Abstract
Previously we demonstrated that muscadine grape skin extract (MSKE), a natural product, significantly inhibited androgen-responsive prostate cancer cell growth by inducing apoptosis through the targeting of survival pathways. However, the therapeutic effect of MSKE on more aggressive androgen-independent prostate cancer remains unknown. This study examined the effects of MSKE treatment in metastatic prostate cancer using complementary PC-3 cells and xenograft model. MSKE significantly inhibited PC-3 human prostate cancer cell tumor growth in vitro and in vivo. The growth-inhibitory effect of MSKE appeared to be through the induction of cell-cycle arrest. This induction was accompanied by a reduction in the protein expression of Hsp40 and cell-cycle regulation proteins, cyclin D1 and NF-kBp65. In addition, MSKE induced p21 expression independent of wild-type p53 induced protein expression. Moreover, we demonstrate that MSKE significantly inhibited cell migration in PC-3 prostate cancer cells. Overall, these results demonstrate that MSKE inhibits prostate tumor growth and migration, and induces cell-cycle arrest by targeting Hsp40 and proteins involved in cell-cycle regulation and proliferation. This suggests that MSKE may also be explored either as a neo-adjuvant or therapeutic for castration resistant prostate cancer.
Keywords: Cancer research, Cell biology, Molecular biology
1. Introduction
Epidemiological studies have demonstrated a link between prostate cancer incidence and diet [1, 2, 3]. Clinical trials with patients consuming dietary phenolic phytochemicals have demonstrated that these agents have potential chemopreventative properties with low toxicity [4]. In a previous study, patients treated with green tea catechins showed a 90% reduction in high-grade prostate intra-epithelial neoplasia incidence [5]. Dietary agents, such as pomegranate and green tea, are rich in polyphenols and have been shown to be effective against cancer [6]. Polyphenols are known to have anti-proliferative and antioxidant effects on prostate cancer cells [3, 7].
MSKE contains components that are like those found in green tea and pomegranate, including polyphenol constituents such as gallic acid, ellagic acid glucosides, catechin, quercetin, kaempferol, and several anthocyanins [8]. We have demonstrated that MSKE, like other plant polyphenols, also has antiproliferative and anti-apoptotic properties. For example, our previous study showed that MSKE inhibited growth, induced apoptosis, and decreased the expression of AKT through AKT degradation in a series of transformed prostate epithelial cell lines [9]. AKT is a client protein of heat shock proteins (Hsp) involved in signal transduction of prostate cancer cell growth. Degradation of client proteins, cell-cycle arrest, and induction of apoptosis are characteristic of Hsp40 inhibition [10]. The effectiveness of MSKE, therefore, can potentially be due to inhibition of Hsp40, leading to degradation and/or inactivity of oncogenic client proteins.
Heat shock proteins are potential targets for prevention and treatment of prostate cancer since they have been linked to poor clinical outcomes in prostate cancer and mediate important biological activities [10]. Heat shock proteins which are classified as small and large proteins are grouped by their molecular weight [11]. Hsp90, Hsp70, and Hsp40 assemble into complexes with co-chaperones and other proteins to mediate the folding of client proteins into their stable tertiary structure which can lead to degradation of client proteins [12]. Hsp40 and Hsp70 form a complex in which Hsp40 functions as a co-chaperone. In this complex, Hsp40 regulates Hsp70 by inducing its ATPase activity, resulting in stabilization of the Hsp70-peptide complex [13]. Together, Hsp40 and Hsp70 are involved in protein folding and prevent formation of cellular aggregates [14]. A subclass of Hsp40 can function independently of Hsp70 to prevent protein aggregation [11]. Studies have shown that upregulation of the different subclass of Hsp40 has been associated with high-grade intraepithelial neoplasia and maintaining stability of client proteins [15, 16].
In this study, we show that MSKE significantly inhibits the growth and migration of metastatic prostate cancer cells, primarily through induction of cell-cycle arrest by targeting Hsp40. These data, therefore, suggest that MSKE has therapeutic potential to inhibit the growth of advanced forms of prostate cancer, and that MSKE warrants further investigation as a treatment for castrate resistant prostate cancer.
2. Materials and methods
2.1. Chemicals and preparation
Ethanol and Propidium iodide (PI) were purchased from Sigma-Aldrich Chemical Co. (Milwaukee, WI). Preparation of MSKE from the Ison cultivar has been previously described [9].
2.2. Reagents
Antibodies used in this study were as follows: Hsp27, Hsp40, Hsp70, Hsp90, NFϰ-B/p65, and Cyclin D1 (Cell Signaling), and VEGF (Santa Cruz), and actin (Chemicon). Plasmids used in this study were HSP40 CRISP/Cas9 KO (Santa Cruz), and Control CRISP/Cas9 (Santa Cruz). Resveratrol was purchased from the Sigma Chemical Company (St. Louis, MO).
2.3. Culture of human prostate epithelial cell lines
PC-3 human prostate cancer cells were obtained as a generous gift from Dr. Kwabi-Addo who purchased the cells from American Type Culture Collection (Manassas, VA). In addition, human LNCaP prostate cancer cells were obtained from the American Type Culture Collection (Manassas, VA). The E006AA, African American human prostate cancer cells were obtained from American Type Culture Collection (Manassas, VA). All three cell lines were maintained using advanced RPMI-1640 supplemented with 10% Fetal Bovine Serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a 5% CO2 atmosphere. For transfection experiments, PC-3 cells were cultured without antibiotics and in Opti-MEM (1X) Reduced Serum Medium (Life Technologies, Carlsbad, CA).
2.4. Proliferation assay
PC-3 and E006AA prostate cancer cells were plated at a density of 1 × 104 cells of complete culture medium in 8 wells of 96-well plates and incubated for 24 hours in two independent experiments. The PC-3 cells were initially synchronized by reducing serum levels and after 24 hours cells were than treated with increasing concentrations of MSKE (0, 2, 5, 10, 20, and 40 μg/ml) in complete medium. Stock solutions of MSKE were prepared in 50% ETOH. Equal volumes of ETOH (final concentrations <0.01%) were added to the control cells. Cell viability was measured using the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] cell proliferation assay kit (Promega, Madison, WI). Sample absorption (indicative of formazan formation) was determined using an ELISA plate reader (OPTImax microplate reader, MTX Labsystems, Vienna, VA) at 490 nm.
2.5. Clonogenic assays
1 × 103 PC-3 cells were plated in RPMI media within 60 mm Petri dishes. Once cells reached 50–60% confluency, they were treated with MSKE at 2.5, 5.0, 10, 20, 40 μg/ml and incubated for 72 hours at 37 °C in a 5% CO2 atmosphere. Cells (1 × 103) were re-plated in triplicate in new 60 mm Petri dishes containing fresh media. After 12 days, colonies were stained with crystal violet (Sigma) and counted. A two-sided t-test was used to compare differences between treatment groups and control.
2.6. Cell-cycle and apoptosis analysis
5 × 105 PC-3 cells were plated in duplicate in a 6-well plate and exposed to MSKE (20 μg/ml and 40 μg/ml) and resveratrol (25 μM) treatment for 12 and 24 hours. After 12 and 24 hours incubation at 37 °C in a 5% CO2 atmosphere, PC-3 cells were centrifuged at 1000 rpm for 5 minutes and the pellet was re-suspended in 200 μl phosphate buffered saline (PBS). The cells were fixed by adding 400 μl of ethanol and incubated on ice for 15 minutes. The cells were then centrifuged at 1500 rpm for 5 minutes and the pellet was re-suspended in 200 μl propidium iodide (PI) solution containing 50 μg/ml PI (Biotium), 0.1 mg/ml RNase A (Sigma-Aldrich), and 0.05% Triton X-100 (Sigma-Aldrich). The PC-3 cells were incubated for 40 minutes at 37 °C before performing imaging cytometric analysis.
2.7. RNA extraction and qRT-PCR
PC-3 and LNCaP cells were grown and extracted at 50–70% confluency, and treated with MSKE for 24 hours. Cells were lysed using Trizol (Invitrogen) and total RNA was extracted. RNA concentrations were determined by NanoDrop (Thermo Scientific). 1 μg of RNA was used for cDNA synthesis, using the iScript cDNA synthesis kit (Bio-Rad). One-tenth of the first strand cDNA reaction was used for RT-PCR amplification. RT-PCR was performed in an iCYCLER real-time PCR machine (Bio-Rad) using SYBR-Green chemistry (Bio-Rad). Test gene Ct values were normalized to Ct values of the housekeeping gene HPRT, and fold differences, as compared to untreated controls, were calculated.
2.8. Protein isolation from prostate cells and xenograft tissue and western blotting analysis
1 × 106 PC-3 and E006AA prostate cancer cells were cultured for 24 hours, washed with cold PBS, and then lysed with SoluLyse-M (Genlantis, San Diego, CA) cell lysis Tris sucrose buffer. In addition to the cells, total protein was isolated from frozen prostate tumors and homogenized using the Millipore extraction kit (Millipore Corporation, Bilerica, MA). Proteins (30 and 50 μg) were separated using 10% or 16% pre-cast Tris-Glycine gels and dry-transferred for seven minutes using iBlot machine (Invitrogen, Gaithersburg, MD) onto PVDF membranes (Invitrogen, Gaithersburg, MD). The membrane was blocked using WesternBreeze Chemiluminescent Immunodetection Kit (Invitrogen) and probed with anti-Hsp27, Hsp40, Hsp60, Hsp70, Hsp90, NF-κB p65, VEGF, p53 (Ser15), p21 and cyclin D1 (1:500 diluted in manufacturer primary antibody diluent buffer) overnight at 4 °C. After washing with Invitrogen buffer wash (Invitrogen), the blots were treated with either Invitrogen Alk-Phos conjugated (anti-Mouse) or (anti-rabbit) for 30 minutes and washed several times. Proteins were detected by the enhanced chemiluminescence system (Invitrogen). Western analysis was performed on total cell lysate.
2.9. Confocal microscopy
The LNCaP cells were cultured on 22 × 40-1 mm glass cover slips and PC-3 cells on 8 chamber tissue culture cover slips (BD Falcon, Bedford MA) and treated with 2.5, 5, 10, 20, 40 μg/ml or ETOH at 70% confluency. After 24 and 48 hours, cells were fixed with 0.125% Triton X-100 in 10% NBF at 37 °C for 20 minutes. Fixed cells were washed two times with PBS and blocked overnight with 1% BSA (Sigma) made in PBS. Cells were then incubated overnight with p53 antibody diluted 1:100, and Hsp40 antibody 1:500 in the blocking buffer at 4 °C. Cells were washed 3X for 5 minutes each with 1% BSA and then incubated with anti-mouse Alexa Fluor-555 (Invitrogen) diluted 1:200 for 1 hour. After washing cells were mounted with Vectashield (Vector Laboratories). Confocal Z-stacks were acquired with a 63 x oil-immersion lens (Numerical Aperture 1.2) on a Zeiss LSM510 microscope using identical excitation, emission, and image acquisition settings for all samples.
2.10. Wound healing assay
PC-3 cells were seeded on 60 mm culture dishes at 90% confluence in growth medium overnight. Several wounds were created using 10-ul micro-pipette tip in 100% confluent cells. The wounded monolayer was then washed twice with PBS to remove cell debris. After culturing to various time points (12, 24, 48, and 72 hours) we supplemented with serum free medium with or without 20 or 40 μg/ml of MSKE. After each time point, migrated cells were analyzed by microscopy.
2.11. Invasion assay
Invasiveness into the basement membrane was assayed using the CytoSelect™ 24-Well Cell Invasion Assay kit (Basement Membrane, Colorimetric Format, Cell Biolabs, INC., San Diego, CA) following the manufacturer's instructions. Suspensions of 300 μl of 5 × 104 cells were incubated with ETOH and MSKE (20 and 40 μg/ml) were added to the upper compartment of transwell cell culture chamber. A total of 10% FBS in 500 μl of media was used as a chemo-attractant in the lower chamber. After 48 hours of incubation at 37° in 5% CO2 atmosphere, cells remaining on the lower surface of the membrane were carefully removed and transferred to a clean well containing 400 μl of the Cell stain solution. They were incubated at 25 °C for 10 minutes, fixed and counted according to the manufacturer's instructions and photographed using the Opelco Olympus 1 × 71® inverted microscope (Optical Elements Cooperation) and DP70 BSW software for Olympus.
2.12. In vivo xenograft bioassay
Male athymic nude mice (BALB/c nu/nu, 20–22 g), 5–6 weeks old, were obtained from Charles River (Frederick, MD). Animals were housed in filter-top cages at Howard University Veterinary Animal Facility and consumed powdered AIN-93M diet (New Brunswick, NJ) prepared weekly and fresh tap water ad libitum. All experimental protocols were in accordance with the National Institutes of Health guidelines and were approved by the Animal Care and Use Committee of Howard University. Food consumption and body weights were recorded bi-weekly. After an acclimation period of 2 weeks, during which mice were fed control AIN-93M diet, the mice were randomly grouped into 5 experimental groups, with 10 animals in the control group and 10 animals in each of the four MSKE treatment groups (50 mg/kg, 100 mg/kg, 200 mg/kg, 400 mg/kg) were administered by gavage twice a week. However, there was a total of seven animals in the highest treatment group (600 mg/kg) because three animals died prior to treatment. Two weeks later, PC-3 human prostate cancer cell xenografts were established in the mice by subcutaneous injection in the flank with PC-3 cells (1 × 106 cells) in 50 μl of phosphate-buffered saline (PBS) plus 50 μl basement membrane extract (BME) (Trevigen, Gaithersburg, MD).
2.13. Transient transfection assay
Transfection of plasmid DNA into PC-3 cells was carried out using UltraCruz transfection reagent according to the manufacture's protocol (Santa Cruz, Dallas, Texas) in Opti-MEM reduced serum media. PC-3 cells were transfected with 1 μg of HSP40 CRISP/Cas9 knockout (KO) (sc-418922, Santa Cruz), and Control CRISP/Cas9 (sc-418922, Santa Cruz) DNA Plasmids. To assess the effect of HSP40CRISP/Cas KO on cell proliferation we plated 5 × 105 PC-3 cells into 6 well plates and transfected the cells with vector control, HSP40 KO plasmid, and treated with ethanol, and 20 μg/ml MSKE compared with untreated control for 24 and 48 hr. Trypan blue exclusion assay used for cell proliferation, western analysis described above was used for detection of proteins, and CytoSelect™ 24-Well Cell Invasion Assay kit used for migration assay.
2.14. Apoptosis phosphorylated signal transduction protein array
The human apoptosis protein array kit was used according to manufacturer's protocol (R&D systems). PC-3 metastatic prostate cells were plated at a density of 6 × 105 cells in a 10-cm dish in complete RPMI medium and then treated with ethanol (control), 5, 10, 20 μg/ml MSKE for 24 h. The cells were rinsed with cold PBS and lysed according to manufacturer's protocol. The lysate (250 ug) was added to the nitrocellulose membrane array, which was incubated with the detection antibody cocktail. After washing, secondary antibody (streptavidin-horseradish peroxidase, 1:2000) was added. Protein signals were detected by the enhanced chemiluminescence system. Western analysis was done on total cell lysates. Duplicate signals on the array were quantitated using Alpha Fluorochem imaging software (IMGEN Technologies).
2.15. Immunohistochemical staining
In brief, slides were incubated 1 hour in a 60° oven and rehydrated using a gradient of xylene and ethanol. Antigen Retrieval was performed with 10 mM Citrate Buffer. The slides were blocked for 20 minutes, then incubated overnight at 4 °C with primary antibody. Detection was performed as previously described [17, 18, 19]. For statistical analyses, an average of three fields of view per sample was used for counting and the average percent positive cells were counted and graphed. Statistical analyses were performed using the Student's t-test with p ≤ 0.05 establishing significance. The following antibody was used: Hsp40 (Cell Signaling cat. 4871).
2.16. Statistical analysis
All statistical procedures were carried out using GraphPad Prism Inc. version 5.0 software package (GraphPad Software, San Diego, CA). Statistical analysis evaluating apoptosis, cell viability, tumor burden, food consumption, and cell migration used ANOVA followed by student-Newman-Keuls post hoc test.
3. Results
3.1. MSKE inhibits PC-3 prostate cancer cell growth in vitro
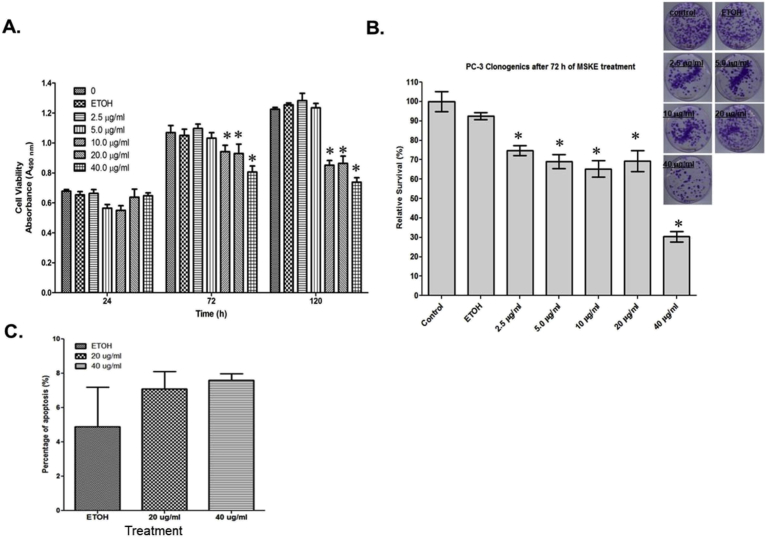
As depicted in Fig. 1A and B, MSKE significantly inhibited PC-3 metastatic prostate cell growth by 50% after 72 hours and was sustained over time. The significant inhibition was not dose dependent. This was confirmed in our Clonogenic assay, as shown in Fig. 1B. We also demonstrated significant growth inhibition in other prostate cancer cells.
Fig. 1.
MSKE inhibits cell growth in PC-3 prostate cancer cells. (A) Cell cultures were treated with various concentrations of MSKE (2.5, 5, 10, 20, 40 μg/ml) during different time intervals (24, 72, 120 hours). The effect of MSKE on growth was determined by MTT proliferation assay in PC-3 cells. Sample absorption (indicative of formazan formation) was determined using an absorbance plate reader (OPTImax microplate reader, MTX Labsystems, Vienna, VA) at 490 nm. Results are expressed as mean absorbance plus or minus standard error of the mean (mean ± S.E. *). (B) Clonogenic assay confirms the inhibition of growth of the PC-3 prostate after treatment with various μg/ml concentrations of MSKE after 72 hours. (C) MSKE (20 and 40 μg/ml) did not significantly induce apoptosis in PC-3 cells after 24 hours using Annexin V-FITC. Columns mean absorbance; bars, ±SE. *, is the significant difference between MSKE treatment and ETOH and untreated control.
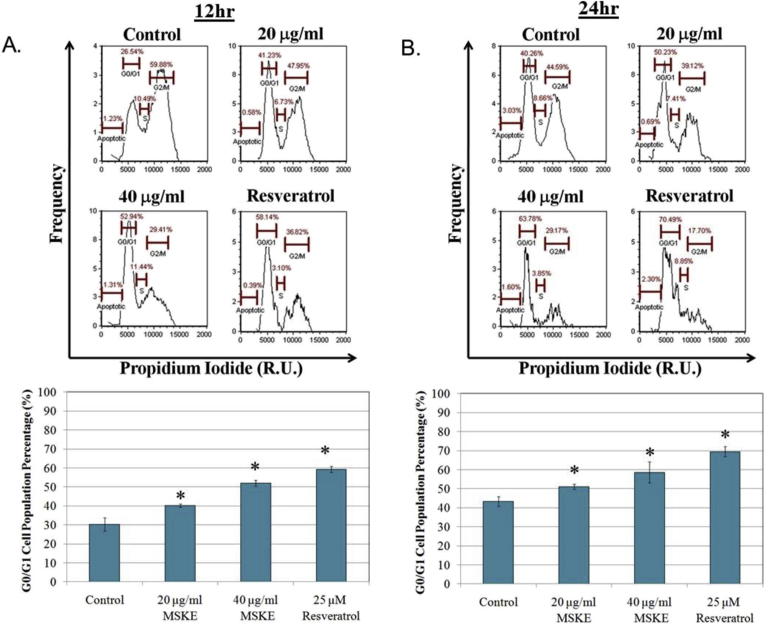
3.2. MSKE induces cell-cycle arrest and not apoptosis in PC-3 prostate cancer cells
The cell cycle analysis demonstrated that MSKE significantly (p ≤ 0.05) arrested cells during the G1 to S phase transition at 20 and 40 μg/ml MSKE compared to control after 12 (Fig. 2A) and 24 hours (Fig. 2B). Resveratrol, which was used as a control, has previously been shown to arrest prostate cancer cells, and confirmed the inhibitory effect of MSKE [9]. However, the PI analysis demonstrated that MSKE did not significantly induce apoptosis. This was confirmed by our Annexin-V FITC analysis (Fig. 1C).
Fig. 2.
MSKE arrested G0/G1 phase transition in PC-3 cells. (A) Describes the relative percentage of cells in the G0/G1 phase of cell cycle after 12 hours of MSKE treatment, (B) after 24 hours of MSKE treatment; Resveratrol treatment (25 μM) used as a control for G0/G1 arrest. The cells were fixed by adding 400 μl of ethanol and incubated on ice for 15 minutes. The cells were then centrifuged at 1500 rpm for 5 minutes and the pellet was re-suspended in 200 μl propidium iodide (PI) solution containing 50 μg/ml PI (Biotium), 0.1 mg/ml RNase A (Sigma-Aldrich), and 0.05% Triton X-100 (Sigma-Aldrich). The cellometer allows simultaneous acquisition of bright-field and fluorescence images for concentration, size, and viability measurement to measure cell cycle transition. The fluorescence intensity and size measurement were recorded for each cell contained within a data set, which was exported from the software into an Excel file, and imported into FlowJo (Tree Star, Oregon) for cytometric data analysis. Columns mean percentage of populated cells in G0/G1 phase of the cell cycle; bars, ±SE. * for three independent experiments, is the significant difference between treated and control.
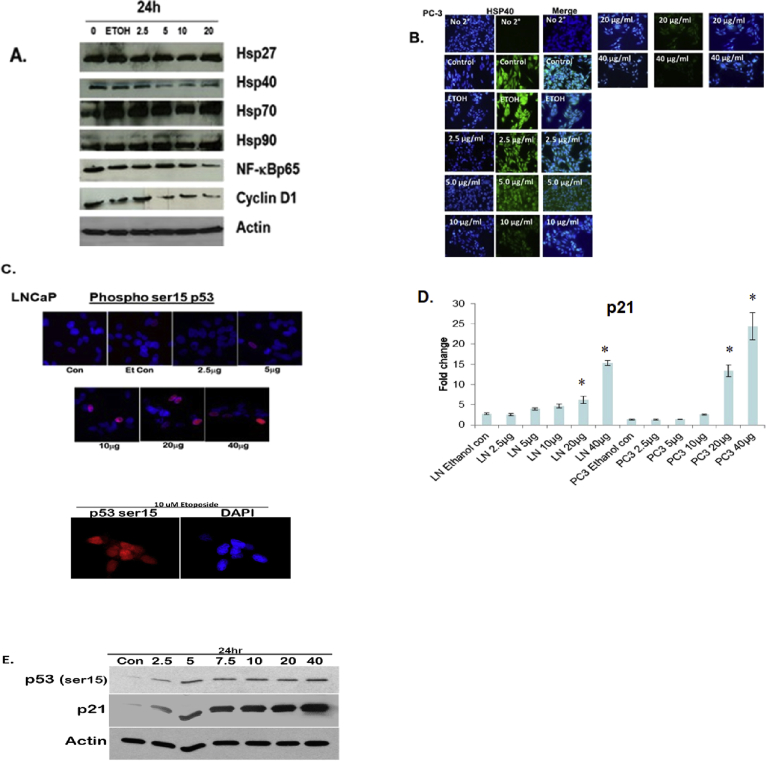
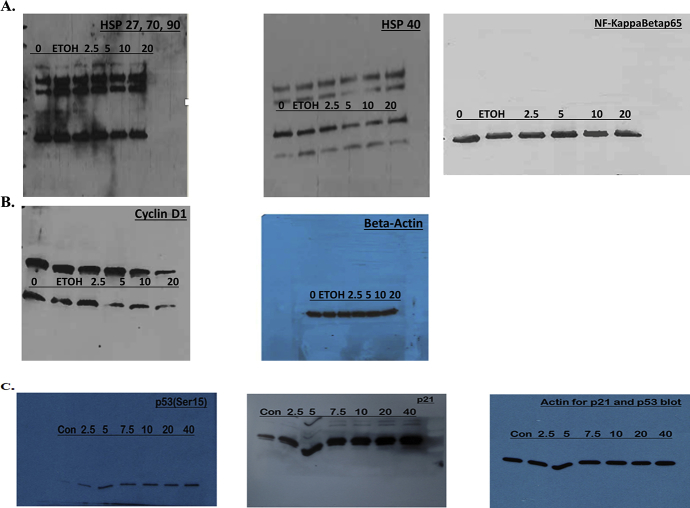
3.3. MSKE inhibits HSP40, NF-ϰB, cyclin D1 protein expression and induces wild-type p53 protein and p21 mRNA
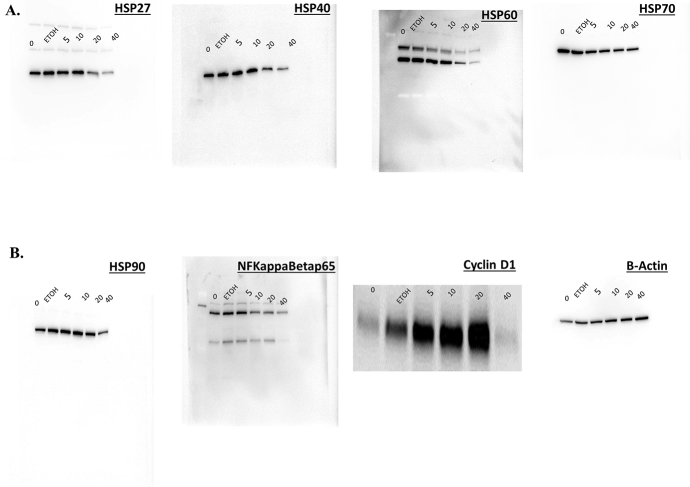
We demonstrated in Fig. 3A and S1A and B Figure that MSKE decreased the expression of Hsp40, NF-ϰB, and Cyclin D1 which play a key role in cell survival [10, 14]. However, the expression of other Hsps were not affected after 24 hours when compared to control. We also showed that MSKE reduced Hsp40 and other Hsp expression in other prostate cancer cells (Fig. 7B and S4A and S4B Figure). Confocal imaging confirmed that MSKE inhibited protein expression of Hsp40 (Fig. 3B). In addition, we showed that MSKE induced p53 (Fig. 3C, and S1C Figure), a marker involved in cell growth and mediation of p21 [20]. However, in our model p21 induction by MSKE in the LNCaP cells was independent of the increase in PC-3 cells (possess mutated p53) (Fig. 3D) The p21 increased was confirmed in our protein phosphorylated array (Fig. 7C) and western blot analysis (Fig. 3E and S1C Figure). Overall, our results confirm the inhibitory effect of MSKE in PC-3 cells through pronounced cell-cycle arrest in G1-S phase transition.
Fig. 3.
MSKE treatment decreases the expression of antibodies, Hsp40, NFKB/p65, and cyclin D1 (diluted 1:500) while increasing the mRNA expression of p21 independent of induced p53 in vitro. (A) Effects of MSKE treatment on protein expression of cell cycle markers by western blot in PC-3 cells. Proteins (50 μg) were separated using 10% or 16% pre-cast Tris-Glycine gels and dry-transferred for seven minutes using iBlot machine (Invitrogen, Gaithersburg, MD) onto PVDF membranes (Invitrogen, Gaithersburg, MD). (B) Confocal microscopy of PC-3 cells fixed and stained with Hsp40 antibody and Alexa Fluor-555 to detect protein expression, (C) Confocal microscopy of LNCaP cells fixed and stained with p53 antibody and Alexa Fluor-488 to detect protein expression of wild-type p53, (D) Fold-change of p21 mRNA in LNCaP and PC-3 cells determined by qRT-PCR analysis. (E) Effects of MSKE treatment on protein expression of p53ser15 and p21 in LNCaP cells. Columns represent mean of fold-change; bars, SE. *, is the significant difference between treatment and control.
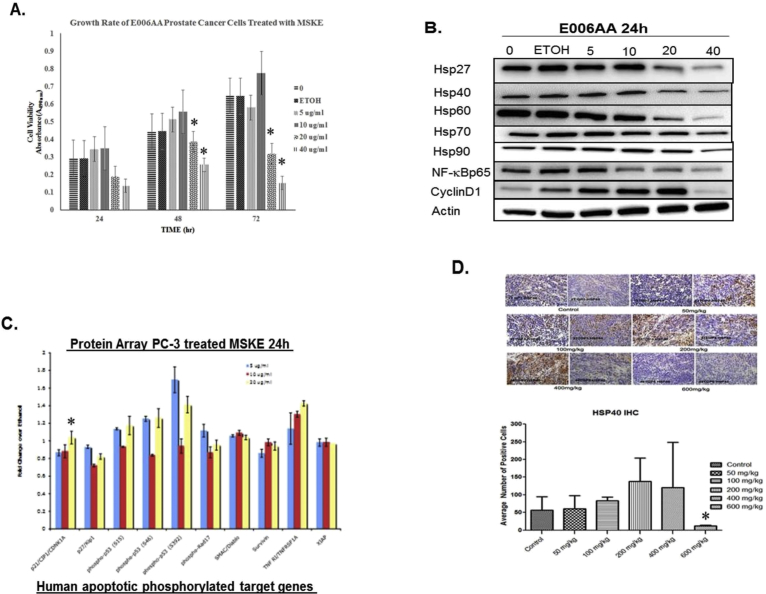
Fig. 7.
(A) MSKE inhibits cell growth in E006AA prostate cancer cells. Cell cultures were treated with various concentrations of MSKE (2.5, 5, 10, 20, 40 μg/ml) during different time intervals (24, 48, and 72 hours). At the end of each experiment MTT assays were performed as described in the methods section to estimate cell numbers. (B) MSKE treatment decreases protein expression of Hsp27, Hsp40, Hsp60, hsp70, hsp90, NF-κB, and cyclin D1 in E006AA after 24 hours. (C) Protein array used to examine the effect of MSKE (10, 20 μg/ml) on proteins involved in cell cycle and apoptosis compared to an ethanol control after 24 hours. Results presented as columns represent mean fold-change over ethanol, bars ± SE, *. (D) IHC analysis of Hsp40 was performed in paraffin-embedded tumor samples from control and MSKE-treated mice. Representation of Hsp40 from three IHC samples from tumor specimen. For statistical analyses, an average of three fields of view per sample was used for counting and the average percent positive cells were counted and graphed. Statistical analyses were performed using the Students' t-test with p ≤ 0.05 establishing significance. Values are means ± SE. *, is the significant difference between MSKE treatment and control.
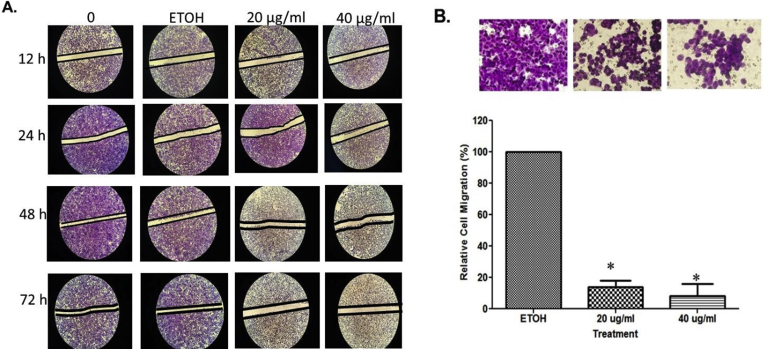
3.4. MSKE inhibits prostate cancer cell migration and invasion
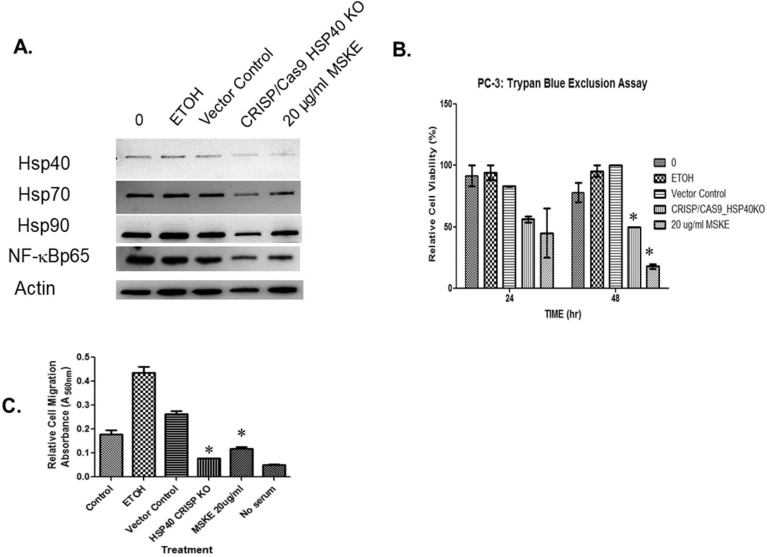
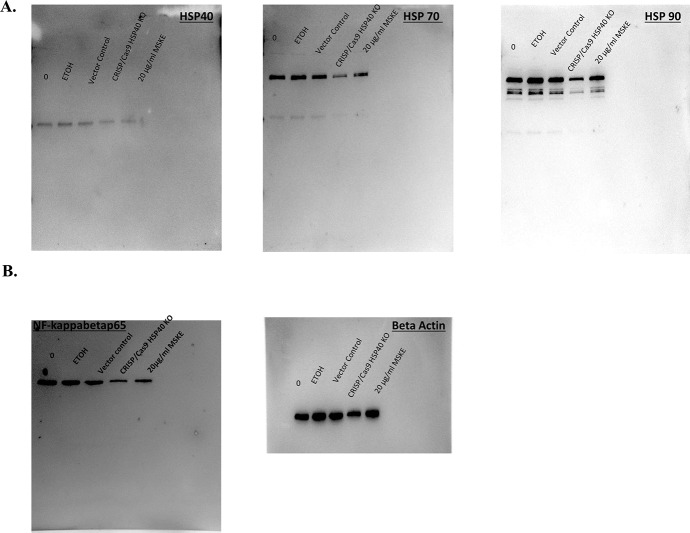
Cancer cells usually exhibit enhanced cellular motility to facilitate metastasis [21]. We demonstrated that under control conditions, and with maintenance of the monolayer wound area for 24, 48, and 72 hours a concentration-dependent recovery of the area occurred after 72 hours (Fig. 4A). However, when the wounded cell area was treated with MSKE at 20 and 40 μg/ml for 24, 48, and 72 hours there was a significant decrease in prostate cancer cell recovery. The migration assay as shown in Fig. 4B further confirmed that MSKE at 20 and 40 μg/ml inhibited migration activity of PC-3 cells after 48 hours. Treatment with 20 and 40 μg/ml MSKE prevented respectively ∼82% and 91% of cells from migrating. Importantly, we showed that knocking out Hsp40 (Fig. 5A and S2A Figure) using CRISP/Cas9 system influenced other cell cycle proteins, specifically, NF-κBp65 (S2B Figure) and decreased metastatic prostate cancer cell growth (Fig. 5B) and migration (Fig. 5C).
Fig. 4.
MSKE decreased migration and invasiveness of PC-3 cells. (A) Representative photographs of the wounded PC-3 cell monolayer. Typical result from two independent experiments conducted in triplicates (Magnification power [di1] 200X, Opelco Olympus 1 × 71® inverted microscope (Optical Elements Cooperation) and DP70 BSW Olympus software Opelco Olympus 1 × 71® inverted microscope (Optical Elements Cooperation) and DP70 BSW Olympus software), (B) Quantitative assessment of relative cell migration inhibition rate of PC-3 prostate cancer cells. Error bar indicates the standard error of the mean of two independent experiments. Bars, SE*.
Fig. 5.
Hsp40 knockout attenuates growth and migration. (A) The transfected PC-3 cells of Hsp40 knockout CRISP/Cas9 plasmid were prepared for evaluating protein expression of Hsp40, Hsp70, Hsp90, and NF-κBp65 in a western analysis. (B)The transfected cells were than trypsanized and the percentage of live and dead cells were counted using trypan blue exclusion assay for cell viability. (C) Moreover, transfected cells were prepared for examining migration using Basement Membrane, Colorimetric Format (Cell Biolabs, INC., San Diego, CA) following the manufacturer's instructions. Columns mean relative percentage of cells that migrate; bars, ±SE. *, is the significant difference between treatment and control.
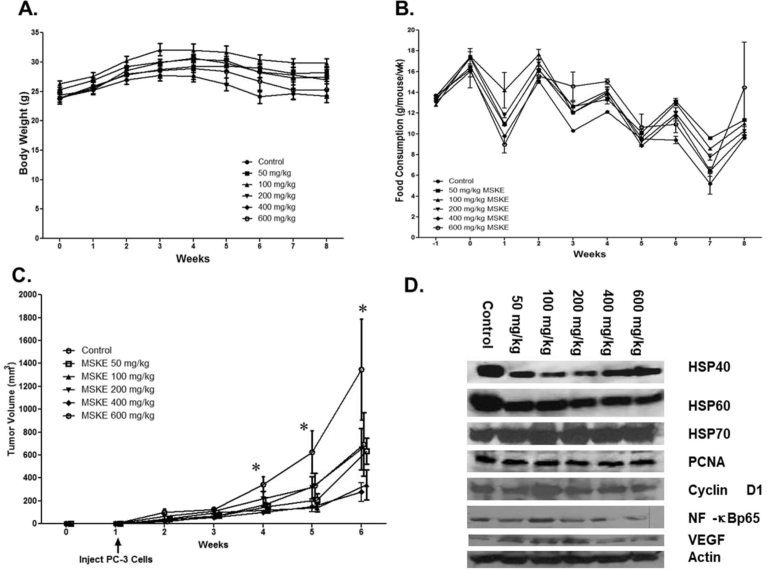
3.5. MSKE treatment inhibits tumor burden
Because of the inhibitory effects demonstrated in vitro, we conducted preclinical studies to evaluate the growth inhibitory effects of MSKE in a PC-3 xenograft mouse model. The results showed that dietary administration of MSKE at the various concentrations did not significantly affect the body weights (Fig. 6A) or food consumption (Fig. 6B) of the treated mice compared to the control. There was significant difference (p < 0.05) between the tumor burden of the treated groups and the control at weeks 5 and 6 after injection of PC-3 tumor cells (Fig. 6C) at 5, 10, 100, 200, and 400 mg/kg MSKE consumption, lower tumor volume was observed (p < 0.5) compared to control mice. The reduction in the tumor burden between the 600 mg/kg group and the control group was marginally significant, which was probably due to small numbers in this treatment group because of death prior to treatment and the large variation in tumor size observed in the control group. However, the decrease in tumor burden was not dose-dependent.
Fig. 6.
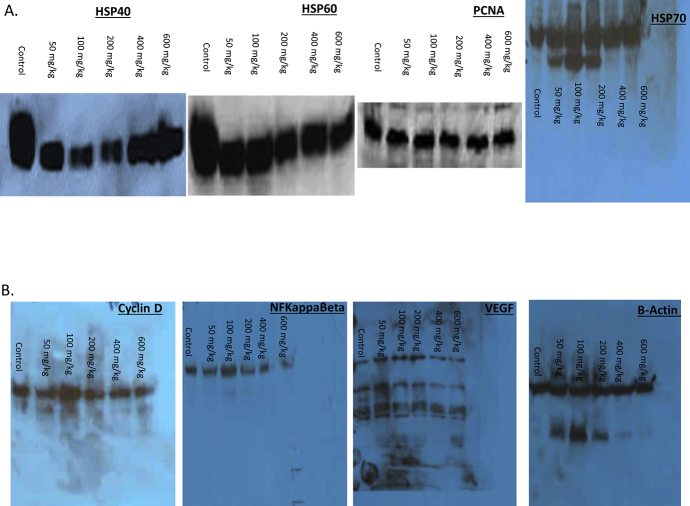
Dietary administration of MSKE decreased growth of PC-3 prostate cancer cell in vivo. (A) PC-3 cells were injected two week after the acclimation period. Animals remained on their respective diets for seven weeks after cell injection, until tumors reached 2–3 cm3 in volume, and were then sacrificed. Athymic nude mice were administered by gavage either control or MSKE diet (50 mg/kg/week) after injection of PC-3 human prostate cancer cells at week 1. This produced palpable tumors within 5 days. Cancer preventive efficacy of the treatments was assessed once a week by measuring tumor volume (cm3) = 0.523 × [length × width2 (cm2)]. Mice were killed after seven additional weeks on their respective diets. (B) Mean weekly food consumption of control and MSKE treated mice was measured weekly. (C) Tumor volume of PC-3 xenografts after dietary administration was measured weekly. Values are means ± SE. *, is the significant difference between MSKE treatment and control at p < 0.05. (D) MSKE decreased protein expression of Hsp40, Hsp60, and NF-κB under in vivo conditions. Equal loading of protein was confirmed by probing blots for beta actin.
3.6. MSKE inhibits HSP40, HSP60, and NF-ϰB protein expression in vivo
The expression of proliferation markers in xenograft tumors was analyzed by IHC and western analysis. As shown in Fig. 6D and S3A Figure, western blot analysis from the tumors showed a decreased in protein expression of Hsp40 marker in 50, 100, 200, 400, and 600 mg/kg MSKE treatment groups compared to the control group. This reduction in Hsp40 protein expression was confirmed at the highest dose level (600 mg/kg) of MSKE treatment in vivo (Fig. 7D). There was also a decrease in the expression of NF-ϰBp65 expression in 600 mg/kg groups (Fig. 6D and S3B Figure). This decrease in protein expression was consistent with that observed in vitro. However, we did not see a decrease in Hsp70, PCNA, and VEGF (Fig. 6D and S3A and S3B Figure).
4. Discussion
This study examined whether MSKE is an effective inhibitor of metastatic prostate cancer, in vitro and in vivo. This study demonstrated that MSKE significantly inhibited cell growth in a non-dose-dependent manner in PC-3 metastatic prostate cancer cells in vitro. This was accompanied by induction of cell cycle arrest. Other studies that have demonstrated significant growth-inhibitory effects by naturally-occurring compounds on advanced prostate cancer cell lines. For example, Gupta et al. (2000) showed that EGCG induced G1 phase arrest in human prostate carcinoma cells [22]. Comparable results were also observed after treatment with resveratrol in PC-3 prostate cancer cells and quercetin in human osteosarcoma cells [23, 24].
The reduction in cell growth and induction of cell cycle arrest by MSKE was accompanied by a decreased in Hsp40, and cyclin D1 protein expression. The decrease expression of Hsp40 and cyclin D1 was confirmed in our in vivo studies. In addition, we demonstrated that MSKE induces expression of cyclin-dependent kinase inhibitor p21 which is known regulator of cyclin D1 [25, 26], a regulator of cells transitioning through G1 and S phase of the cell cycle [27]. The induction of p21 in LNCaP cells was independent of p53 considering we showed an induction of p21 in PC-3 cells which possess a mutated p53. However, it has been shown that Hsp40 has a special relationship with p53 in protecting the cells, but known to lose its protection in the presence of a mutated p53 [28, 29, 30, 31, 32, 33, 34, 35, 36, 37]. Importantly, Akalin et al. (2001) demonstrated that Hsp40 plays a role in prostate cancer progression [38].
These data, along with the current study, provide evidence of a connection between Hsp40 and other signaling molecules involved in cell cycle progression. The decrease in cyclin D1 described above was also accompanied by a decrease in NF-kB protein expression, whose transcriptional activity leads to the activation of cyclin D1 and is also involved in migration and prostate cancer progression [26, 39, 40]. We confirmed the reduction of NF-κB expression by MSKE in vivo. Accordingly, we investigated the role of MSKE on cell migration and invasion, which are important hallmarks of cancer progression.
The invasive behavior of prostate cancer cells is associated with their ability to migrate and invade the surrounding tissue [41]. This study provides evidence that MSKE exerts an inhibitory effect on the motility and invasiveness of PC-3 prostate cancer metastatic cells. As shown in the results, the adhesion of PC-3 cells was markedly suppressed and invasion significantly inhibited by MSKE treatment and Hsp40 knockout. Previous studies have also shown that other natural polyphenols present in grapes, green tea, and tumeric can suppress invasion [42, 43]. Overall, our results indicate that MSKE inhibits metastatic prostate cancer cell growth by inhibiting cell migration and invasion and retarding cells transitioning from the G1 to S phase of the cell cycle through the targeting Hsp40 which is involved in cell growth, cell arrest, and cell invasion.
Importantly, we demonstrated in vivo MSKE ability to significantly reduced tumor burden as early as four weeks after initial subcutaneous injection of cells, while not significantly affecting body weight or food consumption. This anti-tumor effect is similar to previous in vivo studies that examined the effect of purified muscadine grape polyphenols on PC-3 prostate tumor cell growth [44, 45].
In summary, MSKE treatment significantly inhibited the growth of metastatic prostate tumor cells in vitro and in vivo by inducing cell-cycle arrest through the targeting of Hsp40 which is involved in cell-cycle progression and cell migration. Furthermore, we demonstrated that MSKE was safe at high concentrations and had a beneficial effect on metastatic prostate cancer. The safety of MSKE was confirmed by a Phase I clinical trial of a related noble cultivar of muscadine grapes where safety of MPX (muscadine grape skin powder) on PSA doubling time in men with biochemical recurrence was studied [46]. Based on the present comprehensive findings, MSKE could be developed as a potential anticancer agent against castrate resistant prostate cancer.
Declarations
Author contribution statement
Diane N. Ignacio: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Kimberly Mason, Ezra Hacket: Performed the experiments.
Chris Albanese, Diane K. Hartle: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Lymor Ringer, Sushant Kachhap, Channing Paller: Performed the experiments; Analyzed and interpreted the data.
William D. Wagner, Paul Wang, Michael Carducci, Jeffrey E. Green, Collis Brown: Contributed reagents, materials, analysis tools or data.
Janet Mendonca, Leo Li-Ying Chan, Bo Lin: Analyzed and interpreted the data.
Tamaro S. Hudson: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported in part by the National Cancer Institute of National Institute of Health, United States, Howard-Hopkins Partnership Grant Number U54CA091409, and Veterans Administration-Historically Black Colleges and University Research Training Award, United States under Grant number 1lK2RX001114-01. It was also supported in part by the National Institute on Minority Health and Health Disparities of the National Institutes of Health, United States under Award Number 8G12MD007597. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Competing interest statement
The authors declare the following conflict of interests: William D. Wagner owns Muscadine Naturals, Inc., the manufacturer of muscadine grape skin powder, and holds a patent on the manufacturing process. However, the Ison cultivar used in this study was not from Dr. Wagner's cultivar. The remaining authors declare no potential conflicts of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors gratefully acknowledge Dr. Kwabi-Addo, who generously donated the PC-3 human prostate cancer cells. In addition, we would like to thank the Howard University veterinary staff for their animal care-taking assistance.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Figure 1.
Supplementary Figure 2.
Supplementary Figure 3.
Supplementary Figure 4.
References
- 1.Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. https://www.sciencedirect.com/science/article/pii/S0024320599004105?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 2.Clinton S.K. Diet, nutrition, and prostate cancer. Annu. Rev. Nutr. 1998;18:413–440. doi: 10.1146/annurev.nutr.18.1.413. https://www.annualreviews.org/doi/10.1146/annurev.nutr.18.1.413 [DOI] [PubMed] [Google Scholar]
- 3.Syed D.N., Khan N., Afaq F., Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol. Biomark. Prev. 2007;16:2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. http://cebp.aacrjournals.org/content/16/11/2193 [DOI] [PubMed] [Google Scholar]
- 4.Thomasset S.C., Berry D.P., Garcea G., Marczylo T., Steward W.P., Gescher A.J. Dietary polyphenolic phytochemical – promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer. 2006;120:451–458. doi: 10.1002/ijc.22419. https://onlinelibrary.wiley.com/doi/abs/10.1002/ijc.22419 [DOI] [PubMed] [Google Scholar]
- 5.Bettuzzi S., Brausi M., Rizzi F., Castagnetti G., Peracchia G., Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high grade prostate intraepithelial neoplasia: a preliminary report from a one year proof-of-principle study. Can. Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. http://cancerres.aacrjournals.org/content/66/2/1234 [DOI] [PubMed] [Google Scholar]
- 6.Pantuck A.J., Leppert J.T., Zomorodian N., Aronson W., Hong J., Barnard R.J., Seeram N., Liker H., Wang H., Elashoff R., Heber D., Aviram M., Ignarro L., Belldegrun A. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin. Cancer Res. 2006;12:4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. http://clincancerres.aacrjournals.org/content/12/13/4018 [DOI] [PubMed] [Google Scholar]
- 7.Cimino S. Polyphenols: key issues involved in chemoprevention of prostate cancer. Oxid. Med. Cell Longev. 2012:632959. doi: 10.1155/2012/632959. https://www.hindawi.com/journals/omcl/2012/632959/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You Q., Chen F., Sharp J.L., Wang X., You Y., Zhang C. High-performance liquid chromatography-mass spectrometry and evaporative light-scattering detector to compare phenolic profiles of muscadine grapes. J. Chromatogr. 2012;1240:96–103. doi: 10.1016/j.chroma.2012.03.086. [DOI] [PubMed] [Google Scholar]
- 9.Hudson T.S., Hartle D.K., Hursting S.D., Nunez N.P., Wang T.T., Young H.A., Arany P., Green J.E. Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res. 2007;67:8396–8405. doi: 10.1158/0008-5472.CAN-06-4069. http://cancerres.aacrjournals.org/content/67/17/8396 [DOI] [PubMed] [Google Scholar]
- 10.Ciocca D.R., Calderwood Stuart K. Heat shock proteins in cancer: diagnostic, prognostic, predictive and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitra A., Shevde L.A., Samant R.S. Multi-faceted role of Hsp40 in cancer. Clin. Exp. Metastasis. 2009;26:559–567. doi: 10.1007/s10585-009-9255-x. https://link.springer.com/article/10.1007%2Fs10585-009-9255-x [DOI] [PubMed] [Google Scholar]
- 12.Neckers L. Heat shock protein 90 inhibition by 17-Allylamino-17-demethoxygeldanamaycin: a novel therapeutic approach for treating hormone-refractory prostate cancer. Clin. Cancer Res. 2002;8:962–966. [PubMed] [Google Scholar]
- 13.Fewell S.W., Smith C.M., Lyon M.A., Dumitrescu T.P., Wipf P., Day B.W., Brodsky J.L. Small molecule modulators of endogenous and co-chaperone-stimulated Hsp70 ATPase activity. J. Biol. Chem. 2004;279:51131–51140. doi: 10.1074/jbc.M404857200. http://www.jbc.org/content/279/49/51131 [DOI] [PubMed] [Google Scholar]
- 14.Sreedhar A.S., Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol. Ther. 2004;101:227–257. doi: 10.1016/j.pharmthera.2003.11.004. https://www.sciencedirect.com/science/article/pii/S0163725804000038?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 15.Goldstein M.G., Li Z. Heat-shock proteins in infection-mediated inflammation-induced tumorigenesis. J. Hematol. Oncol. 2009;2:5. doi: 10.1186/1756-8722-2-5. https://jhoonline.biomedcentral.com/articles/10.1186/1756-8722-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goetz M.P., Toft D.O., Ames M.M., Erlichman C. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann. Oncol. 2003;14:1169–1176. doi: 10.1093/annonc/mdg316. [DOI] [PubMed] [Google Scholar]
- 17.Casimiro M., Rodriguez O., Pootrakul L., Aventian M., Lushina N., Cromelin C., Ferzli G., Johnson K., Fricke S., Diba F., Kallakury B., Ohanyerenwa C., Chen M., Ostrowski M., Hung M.-C., Rabbani S.,.A., Datar R., Cote R., Pestell R., Albanese C. ErbB-2 induces the cyclin D1 gene in prostate epithelial cells in vitro and in vivo. Cancer Res. 2007;67:4364–4372. doi: 10.1158/0008-5472.CAN-06-1898. http://cancerres.aacrjournals.org/content/67/9/4364 [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez O.C., Lai E.W., Vissapragada S., Cromelin C., Avetian M., Salinas P., Ramos H., Kallakury B., Casimiro M., Lisanti M.P., Tanowitz H.B., Pacak K., Glazer R.I., Avantaggiati M., Albanese C. A reduction in PTEN tumor suppressor activity promotes ErbB-2-induced mouse prostate adenocarcinoma formation through the activation of signaling cascades downstream of PDK1. Am. J. Pathol. 2009;174:2051–2060. doi: 10.2353/ajpath.2009.080859. https://www.sciencedirect.com/science/article/pii/S0002944010610656?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sirajuddin P., Das S., Ringer L., Rodriguez O.C., Sivakumar A., Lee Y.C., Üren A., Fricke S.T., Rood B., Ozcan A., Wang S.S., Karam S., Yenugonda V., Salinas P., Petricoin E., 3rd, Pishvaian M., Lisanti M.P., Wang Y., Schlegel R., Moasser B., Albanese C. Quantifying the CDK inhibitor VMY-1–103's activity and tissue levels in an in vivo tumor model by LC-MS/MS and by MRI. Cell Cycle. 2012;11:3801–3809. doi: 10.4161/cc.21988. https://www.tandfonline.com/doi/abs/10.4161/cc.21988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gan L., Wang J., Xu H., Yang X. Resistance to docetaxel-induced apoptosis in prostate cancer cells by p38/p53/p21 signaling. Prostate. 2011;71:1158–1166. doi: 10.1002/pros.21331. https://onlinelibrary.wiley.com/doi/abs/10.1002/pros.21331 [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. https://www.sciencedirect.com/science/article/pii/S0092867400816839?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 22.Gupta S., Ahmad N., Nieminen A.L., Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (-)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol. Appl. Pharmacol. 2000;164:82–90. doi: 10.1006/taap.1999.8885. https://www.sciencedirect.com/science/article/pii/S0041008X99988853?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 23.Kuwajerwala N., Cifuentes E., Gautam S., Menon M., Barrack E.R., Reddy G.P. Resveratrol induces prostate cancer cell entry into S phase and inhibits DNA synthesis. Cancer Res. 2002;62:2488–2492. [PubMed] [Google Scholar]
- 24.Suh D.K., Lee E.J., Kim H.C., Kim J.H. Induction of G1/S phase arrest and apoptosis by quercetin in human osteosarcoma cells. Arch. Pharm. Res. 2010;33:781–785. doi: 10.1007/s12272-010-0519-4. [DOI] [PubMed] [Google Scholar]
- 25.Ni J., Chen M., Zhang Y., Li R., Huang J., Yeh S. Vitamin E succinate inhibits human prostate cancer cell growth via modulating cell cycle regulatory machinery. Biochem. Biophys. Res. Commun. 2002;300:357–363. doi: 10.1016/s0006-291x(02)02851-6. [DOI] [PubMed] [Google Scholar]
- 26.Li R., Hannon G.J., Beach D., Stillman B. Subcellular distribution of p21 and PCNA in normal and repair-deficient cells following DNA damage. Curr. Biol. 1996;6:189–1992. doi: 10.1016/s0960-9822(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 27.Tashiro E., Tsuchiya A., Imoto M. Functions of cyclin D1 as an oncogene and regulation of cyclin D1 expression. Can. Sci. 2007;98:629–635. doi: 10.1111/j.1349-7006.2007.00449.x. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1349-7006.2007.00449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stangelberger A., Schally A.V., Rick F.G., Varga J.L., Baker B., Zarandi M., Halmos G. Inhibitory effects of antagonists of growth hormone releasing hormone on experimental prostate cancers are associated with upregulation of wild-type p53 and decrease in p21 and mutant p53 proteins. Prostate. 2012;72:555–565. doi: 10.1002/pros.21458. https://onlinelibrary.wiley.com/doi/abs/10.1002/pros.21458 [DOI] [PubMed] [Google Scholar]
- 29.Hollstein M., Rice K., Greenblatt M.S., Soussi T., Fuchs R., Sørlie T., Hovig E., Smith-Sørensen B., Montesano R., Harris C.C. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids. 1994;22:3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 30.McDonnell T.J., Navone N.M., Troncoso P., Pisters L.L., Conti C., von Eschenbach A.C., Brisbay S., Logothetis C.J. Expression of bcl-2 oncoprotein and p53 protein accumulation in bone marrow metastases of androgen independent prostate cancer. J. Urol. 1997;157:569–574. [PubMed] [Google Scholar]
- 31.Navone N.M., Troncoso P., Pisters L.L., Conti C., von Eschenbach A.C., Brisbay S., Logothetis C.J. p53 protein accumulation and gene mutation in the progression of human prostate carcinoma. J. Natl. Cancer Inst. 1993;85:1657–1669. doi: 10.1093/jnci/85.20.1657. [DOI] [PubMed] [Google Scholar]
- 32.Verma I.M., Stevenson J.K., Schwarz E.M., Van Antwerp D., Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 33.Lam K.T., Calderwood S.K. hsp70 binds specifically to a peptide derived from the highly conserved domain (I) region of p53. Biochem. Biophys. Res. Commun. 1992;184:167–174. doi: 10.1016/0006-291x(92)91174-o. [DOI] [PubMed] [Google Scholar]
- 34.Zylicz M., King F.W., Wawrzynow A. Hsp70 interactions with the p53 tumour suppressor protein. EMBO J. 2001;20:4634–4638. doi: 10.1093/emboj/20.17.4634. http://emboj.embopress.org/content/20/17/4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sreedhar A.S., Pardhasaradhi B.V., Khar A., Srinivas U.K. A cross talk between cellular signaling and cellular redox state during heat-induced apoptosis in a rat histiocytic tumor cell. Free Radic. Biol. Med. 2002;32:221–227. doi: 10.1016/s0891-5849(01)00796-1. https://www.sciencedirect.com/science/article/pii/S0891584901007961?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 36.Sreedhar A.S., Pardhasaradhi B.V., Khar A., Srinivas U.K. Effect of C-terminal deletion of p53 on heat-induced CD95 expression and apoptosis in a rat histiocytoma. Oncogene. 2002;21:4042–4049. doi: 10.1038/sj.onc.1205504. https://www.nature.com/articles/1205504 [DOI] [PubMed] [Google Scholar]
- 37.King F.W., Wawrzynow A., Höhfeld J., Zylicz M. Co-chaperones Bag-1, Hop, and Hsp40 regulate Hsc70 and Hsp90 interactions with wild-type or mutant p53. EMBO J. 2001;20:6297–6305. doi: 10.1093/emboj/20.22.6297. http://emboj.embopress.org/content/20/22/6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akalin A., Elmore L.W., Forsythe H.L., Amaker B.A., McCollum E.D., Nelson P.S., Ware J.L., Holt S.E. A novel mechanism for chaperone-mediated telomerase regulation during prostate cancer progression. Cancer Res. 2001;61:4791–4796. [PubMed] [Google Scholar]
- 39.Bharti A.C., Aggarwal B.B. Chemopreventive agents induce suppression of nuclear factor-kappaB leading to chemosensitization. Ann. N. Y. Acad. Sci. 2002;973:392–395. doi: 10.1111/j.1749-6632.2002.tb04671.x. [DOI] [PubMed] [Google Scholar]
- 40.Shukla S., MacLennan G.T., Fu P., Patel J., Marengo S.R., Resnick M.I., Gupta S. Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia. 2004;6:390–400. doi: 10.1593/neo.04112. https://www.sciencedirect.com/science/article/pii/S1476558604800848?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson W.E., Price J.T. Mechanisms of tumour invasion and metastasis: emerging targets for therapy. Expert Opin. Ther. Targets. 2002;6:217–233. doi: 10.1517/14728222.6.2.217. https://www.tandfonline.com/doi/abs/10.1517/14728222.6.2.217 [DOI] [PubMed] [Google Scholar]
- 42.Sliva D. Suppression of cancer invasiveness by dietary compounds. Mini Rev. Med. Chem. 2008;8:677–688. doi: 10.2174/138955708784567412. http://www.eurekaselect.com/82804/article [DOI] [PubMed] [Google Scholar]
- 43.Thangapazham R.L., Passi N., Maheshwari R.K. Green tea polyphenol and epigallocatechin gallate induce apoptosis and inhibit invasion in human breast cancer cells. Cancer Biol. Ther. 2007;6:1938–1943. doi: 10.4161/cbt.6.12.4974. [DOI] [PubMed] [Google Scholar]
- 44.Ganapathy S., Chen Q., Singh K.P., Shankar S., Srivastava R.K. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription ractor. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0015627. http://www.ncbi.nlm.nih.gov/pubmed/?term=Srivastava%20RK%5Bauth%5D Published online 2010 December 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hafeez B.B., Siddiqui I.A., Asim M., Malik A., Afaq F., Adhami V.M., Saleem M., Din M., Mukhtar H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kB signaling. Cancer Res. 2008;68:8564–8572. doi: 10.1158/0008-5472.CAN-08-2232. http://cancerres.aacrjournals.org/content/68/20/8564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paller C.J., Rudek M.A., Zhou X.C., Wagner W.D., Hudson T.S., Anders N., Hammers H.J., Dowling D., King S., Antonarakis E.S., Drake C.G., Eisenberger M.A., Denmeade S.R., Rosner G.L., Carducci M.A. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: safety, tolerability, and dose determination. Prostate. 2015;75:1518–1525. doi: 10.1002/pros.23024. https://onlinelibrary.wiley.com/doi/abs/10.1002/pros.23024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.