communities of workers that academically or professionally identify with human and veterinary medicine, public health and global health are arguably in the early stages of teamwork to better explore, understand and address the human-animal-ecosystem health nexus.
Transdisciplinarity or transdisciplinary (TD) thinking emerged in response to the essential need to move beyond parochial professional perspectives toward a more holistic view of health and the environment. In today's world, complex and interacting problems demand shared understanding of the issues at hand among medical professionals, epidemiologists, environmental scientists, geographers, and specialists in economics, business, law, social and political sciences, and religion under a single umbrella. Collaborations in research, practice, policy, planning, and adaptive management are needed to ensure simultaneous gains in the health of people, domestic and wild animals and plants, soils, and ecosystems. The essential systems and TD thinking, in concert with participatory processes and relevant demonstration projects, will logically offer ways to accommodate reasonable human demands and expectations while simultaneously enabling interdependent life forms to recover so that they not only regain the capacity for self-renewal but also provide a foundation for sustainable social-ecological services (Huntsinger and Oviedo, 2014).
Protecting the health of humans, animals and across species and ecosystems has a long history (Cook et al., 2004; Mi et al., 2016). From ancient Egypt, Greece, Rome and China through the Renaissance and into the current era, exploring similarities and differences among humans and other animals has enabled biomedical progress (Bresalier et al., 2015; Zinsstag et al., 2011). In the 1700s, physicians helped establish veterinary medical schools and methods for animal disease control. In the 1800s, Rudolf Virchow, founder of pathology, coined the term “zoonosis,” Louis Pasteur established the germ theory of disease, Robert Koch developed Koch's Postulates, and collaborations among experts in human and animal health identified epidemiologic determinants of water-, waste-water-, and food-borne infections, pointing the way to rational infrastructure and astute hygiene (Kahn et al., 2012). Nevertheless, by the early 1900s, veterinarians were asserting themselves to gain control in educating their students, and a schism between professions was underway.
Despite separation into different professional groups and physical facilities, the value of sharing knowledge and capabilities persisted and, in the 20th and 21st centuries, human and animal health specialists have intermittently collaborated. In 1947, the Veterinary Public Health Division at the Centers for Disease Control was established. In his book, Veterinary Medicine and Human Health, Dr. Calvin Schwabe defined “One Medicine,” emphasizing the similarities between human and veterinary medicine and the need for effective collaboration for treatment, prevention and control of zoonotic diseases. Today, recently developed genetic, epigenetic, transcriptomic, metagenomic, and analytical tools for exploring nutritional, immunologic, infectious, and toxicological diseases are being linked to epidemiological and ecological models to enable a new era of collaborative analyses and interventions to more effectively protect and improve health (Bresalier et al., 2015; http://www.cdc.gov/onehealth/people-events.html).
Today's problems arise from increasing human population size and consumption, a poorly-designed, expanding human ecological footprint, absent or outdated infrastructure, deforestation, chemical and pathogen pollution, mismanagement of domestic and wild animals, species invasions through global transport and the exotic pet trade, and global environmental change. Ecosystem Health, Conservation Medicine, EcoHealth, One Health, Planetary Health and GeoHealth are overlapping approaches to health that bring specialists together to identify and meet the functional prerequisites of sustainable health and wellbeing. In 1979, the term, Ecosystem Medicine, was used in relation to similarities among concepts of human health and the health of ecosystems (Rapport et al., 1979). Rapport et al. (1985) used the term Ecosystem Health in discussing similarities and differences from human health as well as economic relevance. Soon, related terms, Conservation Medicine, EcoHealth, and One Health were introduced in an effort to unify broader transdisciplinary efforts. The recent emergence of Planetary Health and GeoHealth signal the recognition that human demands undermining all life-support systems are an urgent global health priority (Almada et al., 2017; Whitmee et al., 2015). Herein, we focus on the challenges of operationalizing TD, systems thinking and participatory processes. We highlight key aspects of such concepts and provide a bibliometric analysis of references to them to gauge usage of a subset of them among different experts. We purposely excluded the terms Public Health, Environmental Health and Global Health due to their long-term establishment as human health disciplines. Finally, we illustrate the need for TD and social-ecological health frameworks for better prediction, control, and prevention of parasitic and vector-borne diseases, using the complex examples of malaria, toxoplasmosis, baylisascariasis, and Lyme disease.
2. Transdisciplinary, systems thinking and participation
The ecosystem approach to parasitic and vector-borne diseases requires an integrative process that is transdisciplinary, that employs systems thinking, and that engages “stakeholders” as equal participants or collaborators to enable adaptive capacity (Richter et al., 2015b; Wilcox et al., 2019). TD employs perspectives and methods that transcend traditional disciplines and engage both researchers and practitioners in addressing real-world problems. TD requires the team members to share roles and systematically cross disciplinary boundaries. This approach includes but goes beyond the pooling and integration of disciplinary expertise to include knowledge held by stakeholders who are affected by, or otherwise have an interest in, a problem area. The communication style in TD involves continuous give-and-take among all members on a regular, planned basis. The contributions of different disciplines depend on the needs of the given challenge. Assessment, intervention, and evaluation are carried out jointly. Thus, the objective of TD is to bring together research scientists, other academic experts, field practitioners, community members, business professionals, and political leaders from local to global scales to address pressing issues (Aguirre and Wilcox, 2008; Aguirre et al., 2016; González-Astudillo and Aguirre, 2014).
Systems thinking comprises the identification of appropriate system boundaries and relevant scales considering environmental, social–cultural and economic factors. It involves a description of the system's components, their connections and relationships, and assessments of tradeoffs among systems' (socio-ecological) functions and services (Richter et al., 2015b). This provides a more integrative mitigation strategy toward problem solving and increases the system's adaptive capacity in the sense of its resilience (social and ecological) to the impacts of change, not just related to the specific problem being addressed (e.g. a particular disease eradication), but also keeping an “eye” on the desired general trajectory (e.g. sustainable development) the system at large should follow. Systems thinking thus recognizes the complexity of problems within “real-world wholes” and the necessity of multiple disciplines to collaborate in deriving TD concepts and methods through a continuous learning process, referred to as “generative knowledge” (Richter et al., 2015a, Richter et al., 2015b). In the case of zoonoses, this involves investigating disease dynamics and associated drivers and impacts by emphasizing the entirety of a system's components and the complexity of their interrelated behaviors (Xia et al., 2017).
Enabling knowledge sharing and equitable communication through project stakeholders' participation is essential. The adjective participatory is often used, as in “community-based participatory action,” for projects labeled as an “ecosystem approach.” Participation, or preferably partnership, involves clearly defined intentions and the capacity by the facilitators and the project's investigators to loosen their epistemological boundaries, in order to accept and honestly assess views and perceptions that are not theirs. This is particularly difficult when a project involves stakeholders from various socioeconomic classes (e.g. university professors, public health practitioners, social anthropologists, and villagers). Therefore, there is a need for tools and methodologies to facilitate disparate knowledge merging and the co-creation of new knowledge, employing for example participatory systems modeling (Binot et al., 2015; Etienne, 2013).
3. Bibliometric analysis
We conducted a bibliometric and content analysis using Scopus, one of the largest abstract and citation databases of peer-reviewed literature and quality web sources. Using the following terms ‘Ecosystem Health’, ‘Conservation Medicine’, ‘Ecohealth’, ‘One Health’, ‘Planetary Health’ and ‘GeoHealth’ in the title, abstract and keywords fields. The analysis covered the period 1945 to 2018. Documents obtained were classified by year, affiliation, country/territory and subject area. Subject areas were classified as Environmental Sciences, Agricultural and Biological Sciences, Veterinary and Medicine and Human Medicine. The Veterinary Medicine and Human Medicine categories include their respective journals focused on Clinical Medicine, Biomedical Sciences, Public Health and Epidemiology. Trends clearly relate to disciplinary niches using the above terms; however, it was difficult to exclude the use of some terms as intended; for example, One Health had applications for example “the number one health issue…”, “one's health responses”. All publications began to increase geometrically when terms where first used to establish programs, associations or conferences (Fig. 1.1). Ecosystem Health increased following the seminal paper by Rapport (1989). Conservation Medicine increased when the Center for Conservation Medicine was established and the first book was released (Aguirre et al., 2002). Usage of Ecohealth accelerated after establishment of the journal EcoHealth. One Health sharply increased in 2007 after establishment of the One Health Initiative Task Force and the associated report by the AVMA (2008). Planetary Health and GeoHealth gained ground very recently in concert with few papers published on each of them. The bibliometric scan shows that the various terms have been employed most often within specific disciplines, which likely limits transdisciplinary understanding, cooperation and impact (Fig. 1.2). Ecosystem Health is most often employed by environmental, agricultural, and biological scientists. Conservation Medicine is found across subject areas and EcoHealth is mainly found in the environmental field. It is clear that One Health is mostly used in human medicine by public health and epidemiology journals and by professionals in veterinary medicine, epidemiology, and preventive medicine. The same applied to the emerging Planetary Health and GeoHealth. This analysis suggests that users of different terms for transdisciplinary health are self-segregating in accord with professional groups and respective journals, which is likely to perpetuate isolation from needed collaborators and funding sources.
Fig. 1.
1. Scopus bibliometric analysis of number of publications over time and 2. Percentage of publications by subject area using the terms Ecosystem Health, Conservation Medicine, EcoHealth, One Health, Planetary Health and GeoHealth from 1945 to 2018. Since the book released on Ecosystem Health in 1991, the concept has widely used to date. Conservation Medicine has been used across subject areas since its emergence in the mid 1990s. EcoHealth is a concept commonly used in the environmental fields. One Health has grown exponentially since the establishment of the One Health Commission in 2007. It is clear that Planetary Health and GeoHealth recently emerged primarily within the human health domain during the past decade.
4. Ecosystem Health
Rapport, Costanza, and a number of collaborators produced seminal books on Ecosystem Health, founded the International Society for Ecosystem Health (ISEH), and established the journal Ecosystem Health (Rapport et al., 1998, Rapport et al., 1999, Rapport et al., 2003). The journal and society's founders described their purpose as to “explore potential transfers from the fields of human and veterinary medicine into ecology” (Rapport et al., 1999). Despite this original intention, the field of ecology did not significantly embrace Ecosystem Health. By contrast, others focused on wildlife infectious and toxicological diseases were quicker to see the value of Ecosystem Health as a new, more holistic and potentially practical way of problem framing (Fig. 2). For example, David Schaeffer, and coworkers published articles on Ecosystem Health, emphasizing ecotoxicology and wildlife epidemiologic data in relation to human health (Ellis et al., 1982; Schaeffer and Novak, 1988; Schaeffer et al., 1988). Cairns et al. (1993) described evaluation of Ecosystem Health, highlighting linkages among wild species and human health, and wrote, “Integrative science requires that complex multivariate systems be considered in their entirety, not fragment by fragment.”
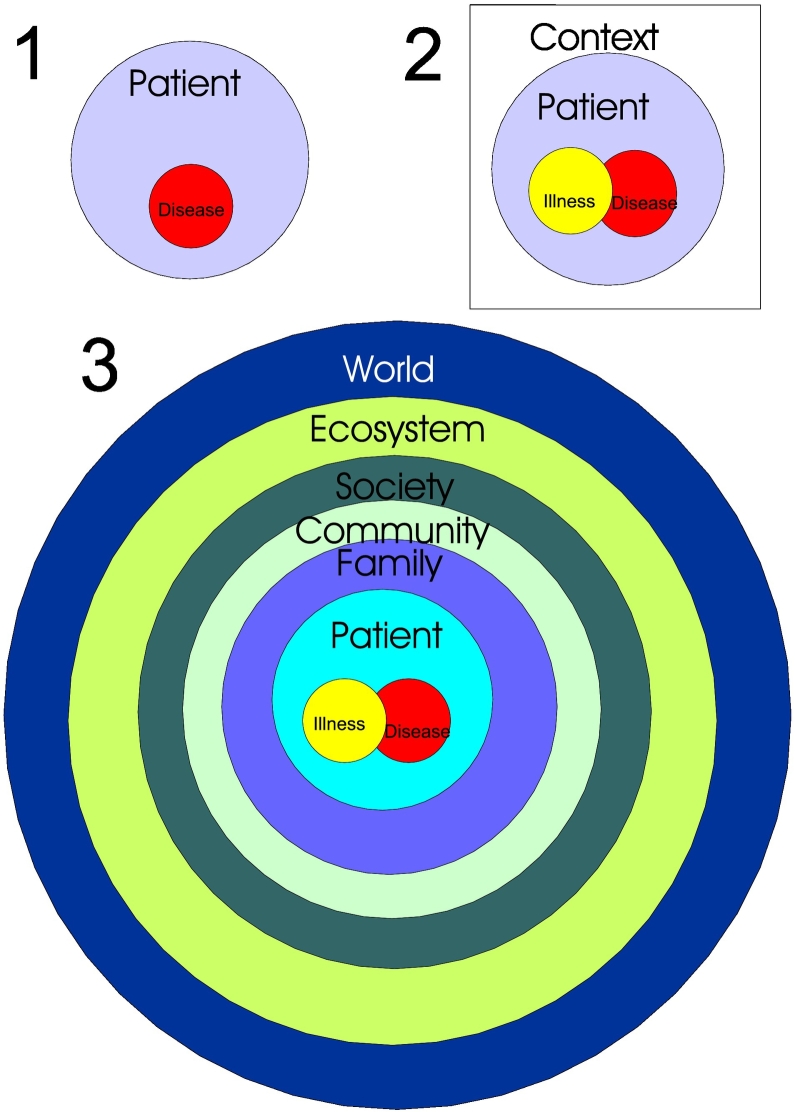
Fig. 2.
1. Diagram depicting a traditional focus on health and the disease-causing agent. 2. A slightly more patient-centered model, including his/her illness and the disease-causing agent. 3. An Ecosystem Health model where patients with illnesses and related disease-causing agent are encountered in the context and influence of family, community, society, ecosystem, and the world as a system.
(Modified from Rapport et al. (2002).)
Beasley (1993) held that the concept of Ecosystem Health was needed for prioritized policy and research to achieve suitable environments for human beings while maintaining biological diversity. He urged students to take courses in ecology, conservation biology, epidemiology, environmental toxicology, risk assessment, environmental law, environmental chemistry, statistics, and modeling. In 1991, the Universities of Illinois, Wisconsin and Minnesota established the Envirovet Program in Wildlife and Ecosystem Health (Gilardi et al., 2004; Schwind et al., 2016). With other universities, U.S. Geological Survey's National Wildlife Health Center, and U.S. Environmental Protection Agency (EPA) National Health Environmental Effects Research Laboratory's Mid-Continent Ecology Division, they provided courses that integrated aquatic animal health with ecotoxicology, ecological monitoring, disease diagnostics, engineering, law, economics, and the conservation of endangered species. During 2000–2011, Envirovet collaborated with the University of California-Davis, White Oak Conservation Center (WOCC), Harbor Branch Oceanographic Institute, and leaders in Brazil, Kenya, South Africa and Tanzania to offer Summer Institutes, emphasizing aquatic and terrestrial wildlife and ecosystem health in developed and lower income, biodiverse country contexts. Envirovet also worked with European universities in Envirovet Baltic workshops and the Baltic University Programme, a network of about 225 universities, to help produce a free-online book series, entitled Ecosystem Health and Sustainable Agriculture (http://www.balticuniv.uu.se/index.php/ecosystem-health-and-sustainable-agriculture). The National Academies' Workforce Needs in Veterinary Medicine, drew attention to additional ecosystem health-related educational, research, and service endeavors (National Research Council, 2011).
5. Conservation Medicine
Conservation Medicine evolved from the realization that protection of declining and endangered species depends upon the health of the affected species, the health and wellbeing of neighboring domestic animal and human populations, and the integrity and resilience of shared ecosystems. In 1996, Wildlife Trust (rebranded EcoHealth Alliance in 2010), Tufts University's Cummings School of Veterinary Medicine, and Harvard University's Center for Health and the Global Environment established the Center for Conservation Medicine based in North Grafton, Massachusetts. In 1999, the Center sponsored an international conference at WOCC, assembling scientists and practitioners in conservation biology, ecology, toxicology, veterinary medicine, epidemiology, and human, public and environmental health. The meeting forged links among experts in climate change, toxicology, emerging infectious diseases, conservation biology, and ecosystem health. The group launched a Symposium on Conservation Medicine at the 2000 meeting of the Society for Conservation Biology and the seminal book, Conservation Medicine: Ecological Health in Practice (Aguirre et al., 2002). Conservation Medicine addresses impacts that ripple through the web of life by linking professions and institutions responsible for the examination and care of humans, animals, and ecosystems (Fig. 3.1). Building upon the time-sensitive, site-specific interventionist necessities of conservation biology, that is, species can become extinct if needed interventions are delayed, Conservation Medicine focuses on concurrent remediation of ecological and wildlife health problems. Aims include protecting genetic diversity and ecosystem services, and avoiding significant infectious, parasitic, microbial and toxicant-induced diseases. Practitioners of Conservation Medicine developed new non-invasive health monitoring techniques, trained veterinarians, physicians and conservation biologists in ecological health practice techniques, and established transdisciplinary teams of professionals to investigate and confront spatially overlapping health threats to species and ecosystems (Aguirre et al., 2012).
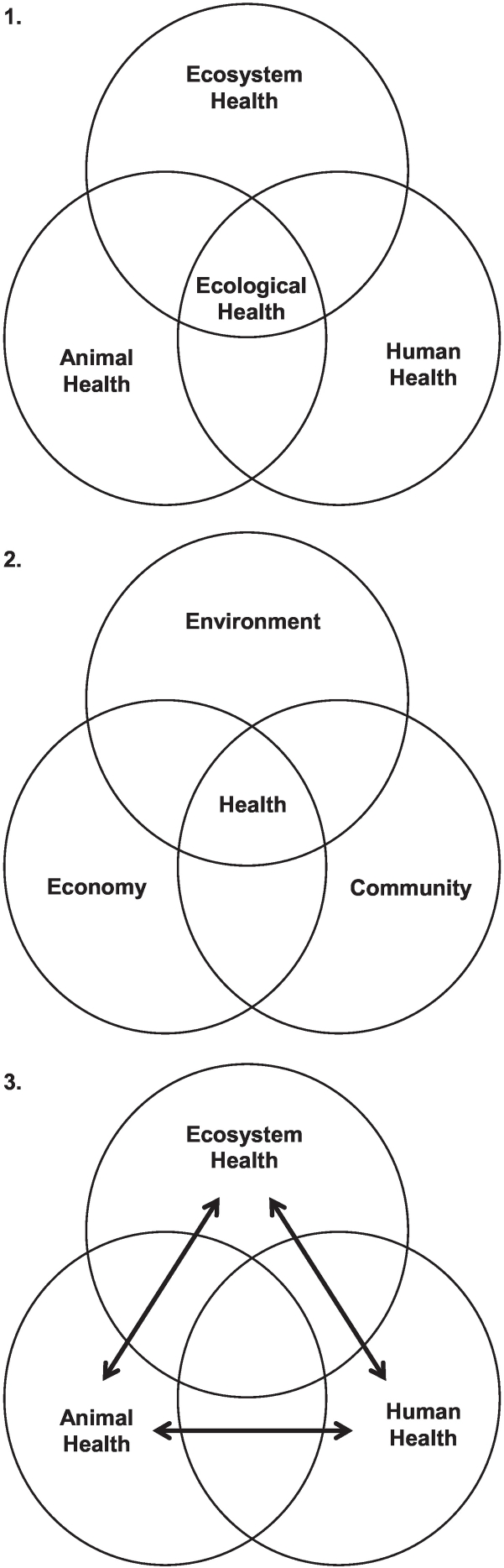
Fig. 3.
1. Conceptually, conservation medicine, the practice of ecological health, is at the nexus of the fields of ecosystem health, animal health and human health (modified from Aguirre et al., 2002). 2. Ecohealth (the ecosystem approach to heath) gives equal importance to environmental management, economic factors, and community aspirations (modified from Lebel, 2003). 3. More recently, the domains and forces of One Health were interconnected with bidirectional arrows (modified from King, 2014).
6. EcoHealth
In 1997, after decades of experience in health and environmental research, Canada's International Development Research Centre (IDRC) initiated the Ecosystem Approaches to Human Health (or Ecohealth) research program (Charron, 2012a). By the time of ISEH's 3rd biennial meeting in 2002, titled “Healthy Ecosystems, Healthy People” in Washington, D.C., despite an impressive showing and a growing following gained during its start-up years, the Ecosystem Health journal and the society had not grown sufficiently to remain financially viable. Journal and society co-founders, Rapport and Costanza, recruited one of the coauthors (BAW) to take over management of the journal and he was officially installed to replace Rapport as the new Editor-in-Chief. Wilcox led a new editorial advisory and management team in the design and launches the journal EcoHealth in 2004 with the purpose of publishing content aligned with “the ecosystem approach to health,” which aimed more toward the health sciences than ecology. The International Association for Ecology and Health was established two years later. The leaders highlighted needs for disciplinary integration at interfaces of ecological and human health sciences (Parkes, 2012). EcoHealth fosters systems-based, participatory approaches in environmental education, research, and stewardship to understand and promote health and wellbeing in the contexts of social and ecological interactions (Fig. 3.2). The initial vision of “ecosystem approaches to health,” or an ecosystem-based approach to health, was to set out frameworks and projects that would enable greater understanding of how social, economic, and ecological dynamics affect health (Forget and Lebel, 2001). Ideally, researchers from different disciplines would collaborate with stakeholder communities to examine current trade-offs and health-related problems, and then generate evidence and test strategies to serve as a foundation for policies and actions to improve health, environments, and livelihoods in ways that are locally sustainable and equitable (Charron, 2012b). Three “pillars” of Ecohealth were stated as transdisciplinarity, participation and gender equity (Charron, 2012a; Lebel, 2003). While IDRC's and other associated initiatives labeled Ecohealth have increased knowledge and yielded health benefits, they arguably failed to achieve their full potential due a lack of operation criteria in relation to their design, implementation and evaluation (Nguyen-Viet et al., 2015; Richter et al., 2015b). Part of meeting the enormous challenge has been the need to scale up sufficiently to address widespread impacts and interactions among pathogens, pesticides, and other pollutants in areas of the world characterized by burgeoning human populations, extraordinary agricultural intensification, and unbridled, poorly regulated industrial growth (Richter et al., 2015a).
7. One Health
One Health, which has captured wider international interest than the other terms, has been described as “the collaborative effort of multiple disciplines—working locally, nationally, and globally—to attain optimal health for people, animals and our environment” (AVMA, 2008). Its aim is to facilitate collaborations in research and sharing of knowledge at multiple levels across human, animal, plant, soil, environmental and ecosystem health disciplines in ways that improve, protect, and defend the health of all species (Fig. 3.3).
Addressing linked health and ecological problems that today's disciplinary experts are neither solving nor preventing calls for creative and purposeful One Health leadership, education, research, planning and action (Johnson-Walker and Kaneene, 2018; Sleeman et al., 2017). The isolation of professions and explosion of medical knowledge in the 20th century led to silos of specialization that have value in fostering targeted discoveries and professional competence, but such compartmentalization left many problems, such as infectious disease emergence and re-emergence, recurrent and chronic poisoning of plants, animals, and human beings, harmful algal blooms, dead zones, climate change, and species declines and extinctions inadequately addressed (Beasley, 2009; Beasley and Adkesson, 2012; Manlove et al., 2016).
The One Health Initiative (OHI) was developed to unite human and veterinary medical, and environmental professionals (One Health Initiative, 2018; Togami et al., 2018). In 2009, the OHI organized a volume of Veterinaria Italiana devoted to One Health that included plant health, vector-borne and other infectious diseases, and chemical contaminants, including how they cause direct harm and how they may increase parasitic and vector-borne diseases (Beasley, 2009; Fletcher et al., 2009; Marcotić et al., 2009; Rabinowitz et al., 2009). The One Health Commission (OHC), sponsored by universities, foundations, and corporations, has similar aims and abundant student engagement. Its mission is, “to ‘educate’ and ‘create’ networks to improve health outcomes and well-being of humans, animals and plants and to promote environmental resilience through a collaborative, global One Health approach.” Its goals include “connecting One Health stakeholders, creating strategic networks/partnerships, and educating about One Health issues to support a paradigm shift in information sharing, active health interventions, collaborations, and demonstration project[s].”
In 2010, the Food and Agriculture Organization of the United Nations (FAO), the World Health Organization of the United Nations (WHO), and the World Organisation for Animal Health (OIE) published a tripartite report calling for coordinated One Health activities. The authors highlighted the importance of shared responsibility and efforts to address zoonotic and other high impact diseases. FAO, WHO and OIE created governance structures, established early warning systems and developed mechanisms to enhance coordination and support by member countries. Additional examples of how university-based, governmental, non-governmental, and international One Health groups detect, control, eliminate and prevent health and ecological problems were described by Uchtmann et al. (2015).
Parallel to these international coordinated efforts on One Health, the U.S. Agency for International Development (USAID) has become a major investor in the global response to the emergence and spread of infectious diseases using the One Health approach. For example, since mid-2005, it has supported building local capacity in more than 50 countries for monitoring the spread of H5N1 Highly Pathogenic Avian Influenza (HPAI), contributing to containment of the virus globally. The USAID Bureau for Global Health, Office of Health, Infectious Disease and Nutrition developed the Emerging Pandemic Threats (EPT) program granting, in 2009, two sizeable cooperative agreements, PREDICT and RESPOND, among others. The goal of PREDICT was to establish a global early warning system for zoonotic disease emergence that is capable of detecting, tracking, and predicting the emergence of new infectious diseases in high-risk wildlife (e.g. bats, rodents, and non-human primates) that could pose a major threat to human health. The goal of RESPOND was to improve the capacity of countries in high-risk areas to respond to outbreaks of emergent zoonotic diseases that pose a serious threat to human health. The geographic scope of these expanded efforts was directed to those zoonotic “hotspots” of wildlife and domestic animal origins (Jones et al., 2008; Morse et al., 2012). More recently, EPT2 has focused on cross-sectoral disease surveillance, training, and outbreak response. PREDICT2, led by the University of California-Davis, is enabling global surveillance for pathogens that can spillover from animal hosts to people by building capacities to detect and discover viruses of pandemic potential. To date, partners of PREDICT2 have detected more than 1000 unique viruses in humans and animals, including strains of Ebola and SARS, and they have established 60 labs to optimize low-cost viral detection methods in 30 countries (https://www2.vetmed.ucdavis.edu/ohi/predict/predict_what-weve-found.cfm).
The One Health Workforce (RESPOND2) Teams at the University of Minnesota and Tufts University provide support for two regional university networks, the One Health Central and Eastern Africa (OHCEA) network and the Southeast Asia One Health University Network (SEAOHUN). These established university networks are striving to create a sustainable transformation in the regions' health workforces (https://www.usaid.gov/sites/default/files/documents/1864/OHW_Overview_Handout_2016-ct-508-1.pdf).
8. Planetary Health
Planetary Health emerged from the Public Health and Global Health communities in response to growing recognition of pervasive and sometimes irreversible anthropogenic changes that transform Earth's ecosystems into landscapes that adversely affect human health. Planetary Health advocates for many of the similar approaches supported by Conservation Medicine, EcoHealth and One Health, including transdisciplinary collaborations, systems thinking, expanding to governance and policy to address inequality, inequity, and the protection of cultural identity and historical and current global values. The need to bridge disciplines incorporating both human and environmental health outcomes are key (Almada et al., 2017; Stone et al., 2018). Among the prominent achievements in this domain are publication of The Lancet Commission on pollution and health, and establishment of the journal The Lancet Planetary Health. The introductory issue explained that The Rockefeller Foundation had played a role in establishing the concept. Planetary Health was defined as the health of human civilizations and the natural systems on which they depend.
9. GeoHealth
GeoHealth emphasizes human health impacts related to environmental stressors. Included are chemical and pathogen pollution as well as impacts of climate change. The Fogarty International Center of the U.S. National Institutes of Health has a program named Global Environmental and Occupational Health (GEOHealth). It supports the development of institutions in low- or middle-income nations that serve as “hubs for collaborative research, data management, training, curriculum and outreach material development and policy support around high-priority local, national and regional environmental and occupational health threats.”
Launched in August 2016, by the now 100-year-old American Geophysical Union (AGU), GeoHealth is an open access journal that publishes research articles and commentaries focused on environmental and occupational health, outdoor and indoor air pollution, food safety and security, water abundance and quality, wastewater treatment, climate change in relation to human, agricultural and environmental health and diseases, soil health and services, ecosystem health and services, environmental epidemiology, geoethics, national and international laws and policies related to remediation of GeoHealth issues, global public health, climate change and exposures to pathogens, harmful agents in aquatic environments encountered via food chains, remote sensing and infectious diseases, and hydroepidemiology (Almada et al., 2017; American Geophysical Union, 2018).
Another development under this banner is the GeoHEALTH Platform (https://geohealth.hhs.gov/arcgis/home/), which is managed by the U.S. Department of Health & Human Services (DHHS). This web platform offers access to datasets and information feeds including local data to help enable more coordinated decision making and responses to specific events and needs. Users are tracked as they rely on that system. A private sector web-based tool under the name GeoHealth US Corp. is accessible at http://geohealth.us/index.html. Its focus is on specific environmental locales and their environmental health risks. The site indicates that the company relies on data from the U.S. National Library of Medicine, EPA, and DHHS.
10. Complexity of parasitic and vector-borne diseases
The complex life cycles and ecology of parasites, including those using multiple hosts, are fertile ground for transdisciplinary insights, study, and interventions. For example, variable susceptibility to pesticides can increase parasite impacts in multiple ways (Jayawardena et al., 2016). Trematode infections increase after nutrient enrichment or herbicide-induced nutrient shifts from macrophytes and phytoplankton to periphyton. Periphyton feeds snail intermediate hosts, and pesticides reduce resistance of vertebrates to parasite infections (Johnson et al., 2007). Also, micropredators, especially aquatic insects, are potent consumers of cercariae (Schotthoefer et al., 2007; Rohr et al., 2015), and therefore selective toxicity affecting such regulators of parasitism prompts more severe infections. Anthropogenic changes that impact ecological communities, impacting other predators can also increase parasitism. For example, schistosomiasis increases after dams are installed and native prawns that had regulated snail host populations can no longer migrate. Conversely, measures that restore prawns can reduce snail populations and human infections with Schistosoma (Sokolow et al., 2017).
10.1. Malaria
Transdisciplinary research, informed environmental management, and purposeful engagement of human populations are critical in malaria avoidance. Malaria caused major losses in indigenous American populations following introductions to the Western Hemisphere with the slave trade (Rodrigues et al., 2018). Today, malaria, transmitted by Anopheles mosquitoes, kills nearly 500,000 people each year. Most deaths occur in children of tropical nations with cerebral malaria caused by Plasmodium falciparum. Avoiding mosquito bites through protective clothing, screens, insect repellents, and insecticides on walls and bed nets reduces malaria. Nevertheless, areas periodically cold or dry enough to greatly limit mosquito populations that later become warm and wet witness large numbers of humans with limited acquired immunity and thus high morbidity and mortality when epidemics arise (Stryker and Bomblies, 2012). Deforestation can increase temperatures, humidity, vector populations, and Plasmodium in mosquitoes (Afrane et al., 2008; Pongsiri et al., 2009; Burkett-Cadema and Vittor, 2017). Migrations of non-immune people into areas with infected people, migrations of infected people into areas that lacked malaria, establishing irrigation canals and/or rice cultivation, and altering hydrology in ways that desalinate coastal estuarine waters can greatly increase human infections and death losses (Lynch and Roper, 2011). Repeated use of the same insecticides or applications of insecticides with high environmental persistence fail due to selection of resistant mosquitoes (Dijoaka et al., 2016; Riveron et al., 2016). Conversely, indigenous plants that take up water drying pools that propagated mosquitoes and maintaining homes away from standing water and a few kilometers from forests that harbor infected mosquitoes substantially reduce malaria. Chemoprophylaxis and early treatment of infected individuals also reduce human malaria.
Birds are impacted by other malarial parasites, such as Plasmodium relictum. The vector of P. relictum, Culex quinquefasciatus, was introduced to the Hawaiian Islands in 1826, and P. relictum apparently arrived there around 1900 (Samuel et al., 2015). Since then, infections have caused multiple extinctions in native birds, including several honeycreeper species (LaPointe et al., 2010). Also, exotic invasive feral swine produce wallows yielding many Culex, and warmer temperatures increase mosquito and malaria exposures of evolutionarily and immunologically naïve birds. In addition to hemolysis, inflammation, and organ damage, infected birds have fewer young; survivors are genetic predisposed to shortened telomeres; and their offspring have shortened lifespans and reduced fitness (Ashghar et al., 2015).
In natural environments, mosquito populations are reduced by native aquatic invertebrates, fish, and larval salamanders (Brodman and Dorton, 2006; Kroeger et al., 2013). Integrated pest management (IPM) that included Daphnia spp., which compete with mosquito larvae, and the biological insecticide, Bacillus thuringiensis serotype israelensis (Bti), was more effective than the crustacean or Bti alone (Kroeger et al., 2013). In concert with responsible actions by mosquito control agencies, integrated biological management, involving more species than IPM, should be studied to develop mosquito control options. Research should examine reliance on locally evolved and adapted species in wild and human-made (e.g. rice field) aquatic ecosystems (Mogi, 2007). Moreover, genetically-modified Anopheles mosquitoes that breed with wild type mosquitoes and pass on their genetic and microbiome-related resistance to malaria infection warrant further study (Pike et al., 2017).
Efficient prevention of malaria and other mosquito-borne diseases requires prevention of transport of mosquitoes and pathogens to new areas including with human migrations, informed landscape and household management, counteracting climate change, and intervening at the levels of the parasite, mosquito, and vertebrate host (The malERA Refresh Consultative Panel on Basic Science and Enabling Technologies, 2017). To counteract development of resistant strains of mosquitoes and Plasmodium and protect human and animal health, research should investigate better: treatments for malaria; adulticides, larvicides and landscape management to control mosquitoes; protection of native organisms that compete with and eat larval mosquitoes; and vaccines.
10.2. Toxoplasmosis
Transdisciplinary integration has been involved in studies of toxoplasmosis, but actions based on relevant discoveries are needed to protect humans and domestic and wild vertebrates. According to Kijstra and Jongert (2008), one-third of the world's human population is infected by Toxoplasma gondii. People with HIV/AIDS are especially at risk, but clinical toxoplasmosis is an important cause of preventable human deaths from food-borne infections in the broader population as well (Cummings et al., 2014). From 2000 to 2010, there were an estimated 789 human deaths in the U.S. from toxoplasmosis; cumulative productivity losses totaled almost $815,000,000.
Domestic and wild felids are definitive hosts of T. gondii, and initial infections produce maximal shedding. Felids that ingest infected prey or raw meats, dairy products, and eggs can shed millions of oocysts that sporulate to become infective to people, domestic animals, and wildlife. Humans and other species are also infected by the encysted protozoan in uncooked or rare meats from domestic and wild ruminants, swine, carnivores and birds, unpasteurized milk and milk products, under-cooked eggs, contaminated fresh greens, other vegetables and fruits, and water (Aramini et al., 1999; Jones et al., 2009; Jones and Dubey, 2012; Lass et al., 2012). Hunting, free-range production of food animals, and unpasteurized dairy products present risks of exposure. Infected species may endure significant disease problems, including from ocular or neurologic infections.
Releasing cats to farms or any outdoor area drives Toxoplasma infections. Domestic cats appear to be the main source of oocysts picked up by shellfish that pose risks to marine mammals and people (Conrad et al., 2005; Lindsay et al., 2003; VanWormer et al., 2016). Coastal pollution with oocysts is likely aggravated by degradation of wetlands, creating un-vegetated mudflats (Shapiro et al., 2010). Outdoor domestic cats are exotic invasives that carry other parasitic and microbial diseases, including several zoonoses, and they compete with and kill indigenous species, undermining conservation (Longcore et al., 2009). Coyotes (Canis latrans) kill cats, therefore, humane societies caution owners to keep pets indoors (Grubbs and Krausman, 2009). In the absence of responsible pet ownership, reliance on larger predators to control cat populations and reduce toxoplasmosis warrants investigation (Molsher et al., 2017). Education of the public and small-scale producers of meat, milk, and eggs on ways to avoid Toxoplasma infections is urgently needed.
Rats and mice on farms can cause immense losses of foodstuffs, and in rural and urban areas they transmit important zoonoses. While cats kill rodents, such pests are controllable through tight buildings, proper storage of feed and wastes, traps, and carefully selected and deployed rodenticides. The environmental oocyst load is the most important risk factor for food-borne human toxoplasmosis (Kijstra and Jongert, 2008). To reduce Toxoplasma infections, enhance conservation successes, and minimize human and animal harm, Felis catus should remain indoors. Transdisciplinarity, integrative research, and capacity building are core elements in establishing interventions to prevent and control toxoplasmosis. Participatory methodologies that are shared among stakeholders should address this major problem confronting society, wildlife, and ecosystems globally (Aguirre et al., 2019).
10.3. Baylisascariasis
Baylisascaris procyonis roundworms infect most raccoons (Procyon lotor) in the U.S., and they typically shed thousands of ova daily. Raccoons thrive in human settlements, and populations are increasing in North America and where they were introduced, including Japan, Europe, and Russia (Troyer et al., 2014). The comprehensive report by Kazacos (2016) reflects a need for greater attention to baylisascariasis. People, domestic and wild mammals and birds, including endangered species, are harmed and killed by B. procyonis. Raccoons also carry rabies and leptospirosis, and they prey on eggs, birds, and other small wildlife. Hunting, trapping, malnutrition and starvation can be important in regulating raccoon numbers in rural areas, but diseases such as canine distemper can play a predominant role in crowded urban/suburban raccoons (Riley et al., 1998). Red wolves (Canis rufus) and pumas (Felis concolor) prey on raccoons (Maehr et al., 1990; McVey et al., 2013), but coyotes rarely do so (Gehrt and Clark, 2003; Gehrt and Prange, 2006). Raccoons and ghost crabs (Ocypode quadrata) are predators on endangered sea turtle eggs, but raccoons also feed on the crabs. Raccoon removal to protect sea turtle eggs increased egg predation by ghost crabs (Barton, 2003). The value of more complete sets of predators, including wolves and pumas that help control mesopredators like raccoons (as well as feral pigs and deer), raccoons and other predators that control ghost crabs, and perhaps organisms that eat and digest parasite eggs and infective larvae would seem to be ripe areas for broader ecological studies (MacDonald and Loveridge, 2010). Currently high raccoon populations in urban areas warrant population control, increased distribution of oral rabies vaccines, deployment of anthelmintic-containing baits, and public education as to the rigor needed in cleaning up raccoon feces to prevent Baylisascaris infections.
10.4. Lyme disease
The impacts of Lyme disease necessitate additional transdisciplinary research, communication, and action. Before European settlement, white-tailed deer (Odocoileus virginianus) populations of North America were likely regulated by a lack of ideal habitat, indigenous hunters, wolves (Canis lupus), pumas, diseases, starvation, and weather events (McCabe and McCabe, 1984). By the late 1800s, whitetail populations in the eastern U.S. were decimated by market and subsistence hunting. In those areas, wolves and cougars were hunted and trapped to the point of regional extinction. Around 1900, whitetail hunting was curtailed and populations rebounded. No similar conservation efforts were afforded to wolves or pumas. North American whitetail populations in recent years were estimated at around 30 million, which is slightly higher than estimates relevant to pre-European settlement (Hewitt, 2015). In that earlier era, mature forests dominated eastern North America, and the carrying capacity of such areas is much lower than in today's transitional areas between forests and logged areas, grassy and prairie habitats, agriculture, and suburbs (VerCauteren, 2003). Current deer populations in the northeastern U.S., where Lyme disease has most notably increased, are high. Although deer do not harbor high numbers of Borrelia burgdorferi, the bacterial cause of Lyme disease, they support large numbers of the principal regional vector, blacklegged ticks (Ixodes scapularis), and offspring of those ticks frequently become infected with the Lyme spirochete by feeding on white-footed mice (Peromyscus leucopus), the prominent reservoir host (Kilpatrick et al., 2014). In turn, nymphs of blacklegged ticks transmit the bacteria to humans, dogs, and many other species. Way and White (2014) reported that increased Borrelia infections may be driven by forest fragmentation that increases mouse populations, and milder winters that favor tick survival. Moreover, environmental conditions that yield greater acorn production may increase survival of deer and mice, as well as spatial overlap when feeding on that food source (Clotfelter et al., 2007; McShea and Schwede, 1993; Ostfeld et al., 1996, Ostfeld et al., 2006).
Levi et al. (2012) suggested that fox numbers may be insufficient to control white-footed mouse populations, which could increase Lyme disease. They held that, without wolves, an influx of coyotes may have reduced fox populations. Subsequently, Way and White (2014) raised doubts that coyotes and coyote-wolf hybrids, both of which occur in northeastern states, have reduced fox numbers, and suggested that coyotes might control mouse populations. Setting aside that debate, it seems that increased deer harvest by hunters or large predators, and any predator species or predator community that reduces mouse populations and/or that keep them closer to their burrows so they pick up fewer ticks, as shown with foxes, would reduce Lyme disease in humans and other species (Hofmeester et al., 2017). Conversely, white-footed mice help protect forests from exotic gypsy moths (Lymantria dispar), and are a food source for many predators, such that driving mouse populations to extremely low levels could adversely impact ecosystems (Pongsiri et al., 2009). Insufficient populations of species that ingest large numbers of ticks but are poor hosts for Borrelia, such as opossums (Didelphis marsupialis), could also favor Lyme disease. The need to rely on disease dilution through conservation of more species to lower risks of Lyme disease was highlighted years ago (LoGuidice et al. (2003). Also, grazing cattle reduced risks of Lyme disease, and thus bovine and other livestock, as well as native herbivores warrant further study (Richter and Matuschka, 2006; Ruiz-Fons et al., 2012). Japanese barberry (Berberis thunbergii), an exotic ornamental bush that has invaded 20 states, inhibits forest regeneration and increases humidity, tick survival and Lyme disease. Methods to control it with herbicides, mechanical removal, and directed flame or controlled burns, are being used to reduce tick numbers and Borrelia exposures (Ward et al., 2013). While work continues on improving vaccines against Lyme disease for humans, domestic animals, and mice (to control the bacteria in them and in ticks that feed on them), a combination of hunting and management enabling communities of native predators to help control deer, mice, and ticks is worthy of modeling studies and field trials. Of course, living with biodiverse organisms brings other infectious risks, which may be rare or common and readily preventable or nearly impossible to prevent. Opossums are definitive hosts for Neospora, which cause protozoal myelitis in horses. Wild canids can be hosts for zoonotic roundworms, such as Toxocara, lethal zoonotic tapeworms such as Echinococcus, and rabies. To optimize their contributions to disease control, biodiverse communities of predators require wise management. This includes measures to regulate their numbers and reduce their infection rates. Such approaches seem to be warranted, given that Lyme disease risks today are high and, with climate change, other tick-borne diseases are a growing concern.
11. Future challenges: climate change and toxic pollutants
Anthropogenic climate change is expanding ranges of vectors from more tropical to temperate areas and from lower to higher elevations, increasing the spread of malaria and Chikungunya, Rift Valley fever, Zika, dengue fever, and other diseases. Other impacts of climate change include: heat stress, increased precipitation in some regions and drought in others; increased flooding, erosion and rising sea levels contributing to waterborne infections; more harmful algal blooms; more dead zones (Altieri and Gedan, 2015; Wuebbles, 2018); and failure of species assemblages to adapt.
Not since Rachel Carson's Silent Spring have people around the world been so focused on wide-ranging ramifications of toxic chemicals. The Lancet Commission on Pollution and Health concluded that pollution has been linked with approximately one in six human deaths worldwide, and that deaths caused by pollution are estimated to be three times more than deaths from AIDS, tuberculosis, and malaria combined, and 15 times more than all wars and other forms of violence (Landrigan et al., 2017). Nevertheless, these numbers are likely a gross underestimation of the burden of human disease caused by pollutants, as robust data exist for only a handful of chemical pollutant-health outcome pairings. There are many chemical pollutants for which causal evidence of disease is building rapidly (e.g., endocrine disruptors, immunomodulatory threats, carcinogens); many health outcomes are now being tied to chemical pollutants (e.g., obesity, preterm births); and new and emerging chemical pollutants with adverse effects on human health are being recognized (e.g., pharmaceutical and personal care products, nanoparticles, new classes of pesticides). The Lancet Commission emphasized that low- and middle-income countries are disproportionately affected by pollution, with 92% of pollution-related deaths occurring in such areas, while they are also disproportionately impacted by communicable diseases. Chemical pollutants also undermine the structure and function of ecosystems, and the health of resident invertebrate, fish, wildlife, and other communities. There is an urgent need for toxicologists of many types, practitioners, diagnosticians, engineers, and citizens' groups to collaborate in protecting human-dominated, interfacial, and wilder places from acute and chronic illnesses caused by toxic contamination (Aguirre et al., 2016; Beasley, 2009).
12. Moving beyond brand name: focused science and system-wide care
We agree with Asakura et al. (2015) and Whitmee et al. (2015), who stressed the need for innovation to confront this century's great developmental challenge of meeting human demands without compromising health or ecological sustainability. Unfortunately, some political leaders ignore scientific and even economic data to the detriment of life and health support systems that operate from local to global scales (Costanza et al., 1992). Achieving governance with the wisdom and the will to meet short- and long-term needs will require engaging and involving the public to generate widespread demand for system-wide management that will reliably lead to a brighter future. Human populations should understand the merits of sustainable and equitable patterns of consumption, gender equity to reduce population growth, and coordinated protection of health and natural resources.
Disciplinary excellence is essential, but bridging barriers that divide disciplines is needed to nourish systems-based thinking and empower individuals and institutions to actively collaborate and take needed risks. Insights of epidemiology, ecology, and TD and social-ecological health frameworks will require a scaled up focus on site-specific to global drivers that harm humans and other species. The geographic frameworks will vary from project to project, and may include water bodies, watersheds, airsheds, cities/towns, agricultural areas, managed forests, wild lands, and zones of industry, mining and fossil fuel exploitation. Climate, weather, and human, animal, and plant health indicators revealed by syndromic and diagnostic surveillance should be included in analyses (Uchtmann et al., 2015). Data entry should be nearly effortless and spatially explicit databases should be widely accessible so that maps point to existing and emerging infectious and toxicological diseases, contributory stressors, and future risks. Related analyses should reveal proximate etiologies, respective societal drivers, the effectiveness of implemented countermeasures, and data gaps, including those caused by a paucity of professionals and paraprofessionals in underserved regions. A strong focus should be on comparing different intervention scenarios with skillful modeling and validation studies.
Shared health challenges abound, and additional ones will arise in the short-term from crowded and stressed human, domestic animal, and wildlife populations, and flawed incentive/disincentive systems that result in myriad cases of poor environmental design and management, water shortages, and climate change. Transdisciplinary perspectives and scientific findings should inform education, research, law, policy and management to trigger changes that benefit species across the board. Accommodation of the common sense inherent in this transdisciplinary efforts should: 1) increase biosecurity and other disease control practices and infrastructure in towns and cities, concentrated animal production units, and agricultural fields; 2) protect and restore wild places to the greatest extent possible; 3) carefully monitor and manage interfacial areas; and, 4) expand research assessing adaptive management to ensure that people, animals, plants, and ecosystems increasingly thrive together.
References
- Afrane Y.A., Little T.J., Lawson B.W., Githeko A.K., Yan G. Deforestation and vectorial capacity of Anopheles gambie Giles mosquitoes in malaria transmission, Kenya. Emerg. Infect. Dis. 2008;14:1533–1538. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A.A., Wilcox B.W. EcoHealth: envisioning and creating a truly global transdiscipline. EcoHealth. 2008;5:238–239. doi: 10.1007/s10393-008-0197-6. [DOI] [PubMed] [Google Scholar]
- Aguirre A.A., Ostfeld R.S., Tabor G.M., House C.A., Pearl M.C., editors. Conservation Medicine: Ecological Health in Practice. Oxford University Press; New York: 2002. [Google Scholar]
- Aguirre A.A., Ostfeld R.S., Daszak D., editors. New Directions in Conservation Medicine: Applied Cases of Ecological Health. Oxford University Press; New York: 2012. [Google Scholar]
- Aguirre A.A., Beasley V.R., Augspurger T., Benson W.H., Whaley J., Basu N. One Health—trans-disciplinary opportunities for SETAC leadership in integrating and improving the health of people, animals, and the environment. Environ. Toxicol. Chem. 2016;35:2383–2391. doi: 10.1002/etc.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A.A., Longcore T., Barbieri M., Dabritz H., Hill D., Klein P.N., Lepczyk C., Lilly E.L., McLeod R., Milcarsky J., Murphy C.E., Su C., VanWormer E., Yolken R., Sizemore G.C. The One Health approach to toxoplasmosis: epidemiology, control and prevention in humans, animals, and ecosystems. EcoHealth. 2019 doi: 10.1007/s10393-019-01420-8. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almada A.A., Golden C.D., Osofsky S.A., Myers S.S. A case for Planetary Health/GeoHealth. GeoHealth. 2017;1:75–78. doi: 10.1002/2017GH000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri A.A., Gedan K.B. Climate change and dead zones. Glob. Chang. Biol. 2015;21:1395–1406. doi: 10.1111/gcb.12754. [DOI] [PubMed] [Google Scholar]
- American Geophysical Union . 2018. GeoHealth Online.https://agupubs.onlinelibrary.wiley.com/journal/2471140 [Google Scholar]
- American Veterinary Medical Association (AVMA) One Health Initiative Task Force; 2008. One Health: A New Professional Imperative.https://www.avma.org/KB/Resources/Reports/Documents/onehealth_final.pdf [Google Scholar]
- Aramini J.J., Stephen C., Dubey J.P., Engelstoft C., Schwantje H., Ribble C.S. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiol. Infect. 1999;122:305–315. doi: 10.1017/s0950268899002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura T., Mallee H., Tomokawa S., Jomi K., Kobayashi J. The ecosystem approach to health is a promising strategy in international development: lessons from Japan and Laos. Glob. Health. 2015;11:3. doi: 10.1186/s12992-015-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashghar M., Hawwelquist D., Hansson B., Zehtindjiev P., Westerdahl H., Bensch S. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science. 2015;347:436–438. doi: 10.1126/science.1261121. [DOI] [PubMed] [Google Scholar]
- Barton B.T. University of Central Florida; Orlando: 2003. Cascading Effects of Predator Removal on the Ecology of Sea Turtle Nesting Beaches. (M.S. thesis) [Google Scholar]
- Beasley V.R. Ecotoxicology and ecosystem health: roles for veterinarians; goals of the Envirovet program. J. Am. Vet. Med. Assoc. 1993;203:617–628. [PubMed] [Google Scholar]
- Beasley V.R. ‘One Toxicology,’ ‘Ecosystem Health,’ and ‘One Health’. Vet. Ital. 2009;45:97–110. [PubMed] [Google Scholar]
- Beasley V.R., Adkesson A.M. In: Wildlife and Ecosystem Health. Ecosystem Health and Sustainable Agriculture, Volume 3: Ecology and Animal Health. Norrgren L., Levengood J.A., editors. Baltic University Press; Uppsala, Sweden: 2012. (pp. 13–26 and 329) [Google Scholar]
- Binot A., Duboz R., Promburom P., Phimpraphai W., Cappelle J., Lajaunie C., Goutard F.L., Pinyopummintr T., Figuié M., Roger F.L. A framework to promote collective action within the One Health community of practice: using participatory modelling to enable interdisciplinary, cross-sectoral and multi-level integration. One Health. 2015;1:44–48. doi: 10.1016/j.onehlt.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresalier M., Casidy A., Woods A. One Health in history. In: Zinsstag J., Schelling E., Waltner-Toews D., Whittaker M., Tanner M., editors. One Health: The Theory and Practice of Integrated Health Approaches. CABI Publishing; London: 2015. pp. 1–15. [Google Scholar]
- Brodman R., Dorton R. The effectiveness of pond-breeding salamanders as agents of larval mosquito control. J. Freshw. Ecol. 2006;21:467–474. [Google Scholar]
- Burkett-Cadema N.D., Vittor A.Y. Deforestation and vector-borne disease: forest conversion favors important mosquito vectors of human pathogens. Basic Appl. Ecol. 2017 doi: 10.1016/j.baae.2017.09.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J.C., Jr., McCormick P.V., Niederlehner B.R. A proposed framework for developing indicators of ecosystem health. Hydrobiologia. 1993;263:1–44. [Google Scholar]
- Charron D.E., editor. Ecohealth Research in Practice: Innovative Applications of an Approach to Health. International Development Research Centre. Springer; New York: 2012. [Google Scholar]
- Charron D.E. Ecosystem approaches to health for a global sustainability agenda. EcoHealth. 2012;9:256–266. doi: 10.1007/s10393-012-0791-5. [DOI] [PubMed] [Google Scholar]
- Clotfelter E.D., Pederson A.B., Cranford J.A., Ram N., Snajdr E.A., Nolan V., Jr., Ketterson E.D. Acorn mast drives long-term dynamics of rodent and songbird populations. Oecologia. 2007;154:493–503. doi: 10.1007/s00442-007-0859-z. [DOI] [PubMed] [Google Scholar]
- Conrad P.A., Miller M.A., Kreuder C., James E.R., Mazet J., Dabritz H., Jessup D.A. Transmission of Toxoplasma: clues from the study of sea otters as sentinels of Toxoplasma gondii into the marine environment. Int. J. Parasitol. 2005;35:1155–1168. doi: 10.1016/j.ijpara.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Cook R.A., Karesh W.B., Osofsky S.A. Wildlife Conservation Society; Bronx, New York: 2004. About One World, One Health.www.oneworldonehealth.org [Google Scholar]
- Costanza R., Norton B.G., Haskell B.D., editors. Ecosystem Health, New Goals for Environmental Management. Island Press; Washington, DC: 1992. (472 pp.) [Google Scholar]
- Cummings P.L., Kuo T., Javanbakht M., Sorvillo F. Trends, productivity losses, and associate medical conditions among toxoplasmosis deaths in the United States, 2000–2010. Am. J. Trop. Med. 2014;91:959–964. doi: 10.4269/ajtmh.14-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijoaka R.J., Atoyebi S.M., Tchigossou G.M., Riveron J.M., Irving H., Akoton R., Kusimo M.O. Evidence of a multiple insecticide resistance in the malaria vector Anopheles funestus in South West Nigeria. Malar. J. 2016;15:565. doi: 10.1186/s12936-016-1615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D.D., Jone C.M., Larson R.A., Schaeffer D.J. Organic constituents of mutagenic secondary effluents from wastewater treatment plants. Arch. Environ. Contam. Toxicol. 1982;11:373–382. doi: 10.1007/BF01055214. [DOI] [PubMed] [Google Scholar]
- Etienne M., editor. A Participatory Approach to Support Sustainable Development. Éditions Quæ; Versailles, France: 2013. p. 403. [Google Scholar]
- Fletcher J., Franz D., LeClerc J.E. Healthy plants: necessary for a balanced ‘One Health’ concept. Vet. Ital. 2009;45:79–95. [PubMed] [Google Scholar]
- Forget G., Lebel J. An ecosystem approach to human health. Int. J. Occup. Environ. Health. 2001;7(2):S3–38. (Suppl) [PubMed] [Google Scholar]
- Gehrt S.D., Clark W.R. Raccoons, coyotes, and reflections on the mesopredator release hypothesis. Wildl. Soc. Bull. 2003;31:836–843. [Google Scholar]
- Gehrt S.D., Prange S. Interference competition between coyotes and raccoons: a test of the mesopredator release hypothesis. Behav. Ecol. 2006;18:204–214. [Google Scholar]
- Gilardi K.V.K., Else J.G., Beasley V.R. Envirovet Summer Institute: integrating veterinary medicine into ecosystem health practice. EcoHealth. 2004;1(S):50–55. [Google Scholar]
- González-Astudillo V., Aguirre A.A. News from the IAEH: achieving transdisciplinary ecohealth education in early professional development. EcoHealth. 2014;11:152–153. doi: 10.1007/s10393-014-0932-0. [DOI] [PubMed] [Google Scholar]
- Grubbs S.E., Krausman P.R. Observations of coyote-cat interactions. J. Wildl. Manag. 2009;73:683–685. [Google Scholar]
- Hewitt D.G. Hunters and the conservation and management of white-tailed deer (Odocoileus virginanus) Int. J. Environ. Stud. 2015;72:839–849. [Google Scholar]
- Hofmeester T.R., Jansen P.A., Wijnen H.J., Coipan E.C., Fonville M., Prins H.H.T., Sprong H. Cascading effects of predator activity on tick-borne disease risk. Proc. R. Soc. B. 2017;284 doi: 10.1098/rspb.2017.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntsinger L., Oviedo J.L. Ecosystem services are social-ecological services in a traditional pastoral system: the case of California's Mediterranean rangelands. Ecol. Soc. 2014;19:8. [Google Scholar]
- Jayawardena U.A., Rohr J.R., Navaratne A.N., Amerasinghe P.H., Rajakaruna R.S. Combined effects of pesticides and trematode infections on hourglass tree frog Polypedates cruciger. EcoHealth. 2016;13:111–122. doi: 10.1007/s10393-016-1103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P.T.J., Chase J.M., Dosch K.L., Hartson R.B., Gross J.A., Larson D.J., Sutherland D.R. Aquatic eutrophication promotes pathogenic infection in amphibians. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15781–15786. doi: 10.1073/pnas.0707763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Walker Y.J., Kaneene J.B. Epidemiology: science as a tool to inform One Health policy. In: Herrmann J.A., Johnson-Walker Y.J., editors. Beyond One Health: From Recognition to Results. first edition. John Wiley & Sons, Inc.; Hoboken, NJ: 2018. pp. 3–30. [Google Scholar]
- Jones J.L., Dubey J.P. Foodborne toxoplasmosis. Clin. Infect. Dis. 2012;55:845–851. doi: 10.1093/cid/cis508. [DOI] [PubMed] [Google Scholar]
- Jones K.E., Patel N., Levy M., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–994. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.L., Dargelas V., Roberts J., Press C., Remington J.S., Montoya J.G. Risk factors for Toxoplasma gondii infection in the United States. Clin. Infect. Dis. 2009;49:878–884. doi: 10.1086/605433. [DOI] [PubMed] [Google Scholar]
- Kahn L.H., Monath T.P., Bokma B.H., Gibbs E.P., Aguirre A.A., C.M. One Health, One Medicine. In: Aguirre A.A., Ostfeld R.S., Daszak P., editors. New Directions in Conservation Medicine: Applied Cases of Ecological Health in Practice. Oxford University Press; New York: 2012. pp. 33–44. [Google Scholar]
- Kazacos K.R. U.S. Geological Survey Circular 1412. 2016. Baylisascaris larva migrans.https://pubs.usgs.gov/circ/1412/cir1412.pdf (122 pp., 3 appendixes) [Google Scholar]
- Kijstra A., Jongert E. Control of the risk of human toxoplasmosis transmitted from meat. Int. J. Parasitol. 2008;38:1359–1370. doi: 10.1016/j.ijpara.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Kilpatrick H.J., Labonte A.M., Stafford K.C.I.I.I. The relationship between deer density, tick abundance, and human cases of Lyme disease in a residential community. J. Med. Entomol. 2014;51:777–784. doi: 10.1603/me13232. [DOI] [PubMed] [Google Scholar]
- King L.J. Combating the triple threat: the need for One Health approach. In: Atlas R.M., Maloy S., editors. One Health: People, Animals and the Environment. ASM Press; Washington D.C.: 2014. pp. 3–15. [Google Scholar]
- Kroeger I., Liess M., Dziock F., Duquesne S. Sustainable control of mosquito larvae in the field by the combined actions of the biological insecticide Bti and natural competitors. J. Vector Ecol. 2013;38:82–89. doi: 10.1111/j.1948-7134.2013.12012.x. [DOI] [PubMed] [Google Scholar]
- Landrigan P.H., Fuller R., Acosta N.J.R., Adeyi O., Arnold R., Basu N., Balde' A.B. The Lancet Commission on pollution and health. Lancet. 2017 doi: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- LaPointe D.A., Goff M.L., Atkinson C.T. Thermal constraints in the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawa'i. J. Parasitol. 2010;96:318–324. doi: 10.1645/GE-2290.1. [DOI] [PubMed] [Google Scholar]
- Lass A., Pietkiewicz H., Szostakowska B., Myjak P. The first detection of Toxoplasma gondii DNA in environmental fruits and vegetables samples. Eur. J. Clin. Microbiol. 2012;31:1101–1108. doi: 10.1007/s10096-011-1414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel J. Focus Collection. International Development Research Centre; Ottawa, Canada: 2003. Health: an ecosystem approach. [Google Scholar]
- Levi T., Kilpatrick A.M., Mangel M., Wilmers C.C. Deer, predators, and the emergence of Lyme disease. Proc. Natl. Acad. Sci. U. S. A. 2012;109:10942–10947. doi: 10.1073/pnas.1204536109. http://www.pnas.org/content/109/27/10942.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay D.S., Collins M.V., Mitchell S.M., Cole R.A., Flick G.J., Wetch C.N., Lindquist A. Sporulation and survival of Toxoplasma gondii oocysts in seawater. J. Eukaryot. Microbiol. 2003;50(S):687–688. doi: 10.1111/j.1550-7408.2003.tb00688.x. [DOI] [PubMed] [Google Scholar]
- LoGuidice K., Ostfeld R.S., Schmidt K.A., Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. U. S. A. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcore T., Rich C., Sullivan L.M. Critical assessment of claims regarding management of feral cats by trap-neuter-return. Conserv. Biol. 2009;23:887–894. doi: 10.1111/j.1523-1739.2009.01174.x. [DOI] [PubMed] [Google Scholar]
- Lynch C., Roper C. The transit phase migration: circulation of malaria and its multidrug-resistant forms in Africa. PloS Med. 2011;8(5) doi: 10.1371/journal.pmed.1001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D.W., Loveridge A.J. Oxford University Press; New York: 2010. Biology and Conservation of Wild Felids. [Google Scholar]
- Maehr D.S., Belden R.C., Land E.D., Wilkins L. Food habits of panthers in southwest Florida. J. Wildl. Manag. 1990;54:420–423. [Google Scholar]
- Manlove K.R., Walker J.G., Craft M.E., Huyvaert K.P., Joseph M.B., Miller R.S., Nol P., Patyk K.A., O'Brien D., Walsh D.P., Cross P.C. “One Health” or Three? Publication silos among the One Health disciplines. PLoS Biol. 2016;14(4) doi: 10.1371/journal.pbio.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotić S., Krajinović L.C., Margaletić J., Turk N., Miletić-Medved M., Zmak L., Jancović M. Zoonoses and vector-borne diseases in Croatia – a multidisciplinary approach. Vet. Ital. 2009;45:55–66. [PubMed] [Google Scholar]
- McCabe R.E., McCabe T.R. Of slings and arrows: an historical retrospection. In: Halls L.K., editor. White-tailed Deer: Ecology and Management. Stackpole Books; Harrisburg, Pennsylvania: 1984. pp. 19–72. [Google Scholar]
- McShea W.J., Schwede G. Variable acorn crops on annual variation in deer and other mast consumers. J. Mammal. 1993;74:999–1006. [Google Scholar]
- McVey J.M., Cobb D.T., Powell R.A., Stoskopf M.K., Bohling J.H., Waits L.P., Moorman C.E. Diets of sympatric red wolves and coyotes in northeastern North Carolina. J. Mammal. 2013;94:1141–1148. [Google Scholar]
- Mi E., Mi E., Jeggo M. Where to now for One Health and EcoHealth? EcoHealth. 2016;13:12–17. doi: 10.1007/s10393-016-1112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M. Insects and other invertebrate predators. Am. Mosq. Control Assoc. Bull. 2007;23(sp2):93–109. doi: 10.2987/8756-971X(2007)23[93:IAOIP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Molsher R., Newsome A.E., Newsome T.M., Dickman C.R. Mesopredator management: effects of red fox control on the abundance, diet and use of space by feral cats. PLoS One. 2017 doi: 10.1371/journal.pone.0168460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S.S., Mazet A.K.M., Woolhouse M., Parrish C.R., Carroll D., Karesh W.B., Zambrana-Torrelio C., Lipkin W.I., Daszak P. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council Workforce needs in veterinary medicine. 2011. https://www.nap.edu/download/13413
- Nguyen-Viet H., Doria S., Tung D.X., Mallee H., Wilcox B.A., Grace D. Ecohealth research in Southeast Asia: past, present and the way forward. Infect. Dis. Poverty. 2015;4:5. doi: 10.1186/2049-9957-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- One Health Initiative One Health Initiative: uniting human, animal, and environmental studies to wrangle zoonotic and infectious diseases, members of the One Health Initiative explain how their holistic approach aims to synergise health care research and boost public health for the future. 2018. http://www.onehealthinitiative.com/publications/One_Health_EF_03.pdf
- Ostfeld R.S., Jones C.G., Wolff J.O. Of mice and mast. Bioscience. 1996;46:323–330. [Google Scholar]
- Ostfeld R.S., Canham C.D., Oggenfuss K., Woinchcombe R.J., Keesing F. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 2006;4(6) doi: 10.1371/journal.pbio.0040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes M.W. Diversity, emergence, resilience: guides for a new generation of ecohealth research and practice. EcoHealth. 2012;8:137–139. doi: 10.1007/s10393-011-0732-8. [DOI] [PubMed] [Google Scholar]
- Pike A., Dong Y., Dizaji N.B., Gacita A., Mongodin E.F., Dimopoulos G. Changes in microbiota caused genetically modified Anopheles to spread in a population. Science. 2017;357:1396–1399. doi: 10.1126/science.aak9691. [DOI] [PubMed] [Google Scholar]
- Pongsiri M.J., Roman J., Ezenwa V.O., Goldberg T.L., Koren H.S., Newbold S.C., Ostfeld R.S. Biodiversity loss affects global disease ecology. Bioscience. 2009;59:945–954. [Google Scholar]
- Rabinowitz P., Scotch M., Conti L. Human and animal sentinels for shared health risks. Vet. Ital. 2009;45:23–34. [PMC free article] [PubMed] [Google Scholar]
- Rapport D.J. What constitutes ecosystem health? Perspect. Biol. Med. 1989;33:120–132. [Google Scholar]
- Rapport D.J., Thorpe C., Regier H.A. Ecosystem medicine. Bull. Ecol. Soc. Am. 1979;60:180–192. http://www.jstor.org/stable/20166211 [Google Scholar]
- Rapport D.J., Regier H.A., Hutchinson T.C. Ecosystem behavior under stress. Am. Nat. 1985;125:617–640. https://www.jstor.org/stable/2461475 URL: [Google Scholar]
- Rapport D.J., Costanza R., Epstein P.R., Gaudet C., Lewis R. Blackwell Publishing; Malden, Massachusetts: 1998. Ecosystem Health. [Google Scholar]
- Rapport D.J., Böhm G., Buckingham D., Cairns J., Jr., Costanza R., Karr J.R., de Kruijf H.A.M., Levins R., McMichael A.J., Nielsen N.O., Whitford W.G. Ecosystem Health, the concept, the ISEH, and the important tasks ahead. Ecosyst. Health. 1999;5:82–90. [Google Scholar]
- Rapport D.J., Howard J., Lannigan R., McMurtry R., Jones D.L., Anjema C.M., Bend J.R. Introducing Ecosystem Health into undergraduate medical education. In: Aguirre A.A., Ostfeld R.S., Tabor G.M., House C., Peal M.C., editors. Conservation Medicine: Ecological Health in Practice. Oxford University Press; New York: 2002. pp. 345–360. [Google Scholar]
- Rapport D.J., Lasley W.L., Rolston D.E., Nielsen N.O., Qualset C.O., Damania A.B., editors. Managing for Healthy Ecosystems. Lewis Publishers; Boca Raton, Florida: 2003. [Google Scholar]
- Richter D., Matuschka F.-R. Modulatory effect of cattle on risk of Lyme disease. Emerg. Infect. Dis. 2006;12:1919–1923. doi: 10.3201/eid1212.051552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C.H., Custer B., Steele J.A., Wilcox B.A., Xu J. Intensified food production and correlated risks to human health in the Greater Mekon Subregion: a systematic review. Environ. Health. 2015;14:43. doi: 10.1186/s12940-015-0033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C.H., Steele J.A., Nguyen-Viet H., Wilcox B.A. Toward operational criteria for ecosystem approaches to health. EcoHealth. 2015;12:220–226. doi: 10.1007/s10393-015-1028-1. [DOI] [PubMed] [Google Scholar]
- Riley S.P.D., Hadidian J., Manski D.A. Population density, survival, and rabies in raccoons in an urban national park. Can. J. Zool. 1998;76:1153–1164. [Google Scholar]
- Riveron J.M., Osae M., Egyir-Yawson A., Irving H., Ibrahim S.S., Wondji C.S. Multiple insecticide resistance in the major malaria vector Anopheles funestus in southern Ghana: implications for malaria control. Parasit. Vectors. 2016;9:504. doi: 10.1186/s13071-016-1787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues P.T., Valdivia H.O., de Oliveira T.C., Alves J.M.P., Duarte A.M.R.C., Cerutti-Junior C. Human migration and the spread of malaria parasites to the New World. Sci. Rep. 2018;8:1993. doi: 10.1038/s41598-018-19554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr J.R., Civitello D.J., Crumrine P.W., Halstead N.T., Miller A.D., Schotthoefer A.M., Stenoien C. Predator diversity, intraguild predation, and indirect effects drive parasite transmission. Proc. Natl. Acad. Sci. U. S. A. 2015;112:3008–3013. doi: 10.1073/pnas.1415971112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Fons F., Fernandez-de-Mera I.G., Acevedo P., Gortazar C., de la Fuente J. Factors driving the abundance of Ixodes ricinus ticks and the prevalence of zoonotic I. ricinus-borne pathogens in natural foci. Appl. Environ. Microbiol. 2012;78:2669–2696. doi: 10.1128/AEM.06564-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M.D., Woodworth B.L., Atkinson C.T., Hart P.J., LaPointe D.A. Avian malaria in Hawaiian forest birds: infection and population dynamics across species and elevations. Ecosphere. 2015;6:104. [Google Scholar]
- Schaeffer D.J., Novak E.W. Integrating epidemiology and epizootiology information in ecotoxicology studies: III Ecosystem Health. Ecotoxicol. Environ. Saf. 1988;16:232–241. doi: 10.1016/0147-6513(88)90053-x. [DOI] [PubMed] [Google Scholar]
- Schaeffer D.J., Herricks E.E., Kerster H.W. Ecosystem Health I: measuring ecosystem health. Environ. Manag. 1988;12:445–455. [Google Scholar]
- Schotthoefer A.M., Labak K.M., Beasley V.R. Ribeiroia ondatrae cercariae are consumed by aquatic invertebrate predators. J. Parasitol. 2007;83:1240–1243. doi: 10.1645/GE1129R.1. [DOI] [PubMed] [Google Scholar]
- Schwind J.S., Gilardi K.V.K., Beasley V.R., Mazet J.A.K., Smith W.A. Advancing the ‘One Health’ workforce by integrating ecosystem health practice into veterinary medical education: the Envirovet Summer Institute. Health Educ. J. 2016;75:170–183. [Google Scholar]
- Shapiro K., Conrad P.A., Mazet J.A., Wallender W.W., Miller W.A., Largier J.L. Effect of estuarine wetland degradation on transport of Toxoplama gondii surrogates from land to sea. Appl. Environ. Microbiol. 2010;76:6821–6828. doi: 10.1128/AEM.01435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman J.M., DeLiberto T., Nguyen N. Optimization of human, animal, and environmental health by using a One Health approach. J. Vet. Sci. 2017;18(Suppl. 1):216–268. doi: 10.4142/jvs.2017.18.S1.263. (Aug) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolow S.H., Jones I.J., Jocque M., La D., Cords O., Knight A., Lund A. Nearly 400 million people are at higher risk of schistosomiasis because dames block the migration of snail-eating river prawns. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0127. (pii: 20160127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S.B., Myers S.S., Golden C.D. Cross-cutting principles for planetary health education. Lancet Planet. Health. 2018;2:192–193. doi: 10.1016/S2542-5196(18)30022-6. [DOI] [PubMed] [Google Scholar]
- Stryker J.J., Bomblies A. The impacts of land use change on malaria vector abundance in a water-limited, highland region of Ethiopia. EcoHealth. 2012;9:455–470. doi: 10.1007/s10393-012-0801-7. [DOI] [PubMed] [Google Scholar]
- The malERA Refresh Consultative Panel on Basic Science and Enabling Technologies malERA: an updated research agenda for basic science and enabling technologies in malaria elimination and eradication. PLoS Med. 2017;14:e1002451. doi: 10.1371/journal.pmed.1002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togami E., Gardy J.L., Hansen G.R., Poste G.H., Rizzo D.M., Wilson M.E., Mazet J.A.K. Core competencies in One Health education: what are we missing? NAM Perspectives. Discussion Paper, National Academy of Medicine, Washington, DC. 2018. https://nam.edu/core-competencies-in-one-health-education-what-are-we-missing
- Troyer E.M., Devitt S.E.C., Sunquist M.E., Goswami V.R., Oli M.K. Survival, recruitment, and population growth of an important mesopredator: the northern raccoon. PLoS One. 2014 doi: 10.1371/journal.pone.0098535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchtmann N., Herrmann J., Hahn N., Beasley V.R. Barriers to, efforts in, and optimization of integrated One Health surveillance: a review and synthesis. EcoHealth. 2015 doi: 10.1007/s10393-015-1022-7. [DOI] [PubMed] [Google Scholar]
- VanWormer E., Carpenter T.E., Singh P., Shapiro K., Wallender W.W., Conrad P.A., Largier J.L. Coastal development and precipitation drive pathogen flow from land to sea: evidence from a Toxoplasma gondii and felid host system. Sci. Rep. UK. 2016;6:29252. doi: 10.1038/srep29252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VerCauteren K. USDA National Wildlife Research Center – Staff Publications; 2003. The Deer Boom: Discussions on Population Growth and Range Expansion of the White-Tailed Deer; p. 281.http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1276&context=icwdm_usdanwrc [Google Scholar]
- Ward J.S., Williams S.C., Worthley T.E. Japanese barberry control methods. Reference guide for foresters and professional woodland managers. Special Bulletin – February. 2013. http://www.ct.gov/caes/lib/caes/documents/publications/special_bulletins/special_bulletin_feb_2013_ward.pdf
- Way J.G., White B.N. Coyotes, red foxes, and the prevalence of Lyme disease. Northeast. Nat. 2014;20:655–665. [Google Scholar]
- Whitmee S., Haines A., Beyrer C., Boltz F., Capon A.G., Ferreira de Souza Dias B., Ezeh A. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386:1973–2028. doi: 10.1016/S0140-6736(15)60901-1. [DOI] [PubMed] [Google Scholar]
- Wilcox B.A., Aguirre A.A., Horwitz P. EcoHealth: connecting ecology, heath, and sustainability. In: Aguirre A.A., Ostfeld R.S., Daszak P., editors. New Directions in Conservation Medicine: Applied Cases of Ecological Health. Oxford University Press; New York: 2012. pp. 17–32. [Google Scholar]
- Wilcox B.A., Aguirre A.A., De Padua N., Siriaroonrat B., Echaubard P. One Health and the UN Sustainable Development Goals: imperatives toward an integrative science and practice of ecology, health and sustainability. Front. Public Health. 2019 (in press) [Google Scholar]
- Wuebbles D.J. Health impacts in a changing climate. In: Herrmann J.A., Johnson-Walker Y.J., editors. Beyond One Health: From Recognition to Results. first edition. John Wiley & Sons, Inc.; Hoboken, NJ: 2018. pp. 31–59. [Google Scholar]
- Xia S., Zhou X.-N., Liu J. Systems thinking in combating infectious diseases. Infect. Dis. Poverty. 2017;6:144. doi: 10.1186/s40249-017-0339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinsstag J., Schelling E., Waltner-Toews D., Tanner M. From “one medicine” to “one health” and systematic approaches to health and well-being. Prev. Vet. Med. 2011;101:148–156. doi: 10.1016/j.prevetmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]