Summary
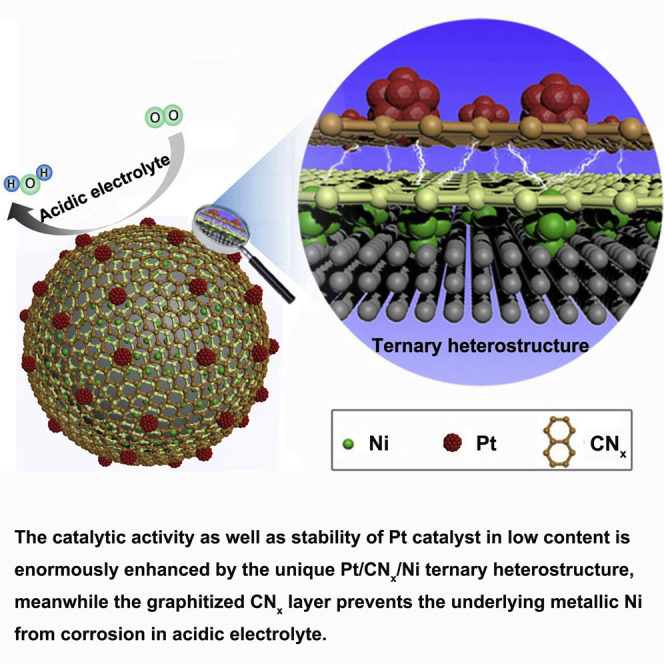
We report here a supercatalyst for oxygen reduction of Pt/CNx/Ni in a unique ternary heterostructure, in which the Pt and the underlying Ni nanoparticles are separated by two to three layers of nitrogen-doped carbon (CNx), which mediates the transfer of electrons from the inner Ni to the outer Pt and protects the Ni against corrosion at the same time. The well-engineered low-Pt catalyst shows ∼780% enhanced specific mass activity or 490% enhanced specific surface activity compared with a commercial Pt/C catalyst toward oxygen reduction. More importantly, the exceptionally strong tune on the Pt by the unique structure makes the catalyst superbly stable, and its mass activity of 0.72 A/mgPt at 0.90 V (well above the US Department of Energy's 2020 target of 0.44 A/mgPt at 0.90 V) after 50,000 cyclic voltammetry cycles under acidic conditions is still better than that of the fresh commercial catalyst.
Subject Areas: Catalysis, Electrochemical Energy Conversion, Energy Materials
Graphical Abstract
Highlights
-
•
Matched band structures among Pt/CNx/Ni enable electron transfer from Ni to Pt
-
•
Enhanced Pt-CNx interaction by underlying Ni prevents Pt from coalescence and oxidation
-
•
Encapsulated Ni is protected from corrosion and maintains the structure stability of THS
Catalysis; Electrochemical Energy Conversion; Energy Materials
Introduction
As a clean energy conversion device, the proton exchange membrane fuel cell (PEMFC) will shoulder the main responsibility together with secondary battery in the coming renewable energy era (Stephens et al., 2016, He et al., 2018, Zhang et al., 2018a, Zhang et al., 2018b, Dong et al., 2018, Mo et al., 2018). However, the high amount of Pt used in the cathode catalyst for oxygen reduction reaction (ORR) is the most serious obstacle for its practical application (Wang et al., 2015, Peng and Yang, 2009). At present, the amount of Pt used in the cathode catalyst of PEMFC is commonly about 0.1–0.5 mg cm−2, approximately an order of magnitude higher than that of the target set by the US Department of Energy (DOE, <0.03 mg⋅cm−2) (Debe, 2012, Zhou et al., 2010). Although great progresses about the designs of low-Pt catalysts have been made continuously (Chen et al., 2007, Chen et al., 2014, Zhang et al., 2015, Wang et al., 2016, Tao et al., 2018), the high-performance ORR catalysts facilely prepared and simultaneously satisfying the demands of low Pt content, high activity, and high stability have been scarcely reported and restricted the commercial applications of PEMFC.
The well-known method to decrease Pt loading by alloying the Pt with one of transition metals (TMs) to form Pt-skin or core-shell bimetallic nanoalloys has been extensively explored, and such catalysts with relatively lower Pt contents often show much enhanced ORR activity due to the downshift of the d-band center of Pt modulated by the inner TMs through electronic effect or strain effect (Lai et al., 2018, Greeley et al., 2009, Hoster et al., 2010, Stamenkovic et al., 2006, Jiang et al., 2017, Du et al., 2015, Li et al., 2018a, Li et al., 2018b). Unfortunately, the devastation of the designed alloys under real operation conditions (corrosive, oxidizing atmosphere, and high potential, >1.5 V under frequent start-up/shut-down operations) (Zhang et al., 2018a, Zhang et al., 2018b, Gasteiger et al., 2005, Banham et al., 2015), leading to the preferred leaching of TMs (the smaller the particle, the faster the leaching), almost surely and rapidly weakens or eliminates the aforementioned modulation (Cui et al., 2012, Cui et al., 2013, Chi et al., 2015). Therefore most of the alloy catalysts failed to meet the DOE 2020 targets on Pt activity and durability (0.44 A/mgPt for membrane electrode assembly (MEA) in mass activity and <40% loss in mass activity after 30,000 cycles) (Li et al., 2018a, Li et al., 2018b). In addition, the nanoparticles (NPs) of metallic alloys are commonly prepared using oleic acid or oleylamine as coordinating and capping ligands, which leads to technical difficulty in the following surface cleaning (Liu et al., 2018a, Liu et al., 2018b, Jiang et al., 2017, Li and Wong, 2018).
We report here a supercatalyst that meets well the abovementioned criteria with a unique ternary structure of Pt/CNx/Ni, in which the Pt and the underlying Ni NPs are separated by two to three layers of nitrogen-doped carbon (CNx), which mediate the transfer of electrons from the inner Ni to the outer Pt and protect the Ni against corrosion at the same time. The well-engineered ternary heterostructure (THS) Pt/CNx/Ni catalyst possesses the following merits: (1) the matched band structures among the Pt, CNx, and Ni enable electrons to transfer from the inner Ni to the outer Pt and significantly promote the ORR activity of the low-Pt catalyst; (2) the graphitized CNx layer prevents the underlying Ni NPs from corrosion and maintains the designed structure enduringly; (3) the strengthened interaction between the Pt NPs and the electron-enriched CNx/Ni support mitigates the agglomeration or detachment of Pt NPs significantly during the electrochemical process; and (4) the Pt NPs are protected from oxidation by the electron-enriched microenvironment, just like the strategy of “cathodic protection with sacrificial anode” in the corrosion prevention system (Abootalebi et al., 2010). Besides, the facile synthetic procedure of the THS Pt/CNx/Ni catalyst is suitable for its scalable manufacture. We think that the novelty of current work makes a solid progress of the low-Pt catalyst for PEMFC toward practical applications.
Results
Synthesis and Characterizations of CNx/Ni and Pt/CNx/Ni
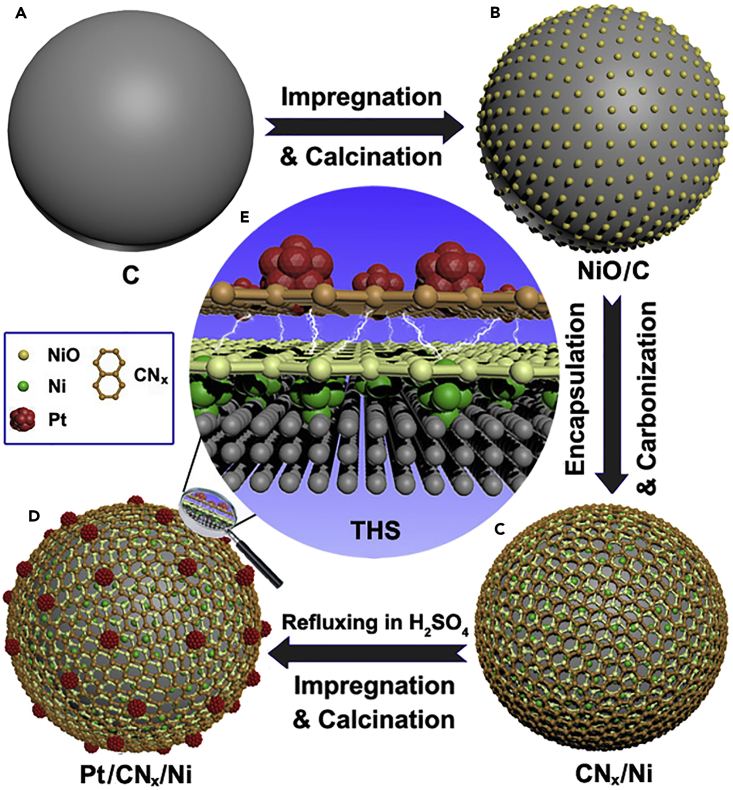
As illustrated in Figure 1, NiO/C was first prepared by loading NiO on a nanocarbon support in globular shape and then encapsulated with the precursor of CNx. After calcination in inert atmosphere, the CNx/Ni/C (denoted as CNx/Ni for clarity) support was obtained. The Pt/CNx/Ni catalyst was prepared by depositing and reducing Pt NPs on the CNx/Ni, which was fully rinsed in acid solution before the deposition (see Transparent Methods). The disappearance of the characteristic peaks of NiO in X-ray diffraction (XRD) pattern and the strong magnetism of CNx/Ni (see Figure S1) reveals that the NiO NPs are reduced to the metallic state after encapsulating with precursor of CNx layer and calcination in N2. Note that the strong magnetism of CNx/Ni was retained after rinsing in acid solution, suggesting that the Ni NPs were encapsulated in and protected by the CNx shell. The transmission electron microscopic (TEM) image of CNx/Ni (Figure 2A) shows that the powders of CNx/Ni are spherical with a diameter of ∼30 nm. The strong characteristic peaks detected by TEM energy-dispersive X-ray spectroscopy (inset in Figure 2A) confirm the coexistence of Ni, C, and N elements. The atomic ratio of C/N is about 11, indicating that the CNx matrix is a nitrogen-doped carbon material. The high-resolution TEM (HRTEM) image depicted in Figure 2B reveals that the Ni NPs are distributed densely and uniformly beneath the CNx layers at an average size of about 1 nm. To reveal the distribution of Ni NPs clearly, a colored image of Ni in CNx/Ni is shown below the HRTEM image (Figure 2B, lower), and the dark-field High-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) image of CNx/Ni with the dense and uniform bright spots shown in Figure 2C further confirms the homogeneous distribution of Ni NPs. Furthermore, the energy dispersive X-ray (EDX) elemental mappings of Ni and N below the STEM image (Figure 2C, lowers) reconfirm their uniformly distributed state.
Figure 1.
Schematic Illustration of the Pt/CNx/Ni Catalyst
(A) Original carbon support (C, Vulcan XC-72R).
(B) Loading NiO NPs onto the carbon support through impregnation and calcination.
(C) Encapsulating the NiO/C with CNx layers and the reduction of NiO NPs to metallic Ni in the meantime.
(D) Depositing Pt NPs on CNx/Ni through impregnation and reduction.
(E) Enlarged THS: the electronic properties of the outer Pt NPs are modulated by the penetrated electrons from the inner Ni NPs through CNx layers; meanwhile the inner Ni NPs are protected by CNx layers from corrosion and oxidation.
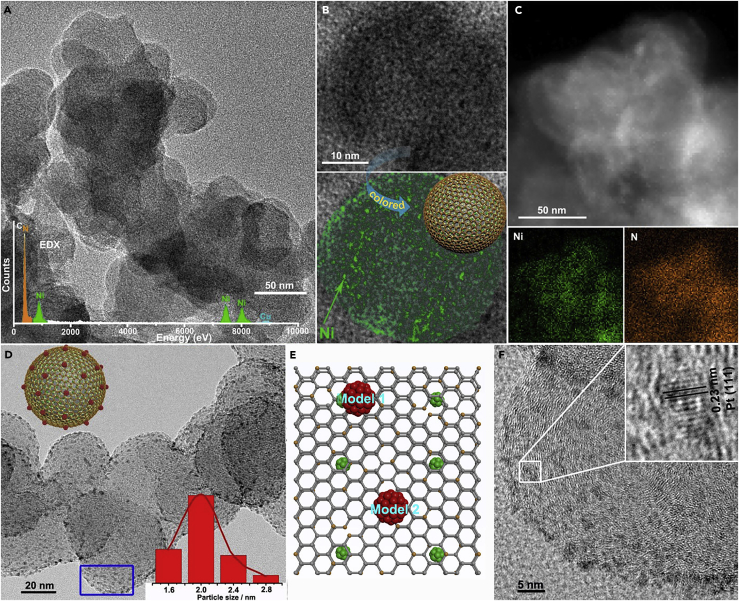
Figure 2.
Morphology and Composition Characterization of the CNx/Ni and Pt/CNx/Ni
(A) Representative TEM image of CNx/Ni. The CNx/Ni are spherical with a diameter of ∼30 nm. Inset: the EDX spectrum of CNx/Ni.
(B) HRTEM image of CNx/Ni. To reveal the distribution of Ni nanoparticles clearly, a colored image of Ni and a cartoon picture of CNx/Ni are shown below the HRTEM image.
(C) Dark-field HAADF-STEM image of CNx/Ni and its corresponding elemental mappings of Ni and N. These dense but uniform bright spots confirm the homogeneous distribution of Ni NPs.
(D) TEM image of the Pt/CNx/Ni. The top left inset shows its cartoon picture, and the lower right inset is the size distribution histogram in the range of 1.6–2.8 nm by statistical analysis of 300 Pt NPs.
(E) The relative positions of Pt and Ni in Pt/CNx/Ni sample: deduced from the structural model of the catalyst. (1) The Pt lies just above the Ni and (2) the Pt lies above but at the mid-position of the two Ni.
(F) HRTEM images of Pt/CNx/Ni. The primary lattice spacing is ∼0.23 nm, consistent with the (111) interplanar distance of cubic Pt.
Because of the similar image characteristics of carbon support and CNx shell, the image information of CNx is difficult to detect in CNx/Ni. A control sample with the CNx layers coated on nano-Al2O3 was prepared using the same method. As shown in Figure S2, the Al2O3 nanorods are ideally encapsulated in CNx shell with a thickness of ∼1.5 nm. The ∼16 wt % weight loss measured by thermogravimetry during heating in air to 1,173 K (see Figure S3) indicates that the thickness of CNx is approximately two to three layers, considering the surface area of precursor NiO/C (116.3 m2/g, see Figure S4 and Equation S1). The results are also coincident with the change of Ni content in the samples before and after CNx coating.
Using the CNx/Ni as support, the THS catalyst of Pt/CNx/Ni (for clarity, the core globular C is omitted) was prepared by impregnating chloroplatinic acid to the support, which was treated by polydopamine before the impregnation to make its surface hydrophobic and to help the dispersion of Pt (Kitagawa and Uemura, 2005, Han et al., 2018, Lee et al., 2007). As shown in Figure 2D, after heat treatment in 5.05 vol % H2/N2 at 673 K, the Pt NPs distribute on the spherical surface of CNx layer evenly in the size range of 1.6–2.8 nm. It should be pointed out that the consistency between the XRD characteristic peaks of Pt in the sample and the standard JCPDS (No. 04-0850) card (see Figure S1) provides evidence to object the formation of Ni-Pt alloy (Cui et al., 2012), indicative of the separation of Ni and Pt NPs by the CNx layer according to the THS, which is further confirmed by the subsequent electrochemical tests. The contents of Ni and Pt in Pt/CNx/Ni measured by X-ray fluorescence (XRF) analyzer are 5.6 and 6.2 wt %, and the same measured by inductively coupled plasma mass spectrometry (ICP-MS) analysis are 5.3 and 6.6 wt %, respectively, which are consistent with each other. Based on these data, i.e., the contents and sizes of Ni and Pt, the surface area of the core nanocarbon and the CNx/Ni (see Figure S5), and the average thickness of the CNx, the distances between the adjacent NPs of Ni and Pt are calculated in the range of 1.5–2.5 nm (see Equation S2), as shown in Figures 1 and 2E. The HRTEM image (Figure 2F) displays the metal NP on the spherical CNx/Ni supports a primary lattice spacing of 0.23 nm, consistent with the (111) interplanar distance of cubic Pt, excluding the formation of Pt-Ni alloy.
X-Ray Photoelectron Spectroscopy
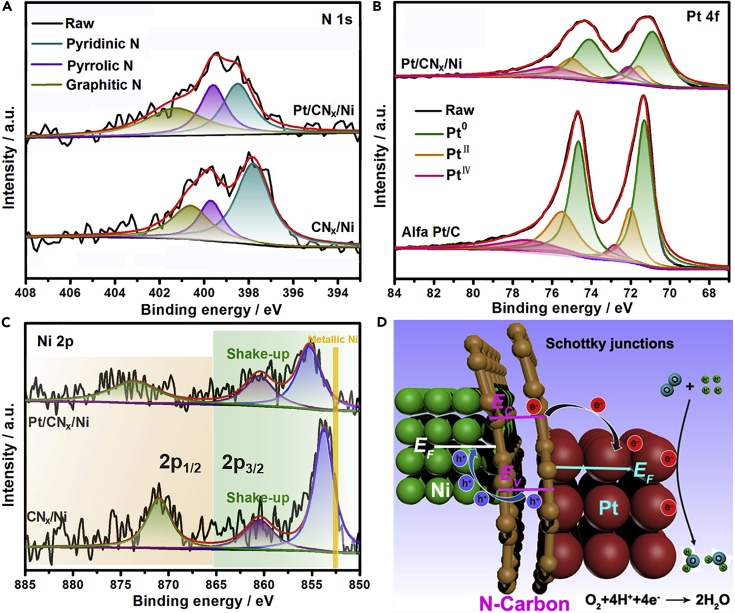
In previous work, we have demonstrated the charge transfer at the interface of CNx/Ni heterojunction because of the different Fermi levels of Ni and CNx matrix (Fu et al., 2014, Chen et al., 2017). The CNx layer is electron-rich after accepting electrons donated from Ni (see Figure S6). When Pt NPs are deposited on such an electron-rich CNx layer, modulation on the electronic property of Pt is also detected. The binding energies of N1s in Pt/CNx/Ni shift positively with respect to those of CNx/Ni (Figure 3A) and there is a clear shift of 4f7/2 and 4f5/2 peaks by ∼ 0.4 eV to lower binding energies for Pt/CNx/Ni with respect to that in commercial Pt/C (20%, Alfa), suggesting the charge transfer from CNx matrix to Pt at the interface of Pt/CN heterojunction (Liu et al., 2018a, Liu et al., 2018b). The electron-deficient state, to some extent, of metallic nickel in CNx/Ni (Figure 3C) is determined by Ni binding energy due to charge transfer from the Ni to CNx matrix (Zhao et al., 2008, Zhu et al., 2018, Fang et al., 2018, Lv et al., 2018), and interestingly, the Ni 2p3/2 peak in Pt/CNx/Ni further increases to a higher binding energy compared with CNx/Ni. This shift suggests that the original equilibrium of Fermi levels between Ni and CNx layer is thrown off with the addition of Pt NPs, and a new one is established among Ni, CNx matrix and Pt in the THS structure (Pt/CNx/Ni). Combined with the binding energy shifts of N1s and Pt 4f, we deduce that the metallic Ni, mediated by the CNx, can increase the electron density of Pt NPs. The surface composition of the catalyst is found as 73.5, 6.4, 8.1, 5.2, and 6.8 wt % for C, N, O, Ni, and Pt, respectively, which seem reasonable when compared with the results of XRF and ICP-MS analysis.
Figure 3.
Electronic Equilibration across the CNx/Ni and Pt/CNx Interfaces and the Results of XPS
(A–C) Deconvoluted spectra of nitrogen 1s (A), platinum 4f (B), and nickel 2p (C) in CN/Ni and Pt/CNx/Ni.
(D) Schematic illustration for Mott-Schottky-type contacts of Pt/CNx/Ni in the THS structure.
The negative shifts of Pt4f binding energies and the more positive shifts of Ni2p binding energies in Pt/CNx/Ni indicating the charge transfer from Ni to Pt through CNx.
Theoretical Calculations
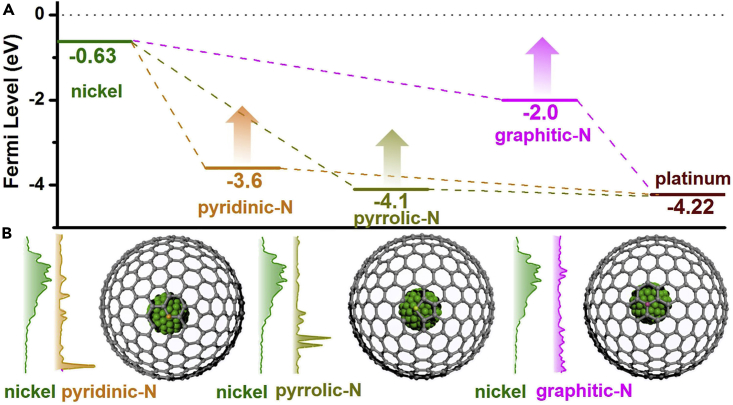
To verify the feasibility of the electron transfer, the density of states (DOS) of the elements were calculated based on the density functional theory and are shown in Figure 4. The higher Fermi level of Ni (−0.63 eV) relative to those of pyridinic N (−3.6 eV), pyrrolic N (−4.1 eV), and graphitic N (−2.0 eV) and the positive integrations for pyridinic N (0.45), pyrrolic N (0.20), and graphitic N (0.12) by integrating the DOS between Fermi levels of Ni and each nitrogen species suggest that Ni can donate its electrons to the CNx matrix. The Fermi levels of each nitrogen species would shift positively along with the increased electron density due to the electron transfer at the interface of the CNx/Ni heterojunction (Subramanian et al., 2004). In addition, the relatively lower Fermi level of Pt (−4.22 eV) also enables it to accept electrons from the CNx matrix easily. Such charge transfer is also corroborated by the shift of conduction band in samples with or without Ni or Pt (Figure S7).
Figure 4.
Electron Transfer Pathway in Pt/CNx/Ni Heterostructures
(A) Fermi level of nickel, pyridinic-N, pyrrolic-N, graphitic-N, and platinum.
(B) The density of states (DOS) of the elements and the local structures of their interfaces. The vacuum level is aligned at 0 eV.
Based on the discussion above, an illustration for Mott-Schottky-type contacts in Pt/CNx/Ni THS is shown in Figure 3D. Owing to the different Fermi levels among the CNx matrix and metallic Ni and Pt, the charges will transfer spontaneously from Ni to Pt, mediated by the CNx, until their Fermi levels reach equilibrium (Deng et al., 2016). The charge transfer will surely affect the electronic properties, and thus the adsorption/desorption properties of the Pt NPs for oxygen species on their surfaces. Redox of O2-H2 titration profiles in Figure S8 supports this viewpoint. The lower onset of reduction temperature of chemisorbed oxygen on Pt/CNx/Ni (∼300°C) than that of Pt/C (∼400°C) suggests the higher reactivity of the oxygen species adsorbed on Pt/CNx/Ni catalyst, which is beneficial to enhance its ORR catalytic activity. The results of the CO stripping measurement (Figure S9) show that the main peaks of CO oxidation on Pt/C, Pt/CNx, and Pt/CNx/Ni are located at 0.95, 0.99, and 0.85 V, respectively. Combined with the above results, it is clear that the Pt with enriched electrons can afford more active oxygen species and can lead to a lower oxidation potential (Van der Vliet et al., 2012, Igarashi et al., 2001).
Electrocatalytic Performance
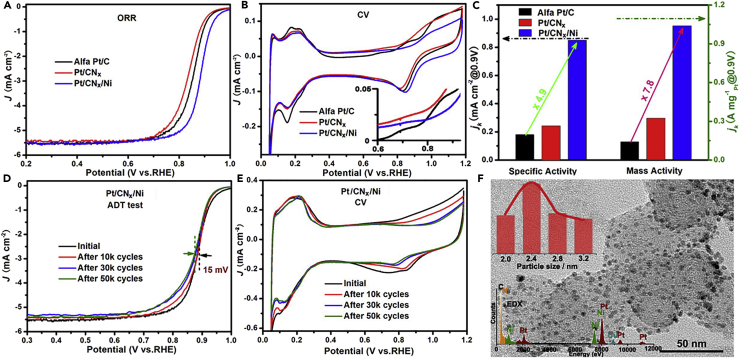
To evaluate the possible relationship between the electronic structure of Pt and its catalytic activity toward ORR, the ORR polarization curves of the Pt/CNx/Ni, Pt/CNx, and the commercial Pt/C were measured and normalized by glassy carbon electrode geometric area (0.246 cm2). The mass loadings of Pt on glassy carbon electrode were 6.2, 6.0, and 20 μg cm−2, for Pt/CNx/Ni, Pt/CNx, and Pt/C, respectively. As shown in Figure 5A, the half-wave potential of the sample Pt/CNx/Ni is 0.886 V, which is higher than those of the corresponding Ni-free sample (Pt/CNx, 0.839 V) and commercial Pt/C (0.858 V). Such positive shift of half-wave potential suggests that the presence of modulation by the underlying Ni can accelerate the separation of the adsorbed oxide species from Pt surface and thereby improve the ORR kinetics. The results of cyclic voltammetry (CV) measurements shown in Figure 5B are in favor of this interpretation. The more positive reduction potential of Pt-OHad (adsorbed hydroxyl species) in Pt/CNx/Ni suggests that the OHad are promoted to react with H+ to form H2O on its electron-enriched surface (Stamenkovic et al., 2007, Wang et al., 2011).
Figure 5.
Electrochemical Performance of Pt/CNx/Ni, Pt/CNx, and Commercial Pt/C Catalysts for ORR
(A–C) (A) ORR polarization curves, (B) cyclic voltammetry (CV) curves, and (C) specific and mass activities of different catalysts at 0.9 V versus RHE.
(D and E) (D) ORR polarization curves and (E) CV curves of Pt/CNx/Ni before and after 50,000 CV cycles between 0.6 and 1.0 V versus RHE. The scan rate for the accelerated durability test (ADT) is 200 mV s−1. The curves in (A, D, and E) were recorded at 298 K in 0.1 M HClO4 aqueous solution at a sweep rate of 20 mV/s, and the curves in (B) were recorded at a sweep rate of 5 mV/s.
(F) TEM image of Pt/CNx/Ni after ADT test. Inset is the corresponding size distribution histogram and EDX spectrum.
Furthermore, the higher initiation potential of Pt oxidation features suggest that the Pt NPs are protected by the surplus electrons on its surface from oxidation, just like “cathodic protection with sacrificial anode” in corrosion prevention system (Abootalebi et al., 2010). Suppression of the formation of Pt oxide will decrease the blockage of active sites by adsorbed oxygen species and then enhance the ORR activity (Snyder et al., 2013). Different from alloy catalysts, of which the Pt electronic property is often overly tuned because of the strong electronic interaction and great lattice mismatch between Pt and parts of TMs, the rate-determining step in the volcano-type activity plots turns from desorption process (left side) to adsorption process (right side in Figure S10) (Greeley et al., 2009, Stephens et al., 2012). The overly tuned Pt catalyst with weak adsorption of oxygen also leads to an undesired decrease in catalytic activity.
To confirm that the Pt and Ni are separated in the THS, the electrochemical performance of Pt/CNx/Ni catalyst before and after refluxing in 1 M H2SO4 at 353 K are measured in alkaline solution (0.1 M KOH). If the Pt and Ni are not separated by CNx, the exposed Ni would be oxidized to NiO partially during the testing in alkaline solution and the formed NiO will dissolve in 1 M H2SO4 in the following refluxing process, which will influence the ORR performance. However, the coincident CV and ORR polarization curves for the samples initially and after refluxing for 3 and 6 hr, shown in Figure S11, provide further evidences of the effective protection of Ni by the CNx shell and also to veto the formation of Ni-Pt alloy (Rudi et al., 2017). Based on the integral charge in underpotentially deposited hydrogen (HUPD) adsorption/desorption region in the CV curves and the mass loading of Pt, the specific electrochemically active surface area (ECSA) of Pt/CNx/Ni, Pt/CNx, and Pt/C are calculated as 122.0, 125.5, and 77.2 m2/gPt, respectively. It should be pointed out that the theoretical specific surface area of the Pt NPs in Pt/CNx/Ni (d = 2 nm) and Pt/C (d = 3.5 nm) are 139.8 and 79.9 m2/gPt (Equation S3), respectively, well consistent with the ECSA results. The higher specific ECSA of the Pt/CNx/Ni catalyst is consistent with the higher H2 consumption in O2-H2 titration measurements (see Figure S8).
Figure 5C shows the specific surface and specific mass activities of the samples, which are normalized by the ECSA or the mass of the loaded Pt, respectively. With 6.2 μg cm−2 loading of Pt, Pt/CNx/Ni exhibits a mass activity of 1.04 A mg−1Pt and a specific activity of 0.85 mA cm−2 (at 0.9 V versus reversible hydrogen electrode (RHE)), which are 7.8 and 4.9 times higher than those of the commercial Pt/C (20 μg cm−2) under the same conditions. It should be pointed out that the onset potential of CNx/Ni support is just 0.75 V versus RHE (see Figure S12) under the same testing conditions, indicating that the catalytic contribution from support is negligible. Hence, the enhanced catalytic activity should be ascribed to the modulated electronic structure and the relatively higher specific ECSA of the Pt/CNx/Ni catalyst. The former is the main cause, for the ECSA of Pt/CNx/Ni is about only 1.5 times that of the Pt/C.
The catalytic stability of the Pt/CNx/Ni catalyst was assessed by accelerated deterioration tests (ADT) at a scan rate of 200 mV s−1 between 0.6 and 1.0 V versus RHE in O2-saturated 0.1 M HClO4. As shown in Figure 5D, a negative shift of mere 15 mV of the half-wave potential of Pt/CNx/Ni is observed after 50,000 sweeping cycles. However, a ∼64-mV negative shift is shown for the commercial Pt/C and ∼50 mV for Pt/CNx under the same condition (Figure S13). The retention of specific ECSA for the samples are measured from HUPD and listed in Table S1. After 50,000 sweeping cycles, the retention of the specific ECSA is ∼90% for Pt/CNx/Ni, much higher than that of Pt/CNx (∼73%) and Pt/C (∼60%). The unique THS of Pt/CNx/Ni mitigates the surface oxidation, detachment, and aggregation of Pt and thereby contributes to the higher ECSA retention and outstanding stability. Indeed, the designed THS of Pt/CNx/Ni is reserved commendably after the ADT test in corrosive and oxidative environment and the TEM images of Pt/CNx/Ni (Figure 5F) after 50,000 cycles reveal the unchanged morphology and size of the Pt NPs. In contrast, a serious aggregation of Pt NPs occurs for the commercial Pt/C, wherein the average size of Pt increases from 3.5 to 5.2 nm (Figure S14).
It should be pointed out that the THS model is also applicable to other TMs occupying suitable Fermi levels, such as cobalt. As shown in Figure S15 and Table S1, the Pt/CNx/Co catalyst exhibits similar physical property and catalytic activity for ORR with respect to Pt/CNx/Ni. The unique Pt/CNx/Ni(Co) catalyst in the THS with high specific activity and superstability provides hope for practical use in fuel cells, which simultaneously satisfies the target set by US DOE of low Pt content, high activity, and high stability.
Discussion
The unique THS of Pt/CNx/Ni catalyst has been designed and synthesized successfully using a facile and scalable method. Based on the matched band structures among the Pt, CNx, and Ni, the electronic properties of the outer Pt NPs are properly modulated by the inner Ni, mediated by the CNx layers, which also act as a protective shield for Ni against the corrosive and oxidative conditions. The modulation of the Pt NPs by the electron-rich CNx/Ni support significantly promotes the activity of the catalyst, and the strong interactions between them mitigates the oxidation, detachment, and aggregation of Pt NPs under the working conditions of electrocatalysis. Benefited from the artful design of the THS, the catalyst exhibits high performance for oxygen reduction, simultaneously satisfying the target set by US DOE of low Pt content, high activity, and high stability. The catalyst will be supplied in quantity to the scientific community of fuel cells in the near future.
Limitations of Study
In the Pt/CNx/Ni THS, the thinner the CNx layers, the easier the electron transfer from the inner Ni to the outer Pt through CNx layers and the higher the ORR performance of Pt catalyst. However, considering the protection of the CNx layers for the Ni particles from corrosion, the CNx layers should not be too thin. To study the electronic modulation of the Pt sites and the stability of Ni particles in acidic electrolyte, the influence of CNx thickness could be explored in detail in the future.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors thank the financial supports from the Ministry of Science and Technology of China (2017YFB0702900), the National Science Foundation of China (91434101, 91745108), and Nanjing Black-Tech Co. Ltd., China.
Author Contributions
W.D. conceived the work. B.Z guided the experiments. T.C. designed and performed main experiments. Y.X., S.G., and D.W. participated in the experimental work. Z.C. performed the theoretical calculations. N.X., L.P., and X.G. carried out the discussion. T.C. wrote the manuscript and B. Z. participated in the writing, and W.D. finalized the work. All authors discussed the results.
Declaration of Interests
The authors declare no competing interests.
Published: January 25, 2019
Footnotes
Supplemental Information includes Transparent Methods, 15 figures, and 1 table and can be found with this article online at https://doi.org/10.1016/j.isci.2018.12.029.
Contributor Information
Bin Zhao, Email: binzhao@nju.edu.cn.
Weiping Ding, Email: dingwp@nju.edu.cn.
Supplemental Information
References
- Abootalebi O., Kermanpur A., Shishesaz M., Golozar M. Optimizing the electrode position in sacrificial anode cathodic protection systems using boundary element method. Corros. Sci. 2010;52:678–687. [Google Scholar]
- Banham D., Ye S., Pei K., Ozaki J.I., Kishimoto T., Imashiro Y. A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources. 2015;285:334–348. [Google Scholar]
- Chen T., Guo S., Yang J., Xu Y., Sun J., Wei D., Chen Z., Zhao B., Ding W. Nitrogen-doped carbon activated in situ by embedded nickel through the Mott-Schottky effect for the oxygen reduction reaction. ChemPhysChem. 2017;18:3454–3461. doi: 10.1002/cphc.201700834. [DOI] [PubMed] [Google Scholar]
- Chen Z., Waje M., Li W., Yan Y. Supportless Pt and PtPd nanotubes as electrocatalysts for oxygen-reduction reactions. Angew. Chem. Int. Ed. 2007;119:4138–4141. doi: 10.1002/anie.200700894. [DOI] [PubMed] [Google Scholar]
- Chen C., Kang Y., Huo Z., Zhu Z., Huang W., Xin H.L., Snyder J.D., Li D., Herron J.A., Mavrikakis M. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science. 2014;343:1339–1343. doi: 10.1126/science.1249061. [DOI] [PubMed] [Google Scholar]
- Chi M., Wang C., Lei Y., Wang G., Li D., More K.L., Lupini A., Allard L.F., Markovic N.M., Stamenkovic V.R. Surface faceting and elemental diffusion behaviour at atomic scale for alloy nanoparticles during in situ annealing. Nat. Commun. 2015;6:8925. doi: 10.1038/ncomms9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C., Gan L., Heggen M., Rudi S., Strasser P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013;12:765–771. doi: 10.1038/nmat3668. [DOI] [PubMed] [Google Scholar]
- Cui C., Gan L., Li H.H., Yu S.H., Heggen M., Strasser P. Octahedral PtNi nanoparticle catalysts: exceptional oxygen reduction activity by tuning the alloy particle surface composition. Nano Lett. 2012;12:5885–5889. doi: 10.1021/nl3032795. [DOI] [PubMed] [Google Scholar]
- Debe M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature. 2012;486:43–50. doi: 10.1038/nature11115. [DOI] [PubMed] [Google Scholar]
- Deng D., Novoselov K.S., Fu Q., Zheng N., Tian Z., Bao X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016;11:218–230. doi: 10.1038/nnano.2015.340. [DOI] [PubMed] [Google Scholar]
- Dong C., Gao W., Jin B., Jiang Q. Advances in cathode materials for high-performance lithium-sulfur batteries. iScience. 2018;6:151–198. doi: 10.1016/j.isci.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M., Cui L., Cao Y., Bard A. Mechanoelectrochemical catalysis of the effect of elastic strain on a platinum nanofilm for the ORR exerted by a shape memory alloy substrate. J. Am. Chem. Soc. 2015;137:7397–7403. doi: 10.1021/jacs.5b03034. [DOI] [PubMed] [Google Scholar]
- Fang Z., Peng L., Qian Y., Zhang X., Xie Y., Cha J.J., Yu G. Dual tuning of Ni-Co-A (A= P, Se, O) nanosheets by anion substitution and holey engineering for efficient hydrogen evolution. J. Am. Chem. Soc. 2018;140:5241–5247. doi: 10.1021/jacs.8b01548. [DOI] [PubMed] [Google Scholar]
- Fu T., Wang M., Cai W., Cui Y., Gao F., Peng L., Chen W., Ding W. Acid-resistant catalysis without use of noble metals: carbon nitride with underlying nickel. ACS Catal. 2014;4:2536–2543. [Google Scholar]
- Gasteiger H.A., Kocha S.S., Sompalli B., Wagner F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005;56:9–35. [Google Scholar]
- Greeley J., Stephens I.E.L., Bondarenko A.S., Johansson T.P., Hansen H.A., Jaramillo T.F., Rossmeisl J., Chorkendorff I., Nørskov J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009;1:552–556. doi: 10.1038/nchem.367. [DOI] [PubMed] [Google Scholar]
- Han A., Chen W., Zhang S., Zhang M., Han Y., Zhang J., Ji S., Zhang L., Wang Y., Gu L. A polymer encapsulation strategy to synthesize porous nitrogen-doped carbon-nanosphere-supported metal isolated-single-atomic-site catalysts. Adv. Mater. 2018;30:1706508. doi: 10.1002/adma.201706508. [DOI] [PubMed] [Google Scholar]
- He J., Chen Y., Manthiram A. MOF-derived cobalt sulfide grown on 3D graphene foam as an efficient sulfur host for long-life lithium-sulfur batteries. iScience. 2018;4:36–43. doi: 10.1016/j.isci.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoster H., Alves O., Koper M. Tuning adsorption via strain and vertical ligand effects. ChemPhysChem. 2010;11:1518–1524. doi: 10.1002/cphc.200900500. [DOI] [PubMed] [Google Scholar]
- Igarashi H., Fujino T., Zhu Y., Uchida H., Watanabe M. CO tolerance of Pt alloy electrocatalysts for polymer electrolyte fuel cells and the detoxification mechanism. Phys. Chem. Chem. Phys. 2001;3:306–314. [Google Scholar]
- Jiang K., Zhao D., Guo S., Zhang X., Zhu X., Guo J., Lu G., Huang X. Efficient oxygen reduction catalysis by subnanometer Pt alloy nanowires. Sci. Adv. 2017;3:e1601705. doi: 10.1126/sciadv.1601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Uemura K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 2005;34:109–119. doi: 10.1039/b313997m. [DOI] [PubMed] [Google Scholar]
- Lai J., Huang B., Tang Y., Lin F., Zhou P., Chen X., Sun Y., Lv F., Guo S. Barrier-free interface electron transfer on PtFe-Fe2C janus-like nanoparticles boosts oxygen catalysis. Chem. 2018;4:1153–1166. [Google Scholar]
- Lee H., Dellatore S., Miller W., Messersmith P. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xi Z., Pan Y.T., Spendelow J.S., Duchesne P.N., Su D., Li Q., Yu C., Yin Z., Shen B. Fe stabilization by intermetallic L10-FePt and Pt catalysis enhancement in L10-FePt/Pt nanoparticles for efficient oxygen reduction reaction in fuel cells. J. Am. Chem. Soc. 2018;140:2926–2932. doi: 10.1021/jacs.7b12829. [DOI] [PubMed] [Google Scholar]
- Li J., Sharma S., Liu X., Pan Y.T., Spendelow J.S., Chi M., Jia Y., Zhang P., Cullen D.A., Xi Z. Hard-magnet L10-CoPt nanoparticles advance fuel cell catalysis. Joule. 2018;3:1–12. [Google Scholar]
- Li L., Wong S.S. Ultrathin metallic nanowire-based architectures as high-performing electrocatalysts. ACS Omega. 2018;3:3294–3313. doi: 10.1021/acsomega.8b00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Liu Y., Ai Y., Li J., Zhou J., Fan Z., Bao H., Jiang R., Hu Z., Wang J. Pd-CuFe catalyst for transfer hydrogenation of Nitriles: controllable selectivity to primary amines and secondary amines. iScience. 2018;8:61–73. doi: 10.1016/j.isci.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Qi J., Liu M., Zhang S., Fan Q., Liu H., Liu K., Zheng H., Yin Y., Gao C. Aqueous synthesis of ultrathin platinum/non-noble metal alloy nanowires for enhanced hydrogen evolution activity. Angew. Chem. Int. Ed. 2018;130:11852–11856. doi: 10.1002/anie.201806194. [DOI] [PubMed] [Google Scholar]
- Lv C., Qian Y., Yan C., Ding Y., Liu Y., Chen G., Yu G. Defect engineering metal-free polymeric carbon nitride electrocatalyst for effective nitrogen fixation under ambient conditions. Angew. Chem. Int. Ed. 2018;57:10246–10250. doi: 10.1002/anie.201806386. [DOI] [PubMed] [Google Scholar]
- Mo R., Rooney D., Sun K. Uniform yolk-shell germanium@ polypyrrole architecture with precision expansion void control for high-performance lithium ion batteries. iScience. 2018;9:521–531. doi: 10.1016/j.isci.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z., Yang H. Designer platinum nanoparticles: control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today. 2009;4:143–164. [Google Scholar]
- Rudi S., Teschner D., Beermann V., Hetaba W., Gan L., Cui C., Gliech M., Schlögl R., Strasser P. pH-induced versus oxygen-induced surface enrichment and segregation effects in Pt-Ni alloy nanoparticle fuel cell catalysts. ACS Catal. 2017;7:6376–6384. [Google Scholar]
- Snyder J., Livi K., Erlebacher J. Oxygen reduction reaction performance of [MTBD][beti]-encapsulated nanoporous NiPt alloy nanoparticles. Adv. Funct. Mater. 2013;23:5494–5501. [Google Scholar]
- Stamenkovic V., Mun B.S., Mayrhofer K.J., Ross P.N., Markovic N.M., Rossmeisl J., Greeley J., Nørskov J.K. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. Int. Ed. 2006;118:2963–2967. doi: 10.1002/anie.200504386. [DOI] [PubMed] [Google Scholar]
- Stamenkovic V.R., Fowler B., Mun B.S., Wang G., Ross P.N., Lucas C.A., Marković N.M. Improved oxygen reduction activity on Pt3Ni (111) via increased surface site availability. Science. 2007;315:493–497. doi: 10.1126/science.1135941. [DOI] [PubMed] [Google Scholar]
- Stephens I.E.L., Rossmeisl J., Chorkendorff I. Toward sustainable fuel cells. Science. 2016;354:1378–1379. doi: 10.1126/science.aal3303. [DOI] [PubMed] [Google Scholar]
- Stephens I., Bondarenko A., Grønbjerg U., Rossmeisl J., Chorkendorff I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 2012;5:6744–6762. [Google Scholar]
- Subramanian V., Wolf E.E., Kamat P.V. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 2004;126:4943–4950. doi: 10.1021/ja0315199. [DOI] [PubMed] [Google Scholar]
- Tao L., Yu D., Zhou J., Lu X., Yang Y., Gao F. Ultrathin wall (1 nm) and superlong Pt nanotubes with enhanced oxygen reduction reaction performance. Small. 2018;14:1704503. doi: 10.1002/smll.201704503. [DOI] [PubMed] [Google Scholar]
- Van der Vliet D., Wang C., Li D., Paulikas A.P., Greeley J., Rankin R.B., Strmcnik D., Tripkovic D., Markovic N.M., Stamenkovic V.R. Unique electrochemical adsorption properties of Pt-skin surfaces. Angew. Chem. Int. Ed. 2012;124:3193–3196. doi: 10.1002/anie.201107668. [DOI] [PubMed] [Google Scholar]
- Wang X., Figueroa-Cosme L., Yang X., Luo M., Liu J., Xie Z., Xia Y. Pt-based icosahedral nanocages: using a combination of {111} facets, twin defects, and ultrathin walls to greatly enhance their activity toward oxygen reduction. Nano Lett. 2016;16:1467–1471. doi: 10.1021/acs.nanolett.5b05140. [DOI] [PubMed] [Google Scholar]
- Wang Y.J., Zhao N., Fang B., Li H., Bi X.T., Wang H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 2015;115:3433–3467. doi: 10.1021/cr500519c. [DOI] [PubMed] [Google Scholar]
- Wang C., Chi M., Li D., Strmcnik D., van der Vliet D., Wang G., Komanicky V., Chang K.C., Paulikas A.P., Tripkovic D. Design and synthesis of bimetallic electrocatalyst with multilayered Pt-skin surfaces. J. Am. Chem. Soc. 2011;133:14396–14403. doi: 10.1021/ja2047655. [DOI] [PubMed] [Google Scholar]
- Zhang L., Roling L.T., Wang X., Vara M., Chi M., Liu J., Choi S., Park J., Herron J.A., Xie Z. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science. 2015;349:412–416. doi: 10.1126/science.aab0801. [DOI] [PubMed] [Google Scholar]
- Zhang G.R., Wolker T., Sandbeck D.J., Munoz M., Mayrhofer K.J., Cherevko S., Etzold B.J. Tuning the electrocatalytic performance of ionic liquid modified Pt catalysts for the oxygen reduction reaction via cationic chain engineering. ACS Catal. 2018;8:8244–8254. doi: 10.1021/acscatal.8b02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zu L., Kong B., Chen B., He H., Lan K., Liu Y., Yang J., Zhao D. Mesoporous TiO2/TiC@ C composite membranes with stable TiO2-C interface for robust lithium storage. iScience. 2018;3:149–160. doi: 10.1016/j.isci.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Wu Z., Zhou L., Zhang M., Li W., Tao K. Synthesis of a nano-nickel catalyst modified by ruthenium for hydrogenation and hydrodechlorination. Catal. Commun. 2008;9:2191–2194. [Google Scholar]
- Zhou Y., Neyerlin K., Olson T., Pylypenko S., Bult J., Dinh H., Gennett T., Shao Z., O'Hayre R. Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports. Energy Environ. Sci. 2010;3:1437–1446. [Google Scholar]
- Zhu Y., Peng L., Fang Z., Yan C., Zhang X., Yu G. Structural engineering of 2D nanomaterials for energy storage and catalysis. Adv. Mater. 2018;30:1706347. doi: 10.1002/adma.201706347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.