Summary
Exercise affects whole-body metabolism through adaptations to various tissues, including adipose tissue (AT). Recent studies investigated exercise-induced adaptations to AT, focusing on inguinal white adipose tissue (WAT), perigonadal WAT, and interscapular brown adipose tissue (iBAT). Although these AT depots play important roles in metabolism, they account for only ∼50% of the AT mass in a mouse. Here, we investigated the effects of 3 weeks of exercise training on all 14 AT depots. Exercise induced depot-specific effects in genes involved in mitochondrial activity, glucose metabolism, and fatty acid uptake and oxidation in each adipose tissue (AT) depot. These data demonstrate that exercise training results in unique responses in each AT depot; identifying the depot-specific adaptations to AT in response to exercise is essential to determine how AT contributes to the overall beneficial effect of exercise.
Subject Areas: Molecular Biology, Molecular Mechanism of Behavior, Cell Biology, Specialized Functions of Cells
Graphical Abstract
Highlights
-
•
This study investigates the effects of exercise on all adipose tissue (AT) depots
-
•
Exercise training induces unique metabolic changes to BAT, scWAT, and vWAT
-
•
Exercise training differentially affects each AT depot within BAT, scWAT, and vWAT
Molecular Biology; Molecular Mechanism of Behavior; Cell Biology; Specialized Functions of Cells
Introduction
AT consists of two primary tissue types, white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is composed of mature adipocytes containing a unilocular fat droplet and the stromal vascular fraction (SVF), which is composed of multiple cell types including progenitor cells and immune cells. WAT is primarily involved in functions of energy storage, hormone production, local tissue architecture, and immune function (Tran and Kahn, 2010). WAT can be broadly categorized into two different types: visceral WAT (vWAT) and subcutaneous WAT (scWAT). vWAT surrounds the internal organs, and an excess accumulation of vWAT has been associated with metabolic impairments (Tran and Kahn, 2010). scWAT is primarily found around the thighs and buttocks, and a predisposition for storage of lipids in this region over storage in vWAT has been linked with insulin sensitivity and a reduced risk of type 2 diabetes (Tran et al., 2008). This suggests that there are distinct physiological functions of these two classes of WAT.
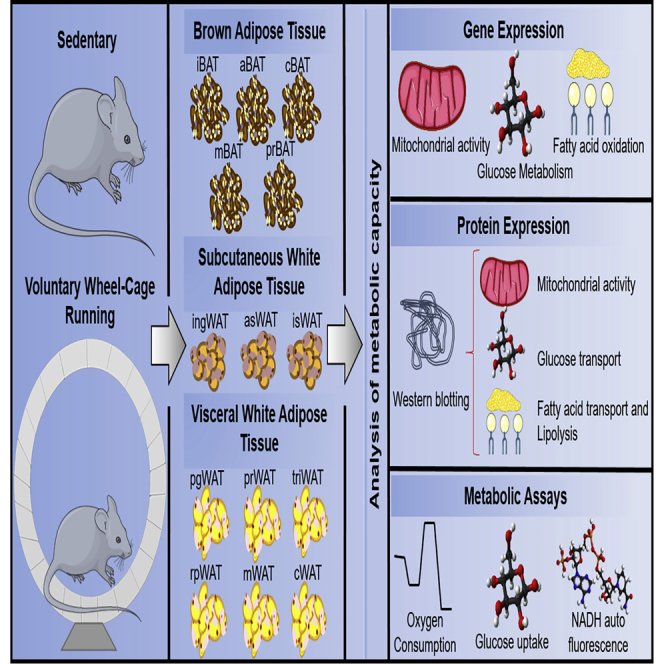
Although WAT is typically classified as vWAT or scWAT, these types of AT can be further classified based on the specific location of the AT depot. In the mouse, which has been widely studied, there are six vWAT depots: perigonadal WAT (pgWAT), mesenteric WAT (mWAT), perirenal WAT (prWAT), retroperitoneal WAT (rpWAT), cardiac WAT (cWAT), and triceps-associated WAT (triWAT). For scWAT, there are three major depots in the mouse: inguinal WAT (ingWAT), anterior subcutaneous WAT (asWAT), and interscapular WAT (isWAT). The function of each scWAT and vWAT depot, as well as how they respond to various stimuli, has not been thoroughly investigated.
BAT is composed of adipocytes that house multilocular fat droplets and an abundance of mitochondria. BAT is a thermogenic energy-expending tissue that helps to regulate body temperature (Rothwell and Stock, 1983). In the mouse there are five major BAT depots: interscapular BAT (iBAT), mediastinal BAT (mBAT), perirenal BAT (prBAT), axillary BAT (aBAT), and cervical BAT (cBAT). The function of each BAT depot has not been investigated. Combining all the types of AT, there are a total of 14 different AT depots in the body (3 scWAT, 6 vWAT, and 5 BAT). The anatomical distribution of these depots can be seen in Figure 1A.
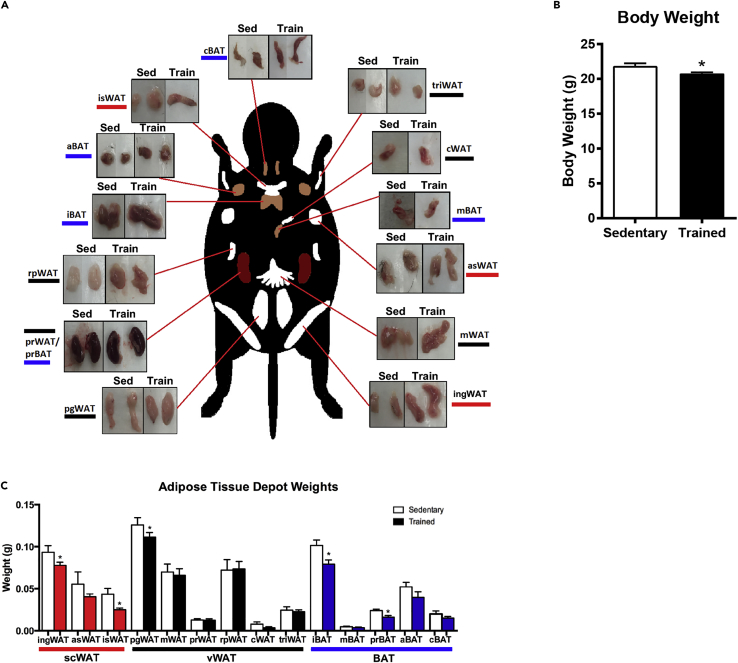
Figure 1.
Changes in Adipose Tissue Depot Mass After Chronic Exercise
(A) Images of each adipose tissue depot from sedentary and exercise-trained mice.
(B and C) (B) Body weight and (C) tissue weight of each adipose tissue depot from sedentary and exercise-trained mice. Data are presented as means ± SEM (n = 8/group; *p < 0.05).
Emerging evidence suggests that exercise results in major adaptations to AT, which play a role in the metabolic effects of exercise on health (Bostrom et al., 2012, Stallknecht et al., 1991, Stanford et al., 2015b, Stanford et al., 2015c, Stanford et al., 2018, Trevellin et al., 2014). Most studies of exercise have focused on the three most commonly studied AT depots in rodents; ingWAT, pgWAT, and iBAT. These investigations suggest that these major classes of AT have a varying response to exercise. Exercise training increases mitochondrial activity in pgWAT and ingWAT (Stallknecht et al., 1991, Stanford et al., 2015c, Sutherland et al., 2009, Trevellin et al., 2014, Wu et al., 2014). Other studies have shown that exercise causes a beiging, or an increased presence of brown-like adipocytes, in ingWAT in rodents (Bostrom et al., 2012, Cao et al., 2011, Petrovic et al., 2010, Stanford et al., 2015c, Trevellin et al., 2014). We found that transplantation of exercise-trained ingWAT improves the glucose metabolism of recipient mice, whereas transplantation of exercise-trained pgWAT has no effect on the whole-body glucose metabolism (Stanford et al., 2015c). Studies investigating the effect of exercise on iBAT have resulted in conflicting findings showing both increases (Yoshioka et al., 1989) and decreases (Ignacio et al., 2012, Wu et al., 2014) in mitochondrial activity with exercise training. Taken together, these data suggest that ingWAT, pgWAT, and iBAT each have distinct responses to exercise training.
The effects of exercise training have only been studied in these three major AT depots, which account for only ∼50% of the AT mass in a mouse. In the current study, we examined the effects of exercise on the expression of genes involved in mitochondrial activity, beiging, glucose metabolism, and fatty acid oxidation in each of the 14 AT depots. We found that exercise induces specific adaptations to scWAT, vWAT, and BAT, and also induces depot-specific effects within each class of AT. These data indicate that each AT depot has a unique metabolic response to exercise training.
Results
Effects of Exercise Training on Body Mass and Adipose Tissue Mass
To determine the effect of 3 weeks of exercise training on each AT depot, mice were euthanized and 14 different AT depots were rapidly dissected from sedentary and exercise-trained mice (Figure 1A). Exercise training significantly decreased body mass in mice by ∼10% (Figure 1B). Exercise training decreased the mass of two scWAT adipose tissue depots (ingWAT and isWAT), one vWAT depot (pgWAT), and two BAT depots (iBAT and prBAT) (Figure 1C). There was no effect of exercise on the mass of the other depots.
Exercise Regulation of scWAT
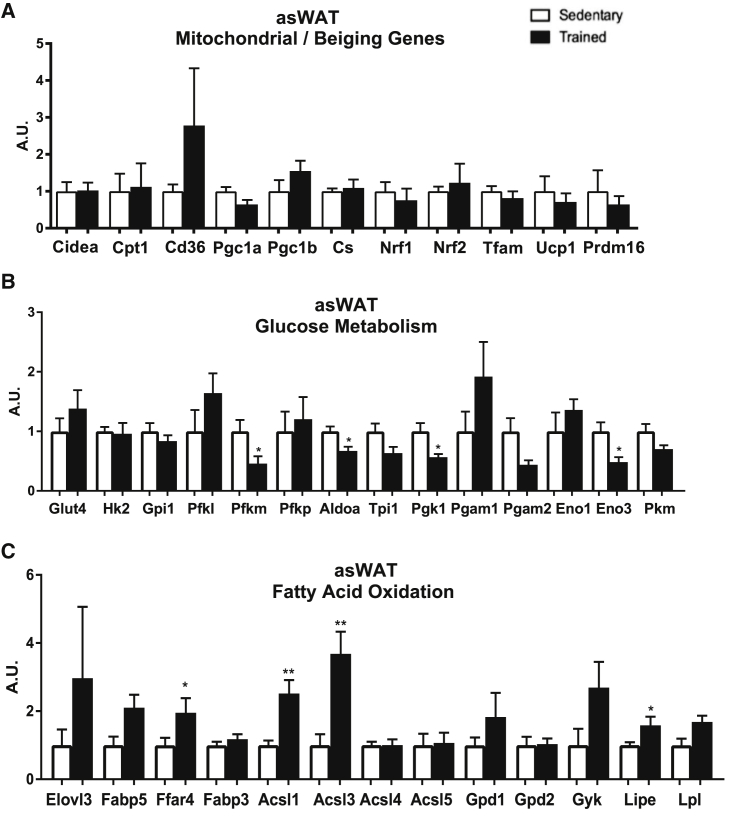
We determined the effects of exercise training on gene expression in the three distinct scWAT depots, asWAT, isWAT, and ingWAT (Figures 2, 3, and S1). For asWAT, there was an interesting response pattern to exercise training. Exercise training had no effect on the expression of mitochondrial or beiging marker genes (Figure 2A), decreased the expression of many genes involved in glucose metabolism (Figure 2B), and increased the expression in multiple genes involved in fatty acid oxidation and metabolism (Figure 2C). In contrast to asWAT, with the exception of a decrease in one gene involved in glucose metabolism (Eno1), there was no effect of exercise training on the expression profile for mitochondrial, beiging, glucose metabolism, and fat metabolism genes in isWAT (Figures S1A–S1C).
Figure 2.
Exercise Decreases Genes Involved in Glucose Metabolism and Increases Genes Involved in Fatty Acid Oxidation in asWAT
(A) Expression of genes involved in mitochondrial activity and beiging.
(B and C) (B) Glucose metabolism and (C) fatty acid oxidation in asWAT. Data are presented as means ± SEM (n = 8/group; *p < 0.05, **p < 0.01).
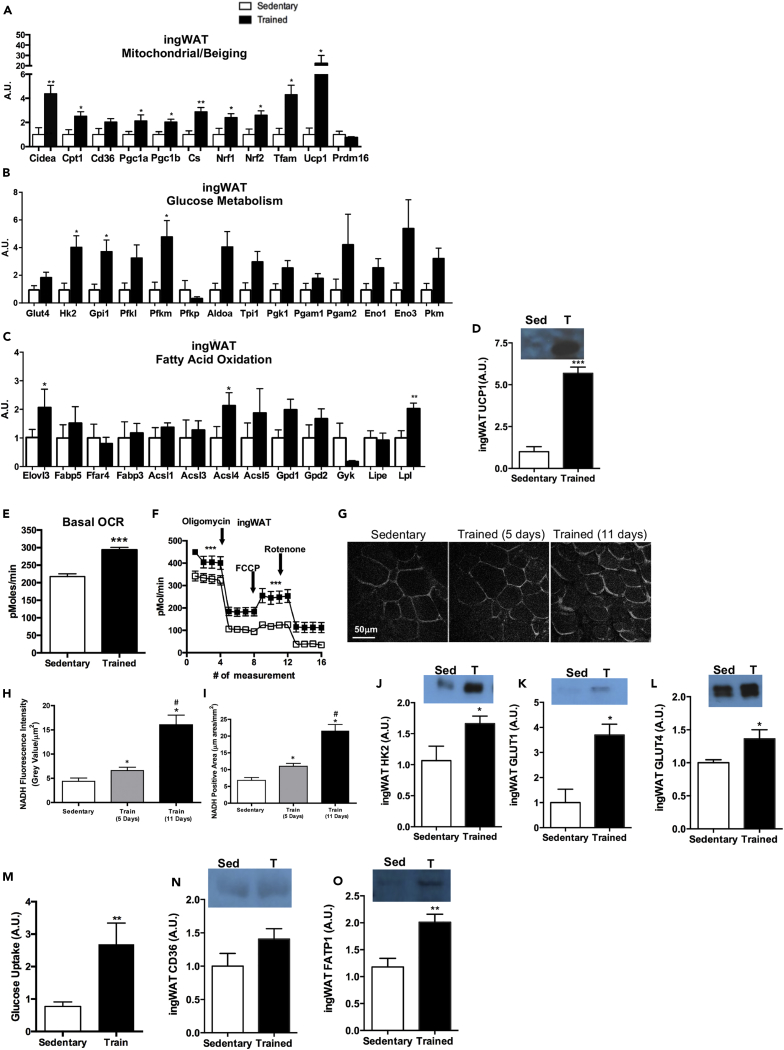
Figure 3.
Exercise Increases Mitochondrial Activity, Glucose Metabolism, and Genes Involved in Fatty Acid Oxidation in ingWAT
(A–D) (A) Expression of genes involved in mitochondrial activity and beiging, (B) glucose metabolism, and (C) fatty acid oxidation. (D) Protein expression of UCP1 in ingWAT. Data are presented as means ± SEM (n = 8/group; *p < 0.05, **p < 0.01, ***p < 0.001).
(E and F) (E) Basal OCR in adipocytes differentiated from sedentary and trained ingWAT and (F) bioenergetic profiles of adipocytes differentiated from sedentary and trained ingWAT. Data are presented as means ± SEM (n = 5/group; ***p < 0.001).
(G–I) (G) Images and (H) quantification of NADH autofluorescence and (I) NADH-positive area in ingWAT. Data are presented as means ± SEM (n = 3/group; *p < 0.05 compared with baseline; #p < 0.05 compared with day 5). Figure 3G: scale bar, 50μm.
(J–L) Protein expression of (J) HK2, (K) GLUT1, and (L) GLUT4 in ingWAT. Data are presented as means ± SEM (n = 8/group; *p < 0.05).
(M–O) (M) Glucose uptake in differentiated adipocytes isolated from sedentary and trained ingWAT. Data are presented as means ± SEM (n = 3/group; **p < 0.01). Protein expression of (N) CD36 and (O) FATP1 in ingWAT. Data are presented as means ± SEM (n = 8/group; **p < 0.01).
We found that the most robust effects of exercise training in the scWAT class occurred in the ingWAT depot. There were significant changes in numerous genes involved in mitochondrial function and beiging (Figure 3A) and glucose metabolism (Figure 3B), and a change in the expression of three genes involved in fatty acid oxidation (Figure 3C). Given the striking changes in this scWAT depot, we used this depot to determine functional adaptations that occur in response to exercise training. Consistent with gene expression changes, we found that exercise training increased UCP1 protein expression in ingWAT (Figure 3D). To determine if changes in mitochondrial gene expression resulted in changes in function, we used three approaches: expression of mitochondrial proteins, oxygen consumption via seahorse, and measurements of NADH autofluorescence in ingWAT. Oxygen consumption rate (OCR) was measured in adipocytes differentiated from the SVF of ingWAT. Exercise-trained ingWAT had a significantly higher basal OCR (Figures 3E and 3F), ATP turnover, and maximal respiratory capacity than sedentary ingWAT (Figure 3F). To determine the effects of exercise training on mitochondrial function in ingWAT in vivo, we measured NADH autofluorescence in mice pre-exercise, and on days 5 and 11 of a voluntary wheel running exercise-training regimen. The NADH-positive area and NADH autofluorescence were significantly increased after 5 days of exercise training in ingWAT. After 11 days of exercise training, the NADH-positive area and NADH autofluorescence doubled in size and intensity and was significantly increased in the ingWAT compared with sedentary mice and mice after 5 days of exercise (Figures 3G–3I).
To determine if changes in genes involved in glucose metabolism resulted in changes in function, we measured the expression of proteins involved in glucose metabolism in ingWAT and glucose uptake in SVF-derived adipocytes. Three weeks of exercise training significantly increased the protein expression of HK2, GLUT1, and GLUT4 in the ingWAT (Figures 3J–3L). Basal glucose uptake was significantly increased in adipocytes differentiated from the SVF of exercise-trained ingWAT compared with sedentary ingWAT (Figure 3M), indicating that exercise training increases glucose uptake and metabolism in SVF-derived adipocytes from ingWAT.
Exercise training significantly increased the expression of three genes involved in fatty acid oxidation in ingWAT (Figure 3C). To determine if exercise increased the expression of proteins involved in fatty acid uptake and oxidation we measured the proteins involved in fatty acid uptake, CD36 and FATP1, in ingWAT. Exercise training did not alter CD36 expression (Figure 3N), but did increase FATP1 in ingWAT (Figure 3O). Together, these data indicate that each scWAT depot responds differently to exercise and asWAT and ingWAT are the scWAT depots most sensitive to exercise-induced adaptations.
Variable Effects of Exercise on vWAT Depots
The effects of exercise training on gene expression were determined in the six vWAT depots (cWAT, rpWAT, triWAT, prWAT, mWAT, and pgWAT) (Figures 4, 5, 6, S2, and S3). For cWAT, exercise training had a minimal effect on the expression of mitochondrial genes, with the only gene increased being Nrf1. There was also no effect of exercise training on the expression of genes involved in glucose metabolism or fatty acid oxidation and uptake in cWAT (Figures S2A–S2C). In rpWAT, exercise training increased the expression of two genes involved in mitochondrial activity (Nrf1, Nrf2), decreased the expression of three genes involved in glucose metabolism (Aldoa, Tpl1, Pgam2), decreased the expression of one gene involved in fatty acid metabolism (Fabp3), and increased the expression of one gene involved in fatty acid metabolism (Acsl5) (Figures S2D–S2F).
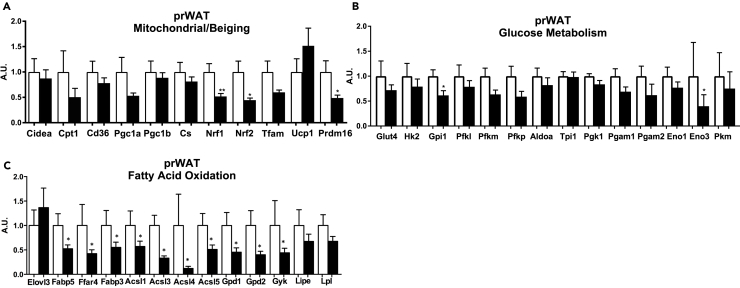
Figure 4.
Effects of Exercise on Gene Expression in prWAT
(A–C) (A) Expression of genes involved in mitochondrial activity and beiging, (B) glucose metabolism, and (C) fatty acid oxidation in prWAT.
Data are presented as means ± SEM (n = 8/group; *p < 0.05, **p < 0.01).
Figure 5.
Exercise Increases Genes Involved in Fatty Acid Oxidation in mWAT
(A–C) (A) Expression of genes involved in mitochondrial activity and beiging, (B) glucose metabolism, and (C) fatty acid oxidation in mWAT.
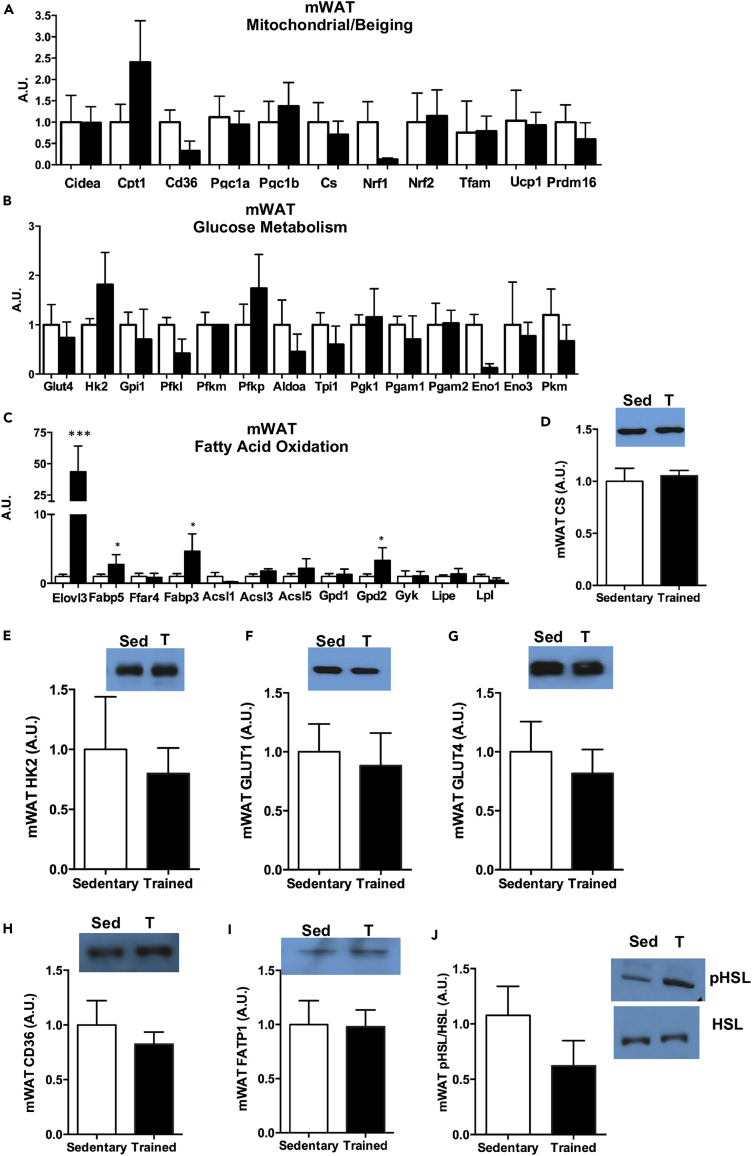
(D–J) Protein expression of (D) CS, (E) HK2, (F) GLUT1, (G) GLUT4, (H) CD36, (I) FATP1, and (J) pHSL/HSL in mWAT.
Data are presented as means ± SEM (n = 8/group; *p < 0.05, ***p < 0.001).
Figure 6.
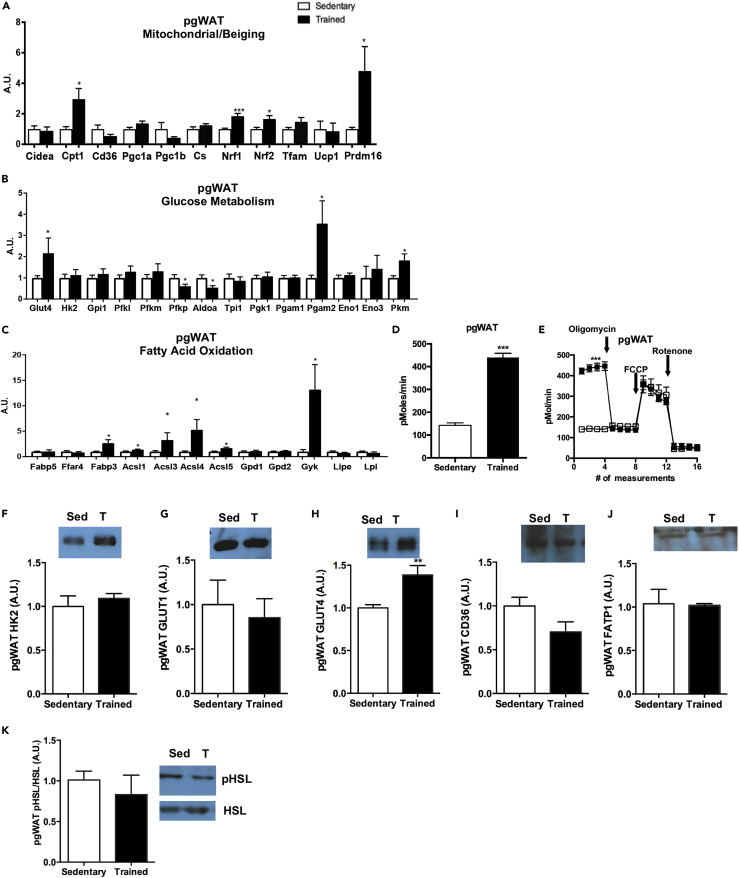
Exercise Increases Mitochondrial Activity and Genes Involved in Fatty Acid Oxidation in pgWAT
(A–C) (A) Expression of genes involved in mitochondrial activity and beiging, (B) glucose metabolism, and (C) fatty acid oxidation in pgWAT. Data are presented as means ± SEM (n = 8/group; *p < 0.05, ***p < 0.001).
(D and E) (D) Basal OCR in adipocytes differentiated from sedentary and trained pgWAT and (E) bioenergetic profiles of adipocytes differentiated from sedentary and trained pgWAT. Data are presented as means ± SEM (n = 5/group; ***p < 0.001).
(F–K) Protein expression of (F) HK2, (G) GLUT1, (H) GLUT4, (I) CD36, (J) FATP1, and (K) pHSL/HSL in pgWAT. Data are presented as means ± SEM (n = 8/group; **p < 0.01). Data are presented as means ± SEM (n = 8/group).
triWAT is the vWAT depot closest to the triceps skeletal muscle, a muscle that is highly recruited with numerous adaptations in response to wheel running exercise (Belter et al., 2004, Hokari et al., 2010, Stanford et al., 2015a). Although we hypothesized that the AT depot closest in anatomical proximity to the working muscle would be significantly affected by exercise training, there was no effect of training on the expression of genes involved in mitochondrial activity, beiging, glucose metabolism, or fatty acid oxidation in triWAT (Figures S3A–S3C). Similar to gene expression data, exercise did not alter citrate synthase (CS), HK2, GLUT1, GLUT4, CD36, FATP1, or pHSL/HSL protein expression in triWAT (Figures S3D–S3J). These data suggest that the primary function of the triWAT may not be to provide fuel to the nearby muscle in response to exercise.
The three vWAT depots that were the most affected by exercise were prWAT, mWAT, and pgWAT. Interestingly, these three classes of vWAT each responded to exercise very differently. Exercise training in prWAT decreased the expression of 3 genes involved in mitochondrial activity (Nrf1, Nrf2, Prdm16), 2 genes involved in glucose metabolism (Gpi1, Eno3), and 10 genes involved in fatty acid oxidation (Fabp5, Ffar4, Fabp3, Acsl1, Acsl3, Acsl4, Acsl5, Gpd1, Gpd2, Gyk) (Figures 4A–4C). This depot was the only AT depot that did not have any increase in gene expression in response to exercise training. For mWAT, the AT depot considered to be the most similar to human visceral WAT (Catalano et al., 2010, Chusyd et al., 2016), there was no effect of exercise on altering the expression of mitochondrial or beiging genes (Figure 5A). There was also no exercise-training-induced change in the expression of genes involved in glucose metabolism (Figure 5B). The expression of genes involved in fatty acid oxidation and elongation were significantly increased in mWAT after exercise training (Elovl3, Fabp5, Fabp3, Gpd2) (Figure 5C). Similar to gene expression data, there was no change in CS, HK2, GLUT1, or GLUT4 protein expression (Figures 5D–5G). There was no change in protein expression of fatty acid transporters CD36 or FATP1, or indicators of lipolysis pHSL or HSL (Figures 5H–5J).
The strongest effects of exercise training in the vWAT depots were in the pgWAT depot. Exercise training increased the expression of several mitochondrial and beiging genes (Cpt1, Nrf1, Nrf2, Prdm16), but had no effect on Ucp1 or Cidea in pgWAT (Figure 6A). Unique from other AT depots, we found that exercise training had opposing effects on genes involved in glucose metabolism in pgWAT, with some genes increased by training (Glut4, Pgam2, Pkm) and others decreased (Pfkp, Aldoa) (Figure 6B). Exercise training increased the expression of genes involved in fatty acid oxidation and uptake in pgWAT (Fabp3, Acsl1, Acsl3, Acsl4, Acsl5, Gyk) (Figure 6C).
To determine if exercise training altered mitochondrial function in pgWAT, OCR was measured in adipocytes differentiated from the SVF of pgWAT. Exercise training resulted in a significantly higher basal OCR compared with SVF-derived adipocytes from sedentary mice (Figures 6D and 6E), but there was no difference in ATP turnover or maximal respiratory capacity when comparing trained versus sedentary SVF-derived adipocytes from pgWAT (Figure 6E). Exercise training had no effect on the HK2 or GLUT1 protein, but significantly increased GLUT4 (Figures 6F–6H) in pgWAT. Exercise training did not affect proteins involved in fatty acid uptake (CD36, FATP1) or lipolysis (pHSL, HSL) (Figures 6I–6K). Together these data indicate that exercise training increases basal mitochondrial activity and GLUT4 in pgWAT, but has little effect on genes or proteins involved in fatty acid metabolism.
Exercise Decreases Mitochondrial Activity and Glucose Uptake in iBAT
We determined the effects of exercise training on gene expression in the five distinct BAT depots (mBAT, prBAT, aBAT, cBAT, and iBAT) (Figures 7 and S4). Exercise training had a minimal effect on the expression of genes involved in mitochondrial activity, glucose metabolism, or fatty acid oxidation and metabolism in mBAT (Figures S4A–S4C), prBAT (Figures S4D–S4F), or aBAT (Figures S4G–S4I). For cBAT, we found that there was a minimal effect of exercise training on the expression of mitochondrial or beiging markers and genes involved in glucose metabolism (Figures S4J and S4K), but an increase in several genes involved in fatty acid oxidation in cBAT (Ffar4, Fabp3, Acsl1, Gpd1, Gyk) (Figure S4L).
Figure 7.
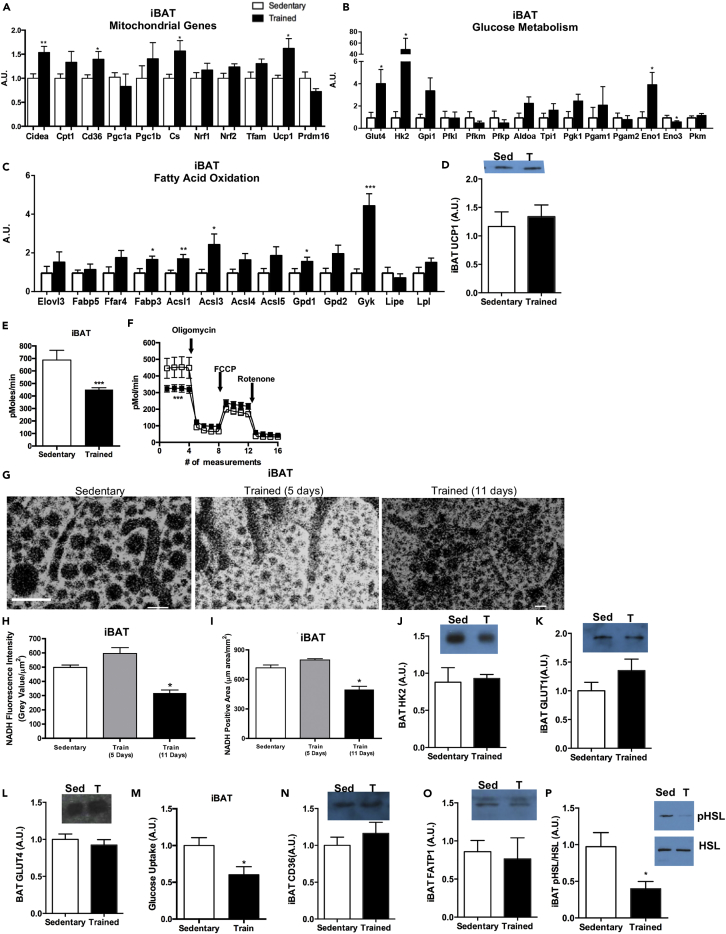
Exercise Decreases Mitochondrial Activity and Glucose Metabolism, and Increases Genes Involved in Fatty Acid Oxidation in iBAT
(A–D) (A) Expression of genes involved in mitochondrial activity and beiging, (B) glucose metabolism, and (C) fatty acid oxidation in iBAT. (D) Protein expression of UCP1 in iBAT. Data are presented as means ± SEM (n = 8/group; *p < 0.05, **p < 0.01).
(E and F) (E) Basal OCR in adipocytes differentiated from sedentary and trained iBAT and (F) bioenergetic profiles of adipocytes differentiated from sedentary and trained iBAT. Data are presented as means ± SEM (n = 5/group; ***p < 0.001).
(G–L) (G–I) (G) Images and (H) quantification of NADH autofluorescence and (I) NADH-positive area in iBAT. Data are presented as means ± SEM (n = 3/group; *p < 0.05). Protein expression of (J) HK2, (K) GLUT1, and (L) GLUT4 in iBAT. Data are presented as means ± SEM (n = 8/group; *p < 0.05). Figure 7G: scale bar, 50μm.
(M–P) (M) Glucose uptake in differentiated adipocytes isolated from sedentary and trained iBAT. Data are presented as means ± SEM (n = 3/group). Protein expression of (N) CD36, (O) FATP1, and (P) pHSL/HSL in iBAT. Data are presented as means ± SEM (n = 8/group; *p < 0.05).
The tissue most affected by exercise training in the BAT class was iBAT. Exercise increased the expression of multiple mitochondrial genes (Cidea, Cd36, Cs, Ucp1), genes involved in glucose metabolism (Glut4, Hk2, Eno1, Eno3), and those involved in fatty acid oxidation (Fabp3, Acsl1, Acsl3, Gpd1, Gyk) in iBAT (Figures 7A–7C). There was no effect of exercise training on UCP1 protein expression (Figure 7D). To determine if changes in mitochondrial gene expression resulted in changes in function, we used three approaches: expression of mitochondrial proteins, oxygen consumption via seahorse, and measurements of NADH autofluorescence in iBAT. OCR was measured in adipocytes differentiated from the SVF, and SVF-derived adipocytes from exercise-trained iBAT had a significantly lower OCR compared with sedentary mice (Figures 7E and 7F), but no difference in ATP turnover or maximal respiratory capacity (Figure 7F). These data suggest that exercise decreases the basal OCR of iBAT, whereas it does not decrease its maximal respiratory capacity or alter its ability to be recruited or influenced by various stimuli. To determine the effects of exercise on iBAT in vivo, we measured NADH autofluorescence in mice pre-exercise, and on days 5 and 11 of a voluntary wheel running. Interestingly, after 5 days of exercise training, the NADH-positive area and NADH autofluorescence tended to be increased compared with the sedentary mice, whereas after 11 days of exercise training the NADH-positive area and NADH autofluorescence were significantly decreased compared with both the pre-exercise and 5 days of exercise-training values. These data suggest that short-term exercise training (5 days) might acutely increase mitochondrial activity, but 11 days of exercise training decreases the basal mitochondrial activity of iBAT (Figures 7G–7I).
Exercise training increased genes involved in glucose metabolism in iBAT (Figure 7B), but had no effect on protein expression of HK2, GLUT1, or GLUT4 (Figures 7J–7L). Basal glucose uptake was significantly decreased in adipocytes differentiated from the SVF of exercise-trained iBAT compared with sedentary iBAT (Figure 7M). Interestingly, and similar to what has been observed in human subjects, these data indicate that exercise training decreases basal glucose uptake in iBAT (Motiani et al., 2017, Vosselman et al., 2015). Expression of genes involved in fatty acid oxidation was increased after exercise training in iBAT (Figure 7C), but proteins involved in fatty acid transport (CD36 and FATP1) were not changed (Figures 7N and 7O). There was a significant decrease in the pHSL/HSL ratio, indicating a decrease in lipolysis (Figure 7P).
Discussion
The beneficial effects of exercise on metabolic health have typically been attributed to adaptations to skeletal muscle, but recent studies have demonstrated that exercise training results in marked adaptations to AT, including dramatic changes in the gene expression profile (Stanford et al., 2015c, Trevellin et al., 2014). Most studies of exercise training have focused on a select few AT depots, whereas this dataset provides a comprehensive analysis of the effects of exercise on the gene expression profile of 14 distinct AT depots in mice. These data demonstrate that training results in very distinct depot-specific adaptations. Even within a single class of AT, the various depots responded quite differently to the exercise-training stimulus. This suggests that the different AT depots function differently to the metabolic demands of regular physical training.
Comparison of the three scWAT depots suggests a very different response to training for each depot. Surprisingly, we did not detect any change in gene expression in isWAT in response to exercise training, although exercise training resulted in a decrease in the mass of this tissue. The other scWAT depots, ingWAT and asWAT, were very responsive to exercise training, albeit very differently. Exercise training in ingWAT significantly increased mitochondrial, beiging, and glucose metabolic markers genes, with little effect on fatty acid metabolism genes. In remarkable contrast to ingWAT, exercise training in asWAT resulted in no change in mitochondrial and beiging genes, decreases in glucose metabolic genes, and increases in fatty acid metabolic genes. The opposing responses may stem from the very different anatomical locations of these depots, with asWAT located between the scapulae descending from the neck to the axillae and the ingWAT spreading from the dorsolumbar region to the gluteal region (Cinti, 2001). These unique locations may lead to differing blood flow responses to exercise, which may also translate to the AT depots supplying fatty acids to different surrounding tissues. These anatomical locations, varying blood flow, and prominence to surrounding tissues may also play a role in the functions of the AT depots across classes. For example, initially we hypothesized that the increases in mitochondrial function and glucose metabolism were prominent in the ingWAT because of its close proximity to the working muscle during exercise. However, when we examined other adipose depots such as the triWAT, there were no changes in these metabolic genes. This suggests that anatomical contiguity to working muscle may not be a primary factor in the response to exercise training.
Multiple studies have indicated an important role of ingWAT in response to exercise (Bostrom et al., 2012, Cao et al., 2011, Stanford et al., 2015b, Stanford et al., 2015c, Trevellin et al., 2014), whereas one recent study indicated that ingWAT could be dispensable for the metabolic health benefits of mice after exercise. Mice that had ingWAT surgically removed still exhibited whole-body metabolic benefits of exercise, including improved glucose tolerance and increased skeletal muscle mitochondrial gene expression (Peppler et al., 2018). These data are intriguing, but it is unclear if there were adaptations to the other scWAT depots that occurred in the absence of ingWAT that may have contributed to the metabolic benefit, particularly because the ingWAT accounts for only ∼30% of the total fat mass in a mouse. It is also difficult to determine how to interpret these data in a clinical setting because a human subject would likely not have a comparable complete loss of ingWAT mass and exercise-induced adaptations to ingWAT are observed in human studies (Stanford et al., 2015b).
Exercise training in rodents increases mitochondrial activity in pgWAT and ingWAT (Stallknecht et al., 1991, Stanford et al., 2015c, Sutherland et al., 2009, Trevellin et al., 2014, Wu et al., 2014) and causes a beiging of ingWAT (Bostrom et al., 2012, Cao et al., 2011, Petrovic et al., 2010, Stanford et al., 2015c, Trevellin et al., 2014). In rodents, beige cells can be induced by a variety of stimuli including β3-selective adrenergic agonists (Ishibashi and Seale, 2010), cold exposure (Petrovic et al., 2010), exercise (Bostrom et al., 2012, Cao et al., 2011, Stanford et al., 2015b, Stanford et al., 2015c, Sutherland et al., 2009, Trevellin et al., 2014), and an enriched environment (Cao et al., 2011). Upon stimulation, beige adipocytes have a high expression of Ucp1, have increased fuel oxidation, and contribute to non-shivering thermogenesis (Shabalina et al., 2013, Wu et al., 2012). The beiging of ingWAT by non-exercise stimuli such as cold exposure, environmental factors, or pharmaceuticals is believed to be induced through a heat-compensatory mechanism in which adrenergic stimulation results in the up-regulation of UCP1 to compensate for heat loss (Cannon and Nedergaard, 2004, Cousin et al., 1992, Ghorbani et al., 1997, Ghorbani and Himms-Hagen, 1997), but this mechanism does not make sense in the context of exercise-induced beiging, as exercise increases heat production (Saugen and Vøllestad, 1995). One explanation for the exercise-induced beiging of ingWAT is that exercise decreases adipocyte size and lipid content in ingWAT, thus decreasing insulation of the body and necessitating heat production, resulting in the beiging of ingWAT (Lehnig and Stanford, 2018, Nedergaard and Cannon, 2014, Stanford and Goodyear, 2016). This is a logical explanation because both ingWAT and isWAT decrease in mass after exercise. However, the exercise-induced beiging of WAT was only observed in the ingWAT, and not the other scWAT depots (asWAT, isWAT). It is possible that because this depot is the scWAT depot with the largest mass, a decrease in ingWAT mass would have the biggest effect on insulation, thus necessitating heat production. Interestingly, in studies looking at the effects of cold exposure on different AT depots (de Jong et al., 2015), some markers of beiging (Ucp1 and Cidea) were also increased in isWAT and asWAT, but other markers, such as Prdm16, were only increased in ingWAT (de Jong et al., 2015). These data suggest that ingWAT has the most significant beiging response after cold exposure. This same study showed that ingWAT and triWAT had a similar response to cold; both ingWAT and triWAT had an increase in Ucp1, Prdm16, and Cidea to nearly the same extent (de Jong et al., 2015). This similar response of ingWAT and triWAT to cold was not seen in response to exercise. Together these data indicate that whereas cold causes similar beiging adaptations to all the scWAT depots and triWAT, exercise induces a differential response to each AT depot.
Multiple studies have examined the effects of exercise on pgWAT and determined that exercise training induces mitochondrial activity (Stallknecht et al., 1991) and expression of GLUT4 and PGC-1α (Craig et al., 1981, Hirshman et al., 1989, Stallknecht et al., 1991, Sutherland et al., 2009). Our data support these findings, as we observed an increase in mitochondrial activity and GLUT4 protein expression. However, humans do not have a pgWAT depot; the most prominent vWAT depot in humans is the mWAT, but this depot is understudied in rodents. We determined that exercise training had a minimal effect on mWAT, with the exception of a 50-fold increase in the expression of Elovl3, an enzyme that regulates the recruitment and elongation of lipids (Westerberg et al., 2006). Although further studies will be necessary, it is possible that the training-induced increase in Elovl3 occurs to increase the de novo lipogenesis as a means to replace the lipids that are utilized during exercise (May et al., 2017).
The prWAT is a vWAT depot that had decreased expression of genes involved in mitochondrial activity, glucose metabolism, and fatty acid oxidation in response to exercise training. Previous studies in rodents indicated that prWAT behaved similarly to pgWAT in response to a high-fat diet (Takahashi and Ide, 2000) or growth factors such as fibroblast growth factor 21 (Fisher et al., 2012). However, our data reveal that pgWAT and prWAT have opposing gene expression responses to exercise training. The reason for the different response of prWAT to exercise training is unclear, but it is interesting to note that exercise training induces a distinct response in prWAT compared with other AT depots. This previously unidentified role for exercise to affect prWAT may indicate that this is an important tissue to examine in human subjects in response to exercise.
Previous studies investigating the effects of exercise on BAT in rodents have only examined iBAT (De Matteis et al., 2013, Ignacio et al., 2012, Motiani et al., 2017, Wu et al., 2014, Yoshioka et al., 1989). Studies on exercise in BAT have often conflicting; some studies show that exercise increases mitochondrial gene expression in BAT (Dewal and Stanford, 2018, Ignacio et al., 2012, Stanford and Goodyear, 2016, Yoshioka et al., 1989), whereas others indicate that exercise decreases mitochondrial activity in BAT (De Matteis et al., 2013, Motiani et al., 2017, Vosselman et al., 2015, Wu et al., 2014). Our data demonstrate that in mice, exercise increases the expression of mitochondrial genes in iBAT but decreases mitochondrial activity. It is not clear why expression of genes involved in mitochondrial activity is increased in iBAT, whereas mitochondrial activity itself is decreased. BAT is a thermogenic tissue involved in heat production and energy expenditure (Rothwell and Stock, 1983). Exercise is also a thermogenic activity, resulting in an increase in both muscle and core body temperature (Saugen and Vøllestad, 1995). As an increase in both BAT activity and exercise can raise the core body temperature, the thermogenic role of BAT might not be needed during exercise. In fact, it has been suggested that BAT is “hypoactive” during exercise (Cannon and Nedergaard, 2004, Dewal and Stanford, 2018). Although this argues against a role for BAT's activation by exercise, BAT is regulated by the sympathetic nervous system (SNS). Exercise also stimulates the SNS and corresponding catecholamine release. It is possible that exercise increases sympathetic input to increase mitochondrial activity, but there is a post-translational regulation in BAT that decreases mitochondrial and thermogenic activity because an increase in thermogenic activity would not be required during exercise at room temperature.
Recent studies have shown that exercise training decreases the ability of iBAT to take up glucose in humans (Motiani et al., 2017, Vosselman et al., 2015). Our data in mice also indicate that exercise training decreases BAT glucose uptake and that exercise decreases lipolysis in iBAT. Although glucose uptake is decreased with training, we find that another aspect of BAT metabolism is upregulated by exercise, that is, iBAT endocrine function. We found that exercise alters the endocrine function of iBAT mice, as both acute and chronic exercise increased the release of the signaling lipid 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME) (Stanford et al., 2018). Thus, whereas exercise training may down-regulate substrate utilization and energy expenditure in iBAT, other functions may be activated in this class of AT.
In conclusion, exercise training induces beneficial effects to AT, and each depot has a unique and specific response to this physiological stimulus. Defining the specific adaptation of each AT depot in response to exercise may help identify novel therapeutic targets to combat obesity and metabolic disease. Future studies will establish the detailed physiological function of each of these exercise-induced adaptations, and also help to understand the role that all these depots play in the metabolic response to exercise training in humans. Given the rapidly increasing prevalence of obesity and type 2 diabetes, as well as the detrimental consequences of a sedentary lifestyle on human health, exercise studies investigating the regulation of AT depots to combat obesity, type 2 diabetes, and other diseases will be critical.
Limitations of Study
An important limitation in this study is that only male mice were investigated, at one age and after a fixed period and type of exercise-training program. It is possible that female mice or mice of a different age would have a difference response to exercise, or that different training periods would elicit different responses in the AT depots. An additional limitation of this study is that gene expression was the only measurement performed in all tissues. Future work will investigate the functional adaptations of each AT depot in response to exercise.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by National Institutes of Health grants R01-HL138738 and K01-DK105109 (to K.I.S.), R01-DK099511 (to L.J.G.), and 5P30 DK36836 (Joslin Diabetes Center DRC). The authors thank Nathan Makarewicz for editorial contributions.
Author Contributions
A.C.L. and K.I.S. conceived and designed the study. A.C.L., R.S.D., L.A.B., K.M.K., V.R.M., P.J.A., F.J.M., and K.I.S. performed mouse experiments. K.I.S. performed cell culture experiments. H.P.M.M.L. performed in vivo microscopy experiments. A.C.L., R.S.D., L.A.B., K.M.K., V.R.M., P.J.A., and D.A.S. performed the gene expression and western blots. L.J.G. provided oversight for the in vitro and in vivo experiments. A.C.L., R.S.D., and K.I.S. interpreted the results, prepared figures, and completed statistical analysis. A.C.L., R.S.D., L.J.G., and K.I.S. wrote the manuscript. All authors approved the final version of the manuscript.
Declaration of Interests
The authors declare no conflict of interests.
Published: January 25, 2019
Footnotes
Supplemental Information includes Transparent Methods, four figures, and one table and can be found with this article online at https://doi.org/10.1016/j.isci.2018.12.033.
Supplemental Information
References
- Belter J.G., Carey H.V., Garland T., Jr. Effects of voluntary exercise and genetic selection for high activity levels on HSP72 expression in house mice. J. Appl. Physiol. (1985) 2004;96:1270–1276. doi: 10.1152/japplphysiol.00838.2003. [DOI] [PubMed] [Google Scholar]
- Bostrom P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C., Rasbach K.A., Bostrom E.A., Choi J.H., Long J.Z. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J.A.N. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cao L., Choi E.Y., Liu X., Martin A., Wang C., Xu X., During M.J. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano K.J., Stefanovski D., Bergman R.N. Critical role of the mesenteric depot versus other intra-abdominal adipose depots in the development of insulin resistance in young rats. Diabetes. 2010;59:1416–1423. doi: 10.2337/db08-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chusyd D.E., Wang D., Huffman D.M., Nagy T.R. Relationships between rodent white adipose fat pads and human white adipose fat depots. Front. Nutr. 2016;3:10. doi: 10.3389/fnut.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. The adipose organ: morphological perspectives of adipose tissues. Proc. Nutr. Soc. 2001;60:319–328. doi: 10.1079/pns200192. [DOI] [PubMed] [Google Scholar]
- Cousin B., Cinti S., Morroni M., Raimbault S., Ricquier D., Penicaud L., Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J. Cell Sci. 1992;103(Pt 4):931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- Craig B.W., Hammons G.T., Garthwaite S.M., Jarett L., Holloszy J.O. Adaptation of fat cells to exercise: response of glucose uptake and oxidation to insulin. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981;51:1500–1506. doi: 10.1152/jappl.1981.51.6.1500. [DOI] [PubMed] [Google Scholar]
- de Jong J.M., Larsson O., Cannon B., Nedergaard J. A stringent validation of mouse adipose tissue identity markers. Am. J. Physiol. Endocrinol. Metab. 2015;308:E1085–E1105. doi: 10.1152/ajpendo.00023.2015. [DOI] [PubMed] [Google Scholar]
- De Matteis R., Lucertini F., Guescini M., Polidori E., Zeppa S., Stocchi V., Cinti S., Cuppini R. Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr. Metab. Cardiovasc. Dis. 2013;23:582–590. doi: 10.1016/j.numecd.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Dewal R.S., Stanford K.I. Effects of exercise on brown and beige adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1864:71–78. doi: 10.1016/j.bbalip.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F., Wu J., Kharitonenkov A., Flier J.S., Maratos-Flier E. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani M., Claus T.H., Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem. Pharmacol. 1997;54:121–131. doi: 10.1016/s0006-2952(97)00162-7. [DOI] [PubMed] [Google Scholar]
- Ghorbani M., Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int. J. Obes. Relat. Metab. Disord. 1997;21:465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- Hirshman M.F., Wardzala L.J., Goodyear L.J., Fuller S.P., Horton E.D., Horton E.S. Exercise training increases the number of glucose transporters in rat adipose cells. Am. J. Physiol. 1989;257:E520–E530. doi: 10.1152/ajpendo.1989.257.4.E520. [DOI] [PubMed] [Google Scholar]
- Hokari F., Kawasaki E., Sakai A., Koshinaka K., Sakuma K., Kawanaka K. Muscle contractile activity regulates Sirt3 protein expression in rat skeletal muscles. J. Appl. Physiol. (1985) 2010;109:332–340. doi: 10.1152/japplphysiol.00335.2009. [DOI] [PubMed] [Google Scholar]
- Ignacio D.L., Fortunato R.S., Neto R.A., da Silva Silvestre D.H., Nigro M., Frankenfeld T.G., Werneck-de-Castro J.P., Carvalho D.P. Blunted response of pituitary type 1 and brown adipose tissue type 2 deiodinases to swimming training in ovariectomized rats. Horm. Metab. Res. 2012;44:797–803. doi: 10.1055/s-0032-1314875. [DOI] [PubMed] [Google Scholar]
- Ishibashi J., Seale P. Medicine. Beige can be slimming. Science. 2010;328:1113–1114. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnig A.C., Stanford K.I. Exercise induced adaptations to white and brown adipose tissue. J. Exp. Biol. 2018;221(Pt Suppl 1):1–8. doi: 10.1242/jeb.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May F.J., Baer L.A., Lehnig A.C., So K., Chen E.Y., Gao F., Narain N.R., Gushchina L., Rose A., Doseff A.I. Lipidomic adaptations in white and brown adipose tissue in response to exercise demonstrate molecular species-specific remodeling. Cell Rep. 2017;18:1558–1572. doi: 10.1016/j.celrep.2017.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiani P., Virtanen K.A., Motiani K.K., Eskelinen J.J., Middelbeek R.J., Goodyear L.J., Savolainen A.M., Kemppainen J., Jensen J., Din M.U. Decreased insulin-stimulated brown adipose tissue glucose uptake after short-term exercise training in healthy middle-aged men. Diabetes Obes. Metab. 2017;19:1379–1388. doi: 10.1111/dom.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J., Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Peppler W.T., Townsend L.K., Knuth C.M., Foster M.T., Wright D.C. Subcutaneous inguinal white adipose tissue is responsive to, but dispensable for, the metabolic health benefits of exercise. Am. J. Physiol. Endocrinol. Metab. 2018;314:e66–e77. doi: 10.1152/ajpendo.00226.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N., Walden T.B., Shabalina I.G., Timmons J.A., Cannon B., Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N.J., Stock M.J. Effects of age on diet-induced thermogenesis and brown adipose tissue metabolism in the rat. Int. J. Obes. 1983;7:583–589. [PubMed] [Google Scholar]
- Saugen E., Vøllestad N.K. Nonlinear relationship between heat production and force during voluntary contractions in humans. J. Appl. Physiol. (1985) 1995;79:2043–2049. doi: 10.1152/jappl.1995.79.6.2043. [DOI] [PubMed] [Google Scholar]
- Shabalina Irina G., Petrovic N., de Jong Jasper M.A., Kalinovich Anastasia V., Cannon B., Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Stallknecht B., Vinten J., Ploug T., Galbo H. Increased activities of mitochondrial enzymes in white adipose tissue in trained rats. Am. J. Physiol. 1991;261:E410–E414. doi: 10.1152/ajpendo.1991.261.3.E410. [DOI] [PubMed] [Google Scholar]
- Stanford K.I., Goodyear L.J. Exercise regulation of adipose tissue. Adipocyte. 2016;5:153–162. doi: 10.1080/21623945.2016.1191307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford K.I., Lee M.Y., Getchell K.M., So K., Hirshman M.F., Goodyear L.J. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes. 2015;64:427–433. doi: 10.2337/db13-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford K.I., Lynes M.D., Takahashi H., Baer L.A., Arts P.J., May F.J., Lehnig A.C., Middelbeek R.J.W., Richard J.J., So K. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab. 2018;27:1111–1120.e3. doi: 10.1016/j.cmet.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford K.I., Middelbeek R.J., Goodyear L.J. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes. 2015;64:2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford K.I., Middelbeek R.J., Townsend K.L., Lee M.Y., Takahashi H., So K., Hitchcox K.M., Markan K.R., Hellbach K., Hirshman M.F. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64:2002–2014. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland L.N., Bomhof M.R., Capozzi L.C., Basaraba S.A., Wright D.C. Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. J. Physiol. 2009;587:1607–1617. doi: 10.1113/jphysiol.2008.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Ide T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br. J. Nutr. 2000;84:175–184. [PubMed] [Google Scholar]
- Tran T.T., Kahn C.R. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat. Rev. Endocrinol. 2010;6:195–213. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T.T., Yamamoto Y., Gesta S., Kahn C.R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevellin E., Scorzeto M., Olivieri M., Granzotto M., Valerio A., Tedesco L., Fabris R., Serra R., Quarta M., Reggiani C. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes. 2014;63:2800–2811. doi: 10.2337/db13-1234. [DOI] [PubMed] [Google Scholar]
- Vosselman M.J., Hoeks J., Brans B., Pallubinsky H., Nascimento E.B., van der Lans A.A., Broeders E.P., Mottaghy F.M., Schrauwen P., van Marken Lichtenbelt W.D. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int. J. Obes. (Lond). 2015;39:1696–1702. doi: 10.1038/ijo.2015.130. [DOI] [PubMed] [Google Scholar]
- Westerberg R., Mansson J.E., Golozoubova V., Shabalina I.G., Backlund E.C., Tvrdik P., Retterstol K., Capecchi M.R., Jacobsson A. ELOVL3 is an important component for early onset of lipid recruitment in brown adipose tissue. J. Biol. Chem. 2006;281:4958–4968. doi: 10.1074/jbc.M511588200. [DOI] [PubMed] [Google Scholar]
- Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H., Khandekar M., Virtanen K.A., Nuutila P., Schaart G. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.V., Bikopoulos G., Hung S., Ceddia R.B. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole-body energy expenditure. J. Biol. Chem. 2014;289:34129–34140. doi: 10.1074/jbc.M114.591008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K., Yoshida T., Wakabayashi Y., Nishioka H., Kondo M. Effects of exercise training on brown adipose tissue thermogenesis in ovariectomized obese rats. Endocrinol. Jpn. 1989;36:403–408. doi: 10.1507/endocrj1954.36.403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.