Highlights
-
•
B. aerius S1 and B. iodinum S2 could tolerate Cr6+ upto 30 and 35 mM.
-
•
Both strains showed promising potential to reduce 99% Cr6+ within 6 days from tannery effluent.
-
•
FTIR and EDX analysis confirmed interaction and biosorption of Cr6+ to the bacterial cells.
-
•
Increased GSH and NPSHs levels played significant role in combating the oxidative stress.
Keywords: Chromium resistant bacteria, Antioxidant enzymes, Glutathione, FTIR, Bioremediation
Abstract
Bacillus aerius S1 and Brevibacterium iodinum S2 showed maximum growth at 37 °C and pH 8. B. aerius and B. iodinum could resist Cr6+ upto 30 and 35 mM and biosorption proficiency (q) of B. aerius S1 was 19, 27, 52 and 34 mM/g while for B. iodinum S2, it was 39, 50, 23 and 16 mM/g mM/g after 2, 4, 6 and 8 days of incubation. A pronounced rise in antioxidant enzymes activities was determined in B. aerius S1 i.e. POX (963%), CAT (717%), APOX (699%), SOD (683%), and GST (792%). However, in B. iodinum S2, relatively a minor increase was estimated. A significant GSH increase was determined in B. aerius S1 (364%) and B. iodinum S2 (663%) cultures under 2 mM Cr6+ stress. Pilot scale study demonstrated that both strains could reduce Cr6+ into Cr3+ within 6 days from the original tannery effluent with efficiency of 99%.
1. Introduction
Industrial revolution and technological advancement where raised the standards of living, is a major cause of the environmental pollution. This is due to the fact that industries discharge tons of hazardous wastes containing heavy metals (chromium, cadmium, and lead), metalloids, and organic pollutants at elevated concentrations that have wreaked severe damage to the environment [1,2]. Continuous release and non-degradability of metalloids and heavy metals make them persistent in the biosphere thus posing serious global health issues. The alarming concentration of heavy metals in the environment and its subsequent detrimental consequences to all life forms underline the need of immediately applying effective techniques to cut down their concentration to acceptable limit [3,4].
Chromium has been extensively used in industrial operations which include metal finishing industry, petroleum refinery, electrolating, leather tanning, iron and stainless steel industries, water cooling and wood preservation and pulp processing industries [5], paint and pigment manufacturing, textile and fertilizer industries [6]. These industries produce huge amounts of solid and liquid waste materials harboring Cr6+ compounds which easily dissolved in water causing toxicity and carcinogenicity in mammals [7]. Environment Protection Agency has listed Cr6+ compounds such as chromate and dichromate as priority pollutants in USA [8]. On the other hand, Cr3+, is comparatively far more less toxic and readily get precipitated at higher pH than 5.5, formatting insoluble oxides and hydroxides that precipitates rapidly in soil and water systems [7,9].
A potential detoxification process comprises conversion of Cr6+ into Cr3+ that could be accomplished via physiochemical or biological methods. To detoxify Cr6+ contaminated sites, conventional technologies could be applied such as land filling, soil washing, flushing, excavation, and physico-chemical extraction, but, these methods utilize chemical reagents and are quite expensive and cumbersome [9]. Therefore, it is need of the day to establish an innovative, economical and eco-friendly method to remove toxic heavy metal from the wastewater. Thus, bioremediation is the approach of choice which utilize the indigenous microbiota to clean-up heavy metals from the contaminated environment thus the reestablishing the polluted area without addition of chemical reagents [10]. Various microbes, for instance, bacteria, fungi, algae and protozoa are habitually residing in water mixed with industrial effluents, and these residing microbes have developed strategies to combat the heavy metal toxicity via processes like metal uptake, methylation, adsorption, oxidation and reduction [11].
The objective of the present study was to isolate, characterize, and determine the Cr-removal potential of indigenous micro-flora from the industrial wastewater. Two Cr6+ resistant gram positive strains Bacillus aerius S1and Brevibacterium iodinum S2 from tannery effluent were isolated and characterized. Besides, the behavior of antioxidant enzymes activities were observed under Cr6+ stress, which scavenge the reactive oxygen species produced under heavy metal stress. Furthermore, Cr6+ adsorption, and its subsequent accumulation in the bacterial cell were observed through FTIR spectroscopy, and SEM-EDX analysis, respectively. The bioremediation potential of these bacterial strains was ascertained on the basis of metal-resistance and reducing Cr6+ into Cr3+.
2. Material and methods
2.1. Isolation and characterization of Cr6+ resistant bacterial isolate
Bacterial isolates were obtained indigenously from the tannery effluent from the industrial sites of Sheikhupura, and Qasoor, Lahore (Pakistan). Tannery effluent samples were diluted before plating onto the Luria-Bertani (LB) agar amended with 1 mM Cr6+ stress in the form of K2Cr2O7 and incubated for 24–72 h at 37 °C. Screening of the bacteria was done on the basis of their ability to resist and reduce higher Cr6+ concentrations.
Molecular characterization of the isolated pure strains S1 and S2 were done. Methods of Masneuf-Pomarède et al. [12] was utilized to isolate genomic DNA of the bacterial isolates S1 and S2 and 16S rRNA gene was amplified using primers 8 F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) [13]. The amplified PCR products were cleaned with the Fermentas purification kit (#K0513) and sent for sequencing from Macrogen, Korea. To align the nucleotide sequences, basic local alignment search tool (BLAST) analysis was used.
2.2. Determination of optimum growth conditions
Optimal cultivation conditions of S1 and S2 were ascertained. Optimal growth temperature of S1 and S2 were determined by growing bacterial strains in 100 ml LB broth contained in 250 ml flask and incubated at four different temperatures i.e. 20 °C, 30 °C, 37 °C and 50 °C, for 24 h. cell growth of the bacterial cultures were obtained by taking OD at 600 nm. For optimal pH of S1 and S2, different pH values of the LB broth (5, 6, 7, 8, 9, and 10) were set, and inoculated with the log phase bacterial isolates. These flasks were placed in shaking incubator at 37 °C for 24 h and cell densities were determined at 600 nm.
Growth profiles of the bacterial isolates S1 and S2 in the absence and presence of Cr6+ was studied. Bacterial strains were cultivated in mineral salt medium (MSM) broth [g/L: FeSO4.7H2O 0.015 g, KH2PO4 4.7 g, MgSO4.7H2O 1 g, CaCl2.2H2O 0.01 g, Na2HPO4 0.12 g, NH4NO3 4 g, MnSO4.4H2O 0.01 g, glucose 10 g and yeast extract 5 g (pH 7–7.2)] without metal (control), and MSM broth containing 2 mM K2Cr2O7 (treated). The cell density was obtained at O.D600 nm after regular intervals until 24 h of incubation.
2.3. Determination of MICs of Cr6+ and other heavy metals
MICs of heavy metals against S1 and S2 were determined. For this, different concentrations of metal salts including K2Cr2O7 (for Cr6+), CdCl2, CuSO4.5H2O, NiCl2.6H20, PbNO3 and ZnSO4.7H2O were separately added to 100 ml modified M9 broth medium [g/L: Na2HPO4, 0.65 g ; KH2PO4, 1.5 g ; NH4Cl, 0.5 g ; NaCl, 0.25 g ; MgSO4.7H2O, 0.12 g; Casamino acid, 10 g; Glucose, 5 g (pH 6.9)]. All the flasks were inoculated with log phase culture of S1 and S2, separately and placed in shaking incubator at 37 °C at 150 rpm for 7 days. Optical density was taken, as cell growth of the bacterial isolates, at OD600 nm. Lowest metal concentration that is able to inhibit bacterial growth was considered as MIC.
2.4. Quantification of antioxidant enzymes and glutathione contents
Behavior of antioxidant enzymes of bacterial strains S1 and S2 was studied under Cr6+ stress. For this, bacterial strains were grown in 100 ml MSM medium in 250 ml flasks and placed in shaking incubator at 37 °C. After 24 h of incubation, 2 mM Cr6+ stress was added in the media and flasks were incubated again for another 24 h. Cultures were centrifuged at 14,000 rpm for 10 min, and pellets were weighed and dissolved in phosphate buffer and sonicated. The aliquots obtained after centrifugation of sonicated pellets were used for assaying antioxidant enzymes. Methods of Habig et al. [14] was used to evaluate glutathione transferase (GST) activity. Peroxidase (POX) enzyme was assayed according to Reuveni et al. [15] with minor modifications. Catalase, ascorbate peroxidase (APOX) and superoxide dismutase (SOD) activities were determined by the methods of Beers and Sizer [16], Israr et al. [17], Nakano and Asada [18], and Ewing and Janero [19], respectively.
Any alteration in the induction of glutathione and other non-protein thiols under chromium stress was determined according to Khan et al. [20]. For each strain, three flasks of medium were prepared and inoculated with bacterial culture. After 24 h of incubation, two culture flasks were supplemented with stress of 2 mM K2Cr2O7 stress, and third flask with no metal act as control. All the flasks were incubated again for another 48 h. After incubation, cultures were centrifuged, washed with 1 mM phosphate buffer, weighed and re-suspended in 1 ml of 5% sulfosalicyclic acid. The cell pellets were subjected to sonication and centrifuged at 14,000 rpm for 10 min at 4 °C and aliquot was separated into two equal parts. One part was used to assess glutathione level and other part was used to estimate non-protein thiol levels. Levels of reduced glutathione (GSH), oxidized glutathione (GSSG) and non-protein thiols were quantified by Khan et al. [20].
2.5. Metal processing potential of the Bacterial isolates S1 and S2
Metal processing ability of the bacterial strains S1 and S2 were evaluated by measuring changes in the quantity of Cr6+ in the culture medium by atomic absorption spectrophotometer according to Rehman et al. [21]. For each strain, two flasks were used for bacterial growth under 2 mM Cr6+ stress while the flask with 2 mM Cr6+ containing no organism served as control. Flasks were placed in shaking incubator at 37 °C and 120 rpm. After regular time interval i.e. 2, 4, 6, and 8 days, 5 ml aliquot were taken from each flask and cell culture was centrifuged at 6000 rpm for 10 min. Both the pellets and supernatants were used for Cr6+ estimation.
The pellets were washed with autoclaved distilled water, weighed and separated into two equal portions. To collect the adsorbed Cr fraction on the cell surface as a soluble fraction, one ration was washed with 0.5 M EDTA three times while the other ration was acid digested to gather the absorbed Cr. Acid digestion of pellet was done by re-suspending it in 1 ml autoclaved distilled water along with 1 ml of 0.2 N HNO3 (1:1) and placed on hot plate for 30 min till the suspension turned yellow. This aliquot was used to calculate intracellular Cr concentration. Standard curve of chromium was employed to calculate concentration of metal.
2.6. Chromate reduction in tannery effluent
The Cr6+ reduction potential of S1 and S2 was determined in tannery effluent. Three plastic containers were used; the first container carried the control 1 (10 l original tannery wastewater) while the second container carried control 2 (10 l distilled water, inoculated with 1.5 l culture), and the third container was filled with tannery wastewater (10 L) with culture. All containers were given 2 mM Cr6+ stress and incubated at room temperature (25 ± 2 °C). Samples (10 ml) were withdrawn after regular time of incubation (2, 4, 6, 8 days). Cells were centrifuged at 4000 rpm for 10 min and residual Cr6+ concentration was determined from supernatants via Diphenylcarbazide method. Alterations in concentration of Cr6+ after bacterial treatment was calculated from the calibration curve established under the same experimental conditions using a standards of Cr6+ solution.
2.7. FTIR, SEM and EDX analysis
FTIR Spectroscopy (Bruker, alpha) was employed to obtain Infrared spectra for S1 and S2 under Cr6+ stress. Specimens were treated as mentioned by Deokar et al. [22]. To determine the mechanism of metal-microbe interaction, it is essential to locate the presence of chromium ions (Cr6+, Cr+3) in the bacterial cells. To confirm the intracellular accumulation of chromium SEM-EDX analysis was done.
For scanning electron microscopy, samples were prepared according to Khan et al. [23]. In short, bacterial culture with and without 2 mM Cr was prepared and a drop of suspension was mounted onto the aluminum stub and treated as described by Khan et al. [23]. With sputter coater, samples were covered with gold film (Denton, Desk V HP) and examined through scanning electron microscope (Nova NanoSEM 450) equipped with Oxford energy dispersive X-ray (EDX) microanalysis system.
2.8. Statistical analysis
All the experiments run in triplicate and observations were made. Each experiment was done in at least three separate flasks. Each time three readings were taken, their mean, and standard error of the mean were calculated.
3. Results
3.1. Screening of chromate resistant bacterial strains
Serial dilutions of the effluent samples were plated on to the LB agar plates supplemented with 2 mM Cr6+. The stress of Cr6+ in the medium was increased gradually, and the isolates resisted high Cr6+ concentration as well as have the ability to reduce Cr6+ were selected. Two selected strains, labeled as S1 and S2 were capable of resisting 30 and 35 mM Cr6+, respectively. Both isolates S1 and S2 are gram positive and 16S ribotyping showed 100% homology of S1 with Bacillus aerius and S2 with Brevibacterium iodinum (Accession number KX941840 and KX941841 respectively) already submitted to NCBI database.
3.2. Optimum growth conditions
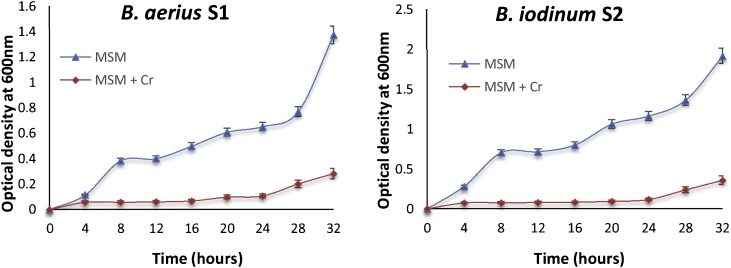
B. aerius S1 and B. iodinum S2 optimum growth temperature was determined as 37 °C and pH 8 for B. aerius S1 while pH 8 for B. iodinum S2. Growth of both strains was substantially declined in presence of Cr6+ (Fig. 1).
Fig. 1.
Growth curves of B. aerius S1 and B. iodinum S2; in mineral salt medium (control) and MSM supplemented with 2 mM K2Cr2O7 (treated) incubated at 37 °C. Optical density was taken at 600 nm after regular time interval.
3.3. Cross metal resistance
B. aerius S1 and B. iodinum S2 could resist Cr6+ up to 30 and 35 mM, respectively. Both strains are capable of tolerating other heavy metals as well, however, the pattern of resistance vary. For B. aerius S1, viz. 23 mM (Pb2+), 17 mM (Zn2+), 2 mM (Cu2+), 5 mM (Cd2+), 21 mM (As3+) and 3 mM (Ni2+). Resistance order according to metal ions concentration was Cr 6+ > Pb2+ > As3+> Zn2+> Cd2+> Ni2+ >Cu2+. For B. iodinum S2, viz., 9 mM (Pb2+), 17 mM (Zn2+), 3 mM (Cu2+), 1 mM (Cd2+), 2 mM (As3+) and 3 mM (Ni2+). Resistance order according to metal ions concentration was Cr 6+ > Zn2+> Pb2+ > Ni2+ / Cu2+ > As3+> Cd2+.
3.4. Quantification of antioxidant enzymes and glutathione
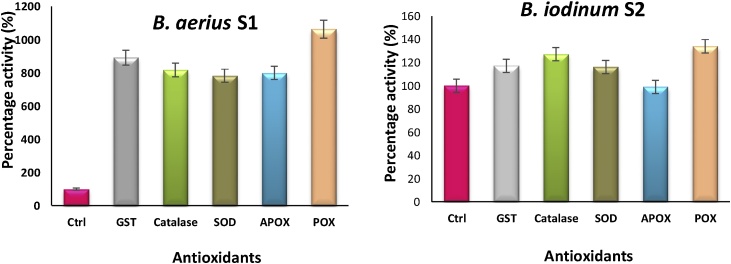
Under Cr6+ stress the antioxidant enzyme profiling of the two bacterial isolates, B. aerius S1 and B. iodinum S2, showed interesting results. In B. aerius S1, a pronounced rise in activities of all the antioxidant enzymes was observed i.e. POX (963%), CAT (717%), APOX (699%), SOD (683%), and GST (792%). However, in B. iodinum S2, relatively only a minor increase in enzyme activities of GST (17%), CAT(27%), SOD (16%), and POX (34%), moreover, 1% decrease in APOX was noticed (Fig. 2).
Fig. 2.
Changes in Antioxidant enzymes activity profile, exhibited by B. aerius S1 and B. iodinum S2 upon exposure of 2 mM Cr 6+.
Cr6+ stress also stimulates GSH and GSSG levels in both B. aerius S1 and B. iodinum S2, (Table 1). In B. aerius S1 and B. iodinum S2, under stress of 2 mM Cr6+, 364% and 663% increase in GSH was determined as compared to the control, respectively. Also, rise in non-protein thiols was observed in B. aerius S1 (275%) and B. iodinum S2 (177%) (Table 1).
Table 1.
Levels of reduced (GSH) and oxidized glutathione (GSSG), total glutathione, reduced and oxidized glutathione ratio, and nonprotein thiols in B. aerius S1 and B. iodinum S2 exposed to Cr6+ at 2 mM.
| Bacterial strains | Cr Conc. (mM) |
GSH (mMg−1 FW) |
GSSG (mMg−1 FW) | GSH + GSSG (mMg−1 FW) | GSH/GSSG ratio | % increase in GSH | Non-protein thiols | % increase in non-protein thiols |
|---|---|---|---|---|---|---|---|---|
| B. aerius S1 | 0 | 15.461 | 3.643 | 19.104 | 4.245 | 364.256 | 13.758 | 275.157 |
| 2 | 19.104 | 8.412 | 27.516 | 2.271 | 16.509 | |||
| B. iodinum S2 | 0 | 15.934 | 7.082 | 23.016 | 2.250 | 663.913 | 17.704 | 177.043 |
| 2 | 22.573 | 3.983 | 26.557 | 5.667 | 19.475 |
3.5. Chromium processing ability of bacterial isolates
3.5.1. Biosorption of Cr6+
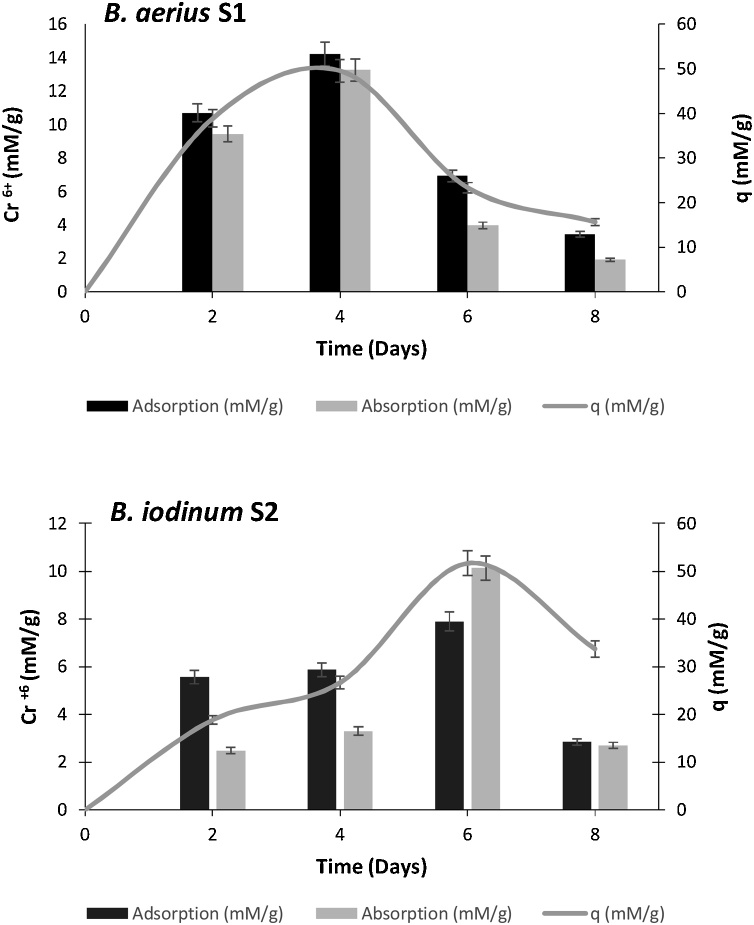
Biosorption potential of B. aerius S1 and B. iodinum S2 was assessed by cultivating them in LB broth supplemented with 2 mM Cr6+ (Fig. 3). Biosorption proficiency (q) of B. aerius S1 was determined after 2, 4, 6 and 8 days which was 19, 27, 52 and 34 mM/g, respectively. While for B. iodinum S2, the q value after 2, 4, 6 and 8 days was estimated as 39, 50, 23 and 16 mM/g, respectively (Fig. 3).
Fig. 3.
Biosorption of Cr 6+ by B. aerius S1 and B. iodinum S2 at lab scale.
3.5.2. Pilot study of Cr6+ bioremediation
Reduction potential of B. aerius S1 and B. iodinum S2 was also determined at pilot scale, where the efficiency of the isolates were determined in 10 l tannery effluent and change in Cr6+ was determined by Diphenylcarbazide method. It was clear that B. aerius S1 and B. iodinum S2 were capable of removing upto 99% Cr6+ from tannery effluent after 6 days of incubation when Cr6+ concentration was maintained at 2 mM (Table 2; Fig. 4).
Table 2.
Concentration of Cr6+ (μM) and the corresponding percentage reduction after regular intervals (2, 4, 6, 8 days) were determined in the original tannery effluent supplemented with 2 mM Cr6+ after treatment with B. aerius S1 and B. iodinum S2.
| Bacterial strains | Days | 2 | 4 | 6 | 8 |
|---|---|---|---|---|---|
| S1 (Water) | Cr6+ (μM) | 11.34 | 0.34 | 0.23 | 0.2 |
| % Reduction | 43 | 98 | 99 | 99 | |
| S1 (Effluent) | Cr6+ (μM) | 2.38 | 1.6 | 0.225 | 0.2 |
| % Reduction | 88 | 92 | 99 | 99 | |
| S2 (Water) | Cr6+ (μM) | 10.47 | 0.58 | 0.38 | 0.28 |
| % Reduction | 48 | 97 | 98 | 99 | |
| S1 (Effluent) | Cr6+ (μM) | 1.99 | 0.76 | 0.13 | 0.10 |
| % Reduction | 90 | 96 | 99 | 100 |
Fig. 4.
Change in Cr6+ color with respect to control in flasks containing culture of bacterial isolates B. aerius S1 (a) and B. iodinum S2 (b) from the original tannery effluents.
3.6. FTIR, SEM and EDX analysis
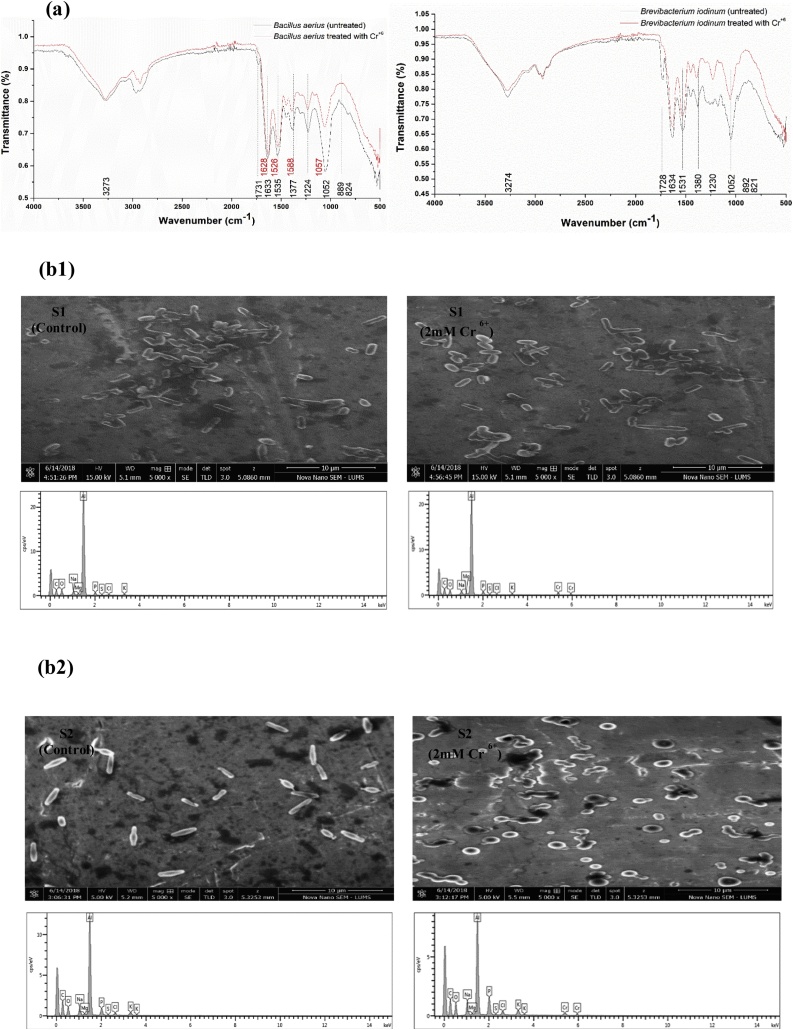
FTIR analysis of B. aerius S1 and B. iodinum S2 with and without chromium is shown in the Fig. 5. Infrared spectra of B. aerius S1 and B. iodinum S2, in the absence of any stress, exhibited characteristic absorption peaks of amino, hydroxyl, carboxyl, and sulphonate groups which confirmed the presence of corresponding groups on the cell surfaces. However, when the strains were subjected to heavy metal stress, changes in peaks and peak intensities were observed in the range of 3275 cm−1 and 1800–1000 cm−1 [24]. Under chromium stress, FTIR peaks of B. aerius S1 shifted from 1633 to 1628, 1535 to 1526, and 1052 to 1057. Also slight shift in hydroxyl group region was observed. Almost similar shift in the absorption peaks of similar regions was observed for the B. iodinum S2. Further confirmation of intracellular uptake of Cr6+ was done through SEM and EDX analysis which also confirmed the changes in cell state under heavy metal stress (Fig. 5).
Fig. 5.
(a) FTIR (b) Energy dispersive X-ray spectroscopy spectra analysis of B. aerius S1 (b1) and B. iodinum S2 (b2) in the presence and absence (control) of hexavalent chromium stress (2 mM Cr 6+).
4. Discussion
Biological procedures for the bioremediation of toxic heavy metals in the tannery effluents utilizing the potential microorganisms have been investigated by numerous researchers worldwide. Growing industrialization cause increased accumulation of Cr6+ to the alarming concentrations in the environment which pose serious environmental concern globally. The toxic nature of Cr6+ threatens all forms of life due to its teratogenic, mutagenic and carcinogenic properties. Therefore, there’s an urgent need to reduce the concentration of Cr6+ in the biosphere to the permissible amount, determined by US-EPA i.e., <0.05 mg L−1 [25].
A lot of work have been done on the utilization of Cr6+ resistant and reducing bacterial strains for the bioremediation purposes [26,27] under aerobic and anaerobic conditions [28,29]. Under chromium ion stress, Cr6+ resistant microbes utilize a number of strategies for their survival; consume a trace amount of metal ions for their metabolism; resist and/or detoxify excessive toxic amount of heavy metal present [30].
Intracellular Cr6+ reduction requires Cr6+ uptake. Once the chromate ions get entered into the cell, some of the ions get adsorbed on the outer surface (adsorption) before making its way into being accumulated inside the cell (absorption). Once accumulated, Cr6+ reduction into Cr3+ was carried out by the intracellular enzymes i.e., chromate reductases, where Cr3+ act as the terminal electron acceptor [6,31,32]. Thus, there are two key methods by which Cr6+ could be reduced into Cr3+ 1) hexavalent chromium bioaccumulation within the microbial cell 2) Cr6+ bioadsorption on the cell wall.
Adsorption leading to absorption of Cr6+ which is a necessary part of uptake process in the bacterial cell which ultimately leads to its reduction into less toxic Cr3+, thus the atomic absorption spectroscopy clearly revealed the amount of Cr6+ being absorbed and adsorbed in the bacterial cells. The biosorption efficiency (q) of B. aerius S1 was as 39, 50, 23 and 16 mM Cr6+/g while the Cr-biosorption efficiency (q) of B. iodinum S2 was as 19, 27, 52 and 34 mM Cr6+/g after 2, 4, 6 and 8 days of incubation. After a certain time (6 days in our case), when the amount of Cr6+ is still exceeding, all the receptors of the bacterial cells get saturated, activating the efflux system for Cr6+ without reducing them.
In this study, two gram positive strains Bacillus aerius S1and Brevibacterium iodinum S2 was isolated from the local tannery wastewater samples; and the behavior of both of the strains were observed and compared under hexavalent chromium stress. Extensive research has been done on the use of chromate resistant Bacillus sp. for the bioremediation of Cr contaminated sites such as Bacillus megatarium TKW3; Bacillus subtilis; Bacillus circulans; Bacillus cereus and Bacillus methylotrophicus [6,11,30]. In contrast only few researchers investigate the potential of Brevibacterium sp. for the Cr bioremediation [[33], [34], [35], [36]].
Generally, a potential microbe removes the heavy metal ions from the aqueous medium through biosorption or bioaccumulation or a combination of both processes. The initial passive uptake of metal ions occurs through biosorption which is followed by chemical bonding of Cr6+ to those sites on the cell surface which exhibit affinity for it. This bondage then leads to the reduction of Cr6+ into Cr3+ [37]. It has been suggested that Cr6+ ions exploit sulphate channels get an entry inside the cell where its reduction takes place [30,38], and the reduction of Cr6+ under aerobic conditions generate short lived lethal intermediates Cr5+ and Cr4+ that stimulate reactive oxygen species (ROS) production and subsequently cause toxicity in the cytoplasm [39].
Metal associated ROS generation promote oxidizing environment in the cell’s vicinity, however, the cell’s defense system comprising antioxidant compounds (superoxide dismutase, glutathione transferase, and catalase) could convert these biologically toxic species in to innocuous compounds [40]. Comparison of antioxidant profiles of Bacillus aerius S1 and Brevibacterium iodinum S2 under 2 mM Cr6+ stress showed very interesting results. Although Cr6+ stress in both strains generally provokes higher levels of antioxidant compounds, however the increase in Bacillus aerius S1 was substantially higher as compared to Brevibacterium iodinum S2 under the same stress conditions. In both strains, the highest increase was observed in POX which is 963 and 34% in Bacillus and Brevibacterium, respectively. Different environmental stress situations stimulate increased produced of peroxidases such as drought-stress, water stress [41], heavy metals stress (Cd, Cu, Al, Zn) [42], and gamma-radiation stress [40]. Suthar et al. [43] also have observed increased production of all the antioxidants under Cr6+ stress. Our results are also in good agreement with Lee and Shin [44] who observed increase in catalase activity under Cd stress. Increase in glutathione reductase activity was also reported by Lenartova et al. [45] and Khan et al. [23] under mercury and Cd stress, respectively. SOD induction was also reported by Lenártová et al. [45] under metal stress.
Wastewater offers a highly inhospitable environment for the propagation of nonindigenous bacteria as it also lacks essential nutrients required for the bacterial life support as well as it is rich in harmful compounds. Although, a lot of work has been done on the bioreduction trials of Cr6+ in LB media, however, only few replicate the same Cr6+ bioremediation trials in the original wastewater. Zahoor and Rehman [26] investigated Cr6+ bioremediation trial directly in original industrial effluent and demonstrated that Bacillus sp. JDM-2-1 and Staphylococcus capitis are capable of reducing Cr6+ (100 mg/l) upto 86% and 89%, respectively, after 144 h of incubation.
Biosorption due to nonspecific binding of Cr6+ or other heavy metal ions on the cell surface of the bacterial strain is highly dependent on the presence of functional groups on the active site of cell wall as well as physiochemical conditions of the solution. When Cr6+ interacts with the functional groups, changes in adsorption peaks were observed through FTIR analysis (Fig. 5). FTIR spectroscopy analysis of the untreated cells of B. aerius S1 and B. iodinum S2 showed presence of the functional group moieties on the cell surface, and changes in absorption peak intensities were observed after treatment with 2 mM Cr6+. Our results are in good agreement with Lameiras et al. [46] and Pandi et al. [47].
Accumulation of Cr6+ within the cells of B. aerius S1 and B. iodinum S2 was determined through SEM/EDX analysis. Changes in cell morphology of both bacterial strains were observed after treatment with 2 mM Cr6+ (treated). SEM analysis of both B. aerius S1 and B. iodinum S2 showed changes in cell morphology after being challenged with 2 mM Cr6+ and EDX also confirmed Cr presence in the cell. Our results are in good agreement with Das et al. [48] and Khan et al. [23].
5. Conclusions
In the present study, two bacterial strains, Bacillus aerius S1 and Brevibacterium iodinum S2, showed maximum growth at 37 °C and pH 8. Both strains were able to resist Cr6+ upto 30 and 35 mM. Biosorption proficiency (q) of B. aerius S1 was 19, 27, 52 and 34 mM/g while for B. iodinum S2, it was 39, 50, 23 and 16 mM/g mM/g after 2, 4, 6 and 8 days of incubation. Cr6+ stress provoked significantly higher production of antioxidant enzymes (APOX, SOD, POX, GST, and CAT) in B. aerius as compared to the B. iodinum. Moreover, a significant GSH increase was determined in B. aerius S1 (364%) and B. iodinum S2 (663%) cultures under 2 mM Cr6+ stress as compared to the non-stressed cultures. Similarly, a rise in non-protein thiols was determined in B. aerius S1 (275%) and B. iodinum S2 (177%) under 2 mM Cr6+ stress. Pilot scale study demonstrated that both strains could reduce Cr6+ into Cr3+ within 6 days from the original tannery effluent with efficiency of 99%. Thus, both strains could be utilized to reclaim the chromium contaminated sites.
Conflict of interest
The authors have declared that no competing interests exist.
Acknowledgements
This is to acknowledge the support of University of the Punjab (Grant no. Env-59), Lahore, Pakistan to accomplish this research work.
References
- 1.Thatoi H., Das S., Mishra J., Bhagwat Prasad R., Das N. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: a review. J. Environ. Manage. 2014;146:383–399. doi: 10.1016/j.jenvman.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Ayangbenro A.S., Babalola O. A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int. J. Environ. Res. Public Health. 2017;14:94. doi: 10.3390/ijerph14010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batta N., Subudhi S., Lal B., Devi A. Isolation of lead tolerant novel species, Achromobacter sp. TL-3: assessment of bioflocculating activity. Indian J. Exp. Biol. 2013;51:1004–1011. [PubMed] [Google Scholar]
- 4.Hookoom M., Puchooa D. Isolation and identification of heavy metal tolerant bacteria from industrial and agricultural areas in Mauritius. Curr. Res. Microbiol. Biotechnol. 2013;1(3):119–123. [Google Scholar]
- 5.Barnhart J. Occurrence, uses and properties of chromium. Regul. Toxicol. Pharmacol. 1997;26:S3–S7. doi: 10.1006/rtph.1997.1132. [DOI] [PubMed] [Google Scholar]
- 6.Cheunga K.H., Gu J.D. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int. Biodeterior. Biodegrad. 2007;59:8–15. [Google Scholar]
- 7.Dhal B., Thatoi H.N., Das N.N., Pandeya B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: a review. J. Hazard. Mater. 2013;250–251:272–291. doi: 10.1016/j.jhazmat.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 8.Polti M.A., García R.O., Amoroso M.J., Abate C.M. Bioremediation of chromium (VI) contaminated soil by Streptomyces sp. MC1. J. Basic Microbiol. 2009;49:285–292. doi: 10.1002/jobm.200800239. [DOI] [PubMed] [Google Scholar]
- 9.Jeyasingh J., Philip L. Bioremediation of chromium contaminated soil: optimization of operating parameters under laboratory conditions. J. Hazard. Mater. 2005;118:113–120. doi: 10.1016/j.jhazmat.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Akcil A., Erust C., Ozdemiroglu S., Fonti V., Beolchini F. A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J. Clean. Prod. 2015;86:24–36. [Google Scholar]
- 11.Mangaiyarkarasi A., Geetharamani D. Bioabsorption of chromium employing microorganism isolated from tannery effluent. Scrutiny Int. Res. J. Biol. Environ. Sci. 2014;1(9):29–36. [Google Scholar]
- 12.Masneuf-Pomarède I., Jeuneb C.L., Durrensc P., Lollierb M., Aigled M., Dubourdieu D. Molecular typing of wine yeast strains Saccharomyces bayanus var. Uvarum using microsatellite markers. Syst. Appl. Microbiol. 2007;30:75–82. doi: 10.1016/j.syapm.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Rehman A., Ali A., Muneer B., Shakoori A.R. Resistance and biosorption of mercury by bacteria isolated from industrial effluents. Pakistan J. Zool. 2007;39(3):137–146. [Google Scholar]
- 14.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 15.Reuveni R., Shimoni M., Karchi Z., Kuc J. Peroxidase activity as a biochemical marker for resistance of muskmelon Cucumis melo to Pseudopernospora cubensis. Phytopathology. 1992;82:749–753. [Google Scholar]
- 16.Beers Jr.R.F., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 17.Israr M., Sahi S.V., Jain J. Cadmium accumulation and antioxidant responses in the Sesbania dummondii callus. Arch. Environ. Contam. Toxicol. 2006;50:121–127. doi: 10.1007/s00244-005-5029-x. [DOI] [PubMed] [Google Scholar]
- 18.Nakano Y., Asada K. Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987;28:131–140. [Google Scholar]
- 19.Ewing J.F., Janero D.R. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem. 1995;232:243–248. doi: 10.1006/abio.1995.0014. [DOI] [PubMed] [Google Scholar]
- 20.Khan Z., Hussain S.Z., Rehman A., Zulfiqar S., Shakoori A. Evaluation of cadmium resistant bacterium, Klebsiella pneumoniae, isolated from industrial wastewater for its potential use to bioremediate environmental cadmium. Pak. J. Zool. 2015;47:1533–1543. [Google Scholar]
- 21.Rehman A., Anjum M.S., Hasnain S. Cadmium biosorption by yeast, Candida tropicalis CBL-1, isolated from industrial wastewater. J. Gen. Appl. Microbiol. 2010;56:359–368. doi: 10.2323/jgam.56.359. [DOI] [PubMed] [Google Scholar]
- 22.Deokar A.R., Lin L.Y., Chang C.C., Ling Y.C. Single-walled carbon nanotube coated antibacterial paper: preparation and mechanistic study. J. Mater. Chem. B. 2013;1:2639–2646. doi: 10.1039/c3tb20188k. [DOI] [PubMed] [Google Scholar]
- 23.Khan Z., Rehman A., Hussain S.Z., Nisar M.A., Zulfiqar S., Shakoori A. Cadmium resistance and uptake by bacterium, Salmonella enterica 43C, isolated from industrial effluent. AMB Express. 2016;6(1):1–16. doi: 10.1186/s13568-016-0225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett N., Croke B.F.W., Jakeman A.J., Newham L.T.H., Norton J.P. Performance evaluation of environmental models. Swayne D.A., Yang W., Voinov A.A., Rizzoli A., Filatova T., editors. Proceedings of 2010 International Congress on Environmental Modelling and Software. 2010 [Google Scholar]
- 25.Mishra R.R., Dhal B., Dutta S.K., Dangar T., Das N.N., Thatoi H. Optimization and characterization of chromium (VI) reduction in saline condition by moderately halophilic Vigribacillus sp isolated from mangrove soil of Bhitarkanika, India. J. Hazard. Mater. 2012;227–228:219–226. doi: 10.1016/j.jhazmat.2012.05.063. [DOI] [PubMed] [Google Scholar]
- 26.Zahoor A., Rehman A. Isolation of Cr (VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J. Environ. Sci. 2009;21:814–820. doi: 10.1016/s1001-0742(08)62346-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X., Krumholz L.R., Yu Z., Chen Y., Liu P., Li X. A novel subspecies of Staphylococcus aureus from sediments of Lanzhou reach of the Yellow River aerobically reduces hexavalent chromium. J. Bioremed. Biodegrad. 2013;4:188. [Google Scholar]
- 28.Pattanapipitpaisal P., Brown N.L., Macaskie L.E. Chromate reduction and 16S rRNA identification of bacteria isolated from a Cr(VI)-contaminated site. Appl. Microbiol. Biotechnol. 2001;57:257–261. doi: 10.1007/s002530100758. [DOI] [PubMed] [Google Scholar]
- 29.Wani R., Kodam K.M., Gawai K.R., Dhakephalkar P.K. Chromate reduction by Burkholderia cepacia MCMB-821, isolated from the pristine habitat of alkaline Crater Lake. Appl. Microbiol. Biotechnol. 2007;75:627–632. doi: 10.1007/s00253-007-0862-7. [DOI] [PubMed] [Google Scholar]
- 30.Mala G., Sujatha D., Rose C. Inducible chromate reductase exhibiting extracellular activity in Bacillus methylotrophicus for chromium bioremediation. Microbiol. Res. 2014;170:235–241. doi: 10.1016/j.micres.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Srinath T., Verma T., Ramteke P.W., Garg S. Chromium (VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere. 2002;48(2002):427–435. doi: 10.1016/s0045-6535(02)00089-9. [DOI] [PubMed] [Google Scholar]
- 32.Cabrera G., Viera M., Gomez J.M., Cantero D., Donati E. Bacterial removal of chromium (VI) and (III) in a continuous system. Biodegradation. 2007;18(2007):505–513. doi: 10.1007/s10532-006-9083-5. [DOI] [PubMed] [Google Scholar]
- 33.Simine D.D., Finoli C., Vecchio A., Andreoni V. Metal ion accumulation by immobilised cells of Brevibacterium sp. J. Indus. Microbiol. Biotechnol. 1998;20:116–120. [Google Scholar]
- 34.Das A., Susmita M. Biodegradation of the metallic carcinogen hexavalent chromium Cr(VI) by an indigenously isolated bacterial strain. J. Carcinog. 2010;9:6. doi: 10.4103/1477-3163.63584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma T., Singh N. Isolation and process parameter optimization of Brevibacterium casei for simultaneous bioremediation of hexavalent chromium and pentachlorophenol. J. Basic Microbiol. 2013;53(3):277–290. doi: 10.1002/jobm.201100542. [DOI] [PubMed] [Google Scholar]
- 36.Ge S., Ge S., Zhou M., Dong X. Bioremediation of hexavalent chromate using permeabilized Brevibacterium sp. and Stenotrophomonas sp. cells. J. Environ. Manage. 2015;157:54–59. doi: 10.1016/j.jenvman.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Lalith Varadhan S., Mohan S. Selection and use of efficient bacterial strains for chromium biosorption in tannery effluent. Int. J. Recent Sci. Res. 2017;08:16230–16233. [Google Scholar]
- 38.Cervantes C., Campos-Garcia J. Reduction and efflux of chromate by bacteria. In: Nies D.H., Silver S., editors. Vol. 6. 2007. pp. 407–419. (Molecular Microbiology of Heavy Metals. Microbiology Monographs). [Google Scholar]
- 39.Bharagava R.N., Mishra S. Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol. Environ. Saf. 2017;147:102–109. doi: 10.1016/j.ecoenv.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 40.Hussein K.A., Joo J.H. Heavy metal resistance of bacteria and its impact on the production of antioxidant enzymes. Afr. J. Microbiol. Res. 2013;7(20):2288–2296. [Google Scholar]
- 41.Zhang J.X., Kirkham M.B. Drought stress induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol. 1994;35:785–791. [Google Scholar]
- 42.Chaoui A., Mazhoudi S., Ghorbal M.H., El-Ferjani E. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in Bean (Phaseolus vulgaris L.) Plant Sci. 1997;127:139–147. [Google Scholar]
- 43.Suthar B., Pansuriya J., Kher M., Patel V., Nataraj M. Biochemical changes under chromium stress on germinating seedlings of Vigna radiata. Not. Sci. Biol. 2014;6:77–81. [Google Scholar]
- 44.Lee M.Y., Shin H.W. Cadmium-induced changes in antioxidant enzymes from the marine alga Nannochloropsis oculata. J. Appl. Phycol. 2003;15:13–19. [Google Scholar]
- 45.Lenártová V., Holovská K.N., Javorský P. The influence of mercury on the antioxidant enzyme activity of rumen bacteria Streptococcus bovis and Selenomonas ruminantium. FEMS Microbiol. Ecol. 1998;27(4):319–325. [Google Scholar]
- 46.Lameiras S., Quintelas C., Tavares M.T. Biosorption of Cr (VI) using a bacterial biofilm supported on granular activated carbon and on zeolite. Bioresour. Technol. 2008;99:801–806. doi: 10.1016/j.biortech.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 47.Pandi M., Shashirekha V., Swamy M. Bioabsorption of chromium from retan chrome liquor by cyanobacteria. Microbiol. Res. 2009;164:420–428. doi: 10.1016/j.micres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Das S., Mishra J., Das S., Pandey S., Rao D.S., Chakraborty A., Sudarshan M., Das N., Thatoi H. Investigation on mechanism of Cr(VI) reduction and removal by Bacillus amyloliquefaciens, a novel chromate tolerant bacterium isolated from chromite mine soil. Chemosphere. 2014;96:112–121. doi: 10.1016/j.chemosphere.2013.08.080. [DOI] [PubMed] [Google Scholar]