Diagnosis of Mycobacterium leprae is often delayed in the United States due to lack of disease prevalence and awareness of symptoms. Pathologic examination via skin biopsy or slit smear bacilloscopy of a cutaneous lesion can aid in diagnosis. Polymerase chain reaction for M leprae DNA can definitively diagnose leprosy, as M leprae is not cultured and no serological test exists.1 We present a case of leprosy confounded with a false-positive amplified Mycobacterium tuberculosis direct (MTD) test that required interdepartmental contributions to definitively diagnose.
Case report
A 57-year-old Guyanese man presented with a year-long history of cutaneous and subcutaneous nodules on the arms and legs that rapidly multiplied within the previous 3 months (Fig 1). The patient denied nerve pain, tingling, burning, or paresthesia. Nodules were nontender and no peripheral nerve thickening was observed or palpated. On review of systems, he stated he had bilateral joint stiffness in the hands and bilateral redness of the eye not alleviated by artificial tears. His medical history was unremarkable. He last visited Guyana 9 months prior. Based on the clinical presentation, differential diagnoses included subcutaneous sarcoidosis, rheumatoid nodules, and possible hematologic malignancy.
Fig 1.
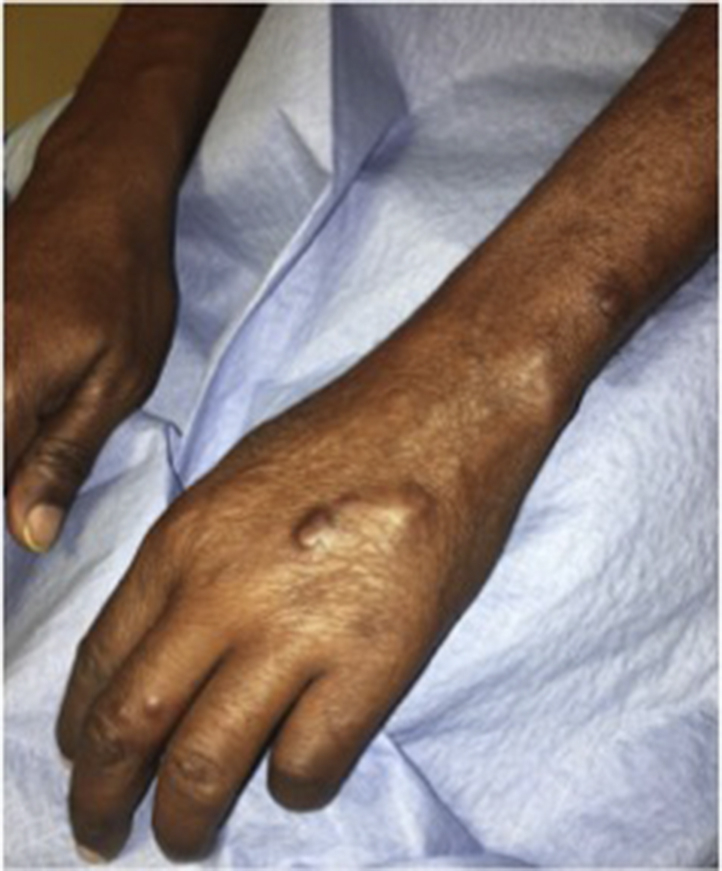
Cutaneous nodules on bilateral dorsal hands and forearms.
Punch biopsy found diffuse nodular dermal infiltrates of histiocytes with rare lymphocytes (Fig 2). Neurovascular bundles were encased with granulomas (Fig 3). Fite stain displayed innumerable bacilli (Fig 4). Periodic acid-Schiff stain was negative. The New York City Department of Health was notified of suspicion for leprosy, and the patient was started on a multidrug treatment. Tissue cultures were performed in house with no growth at 6 weeks. Concurrently, tissue was sent to the New York City Department of Health and to the National Hansen's Disease Laboratory. The Department of Health automatically performed amplified MTD testing on the tissue, the results of which were positive. Upon receiving these results, the pulmonary team immediately changed the patient's drug regimen to a multidrug tuberculosis treatment. Still favoring a diagnosis of leprosy, the departments of dermatology and infectious disease ordered additional tests. Chest computed tomography, induced sputum smears for acid-fast bacillus, tuberculosis skin testing, and QuantiFERON all returned negative. Therefore, the MTD test was deemed a false-positive. The National Hansen's Disease Laboratory reported a positive polymerase chain reaction for M leprae DNA. Borderline lepromatous leprosy was diagnosed, and the patient started on rifampin, clofazimine, and dapsone. Clinically obvious lesions resolved within 2 months.
Fig 2.
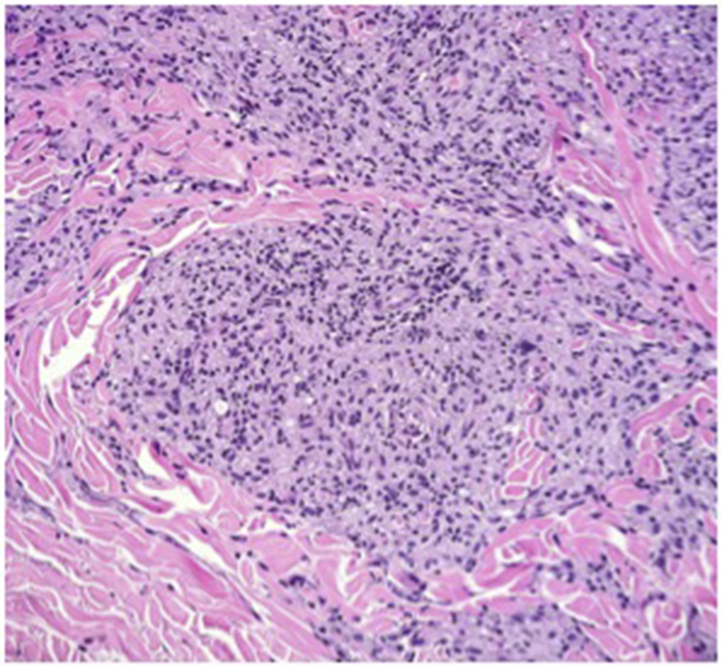
Histiocytes arranged in a nodular pattern. (Hematoxylin-eosin stain; original magnification: ×40.)
Fig 3.
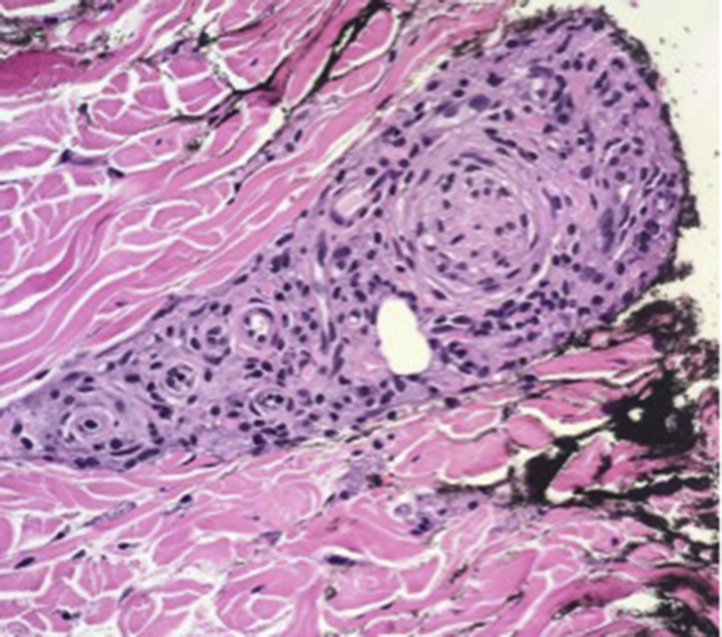
Neurovascular bundle with encased granulomas. (Hematoxylin-eosin stain; original magnification: ×100.)
Fig 4.
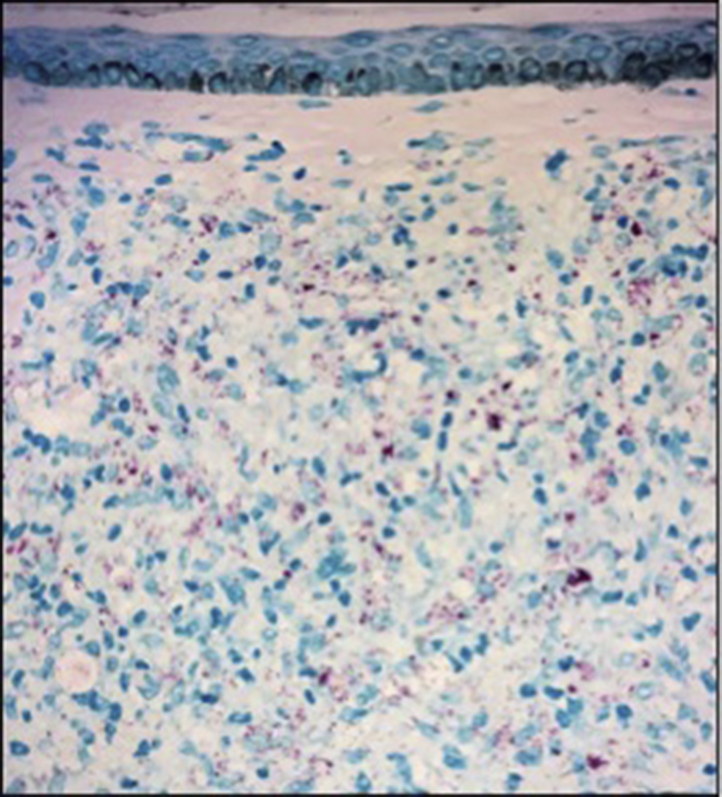
Innumerable acid-fast bacilli. (Fite stain; original magnification: ×40.)
Discussion
M leprae is an obligate intracellular bacillus that infiltrates the skin, peripheral nerves, nasal mucosa, and eyes. The predominance of skin and peripheral nerve involvement is caused by M leprae's ability to replicate best at temperatures of 32° to 34°C, the core temperature of human skin.2 M leprae is very slow growing, dividing every 13 days. Therefore, the incubation period between infection and the appearance of leprosy may be as long as 20 years.3
Clinically, our patient's skin lesions followed the distribution of M leprae infection; however, on physical examination, peripheral nerves were not palpable. Electroneuromyography or ultrasound scan could have been performed to determine whether there was measurable nerve involvement. M tuberculosis was considered in the differential diagnosis, as tuberculosis can uncommonly present with cutaneous manifestations including cutaneous nodules. Cutaneous tuberculosis lesions show a wide range of morphology; lupus vulgaris is a chronic form that may resemble tuberculoid leprosy. Lesions are soft brownish-red plaques with a predilection for the face and are generally asymptomatic. The nodules of leprosy are firmer and accompanied by signs of nerve involvement; peripheral sensory nerves are most commonly affected.
The M leprae bacillus is not easily cultivable, making cultures ineffective for diagnosis. A skin biopsy with an acid-fast stain such as Fite stain or Ziehl-Neelson stain can highlight the mycobacterium bacilli. In patients with visual or palpable nodules, especially on the ear, a slit smear for bacilloscopy can be performed. The gold standard of M leprae detection is to perform a polymerase chain reaction test. This is typically a send-out test, as few laboratories in the Unites States perform this examination, one of which is the National Hansen's Disease Clinical Center in Baton, Rouge, Louisiana.
In patients with suspicion for tuberculosis, amplified MTD testing can detect M tuberculosis RNA. Miyamoto et al4 reported an MTD test false-positive rate of 2% based on 400 clinical samples confirmed with direct microscopy and culture. Sato et al5 reported a sensitivity of 75.8% and a specificity of 93.8% for MTD testing based on 244 clinical specimens. Therefore, the false-positive MTD test result reported in this case is a relatively rare occurrence.
Once a diagnosis is made, paucibacillary leprosy (with no evidence of advanced disease on biopsy) is treated with daily dapsone plus rifampicin once a month. For multibacillary leprosy (with biopsy indicating advanced disease), daily clofazimine is added.3 If the diagnosis is uncertain, the patient should be treated for multibacillary leprosy.
This case highlights the importance of having a high degree of clinical suspicion for M leprae infection in a susceptible patient. It illustrates the steps necessary to workup and confirm the diagnosis of leprosy. Physicians should be aware of the possibility of a false-positive amplified MTD test and only order this test if tuberculosis is in the differential.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.National Hansen's Disease (Leprosy) Program. Health Resources & Services Administration; 2018. https://www.hrsa.gov/hansens-disease/index.html [Google Scholar]
- 2.Kumar V., Abbas A.K., Aster J.C., editors. Robbins and Cotran Pathologic Basis of Disease. Ninth edition. Elsevier/Saunders; Philadelphia, PA: 2015. [Google Scholar]
- 3.Essential Medicines and Health Products Information Portal. World Health Organization; 1998. apps.who.int/medicinedocs/en/d/Jh2988e/2.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyamoto J., Hashimoto A., Mizukane R. The clinical evaluation of MTD false-positive & false-negative results. Kekkaku. 1999;74(8):611–616. [PubMed] [Google Scholar]
- 5.Sato A., Sonobe T., Okazaki M., Umeda B. Evaluations of MTD and Amplicor Mycobacterium for direct detection of Mycobacteria from clinical specimensKekkaku. 1999;74(5):433–439. [in Japanese] [PubMed] [Google Scholar]


